ABSTRACT
The cellular hypoxic response contributes to cell transformation and tumor progression. Hypoxia-inducible factor 1 (HIF-1) is a key transcription factor that mediates transcription of genes whose products are essential for cellular adaptation to hypoxia. The activity of HIF-1 is largely regulated by the abundance of its alpha subunit (HIF-1α), which is primarily regulated by an oxygen-dependent and ubiquitin/proteasome-mediated degradation process. The HIF-1α protein level is also regulated by protein kinases through phosphorylation. Polo-like kinase 3 (Plk3) is a serine/threonine protein kinase with a tumor suppressive function. Plk3 phosphorylates and destabilizes HIF-1α. Plk3 also phosphorylates and stabilizes PTEN, a known regulator of HIF-1α stability via the PI3K pathway. Our latest study showed that the Plk3 protein is suppressed by hypoxia or nickel treatment via the ubiquitin/proteasome system. We discovered that Seven in Absentia Homologue 2 (SIAH2) is the E3 ubiquitin ligase of Plk3 and that Plk3 in turn destabilizes SIAH2. Given the role of SIAH2 in promoting stability of HIF-1α, our work reveals a novel mutual regulatory mechanism between Plk3 and SIAH2, which may function to fine-tune the cellular hypoxic response. Here we discuss the role of Plk3 in the hypoxic response and tumorigenesis in light of these latest findings.
KEYWORDS: Carcinogenesis, HIF-1α, hypoxia, nickel, Plk3, SIAH2, PTEN, tumor angiogenesis
The hypoxic response
The cellular response to hypoxia (the hypoxic response) is a complex intracellular signaling network that coordinates the biological activities in response to low oxygen tension.1,2 The central players of this response are hypoxia inducible factors (HIFs),1,2 with HIF-1 being the most important and best characterized.1,3 As a transcription factor, HIF-1 mediates expression of a group of HIF-response genes, such as the vascular endothelial growth factor (VEGF) and the glucose transporter GLUT1, by binding to the HIF response element (HRE) on the promoters of these genes.1-3 Expression of these genes triggers further signaling cascades leading to profound biological changes in the cell. These changes include metabolic alterations that stimulate angiogenesis and promote cell survival, which are required for adaption to hypoxia.1-3
The overall cellular activity of HIF-1 is primarily dictated by the abundance of its α subunit (HIF-1α), which is regulated mainly at the post-translational level.1-3 HIF-1α is inducible under hypoxia whereas the beta subunit (HIF-1β or ARNT) is constitutively expressed.1-3 Under normoxia, HIF-1α is degraded through oxygen-dependent hydroxylation and the ubiquitin proteasome system mediated by prolyl hydroxylases (PHDs) and Von Hippel-Lindau Factor (pVHL), respectively.1-3 Low oxygen tension reduces hydroxylation and slows degradation of HIF-1α, which lead to higher overall HIF-1 activity in the cell.1-3 The stability of HIF-1α is also regulated by protein kinases. Phosphorylation by a number of protein kinases, including ERKs, GSK3β, and Plk3, has been shown to alter HIF-1α stability and/or localization.4-9
The hypoxic response contributes to both tumorigenesis and tumor progression.3,10,11 An overactive hypoxic response pathway, often manifested as elevated cellular levels of HIF-1α, is a common feature of human cancers.3,11 This characteristic is essential for the survival of rapidly proliferating tumor cells that frequently face oxygen and/or nutrient restrictions due to increase in tumor masses. Elevated hypoxic responses promote survival of tumor cells by triggering angiogenesis which supplies nutrients and oxygen as well as by reprograming the cellular metabolism, all of which are essential for cellular adaptation to the hypoxic condition.1-3,11 Given the importance of the hypoxic response in cancer, inhibition of the hypoxic pathway and/or tumor angiogenesis is considered an important strategy of cancer therapy.12,13
Polo-like kinase 3
