Abstract
Objectives: Pancreatic acinar cell carcinoma (ACC) is a rare tumor that frequently metastasizes to the liver and may present a diagnostic challenge due to its morphologic similarity to hepatocellular carcinoma. We investigated α-fetoprotein (AFP), hepatocyte paraffin antigen 1 (HepPar 1), glypican 3, arginase 1, and albumin messenger RNA (mRNA) in situ hybridization (ISH) in pancreatic neoplasms with ACC differentiation to assess their diagnostic value.
Methods: AFP, HepPar 1, glypican 3, and arginase 1 immunohistochemical staining was performed on 28 ACCs using a tissue microarray. Albumin mRNA ISH was performed on full-faced sections.
Results: Fifteen tumors were positive for at least one marker. Glypican 3 was positive in seven of 28, AFP in five 28, and albumin mRNA ISH in five of 20. None expressed arginase 1.
Conclusions: Hepatocellular differentiation markers, including albumin mRNA ISH, may be positive in ACC, but arginase 1 appears to be uniformly negative. Thus, its use may improve the accuracy in distinguishing these neoplasms from hepatocellular carcinoma. If ACC diagnosis is considered, acinar differentiation can be reliably demonstrated by trypsin/chymotrypsin.
Keywords: Pancreas, Acinar cell carcinoma, Hepatocellular carcinoma, Arginase 1, Glypican 3, Albumin ISH
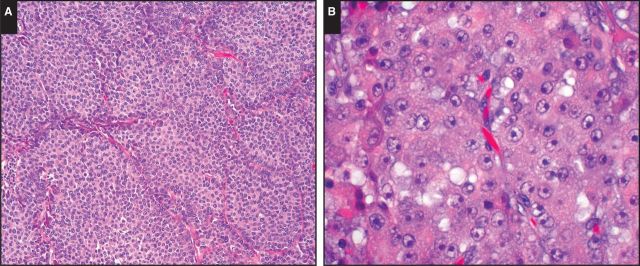
Pancreatic acinar cell carcinoma (ACC) frequently metastasizes to the liver and may mimic hepatocellular carcinoma (HCC) architecturally and cytologically Image 1A. Both tumors are characterized by high cellularity, solid nests, acini or trabecular formations, granular cytoplasm, and prominent single nucleoli Image 1B.1,2 Nonetheless, ACC is a rare neoplasm, and without a broad index of suspicion and understanding of the staining pattern that ACC may display using markers more commonly used for the differential diagnosis of HCC vs cholangiocarcinoma, this diagnostic possibility may not be considered.
Image 1.
A, Pancreatic acinar cell carcinoma closely mimics hepatocellular carcinoma architecturally and cytologically (H&E, ×100). B, Both pancreatic acinar cell carcinomas and hepatocellular carcinomas are characterized by high cellularity, solid nests, acini or trabecular formations, granular cytoplasm, and prominent single nucleoli (H&E, ×400).
Commonly used immunohistochemical markers that can support a diagnosis of HCC include α-fetoprotein (AFP), polyclonal carcinoembryonic antigen (pCEA), CD10, hepatocyte paraffin antigen 1 (HepPar 1), and glypican 3.3-7 However, the utility of each of these markers is limited by suboptimal sensitivity or difficulty in interpretation.8-10 For example, AFP suffers from low sensitivity (30%-50%) and frequent focal staining, limiting its utility in biopsy samples.8 pCEA and CD10 also suffer from low sensitivity (25%-50%) in poorly differentiated HCCs, where the distinction between HCC and other carcinomas is most difficult,9.10 HepPar 1 also has relatively low sensitivity in poorly differentiated HCCs8,10-12 and can exhibit strong cytoplasmic staining in gastric, esophageal, and pulmonary adenocarcinomas.8,11,12 Glypican 3 is more frequently expressed in poorly differentiated HCCs compared with well-differentiated HCCs.13-20 Thus, initially it was regarded as a superior marker in distinguishing poorly differentiated HCCs from metastasis.21-26 However, subsequent studies have shown that gynecologic carcinomas,27,28 pulmonary squamous cell carcinomas,22 and even germ cell tumors24 and malignant melanoma29 can label with glypican 3. Recently, it has also been reported to be expressed by more than 50% of ACCs.30
In contrast, arginase 1 is expressed in normal human liver with a high degree of specificity31 and reported to be a more sensitive (81%) marker for HCC than HepPar 1 or glypican 3.32 Although arginase 1 can also be identified in adenocarcinomas, particularly of pancreatic origin,32 to our knowledge, it has not been studied in ACCs.
