Abstract
Biallelic loss-of-function mutations in the RNA-binding protein EIF4A3 cause Richieri-Costa–Pereira syndrome (RCPS), an autosomal recessive condition mainly characterized by craniofacial and limb malformations. However, the pathogenic cellular mechanisms responsible for this syndrome are entirely unknown. Here, we used two complementary approaches, patient-derived induced pluripotent stem cells (iPSCs) and conditional Eif4a3 mouse models, to demonstrate that defective neural crest cell (NCC) development explains RCPS craniofacial abnormalities. RCPS iNCCs have decreased migratory capacity, a distinct phenotype relative to other craniofacial disorders. Eif4a3 haploinsufficient embryos presented altered mandibular process fusion and micrognathia, thus recapitulating the most penetrant phenotypes of the syndrome. These defects were evident in either ubiquitous or NCC-specific Eif4a3 haploinsufficient animals, demonstrating an autonomous requirement of Eif4a3 in NCCs. Notably, RCPS NCC-derived mesenchymal stem-like cells (nMSCs) showed premature bone differentiation, a phenotype paralleled by premature clavicle ossification in Eif4a3 haploinsufficient embryos. Likewise, nMSCs presented compromised in vitro chondrogenesis, and Meckel’s cartilage was underdeveloped in vivo. These findings indicate novel and essential requirements of EIF4A3 for NCC migration and osteochondrogenic differentiation during craniofacial development. Altogether, complementary use of iPSCs and mouse models pinpoint unique cellular mechanisms by which EIF4A3 mutation causes RCPS, and provide a paradigm to study craniofacial disorders.
Introduction
Richieri-Costa–Pereira syndrome (RCPS; OMIM #268305) is a rare autosomal-recessive acrofacial dysostosis in which mandible development, particularly the distal and medial regions, may be severely affected. It is characterized by midline mandibular cleft usually associated with failure of mandibular symphyseal fusion, Robin sequence, laryngeal abnormalities, radial and tibial defects, among other clinical findings (1). Phenotype expressivity is variable, ranging from only mild defects in mandibular fusion or laryngeal clefts to more severe clinical manifestations associated with agenesis of the mandible (1–3).
RCPS is mainly caused by non-coding expansions in the 5’ UTR of the gene EIF4A3. This region is characterized by multiple allelic patterns that vary in size and organization of repeat motifs. The most prevalent allelic pattern among normal individuals has 5–12 repeats, whilst the majority of affected individuals harbor 14–16 repeats. In addition, a missense mutation has been found in trans to an expansion allele in one RCPS patient (3). These alterations are believed to cause partial loss of function of EIF4A3, as 30–40% reduction of EIF4A3 mRNA expression has been reported in RCPS patients’ lymphocytes and adult mesenchymal cells.
EIF4A3 encodes a DEAD-box helicase that is a core component of the RNA-binding exon junction complex (EJC), which controls post-transcriptional events, including alternative splicing, non-sense-mediated mRNA decay, translation initiation and RNA localization (4). EIF4A3 binding to mRNA is stabilized by two additional core components, MAGOH and RBM8A. Eif4a3 knockdown in zebrafish results in embryos with alterations in craniofacial cartilage/bone development and clefting of the lower jaw (3). Eif4a3 is also required in Xenopus for embryonic development including formation of the peripheral nervous system and melanocytes (5,6). In mice, conditional haploinsufficiency of Eif4a3 or other EJC components in the brain impairs neural progenitor proliferation, differentiation and survival (7). Although a relationship between EIF4A3 loss of function and RCPS has been established, the requirement of Eif4a3 for mammalian embryonic craniofacial development and the pathogenic cellular mechanism responsible for the syndrome is entirely unknown.
The craniofacial structures compromised in RCPS are suggestive of disturbances in neural crest or neural crest-derived tissue development. Neural crest cells (NCCs) are a transient cell population originating from the neuroectoderm located at the neural plate border during neurulation. The foremost segment of the neuraxis gives rise to cranial NCCs, which migrate and populate the mesenchyme of the developing pharyngeal arches. Mandibular arches fuse to form a mesenchymal mandible. The cranial NCC-derived mesenchyme then undergoes proliferation and differentiation, generating most of the cranioskeleton (including Meckel’s cartilage, followed by the mandible), ear components and larynx, amongst others (8–10). NCC defects in apoptosis, proliferation, and cell migration underlie some craniofacial disorders, including Treacher Collins syndrome and Nager syndrome, raising the question as to their potential role in RCPS pathology (11–13). Furthermore, NCC mesenchymal differentiation could also play a role in RCPS (14). In order to understand the etiology of RCPS it is critical to define which, if any, of these cellular mechanisms are relevant.
In this study, we addressed these gaps, using two novel models, induced pluripotent stem cells (iPSCs) and mouse mutants, with a particular focus on mandible development. Owing to the natural limitations in studying human embryos, integration of these approaches offers invaluable insight into human craniofacial malformations; NCCs and their derivatives can be generated from patients and screened for disease-relevant phenotypes (15,16), which can be further examined in mice. We found that RCPS patient-derived iPSCs differentiated toward a NCC lineage exhibited autonomous defects in cell migration and those differentiated into mesenchymal derivatives were prone to premature ossification and altered chondrogenesis. Eif4a3 haploinsufficiency in mice caused altered fusion of mandibular processes, impaired development of Meckel’s cartilage, and premature skeletal ossification. These defects were caused by an autonomous requirement of Eif4a3 in NCC and resulted in significant loss of mandibular structures, thus modelling common malformations of RCPS. Together, complementary use of iPSCs and mouse models pinpoint developmental mechanisms by which EIF4A3 mutation causes RCPS.
Results
Derivation of iPSC cultures
We first set out to establish iPSC lines from RCPS patients. Two of the RCPS patients (F8417-1 and F8417-2) were homozygous for the 16-repeat allele, while a third patient (F6099-1) had a 14-repeat allele in trans with the missense mutation p.Asp270Gly (3); controls were homozygous for the 6-repeat allele (F9048-1) and heterozygous for 7-repeats/6-repeats (F7405-1 and F8799-1) (Supplementary Material, Table S1; see Materials and Methods). Information regarding sample usage in experiments is shown in Supplementary Material, Table S1. After reprogramming, all iPSC lines displayed pluripotent stem cell-like morphology and positive staining for pluripotency markers OCT3/4 and SSEA-4 (Supplementary Material, Fig. S1A), as well as expression of OCT3/4, NANOG and ALP transcripts (Supplementary Material, Fig. S1B–D). Further, iPSCs were able to generate teratoma-containing tissues from all three germ layers in vivo (Supplementary Material, Fig. S1F–H). All iPSCs showed no detectable signs of aneuploidy or genomic integration of the episomal vectors (Supplementary Material, Fig. S1E and I).
NCCs can be derived from RCPS iPSCs and exhibit migration defects
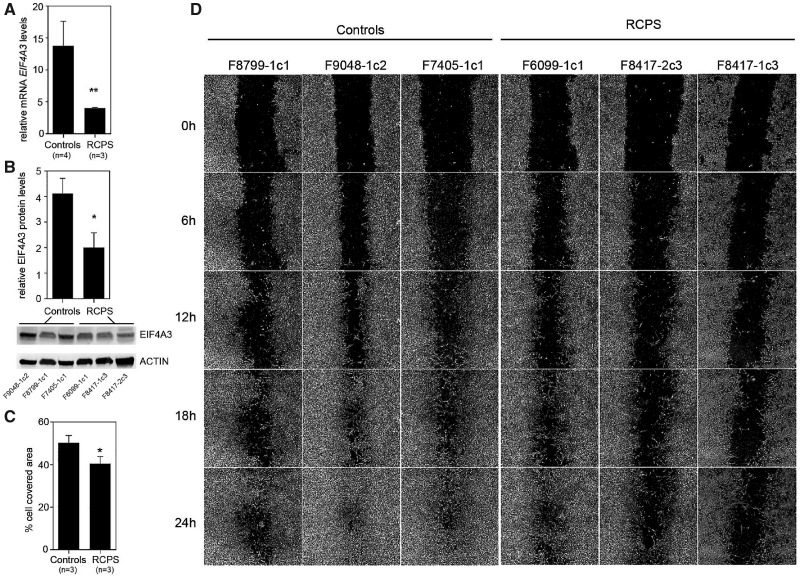
iPSC-derived NCCs (iNCCs) were induced from RCPS and control iPSCs under a methodology based on TGF-β/Activin pathway blockade and WNT pathway activation without the presence of caudalization factors (17,18), which has been shown to favor cranial NCC formation (19). After differentiation, iNCC populations were positively stained for NCC markers p75 and HNK1 with proportions of double-positive cells ranging between 84.7 and 97.6% in both control and patients’ cells (Supplementary Material, Fig. S2A–H). Furthermore, RT-qPCR assays showed upregulation of NCC markers PAX3, SOX10, ZIC1 and TFAP2A, and downregulation of the pluripotency marker OCT3/4, when compared with the originating iPSCs (Supplementary Material, Fig. S2I–M). Moreover, RCPS iNCCs showed reduction of ∼70% in EIF4A3 mRNA and ∼50% in protein expression compared with control iNCCs (Fig. 1A and B).
Figure 1.
Reduced cell migration in RCPS iNCCs. (A, B) RT-qPCR assessment of EIF4A3 mRNA expression (A) and protein levels (B), with western blot assay showing relative EIF4A3/β-actin protein level, in iPSCs from controls and RCPS patients. (C) Bar graph depicting the rate of cell migration [cell-covered area (%)], at 24 h; data shown are representative of two independent assays and three independent measurements in each. (D) Representative phase-contrast micrographs acquired immediately after wounding and at 6, 12, 18 and 24 h afterwards; All values represent mean ± SEM, and sample numbers (n) are indicated *P-value <0.05, **P-value <0.01; Student’s t-test.
