Abstract
About 40,000 American women die from metastatic breast cancer each year despite advancements in treatment. Approximately, 15% of breast cancers are triple-negative for estrogen receptor, progesterone receptor, and HER2. Triple-negative cancer is characterized by more aggressive, harder to treat with conventional approaches and having a greater possibility of recurrence. Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid signaling mediator has emerged as a key regulatory molecule in breast cancer progression. Therefore, we investigated whether cytosolic sphingosine kinase type 1 (SphK1) and nuclear sphingosine kinase type 2 (SphK2), the enzymes that make S1P are critical for growth and PI3K/AKT, ERK-MAP kinase mediated survival signaling of lung metastatic variant LM2-4 breast cancer cells, generated from the parental triple-negative MDA-MB-231 human breast cancer cell line. Similar with previous report, SphKs/S1P signaling is critical for the growth and survival of estrogen receptor positive MCF-7 human breast cancer cells, was used as our study control. MDA-MB-231 did not show a significant effect of SphKs/S1P signaling on AKT, ERK, and p38 pathways. In contrast, LM2-4 cells that gained lung metastatic phenotype from primary MDAMB-231 cells show a significant effect of SphKs/S1P signaling requirement on cell growth, survival, and cell motility. PF-543, a selective potent inhibitor of SphK1, attenuated epidermal growth factor (EGF)-mediated cell growth and survival signaling through inhibition of AKT, ERK, and p38 MAP kinase pathways mainly in LM2-4 cells but not in parental MDA-MB-231 human breast cancer cells. Moreover, K-145, a selective inhibitor of SphK2, markedly attenuated EGF-mediated cell growth and survival of LM2-4 cells. We believe this study highlights the importance of SphKs/S1P signaling in metastatic triple-negative breast cancers and targeted therapies.
Keywords: SphKs, S1P, sphingolipids, signaling, proliferation, EGF, triple-negative metastatic breast cancer
1. Introduction
Breast cancer is most common type of deadly type of cancer in women due to the metastatic process and resistant to treatment [1]. In 2016, it is estimated that there will 246,660 new cases of female breast cancer and estimated 40,450 people will die because of breast cancer worldwide [2]. Approximately, 15% of breast tumors are classified as triple-negative breast cancers (TNBC) have a propensity to metastasize and a poor outcome relative to other breast cancer subtype [3,4]. Finding strategies to cure metastatic TNBC would be beneficial for breast cancer patients. Sphingosine-1-phosphate (S1P) is a potent bioactive lipid mediator produced by the cytosolic SphK1 and the nuclear localized SphK2 from the substrate sphingosine [5], has been known to play important roles to cancer development and progression by regulating tumor proliferation, migration and angiogenesis [6–9]. Although some important roles of intracellular actions of S1P have been discovered [5,10,11], the majority of its biological functions are known to mediated by plasma membrane localized family of five specific G protein coupled receptors (S1PR1-5) [8,12]. The sphingolipid pathway, particularly SphK signaling, has become of increasing interest as a breast cancer therapeutic target [13,14]. SphK1/S1P, in particular, has been implicated in oncogenic roles in cancer, including proliferation, resistance to apoptosis and tumor growth can be inhibited by SphK1 inhibitors [6,13,15,15,16]. In contrast, specific inhibition of SphK1 with a potent and selective inhibitor PF-543 had no effect on the proliferation and survival of various cancer cells, despite a dramatic change in the intracellular levels of S1P [17,18]. However, PF-543 is an essential chemical tool to study whether targeting SphK1/S1P signaling is a viable therapeutic strategy for metastatic TNBC. On the other hand, we and others have demonstrate that SphK2/S1P signaling epigenetically regulates histone acetylation in the nucleus regulates gene transcriptions that are important for cancer progression [5,19]. Growing research evidences also suggest that the two isoforms SphK1 and SphK2 have similar roles in certain cancers, including breast, and promote cancer survival and chemo resistance [9,15,20]. Recent studies suggest that S1P generated from SphK2, may promote cancer cell proliferation and migration, suggesting SphK2 and S1P as potential therapeutic targets in cancer cell [21–23]. Our recent studies demonstrate that a selective SphK2 inhibitor K-145 reduces intracellular S1P [23], reduces specific histone acetylation in the nucleus of breast cancer cells [24], drastically attenuated breast cancer growth in a syngeneic mouse model [23]. Together, SphKs/S1P signaling is critical for growth and survival of estrogen receptor positive breast cancer cells, however the role of SphKs/S1P in TNBC and more importantly in metastatic breast cancer progression was not demonstrated before. Epidermal growth factor (EGF) plays a critical role in progression, invasion, and tumorigenicity of human breast cancers [25]. We and others have demonstrated earlier that EGF activates SphKs by ERK-mediated phosphorylation [26,27], which is required endogenous SphKs-mediated cell motility and growth of estrogen receptor positive breast cancer cells[9,27–29]. Here, we examined the functions of SphKs/S1P signaling in growth and survival of LM2-4 lung metastatic variant of the triple-negative MDA-MB-231 breast cancer cell line [30,31] in comparison with TNBC MDA-MB-231 and estrogen receptor positive MCF-7 human breast cancer cells. For our studies we have used specific pharmacological drugs (PF-543 for SphK1 inhibition [17], K-145 for SphK2 inhibition [23], and DMS for SphKs inhibition [32]) or genetic tools to transiently knock down SphKs to study S1P-mediated survival signaling and proliferation in metastatic breast cancer cell lines. In agreement with our previous observations [20,28,29], we found that SphKs/S1P signaling is essential for growth and survival of estrogen receptor positive MCF-7 human breast cancer cells. Current studies demonstrated that SphKs/S1P signaling is a key component of growth and survival of mainly lung metastatic variant LM2-4 breast cancer cells compared to parental triple-negative MDA-MB-231 human breast cancer cell. A selective and potent inhibitor of sphingosine kinase 1 (SphK1) PF-543 is not effective on original parental triple-negative MDA-MB-231 breast cancer cells to attenuate survival signaling but it markedly attenuated cell growth and PI3K/AKT, ERK & p38 MAP kinases survival signaling of lung metastatic variant LM2-4 human breast cancer cells. We found that SphK2, which is mainly localized in the nucleus of triple-negative MDA-MB-231 human breast cancer cells [24], it also essential for metastatic variant LM2-4 breast cancer cells growth and survival. Our studies demonstrated that triple-negative metastatic human breast cancer progression is dependent on SphKs/S1P signaling.