Branched-chain albumin in situ hybridization (ISH) also offers a robust means of detecting a tumor of liver origin, including HCCs and intrahepatic cholangiocarcinomas, and has been advocated to be particularly valuable when distinguishing metastatic carcinomas from hepatic primaries.33-35 However, positivity of albumin ISH in normal exocrine pancreatic parenchyma and ACCs has recently been noted.35,36
Understanding the extent to which markers of hepatocellular differentiation may label ACC is key for distinguishing these neoplasms. In this study, we evaluated the staining patterns of AFP, HepPar 1, glypican 3, arginase 1, and albumin ISH in a large series of pancreatic ACCs and related acinar cell neoplasms in an attempt to unveil potential pitfalls in separating HCC and these pancreatic primaries.
Materials and Methods
With approval of the Institutional Review Board, we identified 28 tumors (27 primary pancreatic tumors and one metastatic pancreatic ACC to the liver) with acinar differentiation from the archives of the Memorial Sloan Kettering Cancer Center and Emory University Hospital comprising 11 pure ACCs, 11 mixed acinar neuroendocrine carcinomas (MANECs), four mixed acinar ductal carcinomas (MADCs), and two mixed acinar, neuroendocrine, and ductal carcinomas (MANEDCs). Acinar and neuroendocrine differentiation was supported by previously performed immunohistochemical staining for trypsin and/or chymotrypsin and chromogranin and/or synaptophysin, respectively. Mixed acinar neoplasms were defined using World Health Organization 2010 criteria.2,37 A tissue microarray (TMA) of formalin-fixed, paraffin-embedded tumors was created using three 0.6-mm-diameter punches per tumor. For mixed acinar neoplasms, if there were distinct populations of tumor cells, only the acinar component was used for TMA construction. However, for most mixed neoplasms, especially for MANECs, there was a morphologically homogeneous population of tumor cells (see Results section). For these, a representative tumor area was used for TMA construction.
Immunohistochemistry
TMA sections were immunolabeled using the standard avidin-biotin peroxidase method, with antibodies against AFP (Dako, Carpinteria, CA), HepPar 1 (Dako), glypican 3 (Santa Cruz Biotechnology, Dallas, TX), and arginase 1 (Cell Marque, Rocklin, CA). If any staining was seen, the antibody was repeated on a representative full-faced section of the corresponding tumor.
AFP displayed a cytoplasmic staining pattern; HepPar 1, cytoplasmic granular; glypican 3, cytoplasmic and/or membranous; and arginase 1, cytoplasmic and nuclear. The percentage of cell labeling was scored as follows: 1, labeling was observed in less than 5%; 2, 5% to 50%; and 3, more than 50%. Score 1 was regarded as negative; scores 2 and 3 were regarded as positive (patchy and diffuse, respectively).
Albumin Messenger RNA ISH
ISH was performed using automated ViewRNA platform (Affymetrix, Santa Clara, CA). This technology uses a branched DNA structure for signal amplification to enable detection of messenger RNA (mRNA) in formalin-fixed, paraffin-embedded tissue. Automated ISH assays for albumin mRNA were performed using the View-RNA eZ Detection Kit (Affymetrix) on the Bond RX immunohistochemistry and ISH Staining System with BDZ 6.0 software (Leica Biosystems, Buffalo Grove, IL). Paraffin-embedded full-faced (whole) tissue sections were processed automatically from deparaffinization, through ISH staining to hematoxylin counterstaining; sections were coverslipped off-instrument. Briefly, 5-μm-thick sections of formalin-fixed tissue were baked for 1 hour at 60°C and placed on the Bond RX for processing. The Bond RX user-selectable settings were as follows: ViewRNA 1 protocol; ViewRNA Dewax1; View-RNA HIER 10 minutes, ER1 (95); ViewRNA Enzyme1 (20); and ViewRNA Probe Hybridization. With these settings, the RNA unmasking conditions for the liver tissue consisted of a 10-minute incubation at 95°C in Bond Epitope Retrieval Solution 1 (Leica Biosystems), followed by a 20-minute incubation with Proteinase K from the Bond Enzyme Pretreatment Kit at 1:1,000 dilution (Leica Biosystems). Postrun, slides were rinsed with water, air dried for 30 minutes at room temperature, dipped in xylene, and mounted using Histo-Mount solution (Life Technologies, Grand Island, NY). Normal liver served as a positive control substance.33,34
Cytoplasmic dot-like reactivity in more than 5% of tumor cells was regarded as positive. However, if the cytoplasmic dot-like reactivity in the tumor cells was weaker than or equal to that of background, it was interpreted as negative. Nuclear reactivity, a known artifact, was also interpreted as negative.