Alterations involving proliferation, apoptosis, or migration of NCCs or their cellular derivatives are considered to be underlying mechanisms in a number of NCC-related diseases (9,20). To screen for cellular phenotypes associated with RCPS, we examined cell cycle, apoptosis and migration of RCPS iNCCs, compared with controls. No significant differences in cell cycle distribution or basal apoptosis were detected between patients and controls (Supplementary Material, Fig. S3A–G). Wound-healing migration assays, however, revealed significantly decreased migratory capacity in RCPS iNCCs when compared with iNCCs from control individuals (Fig. 1C and D). This phenotypic assessment reveals delayed NCC migration may contribute to early defects of RCPS.
Mesenchymal stem-like cells can be derived from iNCCs and exhibit differentiation defects
One of the hallmarks of the cranial neural crest is the ability to generate mesenchymal precursors that give rise to the craniofacial skeleton and other structures during embryonic development (9,21). Therefore, to screen for cellular phenotypes associated with the RCPS phenotype, we generated NCC-derived mesenchymal stem-like cells (nMSCs) from RCPS and control iNCCs. nMSCs showed significant transcriptional upregulation of mesenchymal marker ENG (CD105) and downregulation of neural crest marker TFAP2A in comparison to iNCCs (Supplementary Material, Fig. S4A and B). Moreover, nMSCs exhibited typical and homogeneous mesenchymal immunophenotype, with positive staining (>82%) for mesenchymal markers CD73, CD166 and CD90, and negative staining (<5%) for endothelial marker CD31 (Supplementary Material, Fig. S4C); accordingly, nMSCs could be differentiated into mesenchymal derivatives in vitro (Supplementary Material, Fig. S4D). These results are in agreement with the expected mesenchymal stem cell phenotype seen in previous work by our group (22–24). Finally, a discrete, significant reduction (∼15%) of EIF4A3 mRNA expression was observed in RCPS nMSCs in comparison to controls (Supplementary Material, Fig. S5A).
EIF4A3 deficiency is associated with premature osteogenic differentiation in vitro
Next, we hypothesized that the craniofacial phenotypes seen in RCPS could also be caused by disturbances in NCC mesenchymal differentiation, leading to alterations in cartilage formation and/or intramembranous ossification, particularly during mandible development. We therefore investigated chondrogenic and osteogenic differentiation in nMSCs from RCPS and control individuals. Since differences in cell density may influence results of in vitro chondrogenic and osteogenic differentiation, all nMSCs were first subjected to proliferation and apoptosis assays. No significant differences in either parameter were detected between RCPS and control nMSCs (Supplementary Material, Fig. S5B and C).
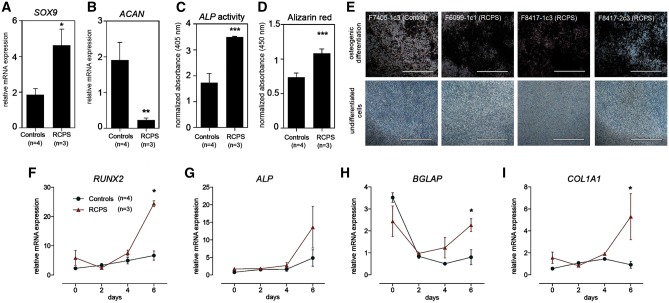
We performed chondrogenic differentiation and then assessed mRNA expression of key chondrogenesis markers, SOX9 and ACAN. In relation to controls, RCPS nMSCs showed augmented expression of early chondrogenesis marker SOX9 and lower expression of late chondrogenesis marker ACAN, after 9 days of differentiation (Fig. 2A and B). During osteogenic differentiation, alkaline phosphatase (ALP) enzymatic activity was significantly increased in RCPS cells after 9 days of osteoinduction, and Alizarin Red staining revealed greater matrix mineralization evident as brown precipitate after 21 days, in comparison to controls (Fig. 2C–E). Next, we assessed expression of key genes involved in osteogenesis during the initial 6 days of differentiation. Compared with controls, at day 6 of osteoinduction, RCPS cells exhibited upregulation of transcription factor RUNX2, as well as upregulation of early osteoblast marker COL1A1, and late-stage osteoblast marker BGLAP. At day 6 of differentiation, ALP expression was higher in RCPS samples, but did not reach statistical significance (Fig. 2F–I). These findings suggest that RCPS nMSCs show premature osteogenic differentiation in comparison to controls. Altogether, use of iNCCs and nMSCs point to defective NCC migration and dysregulation of osteo/chondrogenesis in RCPS pathology.
Figure 2.
Alterations in osteogenic potential in RCPS nMSCs. (A, B) RT-qPCR assessment of SOX9 (A), ACAN (B) in nMSCs from controls and RCPS patients. (C) Quantification of alkaline phosphatase (ALP) enzymatic activity, after 9 days, and (D) alizarin red after 21 days of osteoinduction, in RCPS cells in comparison to controls. Measurements from differentiated cells were normalized to paired, undifferentiated negative staining controls. (E) Representative alizarin red staining micrographs showing matrix mineralization (in dark brown) of RCPS samples vs. one representative control (osteogenic differentiation); micrographs are shown paired to respective negative controls (undifferentiated cells). (F–I) Transcriptional profile of osteogenesis markers (RUNX2, ALP, BGLAP and COL1A1) during the initial 6 days of osteoinduction. All values represent mean ± SEM, and sample numbers (n) are indicated. (A, B) Two-way ANOVA with Bonferroni post-tests; (F-I) Student’s t-test; *P-value <0.05, **P-value <0.01, ***P-value <0.001, Scale bars, (E) 1000 µm.
Eif4a3 germline haploinsufficient embryos are developmentally delayed with craniofacial abnormalities
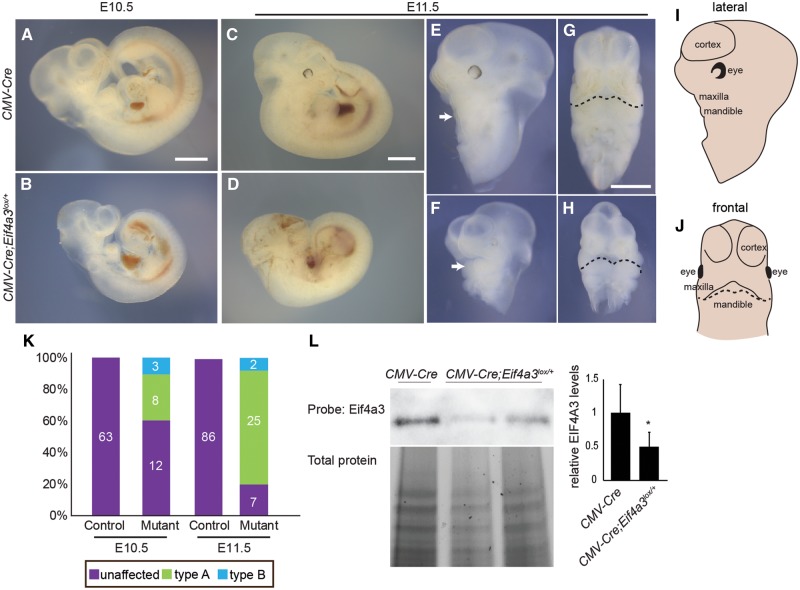
To complement analyses of human iPSCs, we next investigated Eif4a3 function in craniofacial development using mouse models. We generated germline Eif4a3 haploinsufficient mice, by crossing our previously described Eif4a3l°x/+mice (7) to ubiquitous CMV-Cre lines. To examine craniofacial development, embryos were collected at embryonic days (E) 10.5, E11.5 and E12.5 (Table 1). Heterozygous animals were recovered at normal Mendelian ratio at both E10.5 and E11.5; however, lethality was noted by E12.5 (Table 1). Thirty percent of E10.5 Cmv-Cre;Eif4a3l°x/+embryos (n = 8) exhibited marked developmental delay relative to control, as evidenced by reduced body size and delayed eye and limb development (defined as type A mutants with 2 mandibular arches) (Fig. 3A, B and K). Eleven percent of E10.5 Cmv-Cre; Eif4a3l°x/+embryos (n = 3) showed developmental delay and severe facial malformations (defined as type B mutants with 1 apparent mandibular arch) (Supplementary Material, Fig. S6A–C).
Table 1.
Lethality for Cmv-Cre X Eif4a3l°x/+
| E10.5 | Cmv-CreTg/+ | Eif4a3l°x/+ | Cmv-CreTg/+;Eif4a3l°xp/+ | +/+ | P-value |
|---|---|---|---|---|---|
| E10.5 | 19% (n=31) | 24% (n=39) | 25% (n=41) | 32% (n=52) | 0.1377 |
| E11.5 | 29% (n=31) | 16% (n=17) | 27% (n=29) | 28% (n=30) | 0.1860 |
| E12.5 | 33% (n=23) | 29% (n=20) | 8% (n=6) | 30% (n=21) | 0.01587 |
n=number of embryos examined in parentheses.
Figure 3.
Ubiquitous Cre-mediated Eif4a3 haploinsufficiency causes developmental delay and craniofacial defects. Whole mount embryos of indicated genotypes at E10.5 (A, B) and E11.5 (C–H). Both lateral views (E, F) and frontal views (G, H) of head only are shown for CMV-Cre (A, C, E, G) and Cmv-Cre; Eif4a3l°x/+(B, D, F, H). Cmv-Cre; Eif4a3l°x/+embryos shown are type A (developmental delay with two mandibular prominences) (F, H). Mandibles are indicated by arrows (E, F) and a dotted line (G, H). (I, J) Schematics with relevant labels for facial features in the lateral (I) and frontal views (J). (K) Graph depicting quantification of types A and B (more severe with developmental delay, craniofacial abnormality and single mandibular prominence) for indicated ages and genotypes. Total number of embryos examined in each category is indicated. (L) Western blot analysis of whole body from indicated genotypes probed for EIF4A3 and total protein. Relative to control and following normalization for total protein levels, Cmv-Cre; Eif4a3l°x/+embryos have 50% reduction of EIF4A3 (right). n = 5 controls, n = 5 mutants. Error bars, SD, P < 0.05, Student’s t-test. Scales bars, 1 mm.
