Abstract
Background: The mechanisms underlying acute mountain sickness (AMS) and high-altitude pulmonary edema (HAPE) are not fully understood. We hypothesized that regulators of endothelial function, circulatory homeostasis, hypoxia and cell stress contribute to the pathobiology of AMS and HAPE.
Methods: We conducted a prospective case–control study of climbers developing altitude illness who were evacuated to the CIWEC clinic in Kathmandu, compared to healthy acclimatized climbers. ELISA was used to measure plasma biomarkers of the above pathways.
Results: Of the 175 participants, there were 71 cases of HAPE, 54 cases of AMS and 50 acclimatized controls (ACs). Markers of endothelial function were associated with HAPE: circulating levels of endothelin-1 (ET-1) were significantly elevated and levels of sKDR (soluble kinase domain receptor) were significantly decreased in cases of HAPE compared to AC or AMS. ET-1 levels were associated with disease severity as indicated by oxygen saturation. Angiopoietin-like 4 (Angptl4) and resistin, a marker of cell stress, were associated with AMS and HAPE irrespective of severity. Corin and angiotensin converting enzyme, regulators of volume homeostasis, were significantly decreased in HAPE compared to AC.
Conclusion: Our findings indicate that regulators of endothelial function, vascular tone and cell stress are altered in altitude illness and may mechanistically contribute to the pathobiology of HAPE.
Keywords: Acute mountain sickness, high-altitude pulmonary edema, hypoxia, endothelial dysfunction, circulatory homeostasis, endothelin-1, altitude illness
Introduction
Rapid ascent to altitudes of 2500 m and higher may result in altitude illness. Acute illness is often separated into three syndromes: acute mountain sickness (AMS), high-altitude cerebral edema (HACE) and high-altitude pulmonary edema (HAPE).1–4 Individuals with AMS usually display symptoms of headache with/without fatigue, difficulty sleeping, dizziness and loss of appetite, nausea and vomiting. Patients with AMS may progress to HACE, defined by the onset of ataxia and/or altered consciousness, often as a result of continued ascent. HAPE is characterized by the onset of dyspnoea at rest, cough, decreased exercise tolerance and clinically by rales, wheezing, tachypnea and tachycardia.4–6 HAPE may occur by itself or in conjunction with AMS and HACE.
The precise pathophysiological mechanisms resulting in AMS, HAPE and HACE are not fully understood. Therefore, it is often difficult to identify those at risk of altitude illness and progression to HAPE or HACE in the absence of specific markers or models that predict individual susceptibility. A detailed understanding of the underlying pathobiology of these syndromes may identify predictive biomarkers and risk-stratification tools. The primary focus of this study was to therefore elucidate pathways underlying AMS and HAPE. Once validated, biomarkers of these pathways may have utility as predictive or diagnostic markers. It has been proposed that high-altitude hypoxemia induces both humoral and hemodynamic changes, including changes in sympathetic activity and endothelial function that ultimately culminates in pulmonary microvascular leak.7–10 Hypoxia may also induce blood brain barrier dysfunction leading to endothelial cell junction disruption and cerebrovascular leak.8,9
HAPE is a non-cardiogenic hydrostatic pulmonary edema with marked increases in pulmonary artery and capillary pressure due to an exaggerated pulmonary vasoconstrictive response to hypoxia.10,11 Nitric oxide (NO) plays a critical role in regulating endothelial function and vascular tone. Under hypoxic conditions, HAPE-susceptible individuals have reduced exhaled NO levels associated with increased pulmonary artery pressure and capillary leakage.12–15 These data suggest that reduced NO biosynthesis may contribute to increased hypoxic pulmonary vasoconstriction.16 Bioavailable NO has been reported to directly modify the angiopoietin (Ang)-Tie2 pathway, a major regulator of endothelial activation and microvascular leak. The angiogenic factors Ang-1 and Ang-2 play essential roles in endothelial function and integrity mediated through competitive binding of their cognate receptor Tie2.17–20
Regulators of circulatory homeostasis and blood volume, including the renin-angiotensin-aldosterone system, may play mechanistic roles in AMS and HAPE. Mutations in proteins controlling these pathways have been associated with performance and acclimatization at high altitudes.21–23 Modulators of hypoxia and cell stress, such as resistin, may also have effects on susceptibility to AMS and HAPE. Resistin has been shown to induce apoptosis of pulmonary endothelial cells and to have pro-inflammatory effects that may contribute to pulmonary hypertension and HAPE.8,24–26 Neuroprotective factors such as brain-derived neurotrophic factor (BDNF), have also been reported to be altered in individuals at high altitude and under hypoxic conditions.27
In this study, we used a case–control design to test the hypothesis that specific regulators and mediators of endothelial function, circulatory homeostasis and hypoxia may contribute to the pathobiology of AMS and HAPE.