Results
Histology
The TMA comprised 11 (39%) pure ACCs and 17 (61%) mixed acinar neoplasms. MANECs exhibited two histologic patterns. In the first group (n = 9), there was a morphologically homogeneous population of cells, and the divergent differentiation was detected by only immunohistochemical labeling. In the second group (n = 2), there were two distinct populations of neoplastic cells, with acinar and neuroendocrine features, respectively. These distinct populations showed immunophenotypic patterns corresponding to the morphology. Similarly, tumors with combined acinar and ductal differentiation (MADCs/MANEDCs) either exhibited morphologically distinct zones of ductal differentiation (n = 2) or an intimate admixture (n = 4).
Of note, of 26 cases with available information, 14 (54%; seven pure, seven mixed) had distant metastasis: 10 (34%) in the liver, two (8%) in soft tissue, one (4%) in the lung, and one (4%) in bone.
Hepatocellular Differentiation Markers
Thirteen tumors (six pure ACCs, four MANECs, two MANEDCs, and one MADC) did not express any of the markers performed. However, 15 (53%) tumors (five pure ACCs, seven MANECs, and three MADCs) were positive for at least one marker, and one (MANEC) of these tumors was positive for four markers (glypican 3, AFP, HepPar 1, and albumin ISH). The results of immunohistochemistry and albumin mRNA ISH are displayed in Table 1.
Table 1.
Expression of Hepatocellular Differentiation Markers in ACCs
|
Staining Pattern, No.
|
|||||
|---|---|---|---|---|---|
| Marker | Pure ACC | MANEC | MADC | MANEDC | Total No. of Cases |
| Arginase 1 | 0 | 0 | 0 | 0 | 0 |
| Glypican 3 | 2 (1 patchy, 1 diffuse) | 4 (2 patchy, 2 diffuse) | 0 | 0 | 6 |
| AFP | 2 (patchy) | 2 (1 patchy, 1 diffuse) | 1 (diffuse) | 0 | 5 |
| HepPar 1 | 0 | 1 (patchy) | 0 | 0 | 1 |
| Albumin ISH | 1 | 3 | 1 | 0 | 5 |
ACC, acinar cell carcinoma; AFP, α-fetoprotein; HepPar 1, hepatocyte paraffin antigen 1; ISH, in situ hybridization; MADC, mixed acinar ductal carcinoma; MANEC, mixed acinar neuroendocrine carcinoma; MANEDC, mixed acinar, neuroendocrine, and ductal carcinoma.