2. Materials and methods
2.1. Cell Culture, Transfection, and Reagents
MCF-7 human breast cancer cell lines (ATCC, Manassas, VA, USA) were cultured in phenol-red free IMEM (Gibco BRL, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (FBS), 0.25% dextrose (Sigma-Aldrich),100 u/ml penicillin and 100 μg/ml streptomycin (Gibco BRL, Grand Island, NY, USA). The human LM2-4 cells are a metastatic variant of the triple-negative MDA-MB-231 breast cancer cell line derived after multiple rounds of in vivo lung metastasis selection in mice, as previously described [30,31], were a kind gift from John M.L. Ebos, Roswell Park Cancer Institute, and described previously[33]. LM2-4 and MDA-MB-231 cells were maintained in phenol-red free RPMI (Gibco BRL, Grand Island, NY, USA) containing 10% heat-inactivated FBS, 100 u/ml penicillin and 100 μg/ml streptomycin (Gibco). Cells were authenticated by STR profile comparison with ATCC parental cell database (for LM2-4). All cells were incubated at 37°C and 5% CO2 in a humidified incubator. Human SphK1 and SphK2 were downregulated by transfection with ON-TARGETplus SMARTpool siRNA or control scrambled siRNA (Dharmacon, Lafayette, CO, USA). In some experiments, SphK1 and SphK2 expression were down-regulated with sequence-specific siRNAs. siRNA for human SphK1 (sequence targeted: 5′-GGGCAAGGCCTTGCAGCTC-3′, obtained from Qiagen (Valencia, CA)) [28,34] and human SphK2 (sequence targeted: 5′-GCTGGGCTGTCCTTCAACCT-3′, obtained from Qiagen (Valencia, CA)) [20,29], and control siRNA (Qiagen) were used. SphK2 (NM_001204160) human cDNA expression vector (pCMV6-SphK2) was from OriGene Technologies (Rockville, MD, USA). Untagged human SphK2 was overexpressed by transfection of pCMV-SphK2 with Lipofectamine Plus (Invitrogen). S1P and DMS were used from Enzo Life Sciences (Farmingdale, NY, USA), human EGF, K-145 from Sigma-Aldrich (St. Louis, MO, USA), PF-543 from Cayman Chemicals (Ann Arbor, Michigan, USA).
2.2. Immunoblotting
Cells were lysed by freeze-thawing in buffer (20 mM Tris (pH 7.4), 20% glycerol, 1 mM 2- mercaptoethanol, 1 mM EDTA, 5 mM sodium orthovanadate, 40 mM β-glycerophosphate, 15 mM NaF, 0.5 mM 4-deoxypyridoxine, and Sigma protease inhibitor cocktail). Unbroken cells were removed by centrifugation at 700 × g for 10 min. For some experiments, nuclear extracts from cells were prepared and protein expression determined by immunoblotting as previously described (Hait 2009). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween 20. The following primary antibodies were used for immunoblotting: phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), phospho-Akt (Ser473), phospho-p38 MAPK (Thr180/Tyr182), phospho-p70 S6 Kinase (Thr421/Ser424), lamin A/C (Cell Signaling Technology, Danvers, MA, USA), α-tubulin, SphK1, SphK2 (1:1000 dilution, Sigma-Aldrich). Immunopositive bands were visualized with HRP-conjugated secondary antibodies (1:5000 dilution, Jackson ImmunoResearch, West Grove, PA, USA) for 1 h at room temperature and SuperSignal West Pico chemiluminescence substrate (Pierce Chemical Co., Rockford, IL, USA).
