Abstract
Background: Diarrhea is a frequent clinical syndrome affecting international travellers. Bacterial etiologic agents have a long history of emergent antimicrobial resistance against commonly used antibiotics. Current approaches applying first-line antimicrobial therapy are being challenged by increasingly resistant organisms. This review summarizes recent epidemiological and clinical evidence of antibiotic resistance among enteropathogens causing traveller’s diarrhea and the subsequent impact on current treatment recommendations.
Methods: The PubMed database was systemically searched for articles related to antibiotic susceptibility and diarrheal pathogens.
Results: Antibiotic resistance related to travellers’ diarrhea has increased in recent years. Most notably, fluoroquinolone resistance has expanded from the Campylobacter-associated cases well documented in Southeast Asia in the 1990s to widespread occurrence, as well as increases among other common bacterial enteropathogens including, enterotoxigenic and enteroaggregative Escherichia coli, Shigella and non-typhoidal Salmonella. Multidrug resistance among enteropathogenic Enterobacteriacae and Campylobacter species create further challenges with the selection of empiric therapy. Treatment failures requiring early use of alternative agents, as well as delayed recovery comparable to placebo rates emphasize the impact of antimicrobial resistance on effective treatment.
Conclusions: Although there are limitations in the available data, the increasing antibiotic resistance and adverse impact on clinical outcome require continued surveillance and reconsideration of practice guidelines.
Keywords: Traveller’s diarrhea, antimicrobial resistance, Campylobacter, Enterotoxigenic E. coli, Shigella, Salmonella
Evolution of Antimicrobial Use in the Treatment of Traveller’s Diarrhea (TD)
Diarrhea is one of the most common clinical syndromes impacting travellers, of which 80–90% of attacks are the result of bacterial pathogens (e.g., enterotoxigenic and enteroaggregative Escherichia coli [ETEC and EAEC, respectively], Campylobacter spp., Shigella spp. and non-typhoidal Salmonella spp.).1 There are also well-recognized regional differences in the distribution of pathogens,2 so travel destination often influences treatment recommendations. Specifically, Campylobacter spp. is commonly isolated from patients in Southeast Asia (particularly Thailand),3–5 while ETEC is frequently the causative agent for TD among travellers in the Latin America, Africa, south Asia and the Middle East.6–9
Although TD is a self-limited illness commonly resolving within 5 days when left untreated (quicker when antibiotics are prescribed), it frequently results in short-term morbidity adversely impacting travel plans, as well as potential for long-term enteric and extra-intestinal complications.10 Antibiotic therapy has been proved to be effective in treating patients with TD by both significantly reducing associated symptoms and shortening the illness duration.2,11 As antibiotic therapy remains the most effective treatment, it is common for clinicians to prescribe antibiotics to international travellers for self-treatment if they experience diarrheal symptoms while abroad. Over the past two decades, enteropathogens have shown increasing resistance to first-line antibiotics, particularly fluoroquinolones, in endemic regions.1,4,12–17 Decreased susceptibility of preferred antibiotics is an evolving challenge for clinicians requiring interval reconsideration of clinical practice guidelines. This review considers recent information on bacterial enteropathogen antimicrobial resistance against current first-line antibiotics, impact on clinical outcomes, and the effect on modification of clinical practice guidelines.
Factors Impacting Antimicrobial Effectiveness in TD Treatment
The effectiveness of an antibiotic to rapidly lead to clinical resolution with or without microbiological eradication is based on several factors. Randomized clinical trials provide point estimates of treatment efficacy under controlled circumstances, frequently highlighting an important subset of subjects with suboptimal (such as delayed time to resolution approximating placebo recovery rates) or outright treatment failure early in the antibiotic course or late recurrence.18 Antibiotic selection and the relationship to enteropathogen antibiotic susceptibility alone are not the only factors impacting clinical response.