Polo-like kinase 3 (Plk3) is one of the 5 mammalian members (Plk1-5) of an evolutionarily conserved family of serine/threonine protein kinases, which share significant amino acid sequence homology.14-17 All Plks have a highly conserved kinase domain (KD) at the amino–terminus and a polo box domain (PBD) at the carboxyl-terminus.14-17 The KD of Plks confers catalytic activity whereas the PBD is important for their subcellular localization and the substrate recognition.15-17 Plk1 is the best studied member of the Plk family with a well-defined and critical role in cell cycle progression.15-17 Plk4 has an important role in centrosome dynamic during the cell cycle.14-17 Plk5 has a truncated KD rendering it kinase-deficient.14-19 Plk5 functions to suppress cell cycle progression, mediate neuron differentiation, and suppress glioblastoma.18,19 The expression of Plk5 appears to be restricted to the brain.15-19 The functions of Plk2 and Plk3 seem to be more diverse and not restricted to cell cycle progression.14-17,19 Importantly, all members of the Plk kinase family have close association with tumorigenesis and tumor progression.14-17,19-22
PLK3 is considered an immediate early response gene whose mRNA level is inducible by mitogenic stimulation.15,21 Interestingly, the level of Plk3 protein is quite constant in mitogen-stimulated cells throughout the cell cycle,15,21 although it has also been reported that the Plk3 protein level does oscillate during the cell cycle.23,24 The kinase activity of Plk3, on the other hand, appears to oscillate during the cell cycle and is regulated by a variety of stress conditions, including genotoxic insults, hypoxia, and osmotic stresses.25-28 The functional profile of Plk3 is apparently rather diverse. Earlier work indicates that Plk3 is involved in multiple phases of cell cycle progression, including G1/S transition, mitosis, DNA replication, Golgi fragmentation, and centrosomal functions.15,21 Later studies revealed additional functions of Plk3 in stress responses.8,25,27,28 Despite the functional significance of Plk3 discovered in various cell-based studies, PLK3 null mice are rather normal and fertile.9,29 However, these mice tend to be slightly larger and more prone to spontaneous tumor development later in the life.9 This is in sharp contrast with the embryonic lethal phenotypes as result of deletion of Plk1 or Plk4.30,31 The lack of a significant adverse phenotype suggests that cellular functions of Plk3, particularly those associated with cell cycle regulation, can be compensated by other members of the Plk family and therefore largely dispensable. Functional complementation studies by us and others show that both Plk3 and Plk1 are capable of rescuing CDC5 (Plk of budding yeast)-deficiency in budding yeast.32,33 It appears that divergent evolution eventually leads to new functions of Plk3 in higher animals despite its conserved functions in yeast. Thus, regulation of stress responses rather than normal cell cycle progression could be the primary function of Plk3 in mammals.
Plk3 expression is reduced in many human malignancies, including those in the lung, head and neck, colon, kidney, liver, stomach, and rectum.15,21 Expression of Plk3 mRNA and protein is also significantly deregulated in human melanoma cell lines and tissues.15 Plk3 mRNA was found to be significantly downregulated in a majority of more than a dozen human lung carcinoma samples, apparently as a result of reduced PLK3 transcription.34 These data suggest that reduced Plk3 expression may be associated with tumor development. This notion is supported by the observation that although polymorphisms were identified in 40 lung tumor cell lines, no missense or nonsense mutations were found in Plk3.35 These previous observations and the finding that PLK3 null mice are prone to developing tumors in several organs later in the life indicate a tumor suppressive role of Plk3 and that reduced expression is likely the main mechanism that associates Plk3 with increased tumorigenesis.