2.3. Reverse transcription (RT)-PCR and real-time PCR
Total RNA was prepared with Trizol (Life Technologies, Carlsbad, CA, USA). RNA (2 μg) was reverse transcribed with the high-capacity cDNA Archive kit (Life Technologies). cDNAs were diluted 10-fold (target genes) or cyclophilin A 100-fold and amplified with SYBR Green quantitative PCR (qPCR) on CFX96 cycler (Bio-Rad Hercules, CA, USA). Gene expression levels were calculated by the ΔΔCt method and normalized to Cyclophilin A expression. The following primers were used for PCR: human SphK1 sense, F: GCTCTGGTGGTCATGTCTGG-3′ and antisense, 5′-CACAGCAATAGCGTGCAGT-3′; human SphK2 sense, 5′-ATGGCATCGTCACGGTCTC-3′ and antisense, 5′-CTCCCAGTCAGGGCGATCTA-3′; and human Cyclophilin A sense, 5′-CTCGAATAAGTTTGACTTGTGTTT-3′ and antisense, 5′-CTAGGCATGGGAGGGAACA-3′.
2.4. Cell proliferation assay
Cell growth was also measured by adding WST-8 reagent in accordance with the manufacturer’s instructions (Dojindo Molecular Technologies, Rockville, MD, USA). Absorbance was measured at 450 nm with background subtraction.
2.5. Migration assay
For the cell migration assay, cells were seeded into a 24-well or 6–well culture dish. The cells were then maintained in 1–2% serum containing medium for 18 h. The monolayers were carefully scratched using a 20-μl or 200-μl pipette tip. The cellular debris was subsequently removed by washing with PBS, and the cells were incubated in medium with 1–2% serum. Cells were pre-treated with 2 μM PF543 (SphK1 inhibitor), and K145 (SphK2 inhibitor before treatment of EGF (100ng/ml) for 8 to 24 hours. For some experiments, cells were transfected with siRNAs for 24 hours for migration assay. Migrating cells into the wounded area were photographed under a phase contrast microscope.
2.6. Statistical analysis
Statistical analysis was performed using unpaired two-tailed Student’s t-test for comparison of two groups. *P<0.05 and **P<0.005 was considered significant.
3. Results
3.1. Role of SphKs in proliferation of metastatic human breast cancer LM2-4 cells
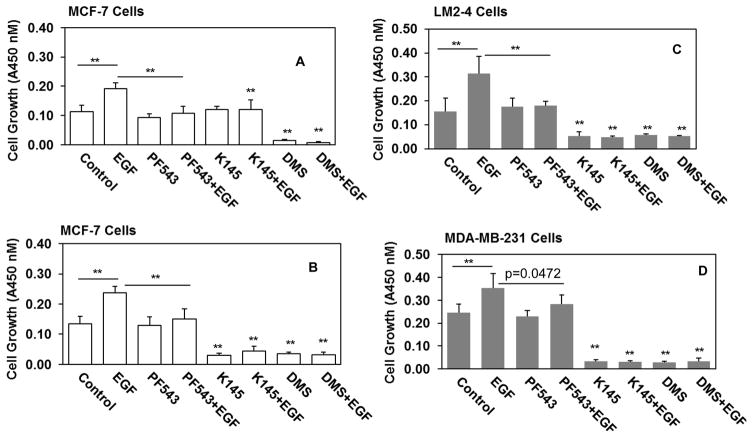
The sphingosine kinases (SphKs) catalyze the conversion of sphingosine to sphingosine 1-phosphate (S1P). The role of cytosolic SphK1 in cancer progression is well characterized with high expression observed in many different cancers including breast cancers. In contrast, the contribution of nuclear localized SphK2 to cancer progression is not yet well established. In this study we wanted to examine whether role of SphKs/S1P is critical for growth and survival of metastatic breast cancer LM2-4 cells in comparison with its parental triple-negative human breast cancer MDA-MB-231 cells and estrogen receptor positive human breast cancer MCF-7 cells. Given the importance of EGF in breast cancer progression [35], we examined whether EGF-induced cell growth is dependent on SphKs/S1P in human breast metastasis model LM2-4 cells. It has been shown that inhibiting SphK1 with a selective potent inhibitor PF-543 reduced intracellular S1P[17] but did not reduce basal growth of human breast cancer cells [17]. In our experiment, we observed that PF-543 markedly attenuated EGF-mediated growth of MCF-7 (Fig. 1A), metastatic breast cancer LM2-4 (Fig. 1B) and may be triple-negative MDA-MB-231 cells (growth, control vs EGF with PF-543, p=0.0472) (Fig. 1C). Inhibiting SphK2 with a selective potent inhibitor K-145 also markedly reduces intracellular S1P [23] or inhibiting SphKs activity with N, N-dimethylsphingosine (DMS) [32,36] has dramatically attenuated basal as well as EGF-mediated cell growth of MCF-7 (Fig. 1A), MDA-MB-231, and most importantly in metastatic breast cancer LM2-4 cells.
Figure 1. Selective inhibitors of SphKs suppress EGF-mediated metastatic breast cancer cell growth.