Additional considerations include host factors, such as immunocompromised states, which may decrease efficacy, as well as add risk for persistent and recurrent illness; adjunctive therapy with antimotility agents (e.g., loperamide), which is well documented to decrease duration of diarrheal episodes;19 patient adherence to prescribed regimen; frequent co-infection challenging interpretation of attribution with resistant pathogens; and potentially non-antimicrobial effects of the therapeutic agents.20 The clinical syndrome encompassing TD includes the most common acute watery diarrhea (AWD) presentation, in addition to dysentery or febrile AWD and even viral gastroenteritis cases that manifest with diarrhea along with the common predominance of vomiting.1 Overall efficacy estimates for first-line therapies are reported by pooling these various clinical presentations into common TD endpoints, which tends to lose discrimination of important differences in time to recovery. Furthermore, the time of clinical presentation from illness onset is known to impact time to resolution in these self-limited illnesses.21 Trials attempt to control for this by restricting duration of illness at time of enrolment; however, this still may fail to consider relative differences given that these illnesses can be quite short lived. Lastly, antimicrobial resistance and the effect on clinical outcomes are extremely important, but should be placed in context with the above considerations.
Current Status of Major TD Enteropathogen Antimicrobial Resistance Epidemiology
The TD bacterial enteropathogen antimicrobial resistance data derive from a number of sources, which have strengths and limitations. Direct assessment of clinical microbiology from a TD treatment trial is most likely to report the relationship of clinical outcomes relative to susceptibility. Other sources of information stem from epidemiological surveys of travellers with TD, either at the travel destination or upon return to their home country, as well as, surveys on non-travellers (e.g., children from developing worlds in their country of residence), which are often extrapolated to inform regional infection threats. Irrespective of the information source, a significant challenge in translating this information to clinical practice guidance is interpretation of the susceptibility criteria.22 Interpretative criteria relies on clinical breakpoint development generated by standardized in vitro susceptibility testing, pharmacological parameters, and, importantly, clinical outcome studies, which are often lacking. Further complicating interpretation is the extremely high faecal antibiotic levels relative to a pathogen’s minimum inhibitory concentration (MIC) for both systemic and non-absorbable antibiotics as follows: azithromycin with a 11–14 h half-life has ∼50% active drug excreted in stool with high tissue levels,23,24 ciprofloxacin has 100- to 1000-fold faecal levels above serum levels with 19% oral dose excreted as metabolites in faeces, as well as high tissue levels,25 and rifaximin is <0.4% absorbed with 4000–8000 µg/g faecal levels at the 400 mg two times daily dose.26
The following summarizes recent reports focusing on traveller populations highlighting the continued evolution of antimicrobial resistance against first-line agents. The summary first considers the TD-causing Enterobacteriaceae species followed by Campylobacter jejuni/coli.
TD-Causing Enterobacteriaceae
Antibiotic resistance among enteric bacterial pathogens has been steadily increasing over the last two decades.4,16,27–32 A recent review of EAEC epidemiology and clinical manifestations summarized the increasing rates of multidrug resistance including loss of fluoroquinolone activity as well as extended-spectrum beta-lactamase (ESBL) production.33 Moreover, a comprehensive study assessed ETEC and EAEC isolate changing antibiotic susceptibility trends over a decade among adult travellers to Mexico, India, and Guatemala with TD.27 The older agents, such as ampicillin, trimethoprim-sulfamethoxazole, and doxycycline, retained high levels of resistance. Fluoroquinolones and azithromycin MICs were significantly higher than when assessed 10 years prior. Regional variation in ETEC fluoroquinolone resistance was also demonstrated with higher rates in India compared with Central America. The EAEC isolates from Central America showed increased resistance across most antibiotics tested. In addition to the observed increases in fluoroquinolone resistance, 4- to 10-fold increases in MIC90 values for ceftriaxone and azithromycin were also seen for both ETEC and EAEC. The non-absorbable antibiotic rifaximin had no significant changes in ETEC or EAEC MICs.