Immunohistochemistry
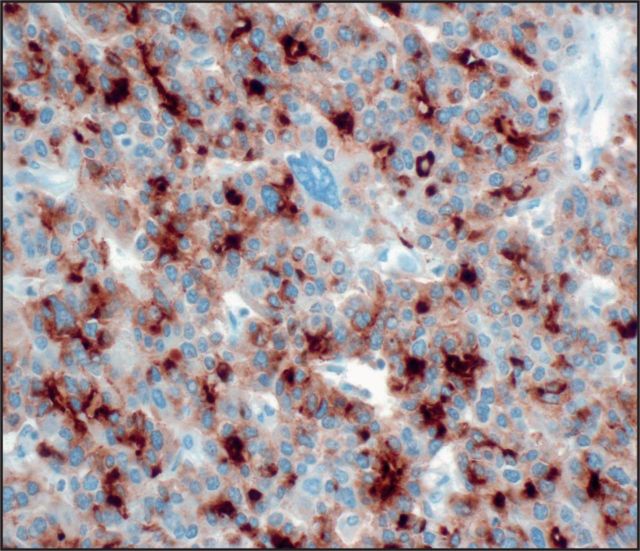
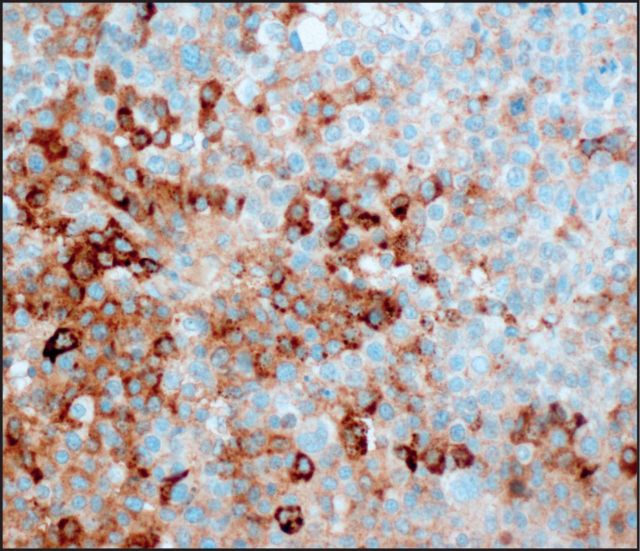
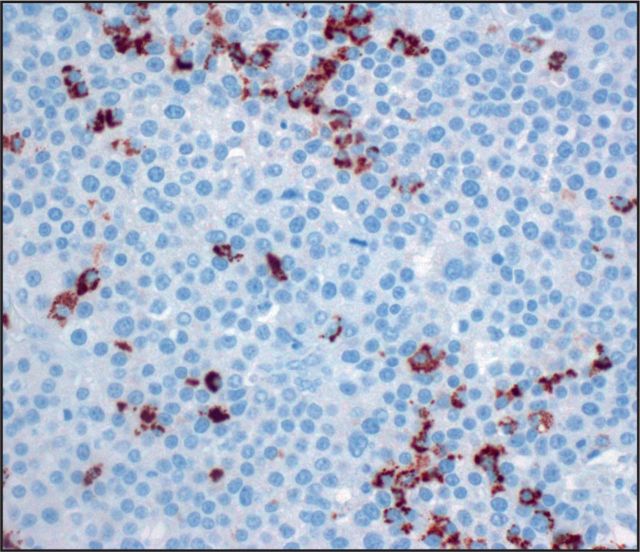
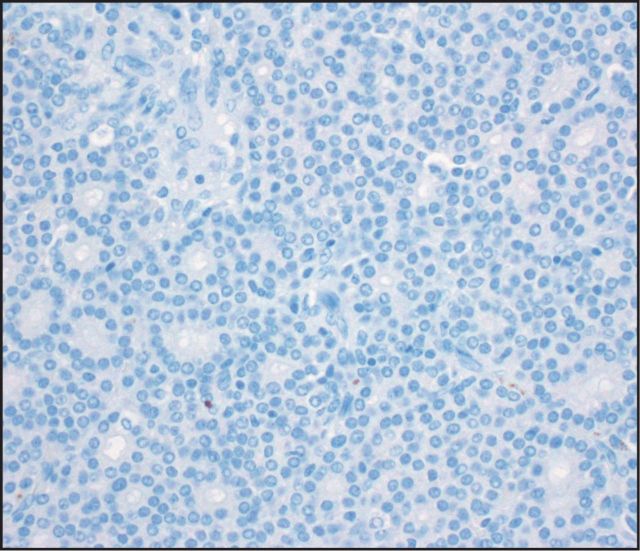
Seven (25%) tumors were immunoreactive for glypican 3 Image 2: three with diffuse staining (one pure ACC, two MANECs) and four with patchy staining (one pure ACC, two MANECs, one MADC). AFP stained five (18%) cases Image 3: two with diffuse staining (one MANEC, one MADC) and three with patchy staining (two pure ACCs, one MANEC). HepPar 1 stained only one MANEC (Image 4, patchy), which was also positive for glypican 3, AFP, and albumin ISH. None of the ACCs demonstrated arginase 1 immunolabeling Image 5.
Image 2.
Seven cases were positive for glypican 3 (×400).
Image 3.
Expression of α-fetoprotein was identified in five acinar cell carcinomas (×400).
Image 4.
Only one of the tested acinar cell carcinomas expressed patchy hepatocyte paraffin antigen 1 (×400).
Image 5.