Human breast cancer MCF-7 cells (A, B), human TNBC MDA-MB-231 (C), and human lung metastatic LM2-4 cells originated from MDA-MB-231 cells (B) were cultured in 1% serum. Cells were pre-treated with 5 μM (A) and 10 μM (B, C, D) PF543 (SphK1 inhibitor), K145 (SphK2 inhibitor), and DMS (SphKs inhibitor) before treatment of 100 ng/ml EGF for 3 days. Cells growth were measured with WST-8 regents according to the manufacture’s protocol. Optical density was measured at 450 nm using Biotek plate reader. Back ground corrected optical densities were plotted. Similar results were obtained in two additional experiments.
3.2. Role of SphKs/S1P in survival signaling of metastatic human breast cancer LM2-4 cells
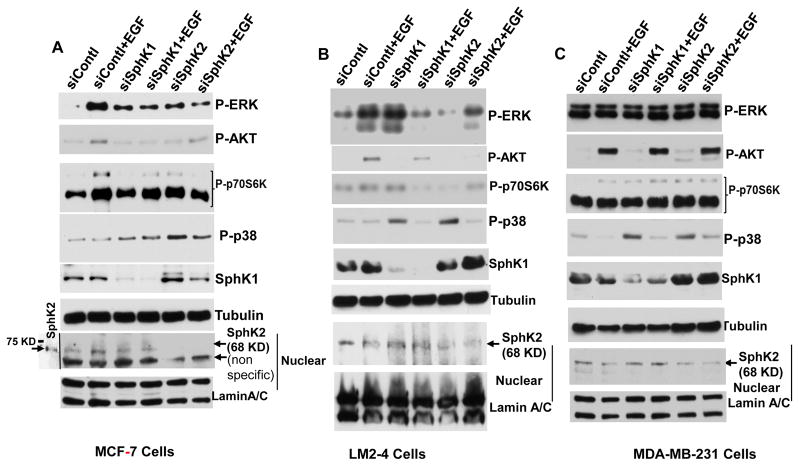
Epidermal growth factor receptor activated by EGF and other ligands triggers signaling cascades leading to cancer cell proliferation, target gene expression [37–39]. Over-expression and aberrant activation of the EGFR signal pathway are also relative to cell proliferation, migration, invasion, and angiogenesis [40,41]. Previous reports have shown that extracellular stimuli elicit a broad spectrum of biological responses through activation of MAPK cascades, including p42/p44 MAPK [42], p38 MAPK [43]. Activated PI3K/AKT initiates a cascade of downstream signaling which promotes cellular growth, metabolism, proliferation, survival, migration, and angiogenesis [44]. The PI3K/AKT pathways are also involved in crosstalk with RAS/RAF/MEK/ERK and estrogen receptor pathways [45]. As S1P has emerged as a new class of lipid messengers that regulate cell proliferation, differentiation, and migration in vitro [6–8] and also in clinical samples, we became interested to understand whether blocking of both sphingosine kinases inhibited MAPK cascades and PI3K/AKT pathways in metastatic breast cancer LM2-4 cells. In agreement with earlier reports [7,46], extracellular S1P or EGF activates P-ERK, P-p38, P-AKT, and P-p70S6K in MCF-7 cells and inhibiting SphKs activity with DMS [36] attenuated EGF-mediated survival signaling (Fig. 2A). Interestingly, inhibition of SphK1 with PF-543 or inhibition of SphK2 with K-145 drastically reduced EGF-mediated P-ERK, P-AKT, and P-p38 in metastatic breast cancer LM2-4 cells (Fig. 2B). Although, it has been demonstrated that SphK1 regulates proliferation and survival in triple-negative breast cancer [47] which supports our cell growth data with EGF in MDA-MB-231 cells (Fig. 1D), while PF-543 is unable to reduce basal P-ERK, P-p38 or P-AKT in MDA-MB-231cells, neither unable to reduces basal growth of any breast cancer tested in these studies (Fig. 2C). Interestingly, inhibiting SphK2 with K-145 inhibits at least basal to some extent but absolutely not the EGF-mediated P-AKT levels in these cells (Fig. 2C) yet inhibits both basal and EGF-mediated growth of triple-negative MDA-MB-231 human breast cancer cells (Fig. 1D). Based on our signaling data in MDA-MB-231 cells (Fig. 2C), it became clear that extracellular S1P did not activate P-ERK, P-AKT, however, it activates P-p38 in MDA-MB-231 cells (Fig. 2C). EGF activates P-AKT and P-p38 independent of SphKs/S1P signaling in these cells (Fig. 2C).
Figure 2. Selective inhibitors of SphKs suppress EGF-mediated signaling of ERK, AKT, p38, and p70S6K in metastatic breast cancer cells.