In recent years, a rise in clinical resistance, often multidrug-resistance, to first-line antibiotics (e.g., fluoroquinolones and azithromycin) and alternative agents (e.g., third-generation cephalosporins) has been reported in Shigella spp. and Salmonella spp. diarrheal patients.34–44 The past 10–15 years have seen higher rates of fluoroquinolone resistance in surveys among industrialized countries with a large proportion associated with foreign travel.36,38,39 Multidrug resistance is also being reported, including decreased azithromycin susceptibility, ESBL production, coupled with loss of fluoroquinolone activity.37 The strong association with quinolone resistance and foreign travel has also been reported with non-typhoidal Salmonella species through the US antimicrobial resistance monitoring systems.43 Furthermore, resistance to ciprofloxacin was reported in 20.9% of Salmonella enterica isolates collected in the UK and associated with treatment failure.42 As seen in Shigella spp.,40 non-typhoidal Salmonella also have increasing evidence in MDR strains of losing available alternative agents, such as cefotaxime.44
Campylobacter jejuni/coli
Although an increasing level of fluoroquinolone resistance has been reported with many enteric pathogens, there is less information connecting resistance to clinical treatment failure. Presently, the association between emergent resistance and adverse clinical outcomes is best understood with Campylobacter spp. (Table 1). The emergence of fluoroquinolone resistance among C. jejuni/coli started earlier (i.e., 1990s)45–47 than the increasing trends over the past decade observed among diarrheagenic E. coli, Shigella, and Salmonella strains. Thailand has been a region with particularly rapid emergence with fluoroquinolone resistance among Campylobacter spp. increasing from zero in 1990 to 84% in 1995.45 In one analysis, travellers with confirmed bacterial enteritis within one month after travel to the tropics were examined. Campylobacter jejuni was the etiologic agent in 41% of cases, of which 53% of isolates were resistant to norfloxacin. Clinical failure occurred in 33% of patients who were prescribed fluoroquinolines.29 Surveillance of Campylobacter spp. isolates over a 10-year period (2001–10) in Peru found ciprofloxacin resistance of C. jejuni and Campylobacter coli in Lima significantly increased from 73.1 to 89.8% and 48.1 to 87.4%, respectively. Resistance of C. jejuni to azithromycin and erythromycin also significantly increased from 2.2 to 14.9% and 3.2 to 14.9%, respectively, in Iquitos, Peru.31
Table 1.
Antibiotic resistance of Campylobacter jejuni/coli and clinical implications
| Region | Location | Antibiotica | Sourceb | Date of isolate collection | Clinical implicationsc | Reference | |
|---|---|---|---|---|---|---|---|
| Asia | |||||||
| Asia | Fluoroquinolones | Travellers | 1994–2000; 2001–2006 | Increased from 36% resistant to 71% resistant (statistically significant) | [49] | ||
| Asia (excluding China) | Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 78% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | 6% resistant | [50] | |||
| East Asia | Fluoroquinolones | Travellers | 1994–2000; 2001–2006 | Increased from 48% resistant to 61% resistant (significance not stated) | [49] | ||
| Southeast Asia (Thailand) | Fluoroquinolones | Travellers | 1990 |
|
[46] | ||
| Travellers | 1993 |
|
[47] | ||||
| Domestic | 1981–1995 | Significant increase from zero in 1990 to 84% resistant in 1995 | [45] | ||||
| Travellers | 1998 |
|
[5] | ||||
| Travellers | 2000–2001 |
|
[4] | ||||
| Travellers | 2000–2006 | 64% resistant | [29] | ||||
| Macrolides | Travellers | 1993 |
|
[47] | |||
| Domestic | 1981–1995 |
|
[45] | ||||
| Travellers | 1998 | No resistance | [5] | ||||
| Travellers | 2000–2001 |
|
[4] | ||||
| Travellers | 2000–2006 | No resistance | [29] | ||||
| China | Fluoroquinolones/ Nalixidic acid | Travellers | 2005–2011 | 100% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | 11% resistant | [50] | |||
| Indian subcontinent | Fluoroquinolones | Travellers | 1994–2000;2001–2006 | Increased from 29% resistant to 79% resistant (statistically significant) | [49] | ||
| Europe and North America | |||||||
| Eastern Europe | Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 79% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | 5% resistant | [50] | |||
| Belgium | Fluoroquinolones | Domestic | 1994–2000; 2001–2006 | Increased from 9% resistant to 43% resistant (statistically significant) | [49] | ||
| Western Europe | Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 50% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | 1% resistant | [50] | |||