Methods
Study Population
Between October 2010 and May 2011, we prospectively enrolled trekkers and climbers at the CIWEC Clinic Travel Medicine Centre in Kathmandu who were medically evacuated to the clinic with altitude illness. Data on patients were entered into the central database of the GeoSentinel Network according to a standardized protocol and were then extracted for this analysis.28,29 This study was approved by the Kathmandu CIWEC regional IRB and participants provided informed consent. At enrolment, data were collected on the ascent profile, demographics, vital signs and a blood sample was collected and stored at −20°C until biomarker analysis. A group of healthy acclimatized hikers/climbers acclimatized controls (ACs) was recruited as a control group. Among the 50 ACs, 29 were co-evacuated by helicopter with a case and co-presented to the clinic. The remaining 21 ACs were recruited from the waiting room, accompanying other climbers who presented with illnesses other than AMS or HAPE. Altitude illness was assessed according to Lake Louise definitions of altitude illness.30 Subjects were classified as having altitude illness if they met the Lake Louise definitions of AMS or HAPE.31
Biomarker Assessment
Biomarkers from selected pathways implicated in the pathogenesis of altitude illness were measured in EDTA plasma by ELISA (Quantikine or DuoSets, R&D Systems, Minneapolis, MN) using dilution factors: Endothelin-1 (ET-1;1:2), sE-Selectin (1:5), Ang-2 (1:2), Ang-1 (1:10), soluble intercellular adhesion molecule-1 (sICAM-1;1:100), soluble receptor tyrosine kinase Tie2 (sTie-2;1:25), soluble endoglin (sEng;1:25), soluble kinase insert domain receptor (sKDR;1:10), angiopoietin-like 4 (Angptl4;1:5), renin (1:5), angiotensin-converting enzyme (ACE;1:25), corin (1:5), cystatin C (1:1000), BDNF (1:25), resistin (1:25) and interleukin-1 receptor antagonist (IL-1Ra;1:2) as described.20,32–34
Statistical Analysis
Statistical analyses were performed using GraphPad Prism v.6.04 and IBM SPSS v.20. All analyses were non-parametric. Comparisons between continuous variables were performed using Mann–Whitney U test with Bonferonni adjustment. Binary outcomes were analysed using Chi-Square or Fisher’s exact test, and correlations were investigated using Spearman’s rho.
Results
Description of Study Population
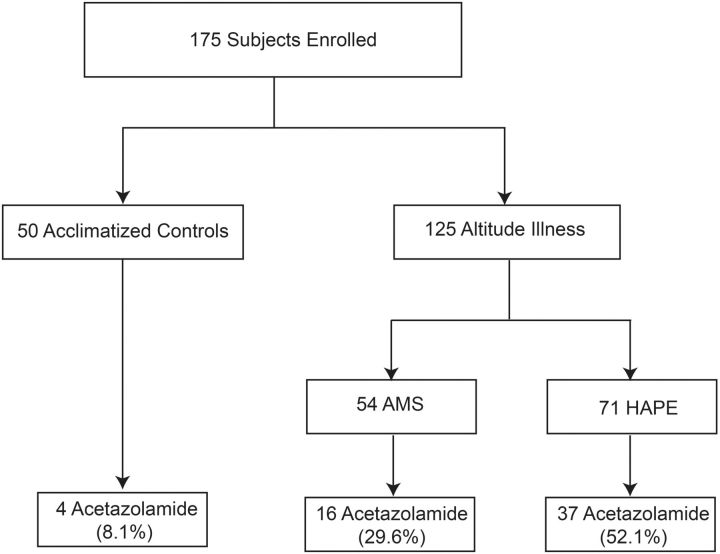
A total of 175 consecutive consenting participants were enrolled in this case–control study to evaluate host response biomarker profiles in cases with altitude illness (n = 125) compared to ACs (n = 50). Baseline characteristics were comparable between the ACs and individuals with altitude illness with median [interquartile range (IQR)] ages of 40.0 (30.0–53.0) and 43.0 (32.0–56.5) years, respectively (P = 0.21). In the ACs, 50% were male compared to 62.4% in cases of altitude illness (P = 0.13). There was no difference in the maximum altitude attained between the two groups with ACs reaching a median altitude of 4240 m (IQR, 3870–5300) and those who developed altitude illness reaching a maximum altitude of 4410 m (3770–4930) (P = 0.57). Acetazolamide use by the study population is shown in Figure 1. Four of the ACs reported taking acetazolamide for prophylaxis, whereas 42.4% (n = 53) of cases reported using acetazolamide but primarily for treatment with 43.4% of cases starting acetazolamide within 24 h before presentation.
Figure 1.