Regulation of HIF-1α by Plk3 through direct phosphorylation
The implication of Plk3 in the cellular hypoxic response was initially revealed in a genetic study showing that PLK3 null mice exhibited an increased tumor incidence later in the life and that the tumors developed in these mice were often larger and more vasculated than those from the wild type animals.9 Biochemical analysis showed that murine embryonic fibroblasts (MEFs) from PLK3 null mice express a much elevated level of HIF-1α in response to hypoxia or nickel, a hypoxia mimic.8,9 Furthermore, ectopically expressed Plk3 suppresses nuclear accumulation of HIF-1α in HeLa cells.9 Inhibition of HIF-1α nuclear translocation appears to be dependent on the kinase activity of Plk3 as overexpression of the Plk3 kinase domain was sufficient to suppress HIF-1α accumulation in the nucleus under hypoxic conditions.9 Consistently, expression of VEGF-A, a major HIF-1α response protein, was also higher in PLK3 null MEFs.9 These results suggest a possible direct regulation of HIF-1α by Plk3. Follow up studies using in vitro kinase assay in combination with mass spectrometry confirmed that Plk3 phosphorylates HIF-1α at two evolutionarily conserved serine residuals: Ser-576 and Ser-657.8 Ser-576 is located within the oxygen-dependent degradation domain (ODDD) whereas Ser-657 residues immediate downstream of the nuclear export signal (NES) of HIF-1α,8 suggesting that Plk3 may regulate degradation and nuclear export of HIF-1α. Further experimentation confirmed that phosphorylation of these residuals reduces the stability of HIF-1α in a hydroxylation- and pVHL-independent manner.8
Previous work demonstrated that ERK MAP kinases phosphorylate HIF-1α at residues Ser-641 and Ser-643 (both are within NES), through which promotes translocation of HIF-1α from the cytoplasm to the nucleus.6,7 Glycogen synthase kinase 3 β (GSK3β) phosphorylates HIF-1α at three serine residues (Ser-551, Ser-555, and Ser-589) located within ODDD,4 through which enhances HIF-1α degradation in a pVHL-independent manner.4,36 The discovery that Plk3 regulates HIF-1α added one more kinase to the short list of protein kinases that directly regulate HIF-1α through direct phosphorylation.
Regulation of HIF-1α by Plk3 through PTEN
Phosphatase and tensin homologue (PTEN) is an important tumor suppressor that inhibits the phosphatidylinositol 3-kinases kinase (PI3K) signaling pathway by dephosphorylating the phosphoinositides.37,38 Activation of the PI3K pathway leads to an elevated AKT activity.38 AKT may increase the HIF-1α protein level by activating mTOR or inhibiting GSK3β, which regulate the protein synthesis and stability of HIF-1α, respectively.4,12,39-41
PTEN can be phosphorylated by a number of kinases.42-49 Phosphorylation of PTEN can affect its activity and/or stability 42-52. The sites of phosphorylation on PTEN are concentrated at the C-terminal region of the protein,42-49 the regulatory domain of PTEN.42,53,54 Phosphorylation of PTEN by Plk3 was discovered based on the observation that PLK3 null MEFs exhibited reduced levels of the PTEN protein.43 In vitro kinase assays followed by mass spectrometry identified Thr-366 and Ser-370 at the C-terminal region of PTEN as the phosphorylation targets of Plk3.43 These two sites were further confirmed using a phospho-specific antibody that recognized p-Thr-366 and p-Ser-370.43 Phosphorylation of these two residues enhances the stability of PTEN, consistent with the reduced PTEN protein level in PLK3 null MEFs.43 Thus, phosphorylation of these two sites by Plk3 may stabilize PTEN and lead to an increased overall PTEN activity in the cell. Given the known effect of the PI3K pathway on HIF-1α stability and that PTEN is a negative regulator of the PI3K pathway, it is conceivable that Plk3 may affect HIF-1α stability indirectly through the PI3K signaling pathway.
Regulation of HIF-1α through mutual regulation between Plk3 and SIAH2
Our most recent work has added additional complexity to the regulation of HIF-1α/the hypoxic response by Plk3. A recent effort to understand the effects of hypoxia and nickel on Plk3 expression reveals that the Plk3 protein is suppressed by hypoxia or nickel through the ubiquitin proteasome system.55 Seven in Absentia Homologue 2 (SIAH2), a RING finger E3 ubiquitin ligase, was identified to catalyze ubiquitination of Plk3 and to promote Plk3 degradation.55 SIAH2 apparently interacts with Plk3 through two domains that closely resemble the consensus SIAH2 binding motif.55-57 One of these domains is located within the KD of Plk3 whereas the other one resides slightly N-terminal of the PBD.55 The domain near the PBD seems to be the main site for the interaction and Plk3 degradation.55 SIAH2 has been shown to be activated and induced by hypoxia and in turn mediates the ubiquitination and degradation of PHDs.58,59 SIAH2 appears to regulate Plk3 in a similar fashion. Given that both PHDs and Plk3 negatively regulate the stability of HIF-1α, SIAH2 may regulate HIF-1α via both PHDs and Plk3. More interestingly, Plk3 also destabilizes SIAH2 in a kinase activity-dependent manner.55 Thus, a mutual regulatory mechanism exists between Plk3 and SIAH2, which may functions to fine-tune the HIF-1 signaling.