Arginase was uniformly negative in acinar cell carcinomas (×400).
Of note, there was no difference between the staining patterns of TMA and full-faced sections or pure ACCs and mixed acinar neoplasms.
Albumin mRNA ISH
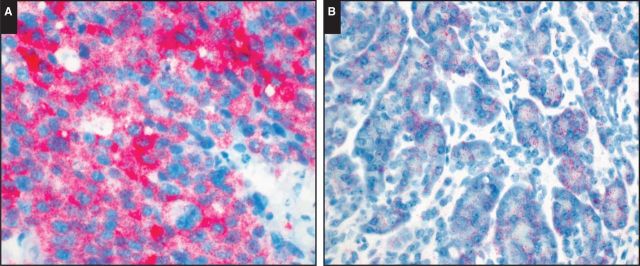
Albumin mRNA ISH, performed on 20 ACCs (six pure ACCs, 14 mixed acinar neoplasms; see Table 1), was positive in five (25%) cases: three with diffuse staining (one pure, two mixed) and two with patchy staining (both mixed) Image 6A. All but one albumin mRNA ISH positive mixed acinar neoplasms were MANEC of homogeneous histology. The exception was an MADC with albumin mRNA ISH reactivity exclusively in the acinar component. Albumin expression was present in both acinar and solid growth pattern areas of ACCs in our series.
Image 6.
A, Tumor cells were diffusely positive for albumin messenger RNA (mRNA) in situ hybridization (ISH). B, Normal pancreatic acini were variably positive for albumin mRNA ISH, but the signal intensity in these cells was less than that seen in tumor cells.
Only one case that was positive for albumin mRNA ISH also showed diffuse glypican 3, diffuse AFP, and patchy HepPAR 1 labeling.
Of note, normal pancreatic acinar cells were also variably positive for albumin mRNA ISH, but the signal intensity in these cells was less than that seen in tumor cells Image 6B.
Discussion
Pancreatic ACC is a rare neoplasm that may be overlooked in the differential diagnosis with HCC. Our study shows that 53% of ACCs and related acinar neoplasms may have patchy or diffuse staining for markers commonly used to interrogate for hepatocellular differentiation, including AFP, HepPar 1, glypican 3, and albumin mRNA (by ISH), creating a potential diagnostic pitfall.
We found that arginase 1 is uniformly negative in ACCs and related acinar neoplasms, highlighting the utility of this stain in this context. Arginase exists in two isoforms—namely, arginase 1 and arginase 2, both of which are responsible for the hydrolysis of arginine to ornithine and urea in the urea cycle. Of the two isoforms, arginase 1 demonstrates high levels of expression within the liver, specifically in periportal hepatocytes,38 whereas arginase 2 levels are highest in the kidneys and pancreas and are very low in the liver.3,39 Arginase 1 was introduced as a sensitive and specific marker for benign and malignant hepatocytes.3,40 Subsequent studies have validated its high sensitivity and specificity, as well as its value in evaluating poorly differentiated HCCs.3,32,39,41,42
ACC labeling for albumin mRNA by ISH is also significant because this marker is reported to have a high level of sensitivity (93%-99%) and specificity (close to 100%)10,34 for hepatocellular differentiation or liver origin due to hepatocellular synthesis of this protein.43,44 Both HCCs and peripheral cholangiocarcinomas express this albumin mRNA, but a wide array of extrahepatic adenocarcinomas, including pancreatic ductal adenocarcinoma, is negative. However, this marker is positive in normal pancreatic acinar cells, and albumin ISH has also been reported to be positive in gastric adenocarcinomas45,46 and extrahepatic germ cell tumors with hepatoid features44 and in rare cases of clear cell carcinoma of the ovary.47 Recently, expression of albumin mRNA in pancreatic ACCs was described. Interestingly, our positivity rate (25%) was about half of what Terris et al35 reported (46%). Details regarding their method are unclear, particularly their positive threshold compared with our series. Also, ACC presents a technical challenge for performing mRNA ISH because of pancreatic enzymes. Therefore, we used the pancreatic ducts and stroma as the judge of background reactivity (ie, if the intensity of cytoplasmic dot-like reactivity in the tumor cells was weaker than or equal to that of background, we did not regard that reactivity as positive). The age of the blocks could also significantly affect the results: paraffin-embedded tissue stored for more than 10 years shows significant deterioration in RNA preservation, and given that some ACCs show a low number of albumin transcripts, the number of ACCs expressing albumin may be higher than observed in this study.