Human breast cancer MCF-7 cells (A), human TNBC MDA-231 (C), and human lung metastatic LM-2-4 cells originated from MDA-231 cells (B) were serum starved 18 hours and pre-treated with DMS (A–C), PF-543 (B–C), and K-145 (B–C) for 1 hour before treatment with or without 100 ng/ml EGF for 15 minutes. For some experiments 100 nM S1P was treated without or with pre-treatment of DMS for 15 minutes. Levels of p-ERK, p-AKT, p-p38, and p-p70S6K proteins were analyzed by immunoblotting with the indicated antibodies. Blots were stripped and re-probed with anti-α-tubulin antibody to show equal loading and transfer. Similar results were obtained in two additional experiments.
3.3. Effect of downregulating SphKs on survival signaling of metastatic human breast cancer LM2-4 cells
We further examined the effect of downregulating SphK1 and SphK2 expression with target specific siRNA on EGF mediated signaling pathways in LM2-4 cells in comparison with its parental MDA-MB-231 and ERα-positive MCF-7 cells. To complement the effect of pharmacological drug used above and to exclude the possible off target effect of drugs, we down regulated individual kinase by using target specific siRNA. We have confirmed that siSphK1 as expected reduced cytosolic SphK1 protein (Fig. 3) and siSphK2 reduced nuclear SphK2 protein in all three breast cancer cell lines (Fig. 3). Consistent with our inhibitors studies (Fig. 2A), downregulation of SphK1 or SphK2 markedly attenuated EGF-mediated P-ERK, P-AKT, and P-p70S6K but not P-p38 in MCF-7 cells and similarly in metastatic variant LM-2-4 breast cancer cells. However, parental triple-negative MDA-MB-231 breast cancer cells did not show any effect on the signaling pathways with down regulation of SphK1 or SphK2 with target specific siRNA. Down regulation of either SphK1 or SphK2 with siRNA markedly enhances P-p38 MAP kinase in all three breast cancer cells, which is also called on of the stress-activated protein kinase pathways in cancer [48].
Figure 3. Downregulation of SphKs with specific siRNAs suppresses EGF-mediated signaling of ERK, AKT, and p70S6K but not p38 in metastatic breast cancer cells.
Human breast cancer MCF-7 cells (A), human TNBC MDA-231 (C), and human lung metastatic LM-2-4 cells originated from MDA-231 cells (B) were transfected with control siRNA and siRNAs targeted to SphKs for 48 hours. Serum starved cells were treated with or without 100 ng/ml EGF for 15 minutes. Levels of p-ERK, p-AKT, p-p38, p-p70S6K, SphK1 proteins in the nuclei-free extracts were analyzed by immunoblotting with the indicated antibodies. Blots were stripped and re-probed with anti-α-tubulin antibody to show equal loading and transfer. Level of SphK2 protein in the nuclear extract was analyzed by immunoblotting with anti-SphK2 antibodies. Nuclear extract from MCF-7 cells transiently transfected with untagged SphK2 was included to indicate the molecular weight of this protein [20]. Blots were stripped and re-probed with anti-lamin A/C antibody to show equal loading and transfer. Similar results were obtained in two additional experiments.
3.4. Effect of downregulating SphKs on cell growth of metastatic human breast cancer LM2-4 cells
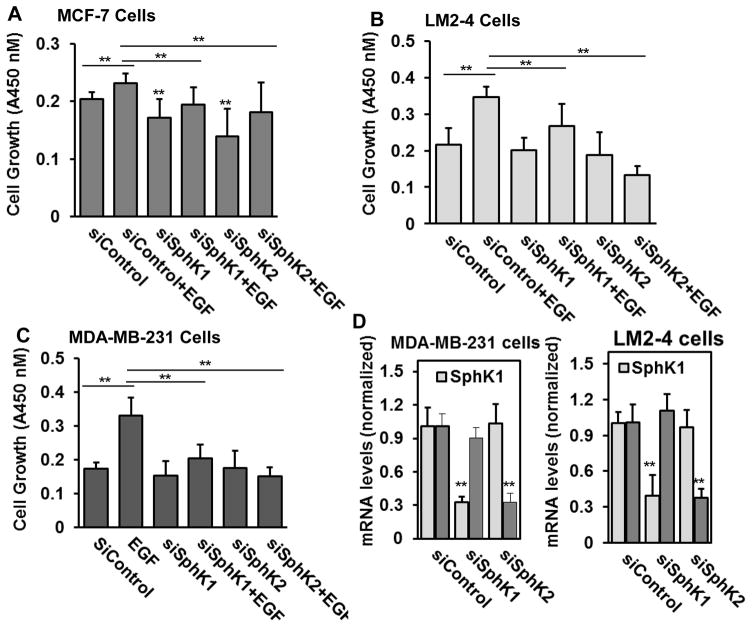
Previous studies demonstrated that SphK1 regulates proliferation and survival responses in MDA-MB- 231 cells [47]. We have demonstrated with others that SphK2 signaling is involved in promoting growth of MDA-MB-231 cells [24,49]. We further confirmed that the effect of downregulating SphK1 and SphK2 expression with target specific siRNA on EGF mediated cell growth in LM2-4 cells in comparison with its parental MDA-MB-231 and ERα-positive MCF-7 cells. Consistent with our inhibitors studies (Fig. 2A), downregulation of SphK1 or SphK2 as expected markedly attenuated EGF-mediated growth of MCF-7 cells (Fig. 4A), MDA-MBA-231 cells (Fig. 4C), and more importantly the metastatic triple-negative breast cancer LM2-4 cells (Fig. 4B). [9]
Figure 4. Downregulation of SphKs with specific siRNAs suppresses EGF-mediated metastatic breast cancer cell growth.