| North America (excluding United States) | Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 12% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | Zero resistance | [50] | |||
| Africa | |||||||
| Africa | Fluoroquinolones | Travellers | 1994–2000; 2001–2006 | Increased from 14% resistant to 32% resistant (statistically significant) | [49] | ||
| Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 37% resistant | [50] | |||
| Macrolides | Travellers | 2005–2001 | 1% resistant | [50] | |||
| Sub-Saharan Africa | Fluoroquinolones | Travellers | 2000–2006 | 31% resistant | [29] | ||
| Macrolides | Travellers | 2000–2006 | No resistance | [29] | |||
| West Africa | Fluoroquinolones | Travellers | 1994–2000; 2001–2006 | Increased from 7% resistant to 29% resistant (statistically significant) | [49] | ||
| Central/Latin America and Tropics | |||||||
| Mexico | Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 62% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | 4% resistant | [50] | |||
| Central America (excludes Mexico) | Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 51% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | 3% resistant | [50] | |||
| South America | Fluoroquinolones/ Nalidixic acid | Travellers | 2005–2011 | 83% resistant | [50] | ||
| Macrolides | Travellers | 2005–2011 | 4% resistant | [50] | |||
| Peru | Fluoroquinolones | Domestic | 2001–2006; 2006–2010 |
|
[31] | ||
| Macrolides | Domestic | 2001–2006; 2006–2010 |
|
[31] | |||
| Caribbean and Central/ South America | Fluoroquinolones | Travellers | 1994–2000;2001–2006 | Increased from 28% resistant to 61% resistant (statistically significant) | [49] | ||
| Combined tropics | Fluoroquinolones | Travellers | 2000–2006 | 33% clinical failure | [29] | ||
| Travellers | 2002–2003 | MIC >0.25 μg/ml in 42% of isolates | [53] | ||||
| Rifaximin | Travellers | 2002–2003 |
|
[53] | |||
aAntibiotics used in the studies are classified as fluoroquinolones, macrolides, and rifaximin. Macrolides includes azithromycin and erythromycin. Fluoroquinolones include norfloxacin, levofloxacin, and ciprofloxacin.
bSource indicates whether isolates were collected from travellers or residents of the location (domestic).
cBolded text in clinical implications column indicates reported clinical outcomes.
Among the confirmed cases of Campylobacter spp. infections affecting people in the United States (2005–11), 18% were associated with international travel. Data also suggest that a significant portion of fluoroquinolone resistance among Campylobacter spp. infections in the United States, as well as other countries,48 may be the result of acquisition through international travel. Specifically, 60% of travel-related Campylobacter spp. infections were found to be fluoroquinolone-resistant, while it was only 13% of domestic cases. Macrolide resistance, which was significantly associated with an increased risk of hospitalization, was observed with 4% of travel-related Campylobacter spp. infections, which has been observed at similar, relatively stable, rates in other surveys.49 In general, the majority of travel-related Campylobacter spp. infections resulted from travel to Asia, Mexico, or South America, and a high proportion were resistant to at least two fluoroquinolones (78, 62, and 83%, respectively).50 Fluoroquinolone resistant C. jejuni/coli has now become a widespread problem among industrialized and developing countries.
Evidence of TD Enteropathogen Treatment Failure Related to Antimicrobial Resistance
Treatment response in a majority of randomized control trials utilizes the time from initial antibiotic dose to the last unformed stool (TLUS). This endpoint does correlate well with overall clinical resolution, but other important outcomes include clearance of associated symptoms along with functional recovery and return to regular activities.18 The eradication of pre-treatment isolates, typically days 5–9 after the last treatment day, has not correlated well with clinical outcome.51 Early studies comparing azithromycin and ciprofloxacin in patients with shigellosis demonstrated high faecal antibiotic levels for both drugs with no evidence of resistance; however, treatment failure rates among S. dysenteriae type 1 were as high as 29 and 17% in the azithromycin and ciprofloxacin groups, respectively.52 In comparison the non-dysenteriae shigellosis cases had overall treatment failure rates, irrespective of drug, of only 6%, suggesting impact of specific pathogen, relative severity of disease at presentation, or both.