Relative to E10.5, E11.5 Cmv-Cre; Eif4a3l°x/+embryos showed a more penetrant and wider spectrum of phenotypes including developmental delay (reduced body size, reduced eye pigmentation, delayed limb development), neural tube kinking or delayed closure, and facial malformations (Fig. 3C–J). In marked contrast to control Cmv-Cre littermates, 74% of Cmv-Cre; Eif4a3l°x/+ embryos were developmentally delayed (type A) (Fig. 3F, H and K). Six percent were especially severe with developmental delay and holoprosencephaly, showing a single mandible formation which lacks two distinct mandibular arches, and has no apparent brain septation (type B) (Fig. 3K;Supplementary Material, Fig. S6D–F). EIF4A3 protein levels were reduced in Cmv-Cre; Eif4a3l°x/+ embryos by ∼50% compared with control (Fig. 3L). As further evidence of a role for EIF4A3 in mandible development, we confirmed EIF4A3 expression in the developing mandible (Supplementary Material, Fig. S6G and H). These findings indicate that reduced EIF4A3 impairs embryonic development and may model mandible malformations of RCPS syndrome.
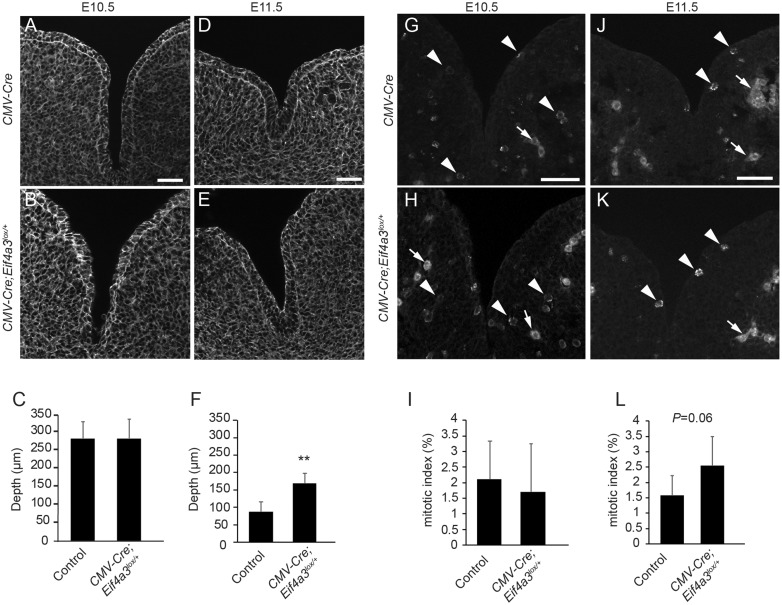
We next examined fusion of the mandibular processes in type A E10.5 and E11.5 tissue sections (Fig. 4A–F). At E10.5, when these processes are just beginning to fuse (25), intermandibular groove depth was equivalent between control and Cmv-Cre; Eif4a3l°x/+mutants (285 and 281 µm, respectively) (Fig. 4A–C). By E11.5, mandibular processes have normally begun to fuse, as evidenced by groove depth of 87 µm in control. In contrast, intermandibular grooves of E11.5 Cmv-Cre; Eif4a3l°x/+embryos were significantly deeper, on average 170 µm (Fig. 4D–F). This phenotype was evident across multiple stage-matched mutant and control embryos, suggesting that developmental delay is insufficient to explain delayed fusion. These results indicate that Eif4a3 is required between E10.5 and E11.5 for mandibular process outgrowth and fusion.
Figure 4.
Mandibular formation defects in ubiquitous Eif4a3 haploinsufficient embryos. Images depicting phalloidin staining to highlight cell architecture of mandibular prominences in CMV-Cre (A, D) and type A Cmv-Cre; Eif4a3l°x/+embryos (B, E), at E10.5 (A, B, n = 6 controls, n = 3 mutants) and E11.5 (D, En = 4 controls, n = 5 mutants). (C, F) Graphs depicting average quantification of mandible clefts at E10.5 (C) and at E11.5 (F) for indicated genotypes. Images depicting PH3 staining in E10.5 CMV-Cre (G) and type A Cmv-Cre; Eif4a3l°x/+embryos (H) at E10.5 (G, H, n = 6 controls, n = 4 mutants) and E11.5 (J, K, n = 4 controls, n = 5 mutants). Arrowheads point to mitotic figures while arrows point to background vasculature. (I, L) Graphs depicting average quantification of mitotic index at E10.5 (I) and at E11.5 (L) for indicated genotypes. Error bars, SD, **P < 0.005, Student’s t-test, scale bars, 50 µm.
We next investigated potential causes of mandibular process fusion defects. Mitosis of mesenchymal progenitors that generate cartilage is important for mandibular development (26). Quantification of mitotic cells within mandibular processes, using phospho-histone 3 (PH3) staining, showed no significant differences between E10.5 control and Cmv-Cre; Eif4a3l°x/+ embryos (Fig. 4G–I). However, we observed a slight, but not quite significant, increase in mitosis in E11.5 mutants (Fig. 4J–L). This suggests mitosis delays could contribute to mandible developmental defects but is unlikely the primary cause. Restriction of apoptosis has also been shown to be critically important for mandibular development, as evidenced in Twsg1 mutants (27). Terminal deoxynucleotidyl transferase UTP nick end labeling (TUNEL) staining of E10.5 whole mount embryos showed evidence of apoptosis only in the most severe type B E10.5 Cmv-Cre; Eif4a3l°x/+ mutants (Supplementary Material, Fig. S7A–I). These results exclude massive apoptosis as a main cause of the mandibular fusion defects in this mouse model.
Neural crest specific haploinsufficiency of Eifa3 disrupts craniofacial development
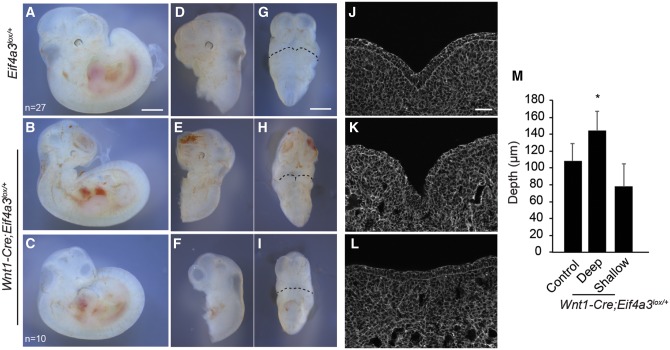
We further probed the mechanism by which Eif4a3 deficiency impairs mandible development by asking what is the cell autonomous requirement of Eif4a3 during fusion of the mandibular processes. Given defects seen in patient-derived iNCCs, we hypothesized that Eif4a3 functions in NCCs in vivo. To deplete Eif4a3 specifically in NCCs, we generated Wnt1-Cre; Eif4a3l°x/+embryos. Compared with control (n = 26), E11.5 Wnt1-Cre; Eif4a3l°x/+ embryos exhibited severe and 100% penetrant craniofacial malformations (n = 10) (Fig. 5A–I). The overall limb development of mutant embryos was relatively normal. Although their body size was slightly reduced, other features of developmental progression such as formation of hair follicles were intact. This argues that unlike Cmv-Cre; Eif4a3l°x/+ embryos there is no significant developmental delay in Wnt1-Cre; Eif4a3l°x/+ embryos. 100% of Wnt1-Cre; Eif4a3l°x/+embryos exhibited micrognathia (Fig. 5B, C, E, F, H and I). Similar to Cmv-Cre; Eif4a3l°x/+embryos, Wnt1-Cre; Eif4a3l°x/+ embryos (n = 3) showed deeper intermandibular grooves than control (n = 6) (Fig. 5J, K and M). Some mutant embryos (n = 4) also had more shallow grooves than control, highlighting a spectrum of phenotypes associated with Eif4a3 haploinsufficiency (Fig. 5J, L and M). Increased apoptosis (CC3+ staining) was primarily evident in lateral facial structures of Wnt1-Cre; Eif4a3l°x/+ embryos, to a slightly greater extent than in Cmv-Cre; Eif4a3l°x/+embryos (Supplementary Material, Fig. S8A–I). This suggests that apoptosis may contribute in part to mandible groove defects, but is likely not the only cause. Importantly, together with the iNCC results, these data argue that Eif4a3 expression in NCCs and its derivatives contributes to RCPS pathogenesis.
Figure 5.
Wnt1-Cre mediated haploinsufficiency of Eif4a3 in NCCs causes craniofacial defects. Whole mount embryos of indicated genotypes at E11.5 showing lateral views (A–F) and frontal views (G–I) shown for Eif4a3l°x/+(A, D, G, n = 27 embryos) and Wnt1-Cre; Eif4a3l°x/+(B, C, E, F, H, I, n = 10 embryos total for B, C). Images of mandibular processes stained for phalloidin for Eif4a3l°x/+(J) or Wnt1-Cre; Eif4a3l°x/+(K, L). Less severe (B, E, H, K) and more severe (C, F, I, L) Wnt1-Cre; Eif4a3l°x/+embryos are depicted. (M) Graph depicting quantification of mandible grooves for indicated genotypes, representing embryos with deeper grooves (n = 3), those with shallow grooves (n = 4) and controls (n = 6). Mean values shown for all embryos. Error bars, SD, P < 0.05, Student’s t-test. Scales bars, (A–I) 1 mm, (J–L) 50 µm.