USP28, a deubiquitinase that suppresses the stability of MYC and HIF-1α,36,60,61 also appears to indirectly contribute to suppression of Plk3 by hypoxia and nickel.55 It has been shown that USP28 can be suppressed by nickel via HIF-, the ubiquitin-proteasome system-, and DNA methylation-dependent mechanisms.62 USP28 prevents the suppression of Plk3 by nickel, suggests that suppression of Plk3 deubiquitination by USP28 in response to nickel could contribute to the elevated degradation of Plk3 by the ubiquitin-proteasome system. However, the effect of USP28 on Plk3 is likely indirect as a direct interaction between Plk3 and USP28 was not detected.55 Of note, although USP28 has been reported to mediate HIF-1α stability, a direct interaction between the two was also undetectable.36
Taken together, the newest findings reveal a novel mutual regulatory mechanism between Plk3 and SIAH2 and support a complex role of Plk3 in regulating the cellular hypoxic response through HIF-1α: under normoxia, Plk3 suppresses the hypoxic response by phosphorylating and destabilizing HIF-1α and SIAH2; under hypoxia or the hypoxia-like condition induced by nickel, the level and/or activity of SIAH2 increases and the level of USP28 decreases, which suppress the protein levels of Plk3 and PHDs; Reduced expression of Plk3 and PHDs in turn helps maintain HIF-1α and SIAH2 proteins at higher levels. This mutual regulatory network highlights a potentially important role of Plk3 in a signaling network that functions to fine-tune the cellular hypoxic response.
Implication of the regulatory network of Plk3, SIAH2, and HIF-1α in tumorigenesis
Evidence collected thus far has established a tumor suppressive role of Plk3 through a mechanism independent of its previously discovered functions on cell cycle regulation. Despite the observed functions of Plk3 in multiple biological processes associated with cell cycle progression at the cellular level, the PLK3 null mouse is largely normal.9 Discernable phenotypes of PLK3 null mice are the slightly larger sizes and the higher tendency of developing highly vasculated tumors in multiple organs.9 These phenotypes are consistent with the findings on the regulation of PTEN and HIF-1 pathways by Plk3 as these pathways are known to regulation cell growth, cell survival, and tumor angiogenesis.1-3,39,63 In vivo data also imply that many Plk3 functions described earlier based on molecular and cellular studies are largely dispensable for normal mouse physiology. This is likely a result of the functional redundancy of Plk3 with other members of the Plk family. However, the phenotypes of PLK3 null mice on tumor burden and the hypoxic response strongly suggest that Plk3 may suppress spontaneous tumorigenesis and tumorigenesis induced by carcinogens, particularly those mimicking hypoxia, such as nickel compounds and other metal carcinogens.
SIAH2 is considered an oncogene in multiple tissues, including the lung.59,64 Elevated expression of SIAH2 has been detected in lung cancers.64,65 SIAH2 promotes tumorigenesis through the Ras signaling pathway by targeting the Ras inhibitor Sprouty 2 for degradation as well as through the hypoxic response pathway.58,64 The finding that Plk3 destabilizes SIAH2 in a kinase activity-dependent manner highlights an additional mechanism underlying the role of Plk3 in tumorigenesis. In conclusion, recent studies have provided new mechanistic insights on how Plk3 may contribute to tumorigenesis and tumor progression (Figure 1).
Figure 1.