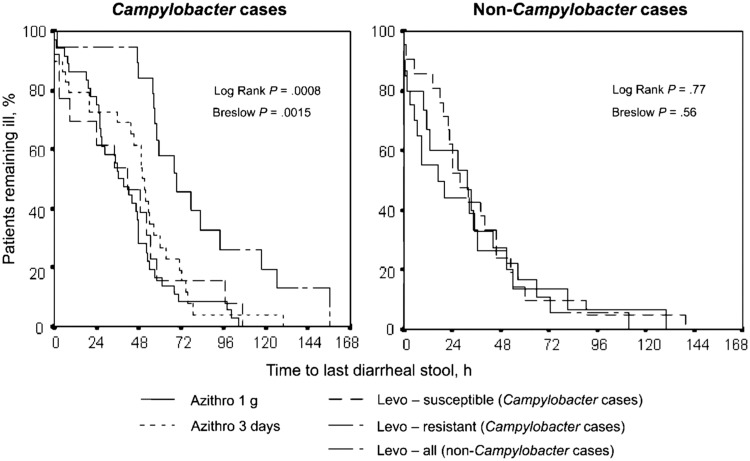
In a randomized, double-blind treatment trial comparing azithromycin and levofloxacin regimens, military personnel presenting with acute diarrhea while serving in Thailand were enrolled. Campylobacter spp. were isolated from 64% of patients, of which C. jejuni was predominant (95%). The Campylobacter spp. isolates were all susceptible to azithromycin; however, 50% and 93% were resistant to levofloxacin and ciprofloxacin, respectively. The TLUS was significantly different among subjects with levofloxacin-resistant Campylobacter-associated diarrhea who were provided the levofloxacin regimen (76.4 h compared with 41.2 h in subjects with levofloxacin-susceptible isolates and 41–47 h in subjects receiving azithromycin; P = 0.008; Figure 1). Clinical cure (i.e., resolution of diarrhea and associated symptoms within 72 h) was 96% for patients receiving single dose of azithromycin and 70% for 3 days of levofloxacin.4
Figure 1.
Time to cure (after receipt of the first antibiotic dose), by treatment group, as stratified by diagnosis of Campylobacter infection. This figure has been reprinted from Tribble et al.4 by permission of Oxford University Press.
While rifaximin has been shown to be effective at treating TD, it should not be prescribed in cases of febrile invasive disease where there have been clearly documented cases of primary treatment failure.53,54 Furthermore, rifaximin should specifically not be prescribed when Campylobacter is the etiologic agent due to its high level of resistance and clinical outcome evidence of treatment failure.55 Rifaximin MICs for ETEC and EAEC have been shown to increase among cases with persistent infection; however, this has not correlated with a decline in treatment efficacy.53
Impact of Resistant Pathogens on Treatment Recommendations
Current clinical practice patterns have been greatly impacted by the increasing levels of antibiotic resistance over the last two decades, as well as regional differences in the profile of resistant pathogens. Specifically, increased resistance of common enteropathogens to older first-line antibiotics, in addition to currently used absorbable systemic antibiotics, created a challenging situation for clinicians leading to past modification of practice guidelines. Although there are significant limitations in the available studies with outcome data, the findings show that the presence of a resistant phenotype often results in suboptimal clinical outcomes, including treatment failure. This is particularly evident with the case of the high level of fluoroquinolone-resistant Campylobacter spp. isolated in Southeast Asia. Further surveillance and assessment of outcomes are needed in order to examine trends in antibiotic resistance and modify treatment regimens to rapidly achieve clinical cure while balancing safety and emergent resistance concerns.
Funding
This work was supported by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health [Inter-Agency Agreement Y1-AI-5072].
Acknowledgement
I would like to thank Leigh Carson for her editorial assistance in the preparation of this manuscript.
Conflict of interest
The views expressed are those of the authors and does not necessarily reflect the official views of the Uniformed Services University of the Health Sciences, National Institute of Health or the Department of Health and Human Services, the Department of Defense or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
References
- 1. Connor BA. Traveler's Diarrhea. In: CDC Health Information for International Travel (Yellow Book): Chapter 2; Atlanta: Centers for Disease Control and Prevention, 2016. http://wwwnc.cdc.gov/travel/page/yellowbook-home-2014 (19 September 2016, date last accessed).