Human breast cancer MCF-7 cells (A), human TNBC MDA-MB-231 (C), and human lung metastatic LM-2-4 cells originated from MDA-MB-231 cells (B) were transfected with control siRNA and siRNAs targeted to SphKs for 24 hours. Cells were grown in 1% serum containing medium and treated with or without EGF (100 ng/ml) for 3 days. Cells growth was measured with WST-8 regents according to the manufacture’s protocol. Optical density was measured at 450 nm using Biotek plate reader. Back ground corrected optical densities were plotted. Similar results were obtained in two additional experiments. Downregulation of SphK1 and SphK2 with siRNAs (D). RNA was isolated and mRNA levels of SphK1 and SphK2, and Cyclophilin A mRNAs were determined by quantitative real-time PCR.
3.5. Role of SphKs in migration of metastatic human breast cancer LM2-4 cells
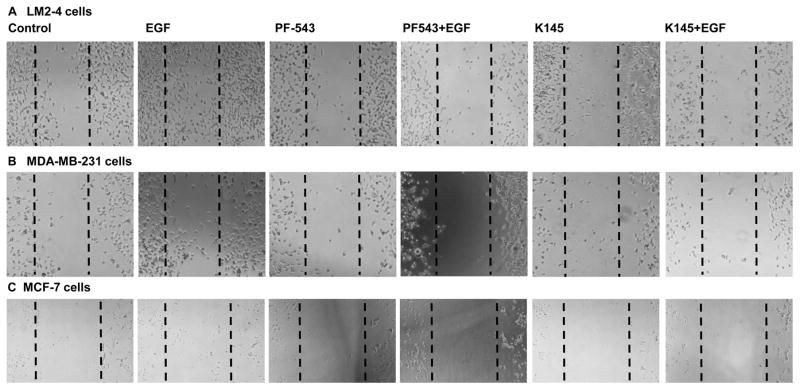
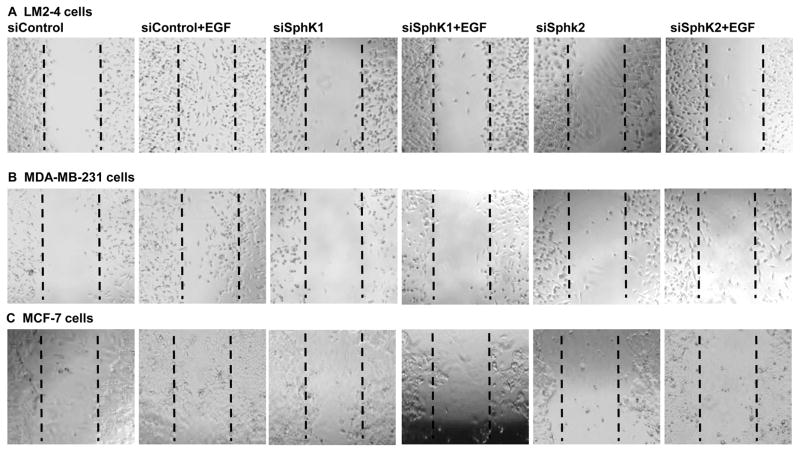
EGF has been reported to activate SphKs [9,27] and to induce cell migration via transactivation of S1PR3 in ER-positive cancer cells [28,50]. We have demonstrated earlier that SphKs signaling is involved in EGF-mediated migration of triple-negative breast cancer cells [29]. Here we have demonstrated that inhibiting SphK1 with PF543 (Fig. 5) or downregulating with siSphK1 as expected inhibits EGF-mediated cell migration of MCF-7 cells (Fig. 5C and Fig. 6C), MDA-MB-231cells (Fig. 5B and Fig. 6B) and more importantly lung metastatic LM2-4 cells (Fig. 5A and Fig. 6A). Furthermore, inhibiting SphK2 with K145 compound or downregulating with siSphK2 not only inhibits EGF-mediated cell migration of MCF-7 cells (Fig. 5C and Fig. 6C), MDA-MB-231 cells (Fig. 5B and Fig. 6B) but also markedly inhibited EGF-mediated cell migration of lung metastatic breast cancer LM2-4 cells (Fig. 5A and Fig. 6A).
Figure 5. Selective inhibitors of SphKs suppress EGF-mediated metastatic breast cancer cell motility.
LM2-4, MDA-MB-231, and MCF-7 cells were grown in 2% serum containing medium for 24 hours. Cells were scratched with a pipette tip and pre-treated with 2 μM PF543 (SphK1 inhibitor), and K145 (SphK2 inhibitor before treatment of EGF (100ng/ml) for 8 hours (A.B) and 24 hours (C). Migrating cells were photographed under a phase contrast microscope.