Flowchart of study subjects evaluated for biomarkers of altitude illness
Of the 125 cases of altitude illness, 54 had AMS, 57 had HAPE and 14 were classified as both AMS and HAPE (considered HAPE for this analysis). HAPE was the predominant manifestation observed and therefore the focus of this study. Of the subjects with HAPE, 29 also had HACE but were classified as HAPE for this analysis. There were no significant differences observed in the maximum altitude reached between the AMS and HAPE groups (median, IQR: 4310 m, 3550–4930 in AMS vs. 4410 m, 3860–4983 in HAPE, P = 0.40). Overall, individuals who developed HAPE were more likely to be male and were older than those with AMS (Table 1).
Table 1.
Characteristics of climbers with altitude illness
| Characteristic | AMS (n = 54) | HAPE (n = 71) | P |
|---|---|---|---|
| Age (years) | 37.0 (27.0–55.8) | 47.0 (37.0–57.5) | 0.054 |
| Sex (M), n (%) | 28 (51.9) | 50 (70.4) | 0.034 |
| Maximum altitude (m) | 4310 (3550–4930) | 4410 (3860–4983) | 0.397 |
| Percentage took Diamoxa | 16 (29.6) | 37 (52.1) | 0.012 |
| Temperature (°C) | 37.0 (36.6–37.3) | 37.4 (37.0–38.0) | 0.001 |
| Systolic BP (mm Hg) | 120 (102–130) | 110 (100–130) | 0.248 |
| Pulse rate (bpm) | 75.0 (64.5–82.0) | 80.0 (74.0–89.5) | 0.034 |
| SpO2 (%) | 97.0 (95.0–98.0) | 91.0 (85.5–96.0) | < 0.001 |
| WBC | 9000 (7100–11 600) | 11 150 (7975–14 188) | 0.064 |
| Hematocrit (%) | 43.5 (40.8–48.0) | 43.0 (42.0–46.0) | 0.943 |
| Lake Louise Score | 11 (9–13) | 14 (11–16) | 0.002 |
Measurements of characteristics were taken upon evacuation/presentation to the clinic for each climber and analysed by Mann–Whitney U test.
aDiamox taken for treatment of symptoms.
Biomarkers Associated with Altitude Illness
We initially compared biomarkers from three pathways implicated in the pathobiology of altitude illness in all participants who developed altitude illness (n = 125) versus ACs (Table 2). Three markers of endothelial activation and vascular permeability were significantly different between cases of altitude illness and ACs; ET-1 (P = 0.006) and angptl4 (P = 0.002) were significant elevated, while sKDR was significantly decreased (P < 0.001). These markers remained significant after correcting for multiple comparisons. Three markers of circulatory homeostasis were significantly different when comparing individuals with altitude illness with ACs; cystatin C was significantly elevated (P = 0.018), while corin and ACE were significantly decreased (P = 0.018; P = 0.036, respectively). Resistin, a marker of hypoxia and cell stress was significantly elevated when comparing cases of altitude illness with AC (P < 0.0001; Table 2).
Table 2.
Biomarker levels in acclimatized controls and climbers who developed altitude illness
| Biomarker | Healthy acclimatized controls, n = 50 | Altitude illness, n = 125 | P |
|---|---|---|---|
| ET-1 | 1.42 (1.09–1.81) | 1.76 (1.27–2.41) | 0.006 |
| sE-Selectin | 18.42 (14.67–25.90) | 19.86 (13.82–24.93) | 0.492 |
| Ang-2 | 0.53 (0.37–1.12) | 0.79 (0.48–1.25) | 0.063 |
| Ang-1 | 11.95 (4.01–27.33) | 12.20 (5.71–24.57) | 0.887 |
| sICAM-1 | 108.75 (95.68–132.30) | 111.80 (97.15–127.30) | 0.865 |
| sTie2 | 16.48 (11.68–20.46) | 15.93 (12.39–18.55) | 0.490 |
| sEng | 10.0 (8.7–12.2) | 9.15 (7.34–11.59) | 0.124 |
| sKDR | 5.44 (4.42–6.69) | 4.69 (3.89–5.73) | 0.001 |
| BDNF | 9.9 (2.9–18.7) | 8.38 (3.38–16.28) | 0.703 |
| Angptl4 | 50.4 (40.6–68.4) | 65.94 (51.63–88.00) | 0.002 |
| Resistin | 8.7 (6.4–13.8) | 12.43 (8.99–16.04) | <0.0001 |
| sIL-1Ra | 0.28 (0.07–0.67) | 0.29 (0.10–0.86) | 0.417 |
| Corin | 1.01 (0.63–1.25) | 0.73 (0.45–1.10) | 0.018 |
| ACE | 91.6 (80.0–123.8) | 88.18 (64.89–109.35) | 0.036 |
| Cystatin C | 832 (733–983) | 910.0 (792.0–1062.0) | 0.018 |
| Renin | 0.28 (0.17–0.38) | 0.29 (0.19–0.43) | 0.666 |
BDNF, brain-derived neurotrophic factor. Data expressed as median (IQR), in ng/ml, analysed by Mann–Whitney U test.