Recently, Mounajjed et al30 reported that most (58.5%) ACCs are glypican 3 positive. Our study corroborates their findings, albeit with a lower positivity rate (25%). This might be due to their use of full-faced sections, whereas we used a TMA section where focal reactivity can be missed. Our study design more closely recapitulates the expected findings in biopsy samples, which would be the most common specimen obtained from a metastatic tumor in the liver.
The expression of AFP in ACCs (identified in 18% of our cases) is well documented in the literature and can also be associated with serum elevations, raising consideration for its use as a tumor marker in those cases. Pediatric acinar cell neoplasms (ie, pancreatoblastomas) are even more likely to express AFP than their adult counterparts. Our results further support that immunohistochemistry for AFP plays no role in distinguishing between ACC and HCC.48 Similarly, HepPar 1 may be positive in a number of non-HCC tumors, and its low sensitivity and specificity limiting its utility in daily practice are becoming well known.8-12 Surprisingly, of the subset of ACCs we tested, only 4% revealed patchy HepPar 1 expression.
Of note, acinar differentiation markers chymotrypsin and trypsin are uniformly negative in HCCs. Recently, we tested 88 HCCs using a TMA, composed of a balanced mixture of well, moderately, and poorly differentiated examples, and none of the HCCs labeled with trypsin and chymotrypsin (unpublished data).
In summary, glypican 3, AFP, HepPar 1, and albumin mRNA ISH are positive in more than half (53%) of ACCs and related mixed acinar neoplasms. Therefore, caution should be exercised when using these markers to explore a diagnosis of HCC. Tumors lacking arginase 1 expression should prompt a careful evaluation of the morphologic and immunophenotypic features to exclude ACC. Acinar differentiation can be reliably demonstrated by using the highly sensitive and specific immunohistochemical markers trypsin and chymotrypsin1,2,49 if the diagnosis of ACC is considered.
References
- 1. Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas: a clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815-837. [DOI] [PubMed] [Google Scholar]
- 2. Klimstra DS, Hruban RH, Kloppel G, et al. Acinar cell neoplasms of the pancreas In: Bosman FT, Carneiro FHHR, Theise ND, eds. WHO Classification of Tumors of Digestive System. Lyon, France: IARC; 2010:314-318. [Google Scholar]
- 3. Yan BC, Gong C, Song J, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol. 2010;34:1147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wee A. Fine needle aspiration biopsy of the liver: algorithmic approach and current issues in the diagnosis of hepatocellular carcinoma. Cytojournal. 2005;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wee A. Fine needle aspiration biopsy of hepatocellular carcinoma and hepatocellular nodular lesions: role, controversies and approach to diagnosis. Cytopathology. 2011;22:287-305. [DOI] [PubMed] [Google Scholar]
- 6. Proca DM, Niemann TH, Porcell AI, et al. MOC31 immunoreactivity in primary and metastatic carcinoma of the liver: report of findings and review of other utilized markers. Appl Immunohistochem Mol Morphol. 2000;8:120-125. [DOI] [PubMed] [Google Scholar]
- 7. Onofre AS, Pomjanski N, Buckstegge B, et al. Immunocytochemical diagnosis of hepatocellular carcinoma and identification of carcinomas of unknown primary metastatic to the liver on fine-needle aspiration cytologies. Cancer. 2007;111:259-268. [DOI] [PubMed] [Google Scholar]
- 8. Kakar S, Gown AM, Goodman ZD, et al. Best practices in diagnostic immunohistochemistry: hepatocellular carcinoma versus metastatic neoplasms. Arch Pathol Lab Med. 2007;131:1648-1654. [DOI] [PubMed] [Google Scholar]
- 9. Lau SK, Prakash S, Geller SA, et al. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. 2002;33:1175-1181. [DOI] [PubMed] [Google Scholar]