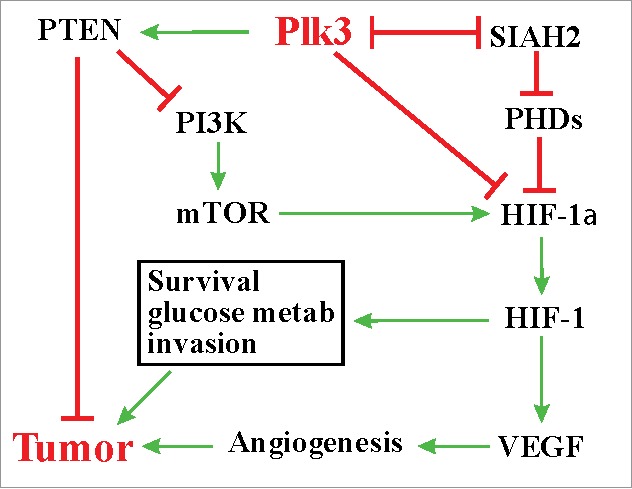
Plk3, hypoxic responses, and tumorigenesis.
Perspectives
Unlike Plk1, the prototype of the mammalian Plk kinase family, the biology of Plk3 and its role in tumor biology is much less studied and understood. Recent discoveries on the new functions of Plk3 in the HIF pathway have shed fresh light on the importance and mechanisms of this protein in tumorigenesis and tumor progression. While Plk1 has been viewed as an attractive target for cancer therapy based on its well define functions in cell cycle progression, the potential of Plk3 in this regard has not been fully appreciated. Given the role of Plk3 in the hypoxic response, it is conceivable that this protein kinase can be a very significant player in tumorigenesis and thus serve as a therapeutic target and/or tumor biomarker. Further studies on the biological significance and detailed mechanisms of Plk3 in regulating the hypoxic pathway and tumorigenesis, particularly in vivo, are highly warranted.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by National Institutes of Health Grant R21ES023862 (DX), a startup fund from NYMC (DX), and funds from the NYMC Castle-Krob Research Endowment Fund under the College's Intramural Research Support Program (DX).
References
- [1].Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393-402. doi: 10.1016/j.molcel.2008.04.009. PMID:18498744 [DOI] [PubMed] [Google Scholar]
- [2].Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614-23. doi: 10.1101/gad.1145503. PMID:14597660 [DOI] [PubMed] [Google Scholar]
- [3].Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551-78. doi: 10.1146/annurev.cellbio.15.1.551. PMID:10611972 [DOI] [PubMed] [Google Scholar]
- [4].Flugel D, Gorlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol. 2007;27:3253-65. doi: 10.1128/MCB.00015-07. PMID:17325032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274:32631-7. doi: 10.1074/jbc.274.46.32631. PMID:10551817 [DOI] [PubMed] [Google Scholar]
- [6].Mylonis I, Chachami G, Samiotaki M, Panayotou G, Paraskeva E, Kalousi A, Georgatsou E, Bonanou S, Simos G. Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1alpha. J Biol Chem. 2006;281:33095-106. doi: 10.1074/jbc.M605058200. PMID:16954218 [DOI] [PubMed] [Google Scholar]
- [7].Mylonis I, Chachami G, Paraskeva E, Simos G. Atypical CRM1-dependent nuclear export signal mediates regulation of hypoxia-inducible factor-1alpha by MAPK. J Biol Chem. 2008;283:27620-7. doi: 10.1074/jbc.M803081200. PMID:18687685 [DOI] [PubMed] [Google Scholar]
- [8].Xu D, Yao Y, Lu L, Costa M, Dai W. Plk3 functions as an essential component of the hypoxia regulatory pathway by direct phosphorylation of HIF-1alpha. J Biol Chem. 2010;285:38944-50. doi: 10.1074/jbc.M110.160325. PMID:20889502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang Y, Bai J, Shen R, Brown SA, Komissarova E, Huang Y, Jiang N, Alberts GF, Costa M, Lu L, et al. . Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008;68:4077-85. doi: 10.1158/0008-5472.CAN-07-6182. PMID:18519666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47. doi: 10.1038/nrc704. PMID:11902584 [DOI] [PubMed] [Google Scholar]
- [11].Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71-103. doi: 10.1080/10409230091169186. PMID:10821478 [DOI] [PubMed] [Google Scholar]
- [12].Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-32. doi: 10.1038/nrc1187. PMID:13130303 [DOI] [PubMed] [Google Scholar]
- [13].Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207-14. doi: 10.1016/j.tips.2012.01.005. PMID:22398146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265-75. doi: 10.1038/nrm2653. PMID:19305416 [DOI] [PubMed] [Google Scholar]