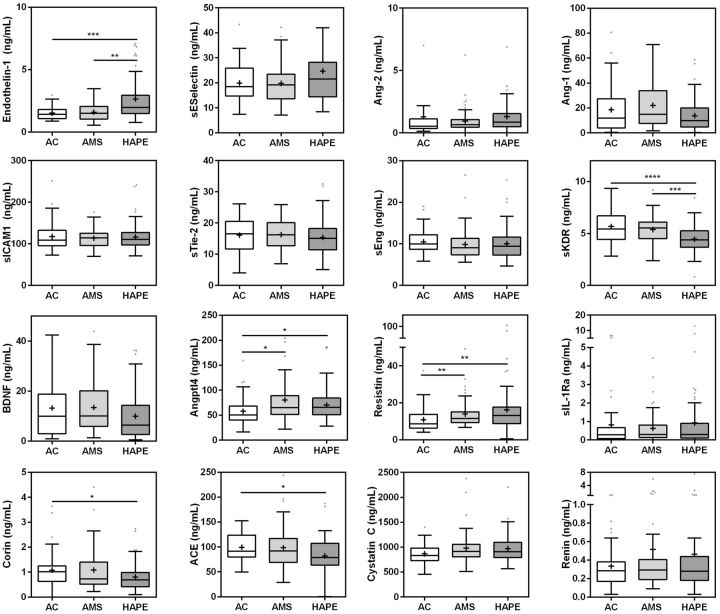
We next explored the association between the biomarkers and pulmonary manifestations of altitude illness by comparing AMS versus HAPE cases (Figure 2). There were three main observations: (i) biomarkers that were significantly elevated in both AMS and HAPE. Compared to ACs, Angptl4 and resistin were elevated in AMS (P = 0.005; P = 0.001, respectively) and HAPE (P = 0.007; P = 0.001, respectively) and remained significant after correcting for multiple comparisons. (ii) Biomarkers associated with HAPE alone. ET-1 was elevated (P < 0.001) and sKDR was decreased (P < 0.001) in ACs and AMS versus HAPE and remained significant after correcting for multiple comparisons. (iii) Biomarkers decreased in HAPE compared to ACs. Corin and ACE were decreased in HAPE compared to ACs (P = 0.002; P = 0.003, respectively) and remained so after correcting for multiple comparisons (Figure 2).
Figure 2.
Box and whisker plots showing the distribution of biomarker levels in climbers returning from altitude. The box represents the median, and IQR, while the whiskers denote the 25 percentile minus 1.5IQR and 75 percentile plus 1.5IQR. Individual data points that fall beyond the whiskers are represented by dots. The group mean is shown by the (+). Data were analysed using the Kruskal–Wallis test with Dunn’s multiple comparisons test comparing acclimatized healthy controls (AC) vs. AMS, AMS vs. high altitude pulmonary edema (HAPE) and AC vs. HAPE. The stars represent significant comparisons by post-hoc testing (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Furthermore, we assessed the impact of acetazolamide on biomarker levels from cases. Corin and sKDR were decreased (P = 0.003, P = 0.023, respectively) in those who reported acetazolamide use (42.4%).
Relationship between Biomarker Levels and Physiological Measures of Severity
We further explored the relationship between biomarkers and physiological and laboratory measures of disease severity to gain insight into possible mechanisms of disease pathogenesis. ET-1 and sKDR were associated with total leukocyte count (WBC) and peripheral oxygen saturation (SpO2). Increased WBC count was positively correlated with ET-1 (rho = 0.287, P = 0.03) and negatively with sKDR (rho = −0.236, P = 0.028), while SpO2 was negatively correlated with ET-1 (rho = 0.336, P = 0.02) and positively with sKDR (rho = 0.227, P = 0.043). sE-Selectin, a marker of endothelial activation, was positively correlated with pulse rate (rho = 0.333, P = 0.04) and negatively correlated with the SpO2 (rho = −0.365, P = 0.03). There was a negative correlation between angptl4 and SpO2 (rho = −0.335, P = 0.002).
Discussion
In this study, we investigated a panel of biomarkers reflective of pathways implicated in the pathobiology of altitude illness, focusing on pathways associated with endothelial dysfunction and for which novel therapeutics are under development (e.g. Ang/Tie2 pathway).32–34 We show that markers and mediators of endothelial activation, circulatory homeostasis and hypoxia are altered in individuals who develop AMS and HAPE compared to healthy acclimatized climbers. We report that increased circulating ET-1 levels were associated with HAPE and measures of disease severity including leukocyte count and SpO2. In contrast, increased systemic sKDR levels were negatively correlated with HAPE and measures of disease severity. Collectively, these observations suggest that these regulators of pulmonary vascular function may mechanistically contribute to the pathobiology of HAPE, correlate with disease severity and may be clinically informative.