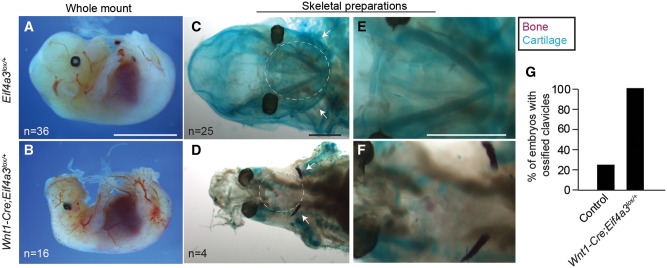
Given that patient nMSCs exhibited dysregulated chondrogenesis and precocious osteogenic differentiation, we next monitored formation of Meckel’s cartilage and clavicle ossification in vivo (Fig. 6). Compared with control, the head structures of E14.5 Wnt1-Cre; Eif4a3l°x/+ embryos were markedly dysmorphic, missing ears, face and skull. E14.5 mutant embryos were also slightly reduced in body size, but had relatively normal limbs, similar to E11.5 embryos (Fig. 6A and B). Meckel’s cartilage serves as the template for the future mandible (28). In skeletal preparations of E14.5 control embryos (n = 25), Meckel’s cartilage was evident as a V-shaped structure, as previously described (Fig. 6C and E). In contrast, this structure was markedly absent or reduced in E14.5 Wnt1-Cre; Eif4a3l°x/+ embryos (n = 4) (Fig. 6D and F). Owing to loss of this structure, to examine bone ossification we focused on the clavicle, which is also affected in a subset of RCPS patients (2,29,30) and shares a NCC origin with the mandible (31). Alizarin red staining of the clavicle revealed ossification of only 25% of control embryos (5/20) compared with 100% of E14.5 Wnt1-Cre; Eif4a3l°x/+ embryos (4/4) (Fig. 6C–G;Supplementary Material, Fig. S9A–D). This indicates that NCC-specific haploinsufficiency of Eif4a3 disrupts formation of Meckel’s cartilage and causes premature clavicle ossification. This parallels observations made with human RCPS nMSCs, and provides a mechanistic explanation for mandibular and clavicular anomalies seen in patients.
Figure 6.
Wnt1-Cre mediated haploinsufficiency of Eif4a3 disrupts Meckel’s cartilage development and causes premature clavicle ossification. (A, B) Whole mount E14.5 Eif4a3l°x/+ (A) and Wnt1-Cre; Eif4a3l°x/+ embryos (B) with number of embryos analyzed indicated. Skeletal preparations of E14.5 Eif4a3l°x/+(C, E) and Wnt1-Cre; Eif4a3l°x/+ embryos (D, F) showing low magnification and higher magnification images. Note cartilaginous and well-formed Meckel’s cartilage (blue staining, white circle) in control but underdeveloped in mutants (C–F). Note controls lack clavicle ossification (purple, white arrows) which is present in mutant clavicles (C–F). (G) Graph depicting quantification of fraction of embryos with ossified clavicles. Scales bars, (A, B) 5 mm, (C–F) 1 mm.
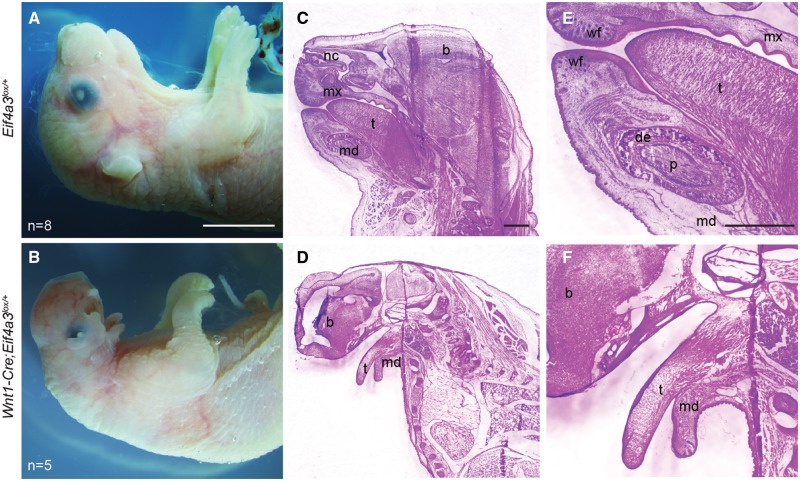
Finally, we assessed the impact of NCC-specific Eif4a3 haploinsufficiency upon craniofacial structures at the end of gestation. Compared with control (n = 8), E18.5 Wnt1-Cre; Eif4a3l°x/+ embryos exhibited severely hypoplastic mandibles (n = 5) (Fig. 7A and B). We observed additional phenotypes associated with defective NCC development, including reduced eyelid closure, loss of outer ear structures, and in some cases exencephaly. Hematoxylin and eosin (H&E) analyses of control mandibles demarcated the tongue, dental papilla, dental epithelium, nasal cavities, whisker follicles and maxillary structures (Fig. 7C and E). Conversely, no defined mandibular structures or nasal passages were evident in mutant embryos, although tongues were present without clefts, similar to RCPS patients (Fig. 7D and F;Supplementary Material, Fig. S10A–H). Taken together, these findings reveal an essential requirement of Eif4a3 in NCCs and their mesenchymal derivatives during craniofacial development.
Figure 7.
Wnt1-Cre mediated Eif4a3 haploinsufficiency causes underdevelopment of the mandible and craniofacial defects. (A, B) E18.5 whole mount Eif4a3l°x/+(A) and Wnt1-Cre; Eif4a3l°x/+ (B) embryos with number of embryos listed. H&E staining of sagittal tissue sections from Eif4a3l°x/+(C, E) and Wnt1-Cre; Eif4a3l°x/+ embryos (D, F) showing low and 3× high magnification images of the same embryos. t, tongue; p, dental papilla; b, brain; de, dental epithelium; nc, nasal cavity; md, mandible; wf, whisker follicle; mx, maxilla. Scales bars, (A, B) 5mm, (C–F) 1 mm.
Discussion
RCPS is a craniofacial syndrome caused by loss-of-function mutations in the RNA-binding protein EIF4A3. The cellular mechanisms by which reduced EIF4A3 causes the phenotypic specificity of this syndrome have been entirely unknown. Here, we employed two complementary approaches to gain fundamental new insights into the underlying pathology of this developmental syndrome. Patient-derived iPSCs highlight delayed NCC migration and precocious bone differentiation in RCPS pathology. Eif4a3 haploinsufficient mice recapitulate mandibular and craniofacial abnormalities associated with RCPS, and establish in vivo requirements for Eif4a3 in NCC development and differentiation. Altogether this points to specific and converging alterations of NCC as an underlying cause of RCPS.
One major unsolved problem concerning craniofacial development and disease is the existence of a number of clinically discernible syndromes caused by mutations in genes that exert basic and ubiquitous functions. Genetic mutations affecting mRNA splicing and ribosome biogenesis, for example, manifest tissue-specific phenotypes restricted to craniofacial structures not only in RCPS but in additional disorders such as Treacher Collins syndrome (11,32–34). Elucidating the pathogenetic mechanism behind RCPS will aid in clarifying the etiology of these other craniofacial syndromes and understanding developmental pathways critical for craniofacial morphogenesis.
Generation of RCPS patient-derived iPSCs and a new mouse model
Here, to elucidate the pathogenetic mechanism responsible for RCPS, we generated patient-specific, iPSC-derived iNCCs and nMSCs. All iPSCs showed hallmarks of pluripotent stem cells, including high expression of pluripotency-associated genes, no aneuploidies and in vivo teratoma formation, showing that reduced EIF4A3 in RCPS patients’ cells does not negatively impact iPSC generation. Moreover, iPSCs were efficiently differentiated into iNCCs exhibiting typical p75(NTR)/HNK1 double-positive staining and high expression of NCC markers. The neural crest identity of all iNCCs was further confirmed through generation of homogeneous nMSC populations with mesenchymal immunophenotype and ability to give rise to mesenchymal derivatives in vitro, as previously reported for other iNCC lines (17,18,35). Taken together, our strategy successfully yielded disease-relevant cell types to study RCPS in vitro.
We also generated two new Eif4a3 mouse models that recapitulate several anomalies seen in RCPS patients, including abnormal mandible development, microstomia, micrognathia, ear anomalies and clavicle defects. RCPS is caused by recessive hypomorphic EIF4A3 alleles, resulting in 15–70% reduction in EIF4A3 levels depending on cell type (3). It is noteworthy that haploinsufficiency in mice reduces EIF4A3 levels by 50% yet results in comparable phenotypes to humans whose EIF4A3 levels are more varied. These potential differences in susceptibility to disease phenotypes could be because that humans are genetically diverse, whereas mice are inbred. In addition, some of the phenotypic variability in the Eif4a3 Cre-lox mouse models could be due to Cre efficiency. The use of Wnt1-Cre enabled us to investigate how Eif4a3 depletion affects craniofacial development; however, this Cre is not active in the developing limb (36). In the future, it will be of interest to employ other Cre drivers to recapitulate additional relevant RCPS phenotypes. Regardless, given that the Eif4fa3 haploinsufficient mice present with the most penetrant phenotypes of RCPS, these models are valuable for gaining mechanistic insights into RCPS pathology. Beyond this, the Eif4a3 haploinsufficient mice also demonstrate new biological functions for Eif4a3 in NCC and craniofacial development. These roles are likely conserved given requirements for Magoh and Eif4a3 in development of NCC-derived cell types in mice and Xenopus, respectively (5,37).
Neural crest defects underlying RCPS
The use of patient-derived iPSCs pinpoints defective NCC migration as one key cellular mechanism to explain RCPS etiology. We speculate EIF4A3 depletion delays NCC migration at early developmental stages, causing defective mandibular fusion and formation, as seen in Eif4a3-deficient embryos. This may be due to reduced mesenchymal progenitors populating the pharyngeal arches and/or impaired cartilage and bone formation. Although migration defects are common in mouse models with micrognathia, they have not been observed in animal models for the craniofacial disorders ribosomopathies (12,38) or spliceopathies (11,13), despite the clinical overlap of RCPS with these conditions. This highlights an interesting and potentially unique role for NCC migration in RCPS etiology.