- [15].Xu D, Wang Q, Jiang Y, Zhang Y, Vega-Saenzdemiera E, Osman I, Dai W. Roles of Polo-like kinase 3 in suppressing tumor angiogenesis. Exp Hematol Oncol. 2012;1:5. doi: 10.1186/2162-3619-1-5. PMID:23210979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zitouni S, Nabais C, Jana SC, Guerrero A, Bettencourt-Dias M. Polo-like kinases: structural variations lead to multiple functions. Nat Rev Mol Cell Biol. 2014;15:433-52. doi: 10.1038/nrm3819. PMID:24954208 [DOI] [PubMed] [Google Scholar]
- [17].de Carcer G, Manning G, Malumbres M. From Plk1 to Plk5: functional evolution of polo-like kinases. Cell Cycle. 2011;10:2255-62. doi: 10.4161/cc.10.14.16494. PMID:21654194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andrysik Z, Bernstein WZ, Deng L, Myer DL, Li YQ, Tischfield JA, Stambrook PJ, Bahassi el M. The novel mouse Polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Res. 2010;38:2931-43. doi: 10.1093/nar/gkq011. PMID:20100802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Carcer G, Escobar B, Higuero AM, Garcia L, Anson A, Perez G, Mollejo M, Manning G, Melendez B, Abad-Rodriguez J, et al. . Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Mol Cell Biol. 2011;31:1225-39. doi: 10.1128/MCB.00607-10. PMID:21245385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267-76. doi: 10.1038/sj.onc.1208273. PMID:15640842 [DOI] [PubMed] [Google Scholar]
- [21].Helmke C, Becker S, Strebhardt K. The role of Plk3 in oncogenesis. Oncogene. 2016;35:135-47. doi: 10.1038/onc.2015.105. PMID:25915845 [DOI] [PubMed] [Google Scholar]
- [22].Liu X. Targeting Polo-Like Kinases: a promising therapeutic approach for cancer treatment. Transl Oncol. 2015;8:185-95. doi: 10.1016/j.tranon.2015.03.010. PMID:26055176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zimmerman WC, Erikson RL. Polo-like kinase 3 is required for entry into S phase. Proc Natl Acad Sci U S A. 2007;104:1847-52. doi: 10.1073/pnas.0610856104. PMID:17264206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zimmerman WC, Erikson RL. Finding Plk3. Cell Cycle. 2007;6:1314-8. doi: 10.4161/cc.6.11.4275. PMID:17568195 [DOI] [PubMed] [Google Scholar]
- [25].Bahassi el M, Conn CW, Myer DL, Hennigan RF, McGowan CH, Sanchez Y, Stambrook PJ. Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene. 2002;21:6633-40. doi: 10.1038/sj.onc.1205850. PMID:12242661 [DOI] [PubMed] [Google Scholar]
- [26].Xie S, Wu H, Wang Q, Kunicki J, Thomas RO, Hollingsworth RE, Cogswell J, Dai W. Genotoxic stress-induced activation of Plk3 is partly mediated by Chk2. Cell Cycle. 2002;1:424-9. doi: 10.4161/cc.1.6.271. PMID:12548019 [DOI] [PubMed] [Google Scholar]
- [27].Wang L, Gao J, Dai W, Lu L. Activation of Polo-like kinase 3 by hypoxic stresses. J Biol Chem. 2008;283:25928-35. doi: 10.1074/jbc.M801326200. PMID:18650425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang L, Payton R, Dai W, Lu L. Hyperosmotic stress-induced ATF-2 activation through Polo-like kinase 3 in human corneal epithelial cells. J Biol Chem. 2011;286:1951-8. doi: 10.1074/jbc.M110.166009. PMID:21098032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Myer DL, Robbins SB, Yin M, Boivin GP, Liu Y, Greis KD, Bahassi el M, Stambrook PJ. Absence of polo-like kinase 3 in mice stabilizes Cdc25A after DNA damage but is not sufficient to produce tumors. Mutat Res. 2011;714:1-10. doi: 10.1016/j.mrfmmm.2011.02.006. PMID:21376736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hudson JW, Kozarova A, Cheung P, Macmillan JC, Swallow CJ, Cross JC, Dennis JW. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr Biol. 2001;11:441-6. doi: 10.1016/S0960-9822(01)00117-8. PMID:11301255 [DOI] [PubMed] [Google Scholar]