Our findings are supported by previous studies showing a role for ET-1 in HAPE and a direct correlation of ET-1 levels with pulmonary artery pressure at high altitudes.35–37 ET-1 is synthesized by the pulmonary endothelium and has been reported to circulate at increased concentrations at high altitudes.37 ET-1 is a key regulator of vascular tone and renal homeostasis and increased plasma levels are associated with pulmonary hypertension and support our findings of an association with HAPE and disease severity.38,39 ET-1 is thought to be regulated via the transcription of ET-1 gene (edn1) and exocytosis of endothelial Weibel–Palade bodies (WPB).40,41 NO controls WPB exocytosis and the expression of edn1 but NO is reported to be reduced in individuals that are susceptible to HAPE.14,40–42 These observations fit a model whereby reduced bioavailable NO would be expected to result in increased WPB exocytosis, release of Ang-2, endothelial dysfunction and higher levels of circulating ET-1. Collectively, these events may exacerbate hypoxic pulmonary vasoconstriction and increase the risk of HAPE.37,43
sKDR (also known as VEGF receptor 2) is the soluble truncated variant of KDR expressed by endothelial cells that binds to and inhibits vascular endothelial growth factor (VEGF), a potent inducer of microvascular leak.44,45 The lower levels of circulating sKDR we observed in cases of HAPE is consistent with the hypothesis that there is less sequestration of VEGF and therefore more free local VEGF to mediate pulmonary vascular leak.
Angptl4 is a hormone involved in glucose and lipid metabolism that is induced under hypoxic conditions.46,47 Angptl4 has been proposed to promote vascular leak through integrin-mediated signalling or via hypoxia-induced apoptosis.48,49 In this study, an increase in Angptl4 levels was associated with altitude illness and negatively correlated with SpO2. Further study will be required to determine if Angptl4 plays a mechanistic role or is merely reflective of hypoxia.
There is considerable evidence supporting a causal role for the Ang-Tie2 pathway in regulating microvascular leak in acute lung injury and other conditions that share pathophysiologic features with HAPE.50–55. Ang-1 promotes endothelial quiescence and stability, whereas Ang-2 completes for Tie2 binding and promotes endothelial activation and permeability.14,56 In this study, there were alterations in the Ang-Tie2 axis suggesting a relationship between HAPE and increased circulating Ang-2 and decreased Ang-1 levels associated with HAPE. However, the associations were not strong, perhaps reflecting that the kinetics of markers of this pathway were not well suited to the timing of sample acquisition in this study. This hypothesis will need to be further investigated in larger prospective studies with sample collection closer to the onset of HAPE.
In this study, markers of circulatory homeostasis were associated with altitude illness and severity. Decreased levels of ACE were associated with HAPE compared to AC. ACE is expressed mainly on the lung endothelium and kidney epithelium and converts angiotensin I into physiologically active peptide angiotensin II, which acts as a potent vasopressor, controlling blood pressure and fluid electrolyte balance. Of note, polymorphisms in the ACE gene have been associated with successful acclimatization to extreme altitudes, increased transcription of ACE and with AMS/HAPE susceptibility in some ethnic backgrounds57–60 but not others.21,22,61 This study is consistent with the hypothesis that an increase in circulating ACE levels are associated with protection from developing HAPE.
Corin is a serine protease that converts pro-ANP into active ANP, regulating blood pressure and volume.62 Active ANP is required to reduce sodium levels, resulting in lower blood pressure. In this study, corin levels were significantly lower in cases of HAPE, consistent with studies that have linked SNPs in human corin that reduce activity or expression, to an increased risk of hypertension or preeclampsia.63–67
One marker of hypoxia and cell stress, resistin, promotes pulmonary endothelial cell apoptosis and has been shown to be up-regulated in hypoxia-induced pulmonary hypertension models.26,68,69 In this study, higher plasma resistin levels were associated with both AMS and HAPE, but have not with disease severity.
This study has limitations including a 1–2 days delay in sample acquisition from disease onset in some cases and limited data on the rate of ascent. However, the time from maximum altitude to blood draws was comparable between groups (P > 0.05; Table 1). Ideally samples should be collected as soon after the onset of symptoms of AMS and HAPE (i.e. before evacuation); however, this presents some challenging logistical constraints. Studies suggest that the rate of ascent is correlated to acclimatisation. Therefore, to test the hypothesis that genetic and physiologic susceptibility are risk factors for developing altitude illness, future studies should ideally examine cases and controls that are on identical ascent schedules. Future studies will also need to confirm and extend these findings by following climbers through their ascent, monitoring the kinetics of biomarkers of endothelial function, vascular homeostasis, hypoxia and cell stress during the onset and progression of altitude illness, as well as investigating polymorphisms in the genes that underlie these pathways.