- 2. Diemert DJ. Prevention and self-treatment of traveler's diarrhea. Clin Microbiol Rev 2006; 19: 583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serichantalergs O, Pootong P, Dalsgaard A. et al. PFGE, Lior serotype, and antimicrobial resistance patterns among Campylobacter jejuni isolated from travelers and US military personnel with acute diarrhea in Thailand, 1998-2003. Gut Pathog 2010; 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tribble DR, Sanders JW, Pang LW. et al. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis 2007; 44: 338–46. [DOI] [PubMed] [Google Scholar]
- 5. Sanders JW, Isenbarger DW, Walz SE. et al. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: presentation and outcome of Campylobacter infection. Am J Trop Med Hyg 2002; 67: 533–8. [DOI] [PubMed] [Google Scholar]
- 6. Nada RA, Armstrong A, Shaheen HI. et al. Phenotypic and genotypic characterization of enterotoxigenic Escherichia coli isolated from U.S. military personnel participating in Operation Bright Star, Egypt, from 2005 to 2009. Diagn Microbiol Infect Dis 2013; 76: 272–7. [DOI] [PubMed] [Google Scholar]
- 7. Porter CK, Riddle MS, Tribble DR. et al. The epidemiology of travelers' diarrhea in Incirlik, Turkey: a region with a predominance of heat-stabile toxin producing enterotoxigenic Escherichia coli. Diagn Microbiol Infect Dis 2010; 66: 241–7. [DOI] [PubMed] [Google Scholar]
- 8. Riddle MS, Rockabrand DM, Schlett C. et al. A prospective study of acute diarrhea in a cohort of United States military personnel on deployment to the Multinational Force and Observers, Sinai, Egypt. Am J Trop Med Hyg 2011; 84: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah N, DuPont HL, Ramsey DJ.. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg 2009; 80: 609–14. [PubMed] [Google Scholar]
- 10. Connor BA, Riddle MS.. Post-infectious sequelae of travelers' diarrhea. J Travel Med 2013; 20: 303–12. [DOI] [PubMed] [Google Scholar]
- 11. De Bruyn G, Hahn S, Borwick A.. Antibiotic treatment for travellers' diarrhoea. Cochrane Database Syst Rev 2000; CD002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humphries RM, Schuetz AN.. Antimicrobial susceptibility testing of bacteria that cause gastroenteritis. Clin Lab Med 2015; 35: 313–31. [DOI] [PubMed] [Google Scholar]
- 13. Meng CY, Smith BL, Bodhidatta L. et al. Etiology of diarrhea in young children and patterns of antibiotic resistance in Cambodia. Pediatr Infect Dis J 2011; 30: 331–5. [DOI] [PubMed] [Google Scholar]
- 14. Novoa-Farias O, Frati-Munari AC, Peredo MA. et al. Susceptibility of bacteria isolated from acute gastrointestinal infections to rifaximin and other antimicrobial agents in Mexico. Rev Gastroenterol Mex 2016; 81: 3–10. [DOI] [PubMed] [Google Scholar]
- 15. Nunes MR, Magalhaes PP, Penna FJ. et al. Diarrhea associated with Shigella in children and susceptibility to antimicrobials. J Pediatr (Rio J) 2012; 88: 125–8. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz-Castillo A, Torres-Sanchez MJ, Aznar-Martin J.. Molecular epidemiology and antimicrobial susceptibility of Campylobacter coli clinical isolates. Enferm Infecc Microbiol Clin 2014; 32: 443–5. [DOI] [PubMed] [Google Scholar]
- 17. Streit JM, Jones RN, Toleman MA. et al. Prevalence and antimicrobial susceptibility patterns among gastroenteritis-causing pathogens recovered in Europe and Latin America and Salmonella isolates recovered from bloodstream infections in North America and Latin America: report from the SENTRY Antimicrobial Surveillance Program (2003). Int J Antimicrob Agents 2006; 27: 367–75. [DOI] [PubMed] [Google Scholar]
- 18. DuPont HL, Ericsson CD, Farthing MJ. et al. Expert review of the evidence base for self-therapy of travelers' diarrhea. J Trav Med 2009; 16: 161–71. [DOI] [PubMed] [Google Scholar]