Although some NCC-related disorders have been attributed to increased cell death or altered proliferation (13,38,39), neither RCPS iNCCs and nMSCs nor mouse models showed massive alterations in either process. This suggests that aberrant apoptosis and proliferation are not a major cause of RCPS, however the possibility remains that low levels of apoptosis and proliferation delays could impair outgrowth and fusion of mandibular arches and thus contribute to RCPS pathogenesis. Indeed, apoptosis was detectable in especially severe Cmv-Cre and Wnt1-Cre Eif4a3 mouse mutants. Additionally, mitotic index was increased, albeit not quite significantly, in Eif4a3 haploinsufficient mandibular processes, consistent with known roles for EIF4A3 and its binding partners in mitosis (40,41,42).
Both in vitro and in vivo experiments also demonstrate that dysregulation of chondrogenesis and osteogenesis is highly relevant for RCPS. Paralleling observations with RCPS-derived nMSCs, NCC-specific depletion of Eif4a3 disrupts formation of Meckel’s cartilage and causes premature clavicle ossification. Development of the cranioskeletal elements affected in RCPS relies upon proper differentiation of NCC and their progeny residing in the craniofacial mesenchyme. In particular, mandible morphogenesis is thought to depend upon proper formation of Meckel’s cartilage before intramembranous ossification (14,43). Altogether this suggests a mechanistic explanation for mandibular and clavicular anomalies seen in RCPS patients.
Eif4a3 deficiency and phenotypic variability
Both iPSC and mouse models established here recapitulate deficiencies in mandibular development, amongst the most relevant craniofacial alterations of RPCS. A large spectrum of phenotypic variability in development of mandibular processes is observed in Eif4a3 haploinsufficient mice. This parallels RCPS, where there is great clinical variability amongst patients, even within the same family (1,3). Several RCPS patients have died in early infancy owing to severe micrognathia associated with severe respiratory distress or required surgical interventions for survival (1,2,44). A fraction of embryos with either ubiquitous or NCC-specific Eif4a3 depletion presented with especially severe phenotypes not so far described in humans, including holoprosencephaly or exencephaly. Likewise, Eif4a3 haploinsufficiency in neural progenitors causes severe microcephaly, also not reported in patients (7). These phenotypic differences could be explained by inbred genetic backgrounds of mice, exposing phenotypes masked in humans. Alternatively, brain malformations in humans could be embryonic lethal. Consistent with this possibility, spontaneous abortions have been reported in RCPS families [reviewed in (1)].
Eif4a3 mechanisms and RCPS etiology
Going forward it will be important to build upon the cellular mechanisms defined here, to further understand how EIF4A3 functions in NCCs and in particular how it influences early ossification. Predictions can be made from studies in the developing brain, where haploinsufficiency of Eif4a3 and other EJC components impair neural progenitor proliferation, leading to precocious neuronal differentiation (7). In the case of the EJC component, MAGOH, a prolonged mitosis of neural stem cells is sufficient to trigger altered neuronal differentiation in these mutants (41). Perhaps Eif4a3 has parallel requirements in mitosis of NCCs, which leads to precocious ossification. In the brain, Eif4a3 haploinsufficiency also triggers aberrant p53 activation and altered expression of ribosomal components. Dysregulation of ribosome biogenesis and p53 signaling are linked to many craniofacial disorders, including Treacher Collins syndrome (12). Given this connection, along with parallel roles for Eif4a3 in bone differentiation, it is fascinating to consider if EIF4A3 regulates ribosome biogenesis and p53 signaling in NCCs, and if this contributes to RCPS pathology. Moreover, craniofacial disorders similar to RCPS can be caused by mutation of spliceosomal components (11). Given its canonical role in the EJC, it will also be of interest to elucidate if EIF4A3 influences NCC via splicing regulation. For example, EIF4A3 may bind and regulate levels and/or splicing of pro-chondrogenic transcripts required for ossification. Investigation of transcriptome-wide RNA regulation and splicing control in Eif4a3 mutants will provide valuable insights into how EIF4A3 influences chondrogenesis at a molecular level.
In summary, we integrate iPSC technology with mouse models to study a craniofacial syndrome, establishing a foundation for characterizing biological mechanisms responsible for craniofacial disorders. Using patient-derived iPSCs we could recapitulate NCC migration and mesenchyme differentiation in vitro, and show both are compromised in EIF4A3 depleted cells. Likewise, Eif4a3 deficient mouse models exposed defective mandible process formation and bone ossification in vivo. Altogether, this points to critical cellular mechanisms within NCCs which help explain aberrant mandible development in RCPS patients. Going forward, the models established here are valuable for further elucidating how EIF4A3 mutations causes RCPS, and more broadly, for establishing an experimental framework to study craniofacial disorders.
Materials and Methods
Dermal fibroblast and erythroblast cultures
Skin biopsies were extracted from the lower posterior flank region with the use of 2-mm punch instruments and stored in DMEM/high glucose (Life Technologies) supplemented with 100 µg/ml of Normocin (Invivogen), at 4 °C. Within 24 h, biopsies were rinsed with PBS and incubated in a Dispase solution (1 µg/ml; Sigma-Aldrich) in DMEM/high glucose, overnight at 4 °C. Specimens were then rinsed two times with PBS, their epidermis was removed and the dermis was minced with a scalpel and seeded into 25 cm2 culture flasks containing fibroblast medium consisting of DMEM/high glucose supplemented with 1% penicillin–streptomycin and 10% fetal bovine serum (FBS, Life Technologies). CD71+ cells from whole blood samples were reprogrammed according to Okita et al. (45). Briefly, peripheral blood mono-nuclear cells (PBMCs) were isolated by adding 2 mM EDTA in PBS to whole blood samples, and after centrifugation at 1000g for 10 min, transferring the diluted blood to a Leucosep tube pre-filled with Ficoll-Paque (GE Healthcare). Then, the tube was centrifuged at 400g for 30 min at 18 °C, the plasma was discarded, and the cells from white ring collected. PBMCs were cultured in Stem Span Medium (Stem Cell) supplemented with 50 ng/ml of recombinant human stem cell factor (SCF; R&D), 500 U/ml of erythropoietin (EPO; R&D), 1 mM dexamethasone (Sigma), 40 ng/ml of IGF-1, 10 ng/ml of interleukin 3 and 10 µg/ml gentamicin (Miltenyi Biotec) in a 24-well ultra-low attachment plate (Corning). After 4 days in culture, magnetic sorting of CD71+ cells was performed with the use of microbeads (Miltenyi Biotec).
Generation of iPSCs
Dermal fibroblast cultures were derived from skin punch biopsies from three RCPS patients (F8417-1, F8417-2 and F6099-1), previously genotyped elsewhere (3), and from two controls (F9048-1 and F7405-1). Erythroblast cultures were derived from peripheral blood collection from one control (F8799-1). Fibroblasts and erythroblasts were reprogrammed with the use of episomal vectors (pCXLE-hOCT3/4-shP53-F, addgene plasmid 27077; pCXLE-hSK, addgene plasmid 27078; pCXLE-hUL, addgene plasmid 27080), as described (45), in an Amaxa Nucleofector II (program U-020 for fibroblasts and T-016 for erythroblasts) with either NHDF (fibroblasts) or CD34+ (erythroblasts) nucleofector kits (Lonza), according to the manufacturer’s recommendations. Two days after nucleoporation, cells were co-cultivated with irradiated murine embryonic fibroblasts (Millipore) in embryonic stem cell medium (DMEM/F12 supplemented with 2 mM GlutaMAX-I, 0.1 mM non-essential amino acids, 100 µM 2-mercaptoethanol, 20% of knockout serum replacement, 0.1% Gentamicin, 10 ng/ml of bFGF (all provided by Life Technologies), 2 µM SB431542, 0.5 mM valproic acid sodium salt, 0.25 mM sodium butyrate (Sigma-Aldrich), 0.5 µM PD0325901 and 2 µM Thiazovivin (Miltenyi Biotec). Typical iPSC colonies were transferred to 60-mm Matrigel (BD-Biosciences)-coated plates and expanded in iPSC medium consisted of Essential 8™ Medium (Life Technologies) supplemented with 100 µg/ml of Normocin (Invivogen).
Total DNA was extracted from iPSC cultures with the use of NucleoSpin Tissue (Macherey-Nagel), following supplier’s instructions. For PCR reactions, primers targeting the OriP gene present in the backbone of the episomal vectors were used (Supplementary Material, Table S2), according to recommendations provided elsewhere (Epi5 Episomal iPSC Reprogramming Kit, Life Technologies). Multiplex ligation-dependent probe amplification (MLPA) analysis was performed with subtelomeric kits (P036 and P070; MRC-Holland) to detect chromosomal imbalances, as previously described (46). One of the control iPSCs used in this study (F7405-1) had been generated with retroviral transduction and has already been described and characterized elsewhere (47).
Differentiation of iNCCs from iPSCs
Procedures for iNCC derivation were based on previously published methodology (17,18). Before differentiation, iPSC colonies were adapted to single-cell passaging by rinsing cells with PBS followed by dissociation with Accutase (Life Technologies) for up to 5 min at room temperature (RT), followed by centrifugation at 200g for 4 min and seeding onto 60-mm Matrigel-coated petri dishes. After two subcultures, single cells were seeded onto 60-mm Matrigel-coated dishes at 1× 104 cells/cm2. Two days post-seeding, medium was changed to iNCC differentiation medium, composed of Essential 6™ Medium (Life Technologies) supplemented with 8 ng/ml bFGF (Life Technologies), 20 µM SB431542 (Sigma-Aldrich), 1 µM CHIR99021 (Sigma-Aldrich) and 100 µg/ml Normocin; differentiation medium was changed every single day. After ∼2–4 days, neural crest-like cells were seen detaching from colony borders. The cells were split before reaching confluence, using Accutase, at RT, until differentiated cells detached. Cell suspensions were centrifuged at 200g for 4 min and re-seeded into new 60-mm Matrigel-coated dishes in fresh iNCC differentiation medium. With this method, passaging was performed whenever necessary, for 15 days, after which morphologically homogeneous iNCC cultures were obtained. Differentiated iNCCs were cultivated for up to eight passages in iNCC differentiation medium, replenished daily. In all procedures involving single-cell passaging, media were supplemented with 5 µM Rock inhibitor (Sigma-Aldrich) upon seeding and maintained for 24 h; after about 4 days of iNCC differentiation, Rock inhibitor was no longer needed to maintain cell viability.