In summary, this study focused on the association of altitude illness with pathways of microvascular leak, circulatory homeostasis and hypoxia. An improved understanding of mechanistic pathways that underlie life-threatening manifestations of altitude illness such as HAPE may help ultimately identify predictive markers of disease severity and outcome and putative new interventions (i.e. pro-ACE, pro-corin, pro-Ang-1 or anti-ET-1 approaches) to improve clinical outcome.
Funding
This study was supported in part by the Canadian Institutes of Health Research [CIHR MOP-13721, MOP-136813 and MOP-115160 to K.C.K.] and a Canadian Research Chair (to K.C.K.). Funding stipend support supported in part by Peterborough K.M. Hunter Charitable Foundation Graduate Awards and The McCuaig-Throop Bursary (to K.R.B.).
Conflict of interest: None declared.
References
- 1. Hackett PH, Shlim DR. Altitude Illness - Chapter 2 - 2014 Yellow Book | Travelers’ Health | CDC In: Brunette GW and Phyllis E. Kozarsky, (eds). CDC Health Information for International Travel 2014. New York: Oxford University Press, 2013. 65–69. http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-2-the-pre-travel-consultation/altitude-illness (December 15, 2014, date last accessed). [Google Scholar]
- 2. Hackett PH, Rennie D, Levine HD. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet 1976; 2:1149–55. [DOI] [PubMed] [Google Scholar]
- 3. Bärtsch P, Swenson ER. Acute high-altitude illnesses. N Engl J Med 2013; 369:1666–67. [DOI] [PubMed] [Google Scholar]
- 4. Singh I, Khanna PK, Srivastava MC et al. Acute mountain sickness. N Engl J Med 1969; 280:175–84. [DOI] [PubMed] [Google Scholar]
- 5. Hackett PH, Roach RC. High altitude cerebral edema. High Alt Med Biol 2004; 5:136–46. [DOI] [PubMed] [Google Scholar]
- 6. Hultgren HN. High-altitude pulmonary edema: current concepts. Annu Rev Med 1996; 47:267–84. [DOI] [PubMed] [Google Scholar]
- 7. Hackett PH, Roach RC. High-altitude illness. N Engl J Med 2001; 345:107–14. [DOI] [PubMed] [Google Scholar]
- 8. Julian CG, Subudhi AW, Wilson MJ et al. Acute mountain sickness, inflammation, and permeability: new insights from a blood biomarker study. J Appl Physiol 2011; 111:392–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ward TN. Migraine diagnosis and pathophysiology. Continuum (Minneap. Minn) 2012; 18:753–63. [DOI] [PubMed] [Google Scholar]
- 10. Scherrer U, Vollenweider L, Delabays A et al. Inhaled nitric oxide for high-altitude pulmonary edema. N Engl J Med 1996; 334:624–9. [DOI] [PubMed] [Google Scholar]
- 11. Bärtsch P, Mairbäurl H, Maggiorini M, Swenson ER. Physiological aspects of high-altitude pulmonary edema. J Appl Physiol 2005; 98:1101–10. [DOI] [PubMed] [Google Scholar]
- 12. Settergren G, Angdin M, Astudillo R et al. Decreased pulmonary vascular resistance during nasal breathing: modulation by endogenous nitric oxide from the paranasal sinuses. Acta Physiol Scand 1998; 163:235–9. [DOI] [PubMed] [Google Scholar]
- 13. Maggiorini M, Mélot C, Pierre S et al. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 2001; 103:2078–83. [DOI] [PubMed] [Google Scholar]
- 14. Duplain H, Sartori C, Lepori M et al. Exhaled nitric oxide in high-altitude pulmonary edema: role in the regulation of pulmonary vascular tone and evidence for a role against inflammation. Am J Respir Crit Care Med 2000; 162:221–4. [DOI] [PubMed] [Google Scholar]
- 15. Busch T, Bärtsch P, Pappert D et al. Hypoxia decreases exhaled nitric oxide in mountaineers susceptible to high-altitude pulmonary edema. Am J Respir Crit Care Med 2001; 163:368–73. [DOI] [PubMed] [Google Scholar]
- 16. West JB, Mathieu-Costello O. Stress failure of pulmonary capillaries: role in lung and heart disease. Lancet 1992; 340:762–7. [DOI] [PubMed] [Google Scholar]
- 17. Lee WL, Liles WC. Endothelial activation, dysfunction and permeability during severe infections. Curr Opin Hematol 2011; 18:191–6. [DOI] [PubMed] [Google Scholar]
- 18. Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 2013; 4:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ricciuto DR, Dos Santos CC, Hawkes M et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med 2011; 39:702–10. [DOI] [PubMed] [Google Scholar]
- 20. Conroy AL, Phiri H, Hawkes M et al. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study. PLoS One 2010; 5:e15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qi Y, Niu W, Zhu T et al. Synergistic effect of the genetic polymorphisms of the renin-angiotensin-aldosterone system on high-altitude pulmonary edema: a study from Qinghai-Tibet altitude. Eur J Epidemiol 2008; 23:143–52. [DOI] [PubMed] [Google Scholar]