- 10. Kakar S, Muir T, Murphy LM, et al. Immunoreactivity of Hep Par 1 in hepatic and extrahepatic tumors and its correlation with albumin in situ hybridization in hepatocellular carcinoma. Am J Clin Pathol. 2003;119:361-366. [DOI] [PubMed] [Google Scholar]
- 11. Chu PG, Ishizawa S, Wu E, et al. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol. 2002;26:978-988. [DOI] [PubMed] [Google Scholar]
- 12. Fan Z, van de Rijn M, Montgomery K, et al. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol. 2003;16:137-144. [DOI] [PubMed] [Google Scholar]
- 13. Midorikawa Y, Ishikawa S, et al. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer. 2003;103:455-465. [DOI] [PubMed] [Google Scholar]
- 14. Man XB, Tang L, Zhang BH, et al. Upregulation of glypican-3 expression in hepatocellular carcinoma but downregulation in cholangiocarcinoma indicates its differential diagnosis value in primary liver cancers. Liver Int. 2005;25:962-966. [DOI] [PubMed] [Google Scholar]
- 15. Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle. 2008;7:2787-2790. [DOI] [PubMed] [Google Scholar]
- 16. Kwack MH, Choi BY, Sung YK. Cellular changes resulting from forced expression of glypican-3 in hepatocellular carcinoma cells. Mol Cells. 2006;21:224-228. [PubMed] [Google Scholar]
- 17. Nishimura Y, Nakatsura T, Senju S. Usefulness of a novel oncofetal antigen, glypican-3, for diagnosis and immunotherapy of hepatocellular carcinoma [in Japanese]. Nihon Rinsho Meneki Gakkai Kaishi. 2008;31:383-391. [DOI] [PubMed] [Google Scholar]
- 18. Lu ZL, Luo DZ, Wen JM. Expression and significance of tumor-related genes in HCC. World J Gastroenterol. 2005;11:3850-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li BD, Zhao QC, Zhu YT, et al. Significance of glypican-3 mRNA expression in hepatocellular carcinoma tissues and peripheral blood cells [in Chinese]. Zhonghua Wai Ke Za Zhi. 2006;44:458-462. [PubMed] [Google Scholar]
- 20. Zhu ZW, Friess H, Wang L, et al. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011-1018. [DOI] [PubMed] [Google Scholar]
- 22. Aviel-Ronen S, Lau SK, Pintilie M. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol. 2008;21:817-825. [DOI] [PubMed] [Google Scholar]
- 23. Zynger DL, Dimov ND, Luan C, et al. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol. 2006;30:1570-1575. [DOI] [PubMed] [Google Scholar]
- 24. Zynger DL, Everton MJ, Dimov ND, et al. Expression of glypican 3 in ovarian and extragonadal germ cell tumors. Am J Clin Pathol. 2008;130:224-230. [DOI] [PubMed] [Google Scholar]
- 25. Esheba GE, Pate LL, Longacre TA. Oncofetal protein glypican-3 distinguishes yolk sac tumor from clear cell carcinoma of the ovary. Am J Surg Pathol. 2008;32:600-607. [DOI] [PubMed] [Google Scholar]
- 26. Hishinuma M, Ohashi KI, Yamauchi N. Hepatocellular oncofetal protein, glypican 3 is a sensitive marker for alpha-fetoprotein-producing gastric carcinoma. Histopathology. 2006;49:479-486. [DOI] [PubMed] [Google Scholar]
- 27. Stadlmann S, Gueth U, Baumhoer D. Glypican-3 expression in primary and recurrent ovarian carcinomas. Int J Gynecol Pathol. 2007;26:341-344. [DOI] [PubMed] [Google Scholar]
- 28. Maeda D, Ota S, Takazawa Y. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009;22:824-832. [DOI] [PubMed] [Google Scholar]
- 29. Nakatsura T, Kageshita T, Ito S. Identification of glypican-3 as a novel tumor marker for melanoma. Clin Cancer Res. 2004;10:6612-6621. [DOI] [PubMed] [Google Scholar]
- 30. Mounajjed T, Zhang L, Wu TT. Glypican-3 expression in gastrointestinal and pancreatic epithelial neoplasms. Hum Pathol. 2013;44:542-550. [DOI] [PubMed] [Google Scholar]
- 31. Multhaupt H, Fritz P, Schumacher K. Immunohistochemical localisation of arginase in human liver using monoclonal antibodies against human liver arginase. Histochemistry. 1987;87:465-470. [DOI] [PubMed] [Google Scholar]