Figure 6. Downregulation of SphKs with specific siRNAs suppresses EGF-mediated metastatic breast cancer cell motility.
LM2-4, MDA-MB-231, and MCF-7 cells were transfected with control siRNA, siRNA targeted to SphK1 or SphK2 for 24 hours. Cells were grown in 1% serum containing medium for another 24 hours. Cells were scratched with a pipette tip and then incubated with EGF (100 ng/ml) for 24 hours (A, B) and 48 hours (C). Migrating cells were photographed under a phase contrast microscope.
4. Discussion
Outcomes have improved significantly for many women diagnosed with breast cancer at the early stage. Metastatic breast cancer mostly kills the advanced breast cancer patients. Triple-negative breast cancers are aggressive and when they recur and metastasize, there is often little response to chemotherapy, and there are a few treatment options. Thus, there is an urgent clinical need to identify new therapeutic targets in order to improve the outcome for these patients. S1P is known as an important signaling sphingolipid mediator that is essential for breast cancer growth and survival. S1P is produced from cytosolic SphK1 and nuclear SphK2. Redundant functions of SphK1 and SphK2 in cell motility, survival, and chemo resistance in estrogen receptor positive breast cancer cells have been reported earlier [9,20,28,29]. Opposing functions of SphK1 and SphK2 in sphingolipid metabolism also have been reported earlier [51]. Given the important role of EGF in progression, invasion, and tumorigenicity of human breast cancers [41], we examined whether SphKs/S1P signaling is the key signaling for EGF-mediated growth of lung metastatic LM2-4 breast cancer cells along with its parental TNBC MDA-MB-231 and MCF-7 breast cancer cells as a control cell line. In agreement with many reports [28,29,47,50,52], SphKs/S1P signaling is important for EGF-mediated growth and cell motility of ERα-positive MCF-7 human breast cancer cells (Fig. 1A–B). Supporting with earlier reports, a selective potent inhibitor of SphK1 PF-543 [17] did not attenuate the basal growth of MCF-7 cells but significantly attenuated EGF-mediated growth of MCF-7 cells (Fig. A–B). Interestingly, inhibiting SphK2 with the selective inhibitor K-145 drastically attenuated basal as well as EGF-mediated growth of MCF-7 cells (Fig. 1A–B). As expected DMS is a potent competitive inhibitor SphKs [36], also known to inhibit protein kinase C in vitro [32,36], completely blunted cell growth of MCF-7 cells (Fig. 1A–B). SphK1 has been shown to be involved in TNBC MDA-MB-231 cell growth in vitro and in vivo xenograft SCID mouse model [47]. We found that SphK2/S1P but not SphK1/S1P signaling is critical for cell growth in MDA-MB-231 cells (Fig. 1D) independent EGF-mediated PI3K/AKT and ERK MAP kinase pathways. Previously we found that selective SphK2 inhibitor K-145 reduces nuclear S1P and specific histone acetylation in TNBC MDA-MB-231 human breast cancer cells [24] and acts as a potent an anticancer agent for breast cancer JC cells in BALB/c mouse model [23]. In agreement with others we found that SphK2/S1P is important for cell growth (Fig. 1D, Fig. 4C and [49]) and motility (Fig. 5B and Fig. 6B) of MDA-MB-231 cells. However, growth, survival, and motility of lung metastatic breast cancer LM2-4 cells which was originally created in vivo from primary MDA-MB-231 cells [30,33] is solely dependent on SphKs/S1P signaling (Fig. 1C, Fig. 4C, Fig. 5A, and Fig. 6A). Particularly PF-543 augmented EGF-mediated and K145 augmented basal and EGF-mediated growth of LM2-4 cells indicating that SphKs/S1P is critical in metastatic breast cancer cell growth. Further, siSphK1 or siSphk2 markedly augmented EGF-mediated cell growth and motility of LM2-4 cells, which concords with our results with PF-543 and K-145 respectively.
S1P signaling is important in PI3K/AKT and MAP kinase pathways in TNBC cells [47]. Raf/MEK/ERK [53], PI3K/AKT [44], p38 MAPK [43] signaling pathways have been documented in cell growth and survival in cancers. We have tested whether SphKs/S1P signaling is critical in survival signaling in metastatic breast cancer LM2-4 cells. Our data revealed that EGF-mediated survival signaling in MCF-7 cells is dependent on SphKs/S1P (Fig. 2A). Supporting our cell growth data (Fig. 1D), PF-543 did not have any effect on survival signaling in MDA-MB-231 cells (Fig. 2C). Consistent with cell growth assay data with K-145 or DMS, these two drugs reduce both basal P-AKT levels in those cells (Fig. 2C). However, targeting either SphK1 or SphK2 with specific pharmacologic drug reduces basal as well as EGF-mediated P-ERK, P-p38, P-AKT, and P-p70S6K in metastatic breast cancer LM2-4 cells (Fig. 2B). Altogether, data indicated that SphKs/S1P signaling is critical for cell growth and survival in metastatic breast cancer while EGF-mediated survival signaling is not dependent on SphKs/S1P in primary TNBC MDA-MB-231 cells.