- 19. Riddle MS, Arnold S, Tribble DR.. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler's diarrhea: a systematic review and meta-analysis. Clin Infect Dis 2008; 47: 1007–14. [DOI] [PubMed] [Google Scholar]
- 20. Jiang ZD, Ke S, Dupont HL.. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrobiol Agents 2010; 35: 278–81. [DOI] [PubMed] [Google Scholar]
- 21. Salazar-Lindo E, Sack RB, Chea-Woo E. et al. Early treatment with erythromycin of Campylobacter jejuni-associated dysentery in children. J Pediatr 1986; 109: 355–60. [DOI] [PubMed] [Google Scholar]
- 22. Ge B, Wang F, Sjolund-Karlsson M, McDermott PF.. Antimicrobial resistance in campylobacter: susceptibility testing methods and resistance trends. J Microbiol Methods 2013; 95: 57–67. [DOI] [PubMed] [Google Scholar]
- 23. Luke DR, Foulds G.. Disposition of oral azithromycin in humans. Clin Pharmacol Ther 1997; 61: 641–8. [DOI] [PubMed] [Google Scholar]
- 24. Rakita RM, Jacques-Palaz K, Murray BE.. Intracellular activity of azithromycin against bacterial enteric pathogens. Antimicrbiol Agents Chemother 1994; 38: 1915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pastel D. Focus on oral ciprofloxacin; clinical and economic considerations. Hosp Pharm 1989; 24: 814–20. 823–816, 842. [PubMed] [Google Scholar]
- 26. Hong KS, Kim JS.. Rifaximin for the treatment of acute infectious diarrhea. Therap Adv Gastroenterol 2011; 4: 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouyang-Latimer J, Jafri S, VanTassel A. et al. In vitro antimicrobial susceptibility of bacterial enteropathogens isolated from international travelers to Mexico, Guatemala, and India from 2006 to 2008. Antimicrobiol Agents Chemother 2011; 55: 874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ince OT, Yalcin SS, Yurdakok K. et al. Salmonella gastroenteritis in children (clinical characteristics and antibiotic susceptibility): comparison of the years 1995-2001 and 2002-2008. Turk J Pediatr 2012; 54: 465–73. [PubMed] [Google Scholar]
- 29. Bottieau E, Clerinx J, Vlieghe E. et al. Epidemiology and outcome of Shigella, Salmonella and Campylobacter infections in travellers returning from the tropics with fever and diarrhoea. Acta Clin Belg 2011; 66: 191–5. [DOI] [PubMed] [Google Scholar]
- 30. Mendez Arancibia E, Pitart C, Ruiz J. et al. Evolution of antimicrobial resistance in enteroaggregative Escherichia coli and enterotoxigenic Escherichia coli causing traveller's diarrhoea. J Antimicrob Chemother 2009; 64: 343–7. [DOI] [PubMed] [Google Scholar]
- 31. Pollett S, Rocha C, Zerpa R. et al. Campylobacter antimicrobial resistance in Peru: a ten-year observational study. BMC Infect Dis 2012; 12: 193.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pickering LK. Antimicrobial resistance among enteric pathogens. Semin Pediatr Infect Dis 2004; 15: 71–7. [DOI] [PubMed] [Google Scholar]
- 33. Hebbelstrup Jensen B, Olsen KE, Struve C. et al. Epidemiology and clinical manifestations of enteroaggregative Escherichia coli. Clin Microbiol Rev 2014; 27: 614–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Seidlein L, Kim DR, Ali M. et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med 2006; 3: e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu B, Zhou M, Ke X. et al. Comparison of resistance to third-generation cephalosporins in Shigella between Europe-America and Asia-Africa from 1998 to 2012. Epidemiol Infect 2015; 143: 2687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lane CR, Sutton B, Valcanis M. et al. Travel Destinations and sexual behavior as indicators of antibiotic resistant Shigella strains–Victoria, Australia. Clin Infect Dis 2016; 62: 722–9. [DOI] [PubMed] [Google Scholar]