- 32. Fujiwara M, Kwok S, Yano H, et al. Arginase-1 is a more sensitive marker of hepatic differentiation than HepPar-1 and glypican-3 in fine-needle aspiration biopsies. Cancer Cytopathol. 2012;120:230-237. [DOI] [PubMed] [Google Scholar]
- 33. Ferrone CR, Ting DT, Shahid M. The ability to diagnose intrahepatic cholangiocarcinoma definitively using novel branched DNA-enhanced albumin RNA in situ hybridization technology. Ann Surg Oncol. 2016;23:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shahid M, Mubeen A, Tse J. Branched chain in situ hybridization for albumin as a marker of hepatocellular differentiation: evaluation of manual and automated in situ hybridization platforms. Am J Surg Pathol. 2015;39:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Terris B, Hergli I, Lin-Marq N, et al. Letter to the editor with regard to the article entitled: “branched chain in situ hybridization for albumin as a marker of hepatocellular differentiation.” Am J Surg Pathol. 2015;39:1156-1157. [DOI] [PubMed] [Google Scholar]
- 36. Ting DT, Deshpande V. Expression of albumin mRNA in primary hepatic neoplasms and acinar cell carcinoma. Am J Surg Pathol. 2015;39:1157-1158. [DOI] [PubMed] [Google Scholar]
- 37. Fukushima N, Hruban R, Kato Y. Ductal adenocarcinoma variants and mixed neoplasms of the pancreas In: Bosman FT, Carneiro F, Hruban RH, et al, eds WHO Classification of Tumors. Lyon, France: IARC; 2010:292-295. [Google Scholar]
- 38. Sekine S, Ogawa R, McManus MT, et al. Dicer is required for proper liver zonation. J Pathol. 2009;219:365-372. [DOI] [PubMed] [Google Scholar]
- 39. Choi S, Park C, Ahn M, et al. Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem. 2012;114:487-494. [DOI] [PubMed] [Google Scholar]
- 40. Geramizadeh B, Seirfar N. Diagnostic value of arginase-1 and glypican-3 in differential diagnosis of hepatocellular carcinoma, cholangiocarcinoma and metastatic carcinoma of liver. Hepat Mon. 2015;15:e30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Radwan NA, Ahmed NS. The diagnostic value of arginase-1 immunostaining in differentiating hepatocellular carcinoma from metastatic carcinoma and cholangiocarcinoma as compared to HepPar-1. Diagn Pathol. 2012;7:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan BC, Hart JA. Recent developments in liver pathology. Arch Pathol Lab Med. 2009;133:1078-1086. [DOI] [PubMed] [Google Scholar]
- 43. Yamaguchi K, Nalesnik MA, Carr BI. In situ hybridization of albumin mRNA in normal liver and liver tumors: identification of hepatocellular origin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:361-365. [DOI] [PubMed] [Google Scholar]
- 44. Krishna M, Lloyd RV, Batts KP. Detection of albumin messenger RNA in hepatic and extrahepatic neoplasms: a marker of hepatocellular differentiation. Am J Surg Pathol. 1997;21:147-152. [DOI] [PubMed] [Google Scholar]
- 45. Supriatna Y, Kishimoto T, Uno T, et al. Evidence for hepatocellular differentiation in alpha-fetoprotein-negative gastric adenocarcinoma with hepatoid morphology: a study with in situ hybridisation for albumin mRNA. Pathology. 2005;37:211-215. [DOI] [PubMed] [Google Scholar]
- 46. Foschini MP, Baccarini P, Dal Monte PR. Albumin gene expression in adenocarcinomas with hepatoid differentiation. Virchows Arch. 1998;433:537-541. [DOI] [PubMed] [Google Scholar]
- 47. Oliveira AM, Erickson LA, Burgart LJ, et al. Differentiation of primary and metastatic clear cell tumors in the liver by in situ hybridization for albumin messenger RNA. Am J Surg Pathol. 2000;24:177-182. [DOI] [PubMed] [Google Scholar]
- 48. Basturk O, Farris AB, Adsay NV. Immunohistology of pancreas, gallbladder, extrahepatic Bile ducts, ampulla and liver In: Dabbs D, ed. Diagnostic Immunohistochemistry . 4th ed.New York, NY: Elsevier; 2013:503-523. [Google Scholar]
- 49. Wood LD, Klimstra DS. Pathology and genetics of pancreatic neoplasms with acinar differentiation. Semin Diagn Pathol. 2014;31:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]