- 22. Hotta J, Hanaoka M, Droma Y et al. Polymorphisms of renin- angiotensin system genes with high-altitude pulmonary edema in Japanese subjects. Chest 2004; 126:825–30. [DOI] [PubMed] [Google Scholar]
- 23. Srivastava S, Bhagi S, Kumari B et al. Association of polymorphisms in angiotensin and aldosterone synthase genes of the renin-angiotensin-aldosterone system with high-altitude pulmonary edema. J Renin Angiotensin Aldosterone Syst 2012; 13:155–60. [DOI] [PubMed] [Google Scholar]
- 24. Hung H-F, Wang B-W, Chang H, Shyu K-G. The molecular regulation of resistin expression in cultured vascular smooth muscle cells under hypoxia. J Hypertens 2008; 26:2349–60. [DOI] [PubMed] [Google Scholar]
- 25. Kubo K, Hanaoka M, Hayano T et al. Inflammatory cytokines in BAL fluid and pulmonary hemodynamics in high-altitude pulmonary edema. Respir Physiol 1998; 111:301–10. [DOI] [PubMed] [Google Scholar]
- 26. Yamaji-Kegan K, Takimoto E, Zhang A et al. Hypoxia-induced mitogenic factor (FIZZ1/RELMa) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014; 306:L1090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helan M, Aravamudan B, Hartman WR et al. BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia. J Mol Cell Cardiol 2014; 68:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freedman DO, Kozarsky PE, Weld LH, Cetron MS. GeoSentinel: the global emerging infections sentinel network of the international society of travel medicine. J Travel Med 1999; 6:94–8. [DOI] [PubMed] [Google Scholar]
- 29. Pandey P, Shlim DR, Cave W, Springer MFB. Risk of possible exposure to rabies among tourists and foreign residents in Nepal. J Travel Med 2002; 9:127–31. [DOI] [PubMed] [Google Scholar]
- 30. Roach C, Batsch P, Hackett PH, Oelz O. The Lake Louise Consensus on the definition and quantification of altitude illness. In: Sutton J, Coates G, Houston C (eds). Hypoxia and Mountain Medicine, 1st ed. Burlington, Vermont: Pergamon Press, 1992. 327–330. [Google Scholar]
- 31. Leshem E, Pandey P, Shlim DR et al. Clinical features of patients with severe altitude illness in Nepal. J Travel Med 2008; 15:315–22. [DOI] [PubMed] [Google Scholar]
- 32. Finney CA, Hawkes CA, Kain DC et al. S1P is associated with protection in human and experimental cerebral malaria. Mol Med 2011; 17:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alfieri A, Watson JJ, Kammerer RA et al. Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis. Crit Care 2012; 16:R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stiehl T, Thamm K, Kaufmann J et al. Lung-targeted RNA interference against angiopoietin-2 ameliorates multiple organ dysfunction and death in sepsis. Crit Care Med 2014; 42:e654–62. [DOI] [PubMed] [Google Scholar]
- 35. Droma Y, Hayano T, Takabayashi Y et al. Endothelin-1 and interleukin-8 in high altitude pulmonary oedema. Eur Respir J 1996; 9:1947–9. [DOI] [PubMed] [Google Scholar]
- 36. Scherrer U, Rexhaj E, Jayet P-Y et al. New insights in the pathogenesis of high-altitude pulmonary edema. Prog Cardiovasc Dis 2010; 52:485–92. [DOI] [PubMed] [Google Scholar]
- 37. Sartori C, Vollenweider L, Löffler BM et al. Exaggerated endothelin release in high-altitude pulmonary edema. Circulation 1999; 99:2665–8. [DOI] [PubMed] [Google Scholar]
- 38. Yanagisawa M, Kurihara H, Kimura S et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332:411–5. [DOI] [PubMed] [Google Scholar]
- 39. Weitzberg E, Ahlborg G, Lundberg JM. Differences in vascular effects and removal of endothelin-1 in human lung, brain, and skeletal muscle. Clin Physiol 1993; 13:653–62. [DOI] [PubMed] [Google Scholar]
- 40. Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 2005; 15:302–8. [DOI] [PubMed] [Google Scholar]
- 41. Doi Y, Kudo H, Nishino T et al. The enhancement of preproendothelin-1 synthesis and the acceleration of endothelin-1 processing in the acute hypoxic rat aorta. J Cardiovasc Pharmacol 2004; 44:S207–S210. [DOI] [PubMed] [Google Scholar]
- 42. Mortimer H, Patel S, Peacock AJ. The genetic basis of high-altitude pulmonary oedema. Pharmacol Ther 2004; 101:183–192. [DOI] [PubMed] [Google Scholar]
- 43. Rossi GP, Seccia TM, Nussdorfer GG. Reciprocal regulation of endothelin-1 and nitric oxide: relevance in the physiology and pathology of the cardiovascular system. Int Rev Cytol 2001; 209:241–72. [DOI] [PubMed] [Google Scholar]