Wound healing assay
For the wound healing assay (48), iNCCs were seeded at 5×105 cells/cm2 into non-coated 24-well plates (Corning), in iNCC medium. When cells reached 90–100% confluence, the monolayer was scratched in a straight line with a p200 pipette tip. Debris was removed and the edge of the scratch was smoothed by washing two times with DPBS (Life Technologies). Then, the culture medium was replaced and cell migration was monitored. Cells were seeded in quadruplicates and three fields were selected in each well, which were photographed every 30 min during 24 h, using the High Content Imaging InCell Analyzer 2200 (GE Healthcare) at the CEFAP facility, Institute of Biomedical Sciences, University of São Paulo, Brazil. All samples were assessed simultaneously in two independent experiments. The percentage of the wound covered by migrating cells after 24 h was quantified in RCPS and controls iNCCs, on ImageJ.
Differentiation of nMSCs from iNCCs
nMSC populations were obtained through culturing of iNCCs with mesenchymal stem cell medium, as previously described (17). In brief, iNCCs were seeded at 2×104 cells/cm2 onto non-coated 60-mm tissue culture dishes in nMSC medium (DMEM/F12 supplemented with 10% FBS, 2 mM GlutaMAX-I, 0.1 mM non-essential aminoacids and 100 µg/ml Normocin. Cells were differentiated for 6 days and passaged with TrypLE™ Express (Life Technologies) when needed. nMSC cultures were expanded in nMSC medium for up to 6 passages, with medium changes every 3 days.
nMSC chondrogenic, osteogenic and adipogenic differentiation
For the osteogenic induction, cells were seeded in 12-well plates (Corning) (104 cells/cm2), in triplicates. After 3 days, medium was replaced with osteogenic induction medium (StemPro Osteogenesis Kit, Life Technologies); in parallel, negative controls were cultivated in nMSC medium. Differentiation and nMSC media were changed every 2–3 days. After 9 days, ALP activity was quantified through incubation with phosphatase substrate (Sigma-Aldrich), and the resulting p-nitrophenol was quantified colorimetrically using a Multiskan EX ELISA plate reader (Thermo Scientific) at 405 nm; after 21 days, extracellular matrix mineralization was assessed through alizarin red staining at 450 nm. In both assays, absorbance data were normalized by subtracting from undifferentiated, negative controls. For chondrogenesis, 1×105 cells/well were plated into 6-well plates and after 3 days nMSC growth medium was replaced with chondrogenic medium (StemPro Chondrogenesis Kit; Life Technologies). To quantify chondrogenic markers, total RNA was extracted after 9 days of differentiation. After 21 days, pellets were fixed and frozen in Tissue-Tek O.C.T (Sakura) and then, cut in 5-µm cryosections which were fixed with 4% paraformaldehyde and stained with Alcian Blue 0.1% in 0.1 M HCl. Adipogenic differentiation was performed on 1×104 cells/cm2 seeded into 6-well plates. After cells achieved 80% confluence, nMSC growth medium was replaced with adipogenesis medium (StemPro Adipogenesis Kit, Life Technologies). Cells were differentiated for 21 days, after which Oil red staining was performed. Cells were washed with PBS, fixed in 4% paraformaldehyde and stained with 0.5% Oil Red in isopropanol. Pictures were taken under an Axiovision microscope (Zeiss).
Apoptosis, proliferation and cell cycle assays in vitro
For the apoptosis assays, a total of 105 cells/well was seeded into 6-well culture plates (Corning). On the next day, apoptotic activity was measured with a kit based on Annexin V and 7-AAD staining (Guava Nexin Reagent; Millipore), following the manufacturer’s instructions. Cells treated with 10 µM H2O2 for 30 min were used as staining controls. Subpopulations were ascertained in a Guava flow cytometer (EMD Millipore) as follows: non-apoptotic cells-AnnexinV(−) and 7-AAD(−); early apoptotic cells-Annexin V(+) and 7-AAD(−); late-stage apoptotic and dead cells-Annexin V(+) and 7-AAD(+). Cell proliferation was assessed with the use of an XTT assay (Cell Proliferation Kit II; Roche), following supplier’s instructions. Briefly, cells were seeded into 96-well culture plates at 2×103 cells/well, in quadruplicates. To quantify metabolically active cells, medium was changed to DMEM/F12 without phenol red (Life Technologies), a solution of XTT was added, and cells were incubated at 37 °C for 3 h. Immediately following incubation, plates were colorimetrically assessed in a microplate spectrophotometer (Epoch; BioTek) at 450 nm. To determine the percentage of cells in G0/G1, S and G2/M phases based on DNA content, a cell cycle assay was performed, using the Guava Cell Cycle Reagent (Millipore), in a Guava easyCyte flow cytometer (Millipore), according to the manufacturer’s instructions.
Flow cytometry
To assess the immunophenotype of iNCCs, cells were detached with Accutase, and washed two times with two volumes of blocking solution (4% BSA in PBS). iNCCs were incubated with the conjugated antibodies in blocking solution in the dark for 1 h at 4 °C, washed two times with PBS, and fixed in 1% paraformaldehyde/PBS. The following antibodies were used: IgM Κ FITC Mouse Anti-Human CD57 (anti-HNK1; BD Pharmingen 561906), IgG1 Κ Alexa Fluor 647 Mouse Anti-Human CD271 (anti-p75NTR; BD Pharmingen 560877), FITC Mouse IgM Κ isotype control (BD Pharmingen 555583) and Alexa Fluor 647 Mouse IgG1 Κ isotype control (BD Pharmingen 557714). Antibody concentrations followed the manufacturer’s recommendations. A minimum of 5000 events were acquired in a FACS Aria II flow cytometer (BD Biosciences) and analyzed on FlowJo (X 10.0.7r2).
nMSCs were dissociated with TrypLE Express and washed two times with two volumes of blocking solution (1% BSA in PBS without Ca2+ and Mg2+). nMSCs were incubated in the aforementioned conditions with the following conjugated antibodies: FITC Mouse Anti-Human CD31 (BD Pharmingen 555445), APC Mouse Anti-Human CD73 (BD Pharmingen 560847), PE Mouse Anti-Human CD90 (BD Pharmingen 555596), PE Mouse Anti-Human CD166 (BD Pharmingen 559263) and FITC, PE and APC Mouse IgG1 Κ Isotype Controls (BD Pharmingen 555748, 554681 and 555749, respectively). Antibody concentrations followed the manufacturer’s recommendations. At least 5000 events were acquired in FACS Aria II equipment and analyzed on FlowJo (X 10.0.7r2).
Real-time quantitative PCR (RT-qPCR)
Total RNA was obtained from cell samples with the use of Nucleospin RNA II extraction kit (Macherey-Nagel) following the manufacturer’s recommendations. Briefly, 1 µg of total RNA was converted into cDNA using Superscript IV (Life Technologies) and oligo-dT primers according to the manufacturer’s recommendations. RT-qPCR reactions were performed with 2× Fast SYBR Green PCR Master Mix (Life Technologies) and 50–400 nM of each primer. Fluorescence was detected using the 7500 Fast Real-Time PCR System (Life Technologies), under standard temperature protocol. Primer pairs were either designed with Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/; date last accessed March 10, 2017) or retrieved from Primer Bank (http://pga.mgh.harvard.edu/primerbank/; date last accessed March 10, 2017) primers are listed in Supplementary Material, Table S2 and their amplification efficiencies (E) were determined by serial cDNA dilutions log10-plotted against Ct values, in which E = 10−1/sl°pe. Gene expression was assessed relative to a calibrator cDNA (ΔCt). NormFinder (49) was used to determine the most stable endogenous control (among ACTB, TBP, HMBS, GAPDH and HPRT1), and calculate normalization factors (E−ΔC) for each sample. The final relative expression values were determined based on a previous method (50) by dividing E−ΔCt of target genes by E−ΔCt the endogenous control. All relative expression values were log-transformed for analysis and graphed in linear scale, unless stated otherwise. Primers were supplied by Exxtend.
Western blot analyses
Cells were grown until 80% confluence in complete culture medium. Total protein lysates were obtained using RIPA buffer (Sigma) supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Protein concentration was determined using a bicinchoninic acid (BCA) assay kit according to the manufacturer’s instructions (Thermo Fisher Scientific). Cell lysates were denatured by adding Tris–glycine SDS sample buffer (Life Technologies) containing 0.3 M 2-mercaptoethanol (Sigma). Then, 20 µg of total cell lysates were separated by SDS-PAGE and dry-transferred to nitrocellulose membranes with the iBlot system (Life Technologies) according to the manufacturer’s recommendations. Membranes were blocked in buffer TBS-T (1 M Tris–HCl pH 7.5, 5M NaCl, 0.1% Tween-20) with 5% BSA under constant stirring for 1 h at RT, washed four times with TBS-T, and incubated with Rabbit anti-EIF4A3 (1:2000 dilution in TBS-T/BSA; sc-33632; Santa Cruz) primary antibody, overnight at 4 °C, under constant stirring. Detection was performed using anti-rabbit IgG, HRP-linked Antibody (1:2500 dilution in TBS-T/BSA; #7074; Cell Signaling) under constant stirring for 1 h at RT, followed by incubation with ECL Prime (GE Healthcare) and capture in the Image Quant LAS 4000 Mini (GE Healthcare). β-Actin was used as a loading control (1:30 000 dilution in TBS-T/BSA; ab49900, Abcam). Densitometric analyses were performed on the Image Quant TL 8.1 software (GE Healthcare). EIF4AIII protein levels were quantified and normalized to the corresponding β-actin levels.