- 37. Poramathikul K, Bodhidatta L, Chiek S. et al. Multidrug-resistant Shigella infections in patients with diarrhea, Cambodia, 2014-2015. Emerg Infect Dis 2016; 22: 1640–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bowen A, Grass J, Bicknese A. et al. Elevated risk for antimicrobial drug-resistant Shigella infection among men who have sex with men, United States, 2011-2015. Emerg Infect Dis 2016; 22: 1613–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shiferaw B, Solghan S, Palmer A. et al. Antimicrobial susceptibility patterns of Shigella isolates in Foodborne Diseases Active Surveillance Network (FoodNet) sites, 2000-2010. Clin Infect Dis 2012; 54 Suppl 5: S458–63. [DOI] [PubMed] [Google Scholar]
- 40. Goel N, Wattal C, Kaul D, Khanna VK.. Emergence of ceftriaxone resistant Shigella. Indian J Pediatr 2013; 80: 70–1. [DOI] [PubMed] [Google Scholar]
- 41. Toro C, Arroyo A, Sarria A. et al. Shigellosis in subjects with traveler's diarrhea versus domestically acquired diarrhea: implications for antimicrobial therapy and Human Immunodeficiency Virus surveillance. Am J Trop Med Hyg 2015; 93: 491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Al-Mashhadani M, Hewson R, Vivancos R. et al. Foreign travel and decreased ciprofloxacin susceptibility in Salmonella enterica infections. Emerg Infect Dis 2011; 17: 123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Donnell AT, Vieira AR, Huang JY. et al. Quinolone-resistant Salmonella enterica serotype Enteritidis infections associated with international travel. Clin Infect Dis 2014; 59: e139–41. [DOI] [PubMed] [Google Scholar]
- 44. Gunell M, Aulu L, Jalava J. et al. Cefotaxime-resistant Salmonella enterica in travelers returning from Thailand to Finland. Emerg Infect Dis 2014; 20: 1214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoge CW, Gambel JM, Srijan A. et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis 1998; 26: 341–5. [DOI] [PubMed] [Google Scholar]
- 46. Petruccelli BP, Murphy GS, Sanchez JL. et al. Treatment of traveler’s diarrhea with ciprofloxacin and loperamide. J Infect Dis 1992; 165: 557–60. [DOI] [PubMed] [Google Scholar]
- 47. Kuschner RA, Trofa AF, Thomas RJ. et al. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin Infect Dis 1995; 21: 536–41. [DOI] [PubMed] [Google Scholar]
- 48. Hakanen A, Jousimies-Somer H, Siitonen A. et al. Fluoroquinolone resistance in Campylobacter jejuni isolates in travelers returning to Finland: association of ciprofloxacin resistance to travel destination. Emerg Infect Dis 2003; 9: 267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vlieghe ER, Jacobs JA, Van Esbroeck M. et al. Trends of norfloxacin and erythromycin resistance of Campylobacter jejuni/Campylobacter coli isolates recovered from international travelers, 1994 to 2006. J Trav Med 2008; 15: 419–25. [DOI] [PubMed] [Google Scholar]
- 50. Ricotta EE, Palmer A, Wymore K. et al. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005-2011. Am J Public Health 2014; 104: e108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ericsson CD, DuPont HL, Mathewson JJ. et al. Test-of-cure stool cultures for traveler's diarrhea. J Clin Microbiol 1988; 26: 1047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khan WA, Seas C, Dhar U. et al. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Ann Intern Med 1997; 126: 697–703. [DOI] [PubMed] [Google Scholar]
- 53. Taylor DN, Bourgeois AL, Ericsson CD. et al. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers' diarrhea. Am J Trop Med Hyg 2006; 74: 1060–6. [PubMed] [Google Scholar]
- 54. Dupont HL, Jiang ZD, Belkind-Gerson J. et al. Treatment of travelers' diarrhea: randomized trial comparing rifaximin, rifaximin plus loperamide, and loperamide alone. Clin Gastroenterol Hepatol 2007; 5: 451–6. [DOI] [PubMed] [Google Scholar]
- 55. Hopkins KL, Mushtaq S, Richardson JF. et al. In vitro activity of rifaximin against clinical isolates of Escherichia coli and other enteropathogenic bacteria isolated from travellers returning to the UK. Int J Antimicrob Agents 2014; 43: 431–7. [DOI] [PubMed] [Google Scholar]