- [31].Lu LY, Wood JL, Minter-Dykhouse K, Ye L, Saunders TL, Yu X, Chen J. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870-6. doi: 10.1128/MCB.00392-08. PMID:18794363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ouyang B, Pan H, Lu L, Li J, Stambrook P, Li B, Dai W. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J Biol Chem. 1997;272:28646-51. doi: 10.1074/jbc.272.45.28646. PMID:9353331 [DOI] [PubMed] [Google Scholar]
- [33].Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci U S A. 1998;95:9301-6. doi: 10.1073/pnas.95.16.9301. PMID:9689075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li B, Ouyang B, Pan H, Reissmann PT, Slamon DJ, Arceci R, Lu L, Dai W. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem. 1996;271:19402-8. doi: 10.1074/jbc.271.32.19402. PMID:8702627 [DOI] [PubMed] [Google Scholar]
- [35].Wiest J, Clark AM, Dai W. Intron/exon organization and polymorphisms of the PLK3/PRK gene in human lung carcinoma cell lines. Genes Chromosomes Cancer. 2001;32:384-9. doi: 10.1002/gcc.1204. PMID:11746980 [DOI] [PubMed] [Google Scholar]
- [36].Flugel D, Gorlach A, Kietzmann T. GSK-3beta regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1alpha. Blood. 2012;119:1292-301. doi: 10.1182/blood-2011-08-375014. PMID:22144179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644-6. doi: 10.1038/332644a0. PMID:2833705 [DOI] [PubMed] [Google Scholar]
- [38].Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627-44. doi: 10.1038/nrd2926. PMID:19644473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hamada K, Sasaki T, Koni PA, Natsui M, Kishimoto H, Sasaki J, Yajima N, Horie Y, Hasegawa G, Naito M, et al. . The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054-65. doi: 10.1101/gad.1308805. PMID:16107612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526-34. doi: 10.1016/j.tibs.2008.08.002. PMID:18809331 [DOI] [PubMed] [Google Scholar]
- [41].Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749-53. doi: 10.1073/pnas.040560897. PMID:10677529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1-9. doi: 10.1016/j.gene.2006.02.024. PMID:16675164 [DOI] [PubMed] [Google Scholar]
- [43].Xu D, Yao Y, Jiang X, Lu L, Dai W. Regulation of PTEN stability and activity by Plk3. J Biol Chem. 2010;285:39935-42. doi: 10.1074/jbc.M110.166462. PMID:20940307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528:145-53. doi: 10.1016/S0014-5793(02)03274-X. PMID:12297295 [DOI] [PubMed] [Google Scholar]
- [45].Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993-8. doi: 10.1074/jbc.M009134200. PMID:11035045 [DOI] [PubMed] [Google Scholar]
- [46].Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem. 2005;280:35195-202. doi: 10.1074/jbc.M503045200. PMID:16107342 [DOI] [PubMed] [Google Scholar]
- [47].Mehenni H, Lin-Marq N, Buchet-Poyau K, Reymond A, Collart MA, Picard D, Antonarakis SE. LKB1 interacts with and phosphorylates PTEN: a functional link between two proteins involved in cancer predisposing syndromes. Hum Mol Genet. 2005;14:2209-19. doi: 10.1093/hmg/ddi225. PMID:15987703 [DOI] [PubMed] [Google Scholar]
- [48].Valiente M, Andres-Pons A, Gomar B, Torres J, Gil A, Tapparel C, Antonarakis SE, Pulido R. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J Biol Chem. 2005;280:28936-43. doi: 10.1074/jbc.M504761200. PMID:15951562 [DOI] [PubMed] [Google Scholar]
- [49].Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, et al. . Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem. 2003;278:40057-66. doi: 10.1074/jbc.M303621200. PMID:12869565 [DOI] [PubMed] [Google Scholar]