- 44. Millauer B. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993; 72:835–46. [DOI] [PubMed] [Google Scholar]
- 45. Quinn TP, Peters KG, De Vries C et al. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A 1993; 90:7533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Belanger AJ, Lu H, Date T et al. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J Mol Cell Cardiol 2002; 34:765–74. [DOI] [PubMed] [Google Scholar]
- 47. Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med 2005; 11:473–9. [DOI] [PubMed] [Google Scholar]
- 48. Hou M, Cui J, Liu J et al. Angiopoietin-like 4 confers resistance to hypoxia/serum deprivation-induced apoptosis through PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells. PLoS One 2014; 9:e85808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Goodin JL, Pizarro-Matos JM, Prasad BM et al. Evaluating the molecular basis for acute mountain sickness: hypoxia response gene expression patterns in warfighters and murine populations. Mil Med 2013; 178:1256–63. [DOI] [PubMed] [Google Scholar]
- 50. Van der Heijden M, van Nieuw Amerongen GP, Koolwijk P et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008; 63:903–9. [DOI] [PubMed] [Google Scholar]
- 51. McCarter SD, Mei SHJ, Lai PFH et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med 2007; 175:1014–26. [DOI] [PubMed] [Google Scholar]
- 52. Van der Heijden M, van Nieuw, Amerongen GP, van Hinsbergh VWM, Groeneveld ABJ. The interaction of soluble Tie2 with angiopoietins and pulmonary vascular permeability in septic and nonseptic critically ill patients. Shock 2010; 33:263–8. [DOI] [PubMed] [Google Scholar]
- 53. Lovegrove FE, Gharib SA, Peña-Castillo L et al. Parasite burden and CD36-mediated sequestration are determinants of acute lung injury in an experimental malaria model. PLoS Pathog 2008; 4:e1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kong J, Zhu X, Shi Y et al. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol 2013; 27:2116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Darwish I, Liles WC. Emerging therapeutic strategies to prevent infection-related microvascular endothelial activation and dysfunction. Virulence 2013; 4:572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fiedler U, Scharpfenecker M, Koidl S et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004; 103:4150–6. [DOI] [PubMed] [Google Scholar]
- 57. Luo Y, Zou Y, Gao Y. Gene polymorphisms and high-altitude pulmonary edema susceptibility: a 2011 update. Respiration 2012; 84:155–62. [DOI] [PubMed] [Google Scholar]
- 58. Puthucheary Z, Skipworth JRA, Rawal J et al. The ACE gene and human performance: 12 years on. Sports Med 2011; 41:433–48. [DOI] [PubMed] [Google Scholar]
- 59. Rupert JL, Devine DV, Monsalve MV, Hochachka PW. Angiotensin-converting enzyme (ACE) alleles in the Quechua, a high altitude South American native population. Ann Hum Biol 1999; 26:375–80. [DOI] [PubMed] [Google Scholar]
- 60. Tsianos G, Eleftheriou KI, Hawe E et al. Performance at altitude and angiotensin I-converting enzyme genotype. Eur J Appl Physiol 2005; 93:630–3. [DOI] [PubMed] [Google Scholar]
- 61. Dehnert C, Weymann J, Montgomery HE et al. No association between high-altitude tolerance and the ACE I/D gene polymorphism. Med Sci Sports Exerc 2002; 34:1928–33. [DOI] [PubMed] [Google Scholar]
- 62. Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A 2000; 97:8525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dong N, Zhou T, Zhang Y et al. Corin mutations K317E and S472G from preeclamptic patients alert zymogen activation and cell surface targeting. J Biol Chem 2014; 289:17909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhou Y, Wu Q. Corin in natriuretic peptide processing and hypertension. Curr Hypertens Rep 2014; 16:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int 2009; 75:142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail 2011; 4:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dong N, Chen S, Yang J et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail 2010; 3:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kougias P, Chai H, Lin PH et al. Adipocyte-derived cytokine resistin causes endothelial dysfunction of porcine coronary arteries. J Vasc Surg 2005; 41:691–8. [DOI] [PubMed] [Google Scholar]
- 69. Bokarewa M, Nagaev I, Dahlberg L et al. Resistin, an adipokine with potent proinflammatory properties. J Immunol 2005; 174: 5789–95. [DOI] [PubMed] [Google Scholar]