Embryos were lysed in RIPA lysis buffer (Thermo Scientific) with protease inhibitors (Pierce). BCA assays were performed (Pierce) and 20 µg of lysate was run on 4–20% gradient precast TGX gels (Biorad), activated following Biorad’s standard protocol, then transferred onto PVDF membranes (Biorad). Membranes were blocked with 5% milk then blotted with rabbit anti-EIF4A3 1:50 (sc67369, Santa Cruz) and protein levels normalized to total protein as determined by TGX activation using UV. Imaging was performed with a Biorad ChemiLab XRS+ machine with ImageLab software.
Immunofluorescence and H and E staining
Cells were fixed in 4% paraformaldehyde for 20 min at RT, followed by permeabilization with PBS 0.2% Triton X-100, for 30 min, at 4 °C. Blocking was carried out with PBS 5% BSA for 1 h at RT, followed by incubation with primary antibodies in blocking solution overnight, at 4 °C, under constant stirring. After washing two times with PBS, cells were incubated with secondary antibodies in blocking solution at 4 °C for 1 h, in the dark, followed by two PBS washes and counterstaining with DAPI solution (Life Technologies) for 2 min at RT. After a final PBS wash, cells were analyzed with a fluorescent microscope (Axiovision; Zeiss). The primary Anti-OCT4 antibody (ab19857; Abcam), secondary Goat anti-Rabbit IgG (H + L) antibody—Alexa Fluor 546 conjugate (A-11010; Life Technologies), primary Anti-SSEA4 antibody [MC813] (ab16287; Abcam) and secondary Goat anti-Mouse IgG (H + L) antibody—Alexa Fluor 488 conjugate (A-11001; Life Technologies) were used. Antibody concentrations followed the manufacturer’s recommendations.
Embryos were fixed overnight in 4% paraformaldehyde (PFA) at 4 °C, followed by submersion in 10% sucrose, then 20% sucrose until sinking, then embedded in NEG50 and kept at −80 °C. Face cryostat sections (20 μm) were prepared and either stored at −80 °C or prepared for staining. Sections were permeabilized with 0.25% Triton X-100 for 10 min and blocked with either 5% NGS or MOM block reagent (Vector laboratories) for 1 h at RT. Primary antibodies were used at the following dilutions: rhodamine phalloidin 1:100 (R415, Molecular Probes), PH3 1:200 (06-570, Millipore), EIF4A3 1:500 (A302-980A, Bethyl), CC3 (9661, Cell Signaling) for 2 h at RT or overnight at 4 °C. Sections were counterstained with Hoescht for 15 min at RT with secondary antibodies (Thermo Scientific) which were used at 1:200. Standard H&E protocols were used, in brief: sections were dehydrated in Xylene for 1.5 min, Flex100 48 s (Thermo Scientific), Flex 95 24 s (Thermo Scientific), running water for 30 s, then put through a series of dyes and enhancers, hematoxylin (Ricca Chemical Company) 1.5 min, water 1 min, clarifier 30 s (Thermo Scientific), running water 30 s, bluing reagent (Thermo Scientific) 24 s, running water 30 s, Flex95 24 s, Eosin Y 24 s (Harleco), then again dehydrated in Flex100 48 s ×2, Xylene 48 s ×2 before mounting. Depth was calculated by drawing a straight line from the two highest points of each arch and measuring downward into the deepest part of the cleft. Mitotic index was calculated by counting the number of mitotic figures (based on both PH3 staining and DNA morphology) and dividing by the total number of cells as determined by Hoescht staining.
Mouse husbandry and genetics
Plug dates were defined as embryonic day (E) 0.5 on the morning the plug was identified. Cmv-Cre mice and Wnt1-Cre2 mice were from Jackson labs (36). As Cmv-Cre is X-linked, we generated embryos for all analyses using both Cmv-Cre females and males. No phenotypic differences were observed in both types of crosses. Animals reported in Table 1 were generated using Cmv-CreTg/+ females crossed to Eif4a3l°x/+ males. Eif4a3l°x/l°x mice were generated as previously described (7). Genotyping primers and conditions are listed in Supplementary Material, Table S3.
TUNEL assay
The ApoTag Fluorescein In Situ Apoptosis Detection Kit (Millipore) was utilized for whole mount TUNEL staining. Standard protocols were slightly modified as follows: embryos were fixed in 1% PFA for 20 min at 4 °C, washed in 1× PBS and forebrain and heart were pierced with a needle. Embryos were dehydrated in a series of Ethanol washes (25% 10 min, 50% 10 min, 70% 10 min, 95% 10 min ×2, 100% 5 min ×3) and then rehydrated in reverse order. Embryos were pretreated with Proteinase K (20 µg/ml) for 15 min at RT then washed with 1× PBS 2 min ×2. Embryos were put into equilibration buffer for 5 min at RT, then working strength TdT Enzyme for 2 h at 37 °C in a humidified chamber. The reaction was completed by putting the embryos into STOP/WASH buffer for 40 min at 37 °C. The embryos were then washed 3 min ×3 in 2 mM levamisole, then 1 min ×3 in 1× PBS. Anti-digoxigenin conjugate was used at working strength for 40 min RT in the dark then the embryos were washed 2 min ×4 in 1× PBS and then imaged.
Skeletal preparations
E14.5 embryos were dissected and fixed in 100% EtOH overnight at 4 °C, then 100% acetone overnight at 4 °C. Alcian blue staining solution stock was prepared as follows: 5 ml 0.4% Alcian Blue 8 GX in 70% EtOH, 5 ml glacial acetic acid, 70 ml 95% EtOH, 20 ml water, then to 10 ml of staining solution 100 μL of 0.5% Alizarin Red was added. Bone only skeletal preps were performed the same way but without 0.4% Alcian Blue 8GX. Embryos were rocked overnight in staining solution at RT then destained with 1% KOH for approximately 2 h. Embryos were then put through an increasing glycerol gradient then stored in 50% glycerol.
Statistics
All iPSC experiments were performed in triplicate, unless stated otherwise herein. Statistical comparisons were performed on the Graphpad Prism software (v6.0). Values were represented as means ± standard error of the mean (SEM). The level of statistical significance was set at P < 0.05. Mouse experiments were represented as means± standard deviation (SD). All P-values were represented as *<0.05, **<0.005. Significance for all experiments was performed using a standard Student’s two-tailed t-test with the exception of the mouse lethality table (Table 1) which was done using Chi-square analysis.
Ethics statement
The experimental procedures involving samples from human subjects were approved by the Ethics Committee of Instituto de Biociências at Universidade de São Paulo, Brazil (accession number 1.463.852). Subjects donated biological samples only after providing signed informed consent. All animal work was approved by Duke University IACUC.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
We thank members of the Silver lab for helpful discussions, Dr. Hiro Matsunami for sharing Wnt1-Cre mice, Dr. Yong-Hui Jiang for sharing Cmv-Cre mice. We thank Naila Lourenço, Andressa Morales, Patrícia Semedo-Kuriki for technical support in MLPA and flow cytometry analysis, and Gabriella Hsia for EIF4A3 molecular diagnosis. We also thank students of the Passos-Bueno lab for support and discussions.
Conflict of Interest statement. None declared.
Funding
Funding for research from NIH (R01NS0830897) to D.L.S.; NSF Graduate Research Fellowship (DGF 1106401) to E.E.M.; FAPESP/CEPID (2013/08028-1) and CNPq (305405/2011-5) to M.R.P.B. and CNPq research fellowship (143094/2011-4) to G.S.K.