- [50].Adey NB, Huang L, Ormonde PA, Baumgard ML, Pero R, Byreddy DV, Tavtigian SV, Bartel PL. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 2000;60:35-7. PMID:10646847 [PubMed] [Google Scholar]
- [51].Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627-30. doi: 10.1074/jbc.C100556200. PMID:11707428 [DOI] [PubMed] [Google Scholar]
- [52].Tolkacheva T, Boddapati M, Sanfiz A, Tsuchida K, Kimmelman AC, Chan AM. Regulation of PTEN binding to MAGI-2 by two putative phosphorylation sites at threonine 382 and 383. Cancer Res. 2001;61:4985-9. PMID:11431330 [PubMed] [Google Scholar]
- [53].Odriozola L, Singh G, Hoang T, Chan AM. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem. 2007;282:23306-15. doi: 10.1074/jbc.M611240200. PMID:17565999 [DOI] [PubMed] [Google Scholar]
- [54].Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci U S A. 2009;106:480-5. doi: 10.1073/pnas.0811212106. PMID:19114656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li C, Park S, Zhang X, Dai W, Xu D. Mutual regulation between Polo-like Kinase 3 and SIAH2 E3 ubiquitin ligase defines a regulatory network that fine-tunes the cellular response to hypoxia and nickel. J Biol Chem. 2017;292:11431-44. doi: 10.1074/jbc.M116.767178. PMID:28515325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].House CM, Frew IJ, Huang HL, Wiche G, Traficante N, Nice E, Catimel B, Bowtell DD. A binding motif for Siah ubiquitin ligase. Proc Natl Acad Sci U S A. 2003;100:3101-6. doi: 10.1073/pnas.0534783100. PMID:12626763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].House CM, Hancock NC, Moller A, Cromer BA, Fedorov V, Bowtell DD, Parker MW, Polekhina G. Elucidation of the substrate binding site of Siah ubiquitin ligase. Structure. 2006;14:695-701. doi: 10.1016/j.str.2005.12.013. PMID:16615911 [DOI] [PubMed] [Google Scholar]
- [58].Nakayama K, Qi J, Ronai Z. The ubiquitin ligase Siah2 and the hypoxia response. Mol Cancer Res. 2009;7:443-51. doi: 10.1158/1541-7786.MCR-08-0458. PMID:19372575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Qi J, Nakayama K, Gaitonde S, Goydos JS, Krajewski S, Eroshkin A, Bar-Sagi D, Bowtell D, Ronai Z. The ubiquitin ligase Siah2 regulates tumorigenesis and metastasis by HIF-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2008;105:16713-8. doi: 10.1073/pnas.0804063105. PMID:18946040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R, Moll R, Elledge SJ, Eilers M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol. 2007;9:765-74. doi: 10.1038/ncb1601. PMID:17558397 [DOI] [PubMed] [Google Scholar]
- [61].Zhang D, Zaugg K, Mak TW, Elledge SJ. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell. 2006;126:529-42. doi: 10.1016/j.cell.2006.06.039. PMID:16901786 [DOI] [PubMed] [Google Scholar]
- [62].Li Q, Kluz T, Sun H, Costa M. Mechanisms of c-myc degradation by nickel compounds and hypoxia. PloS One. 2009;4:e8531. doi: 10.1371/journal.pone.0008531. PMID:20046830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kozma SC, Thomas G. Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. Bioessays. 2002;24:65-71. doi: 10.1002/bies.10031. PMID:11782951 [DOI] [PubMed] [Google Scholar]
- [64].Wong CS, Moller A. Siah: a promising anticancer target. Cancer Res. 2013;73:2400-6. doi: 10.1158/0008-5472.CAN-12-4348. PMID:23455005 [DOI] [PubMed] [Google Scholar]
- [65].Qi J, Kim H, Scortegagna M, Ronai ZA. Regulators and effectors of Siah ubiquitin ligases. Cell Biochem Biophys. 2013;67:15-24. doi: 10.1007/s12013-013-9636-2. PMID:23700162 [DOI] [PMC free article] [PubMed] [Google Scholar]