References
- 1. Favaro F.P., Zechi-Ceide R.M., Alvarez C.W., Maximino L.P., Antunes L.F.B.B., Richieri-Costa A., Guion-Almeida M.L. (2011) Richieri‐Costa–Pereira syndrome: a unique acrofacial dysostosis type. An overview of the Brazilian cases. Am. J. Med. Genet. A, 155, 322–331. [DOI] [PubMed] [Google Scholar]
- 2. Raskin S., Souza M., Medeiros M.C., Manfron M., Chong e Silva D.C. (2013) Richieri-Costa and Pereira syndrome: Severe phenotype. Am. J. Med. Genet. A, 161, 1999–2003. [DOI] [PubMed] [Google Scholar]
- 3. Favaro F.P., Alvizi L., Zechi-Ceide R.M., Bertola D., Felix T.M., de Souza J., Raskin S., Twigg S.R.F., Weiner A.M.J., Armas P., et al. (2014) A noncoding expansion in EIF4A3 causes Richieri-Costa-Pereira syndrome, a craniofacial disorder associated with limb defects. Am. J. Hum. Genet., 94, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Hir H.E., Sauliere J., Wang Z. (2016) The exon junction complex as a node of post-transcriptional networks. Nat. Rev. Mol. Cell. Biol., 17, 41–54. [DOI] [PubMed] [Google Scholar]
- 5. Haremaki T., Weinstein D.C. (2012) Eif4a3 is required for accurate splicing of the Xenopus laevis ryanodine receptor pre-mRNA. Dev. Biol., 372, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haremaki T., Sridharan J., Dvora S., Weinstein D.C. (2010) Regulation of vertebrate embryogenesis by the exon junction complex core component Eif4a3. Dev. Dyn., 239, 1977–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao H., McMahon J.J., Tsai Y.-H., wang Z., Silver D.L. (2016) Haploinsufficiency for core exon junction complex components disrupts embryonic neurogenesis and causes p53-mediated microcephaly. PLoS Genet., 12, e1006282–e1006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maclean G., Dollé P., Petkovich M. (2009) Genetic disruption of CYP26B1 severely affects development of neural crest derived head structures, but does not compromise hindbrain patterning. Dev. Dyn., 238, 732–745. [DOI] [PubMed] [Google Scholar]
- 9. Cordero D.R., Brugmann S., Chu Y., Bajpai R., Jame M., Helms J.A. (2010) Cranial neural crest cells on the move: Their roles in craniofacial development. Am. J. Med. Genet. A, 155, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong S.-G. (2014) Cranial neural crest—migratory cell behavior and regulatory networks. Exp. Cell. Res., 325, 90–95. [DOI] [PubMed] [Google Scholar]
- 11. Lehalle D., Wieczorek D., Zechi-Ceide R.M., Passos-Bueno M.R., Lyonnet S., Amiel J., Gordon C.T. (2015) A review of craniofacial disorders caused by spliceosomal defects. Clin. Genet., 88, 405–415. [DOI] [PubMed] [Google Scholar]
- 12. Jones N.C., Lynn M.L., Gaudenz K., Sakai D., Aoto K., Rey J.-P., Glynn E.F., Ellington L., Du C., Dixon J., et al. (2008) Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat. Med., 14, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devotta A., Juraver-Geslin H., Gonzalez J.A., Hong C.-S., Saint-Jeannet J.-P. (2016) Sf3b4-depleted Xenopus embryos—a model to study the pathogenesis of craniofacial defects in Nager syndrome. Dev. Biol., 415, 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chai Y., Jiang X., Ito Y., Bringas P., Han J., Rowitch D.H., Soriano P., McMahon A.P., Sucov H.M. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development, 127, 1671–1679. [DOI] [PubMed] [Google Scholar]
- 15. Noisa P., Raivio T. (2014) Neural crest cells: from developmental biology to clinical interventions. Birth. Defect. Res. C, 102, 263–274. [DOI] [PubMed] [Google Scholar]
- 16. Neben C.L., Roberts R.R., Dipple K.M., Merrill A.E., Klein O.D. (2016) Modeling craniofacial and skeletal congenital birth defects to advance therapies. Hum. Mol. Genet., 25, R86–R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menendez L., Kulik M.J., Page A.T., Park S.S., Lauderdale J.D., Cunningham M.L., Dalton S. (2013) Directed differentiation of human pluripotent cells to neural crest stem cells. Nat. Protoc., 8, 203–212. [DOI] [PubMed] [Google Scholar]
- 18. Fukuta M., Nakai Y., Kirino K., Nakagawa M., Sekiguchi K., Nagata S., Matsumoto Y., Yamamoto T., Umeda K., Heike T., et al. (2014) Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE, 9, e112291–e112225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang M., Miller M.L., McHenry L.K., Zheng T., Zhen Q., Ilkhanizadeh S., Conklin B.R., Bronner M.E., Weiss W.A. (2016) Generating trunk neural crest from human pluripotent stem cells. Sci. Rep., 6, 19727.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Passos-Bueno M.R., Ornelas C.C., Fanganiello R.D. (2009) Syndromes of the first and second pharyngeal arches: a review. Am. J. Med. Genet. A, 149A, 1853–1859. [DOI] [PubMed] [Google Scholar]
- 21. Bronner M.E., LeDouarin N.M. (2012) Development and evolution of the neural crest: an overview. Dev. Biol., 366, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kobayashi G.S., Alvizi L., Sunaga D.Y., Francis-West P., Kuta A., Almada B.V.P., Ferreira S.G., de Andrade-Lima L.C., Bueno D.F., Raposo-Amaral C.E., et al. (2013) Susceptibility to DNA damage as a molecular mechanism for non-syndromic cleft lip and palate. PLoS ONE, 8, e65677–e65611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Griesi-Oliveira K., Sunaga D.Y., Alvizi L., Vadasz E., Passos-Bueno M.R. (2013) Stem cells as a good tool to investigate dysregulated biological systems in autism spectrum disorders. Autism Res., 6, 354–361. [DOI] [PubMed] [Google Scholar]
- 24. Yeh E., Atique R., Ishiy F.A.A., Fanganiello R.D., Alonso N., Matushita H., da Rocha K.M., Passos-Bueno M.R. (2011) FGFR2 mutation confers a less drastic gain of function in mesenchymal stem cells than in fibroblasts. Stem Cell Rev. Rep., 8, 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chai Y., Sasano Y., Bringas P., Mayo M., Kaartinen V., Heisterkamp N., Groffen J., Slavkin H., Shuler C. (1997) Characterization of the fate of midline epithelial cells during the fusion of mandibular prominences in vivo. Dev. Dyn., 208, 526–535. [DOI] [PubMed] [Google Scholar]
- 26. Jeong J., Mao J., Tenzen T., Kottmann A.H., McMahon A.P. (2004) Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev., 18, 937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacKenzie B., Wolff R., Lowe N., Billington C.J. Jr, Peterson A., Schmidt B., Graf D., Mina M., Gopalakrishnan R., Petryk A. (2009) Twisted gastrulation limits apoptosis in the distal region of the mandibular arch in mice. Dev. Biol., 328, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramaesh T., Bard J.B.L. (2003) The growth and morphogenesis of the early mouse mandible: a quantitative analysis. J. Anat., 203, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golbert M.B., Dewes L.O., Philipsen V.R., Wachholz R.S., Deutschendorf C., Leite J.C.L. (2007) New clinical findings in the Richieri-Costa/Pereira type of acrofacial dysostosis. Clin. Dysmorphol., 16, 85–88. [DOI] [PubMed] [Google Scholar]
- 30. Ogando P.B., Pires F., Krummenauer R.C.P., Collares M.V.M., Lubianca Neto J.F. (2011) [Laryngeal malformations in the Richieri Costa and Pereira syndrome with airway obstruction]. Braz J. Otorhinolaryngol., 77, 138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuoka T., Ahlberg P.E., Kessaris N., Iannarelli P. (2005) Neural crest origins of the neck and shoulder. Nature, 436, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armistead J., Triggs-Raine B. (2014) Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS Lett., 588, 1491–1500. [DOI] [PubMed] [Google Scholar]
- 33. Danilova N., Gazda H.T. (2015) Ribosomopathies: how a common root can cause a tree of pathologies. Dis. Models Mech., 8, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yelick P.C., Trainor P.A. (2015) Ribosomopathies: global process, tissue specific defects. Rare Dis., 3, e1025185.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menendez L., Yatskievych T.A., Antin P.B., Dalton S. (2011) Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci., 108, 19240–19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lewis A.E., Vasudevan H.N., O’Neill A.K., Soriano P., Bush J.O. (2013) The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Dev. Biol., 379, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silver D.L., Leeds K.E., Hwang H.-W., Miller E.E., Pavan W.J. (2013) The EJC component Magoh regulates proliferation and expansion of neural crest-derived melanocytes. Dev. Biol., 375, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dixon J., Jones N.C., Sandell L.L., Jayasinghe S.M., Crane J., Rey J.-P., Dixon M.J., Trainor P.A. (2006) Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc. Natl. Acad. Sci., 103, 13403–13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brugmann S.A., Allen N.C., James A.W., Mekonnen Z., Madan E., Helms J.A. (2010) A primary cilia-dependent etiology for midline facial disorders. Hum. Mol. Genet., 19, 1577–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silver D.L., Watkins-Chow D.E., Schreck K.C., Pierfelice T.J., Larson D.M., Burnetti A.J., Liaw H.-J., Myung K., Walsh C.A., Gaiano N., et al. (2010) The exon junction complex component Magoh controls brain size by regulating neural stem cell division. Nat. Neurosci., 13, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pilaz L.-J., McMahon J.J., Miller E.E., Lennox A.L., Suzuki A., Salmon E., Silver D.L. (2016) Prolonged mitosis of neural progenitors alters cell fate in the developing brain. Neuron, 89, 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mao H., Pilaz L.J., McMahon J.J., Golzio C., Wu D., Shi L., Katsanis N., Silver D.L. (2015) Rbm8a haploinsufficiency disrupts Embryonic cortical development resulting in microcephaly. J. Neurosci., 35, 7003–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parada C., Chai Y. (2015) Mandible and Tongue Development. Curr. Top. Dev. Biol., 115, 31–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Souza J., dal Vesco K., Tonocchi R., Closs-Ono M.C., Passos-Bueno M.R., da Silva-Freitas R. (2011) The Richieri-Costa and Pereira syndrome: report of two Brazilian siblings and review of literature. Am. J. Med. Genet. A, 155A, 1173–1177. [DOI] [PubMed] [Google Scholar]
- 45. Okita K., Yamakawa T., Matsumura Y., Sato Y., Amano N., Watanabe A., Goshima N., Yamanaka S. (2013) An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells, 31, 458–466. [DOI] [PubMed] [Google Scholar]
- 46. Jehee F.S., Takamori J.T., Medeiros P.F.V., Pordeus A.C.B., Latini F.R.M., Bertola D.R., Kim C.A., Passos-Bueno M.R. (2011) Using a combination of MLPA kits to detect chromosomal imbalances in patients with multiple congenital anomalies and mental retardation is a valuable choice for developing countries. Eur. J. Med. Genet., 54, e425–e432. [DOI] [PubMed] [Google Scholar]
- 47. Ishiy F.A.A., Fanganiello R.D., Griesi-Oliveira K., Suzuki A.M., Kobayashi G.S., Morales A.G., Capelo L.P., Passos-Bueno M.R. (2015) Improvement of in vitro osteogenic potential through differentiation of induced pluripotent stem cells from human exfoliated dental tissue towards mesenchymal-like stem cells. Stem Cells Int., 2015, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang C.-C., Park A.Y., Guan J.-L. (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc., 2, 329–333. [DOI] [PubMed] [Google Scholar]
- 49. Andersen C.L., Jensen J.L., Ørntoft T.F. (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res., 64, 5245–5250. [DOI] [PubMed] [Google Scholar]
- 50. Pfaffl M.W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res., 29, e45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.