This indicated that blocking of Sphingosine kinases blocking the major MAPK signaling pathways required for cell proliferation however different cells use one of the other signaling pathways to survive. It is very important to find out which signaling pathways are dominant pathways for that particular type of cells would be critical for treatment. Surprising data arise from our experiment that LM2-4 cells which gain metastatic phenotype from MDA-MB-231 is more dependent on both kinase isoenzymes and S1P for cell growth, survival signaling, and motility while parental cells not responsive to SphKs/S1P in EGF-mediated survival signaling. Similar kind observation was also reported in mouse models by Francia G. et al [54]. They observed that orthotropic 231/LM2-4 primary tumors showed limited growth delay when mice were treated with doublet metronomic CTX and UFT (tegafur plus an oral 5-Fluorouracil) chemotherapy regimen, however, the therapy was remarkably effective against established spontaneous metastases [54]. We think more precisely selective potent SphK1 inhibitor PF-543 is mainly working in metastatic breast cancer not in primary triple-negative breast cancer MDA-MB-231 cells to reduce growth and survival signaling.
Further, we examined the effect of downregulating SphK1 and SphK2 expression with target specific siRNA on EGF mediated same survival signaling pathways. In order to reduce the off target effect of pharmacological drugs used in our experiments and to solidify the S1P mediated effect, we used target specific siRNA to down regulate individual kinase isoenzyme. In both MCF7 and LM2-4 cells, downregulation of SphK1 and SphK2 with siRNAs reduces cytosolic SphK1 and nuclear SphK2 proteins respectively and drastically abolished EGF-mediated phosphorylation of ERK, AKT p70S6K but not p38 (Fig. 3A–B). However, down regulation of SphK1 or SphK2 induces stress MAP kinase p38 in those cells (Fig. 3A–B). MDA-MB-231 cells did not show any effective down regulation of SphKs in EGF-mediated survival signaling (Fig. 3C). Collectively data indicated that SphKs/S1P signaling is critical in metastatic breast cancer LM2-4 cells growth, survival and migration compared to its parental TNBC MDA-MB-231 cells, indicating that SphKs/S1P axis is a target for therapeutic intervention for metastatic breast cancer.
Acknowledgments
Supported by the Roswell Park Alliance Foundation Start-Up Funds (NCH) and National Cancer Institute Grant R01CA160688 (KT). We thank Dr. Ebos (Roswell Park Cancer Institute) for providing lung metastatic LM2-4 cell lines and original TNBC cell lines MDA-MB-231.
The abbreviations used are
- SphKs
sphingosine kinases
- S1P
sphingosine-1-phosphate
- EGF
epidermal growth factor
- DMS
N,N-dimethylsphingosine
- ER
estrogen receptor
- TNBC
triple-negative breast cancer
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
NCH conceived and coordinated the study and wrote the paper with assistance from AM and KT. AM and NCH performed the experiments, data analysis. NCH and AM contributed to the preparation of the figures. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Sledge GW, Mamounas EP, Hortobagyi GN, Burstein HJ, Goodwin PJ, Wolff AC. Past, present, and future challenges in breast cancer treatment. J Clin Oncol. 2014;32:1979–1986. doi: 10.1200/JCO.2014.55.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.O’Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, et al. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013;66:530–542. doi: 10.1136/jclinpath-2012-201361. [DOI] [PubMed] [Google Scholar]
- 5.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyne S, Edwards J, Ohotski J, Pyne NJ. Sphingosine 1-phosphate receptors and sphingosine kinase 1: novel biomarkers for clinical prognosis in breast, prostate, and hematological cancers. Front Oncol. 2012;2:168. doi: 10.3389/fonc.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 9.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J Lipid Res. 2014;55:1839–1846. doi: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoon JW, Beckman BS. Sphingosine kinase: a promising cancer therapeutic target. Cancer Biol Ther. 2011;11:647–650. doi: 10.4161/cbt.11.7.14921. [DOI] [PubMed] [Google Scholar]
- 14.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramidemediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 15.Meacham WD, Antoon JW, Burow ME, Struckhoff AP, Beckman BS. Sphingolipids as determinants of apoptosis and chemoresistance in the MCF-7 cell model system. Exp Biol Med (Maywood) 2009;234:1253–1263. doi: 10.3181/0902-MR-77. [DOI] [PubMed] [Google Scholar]
- 16.Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484–4492. doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- 17.Schnute ME, McReynolds MD, Kasten T, Yates M, Jerome G, Rains JW, et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem J. 2012;444:79–88. doi: 10.1042/BJ20111929. [DOI] [PubMed] [Google Scholar]
- 18.Lynch KR. Building a better sphingosine kinase-1 inhibitor. Biochem J. 2012;444:e1–2. doi: 10.1042/BJ20120567. [DOI] [PubMed] [Google Scholar]
- 19.Wallington-Beddoe CT, Powell JA, Tong D, Pitson SM, Bradstock KF, Bendall LJ. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer Res. 2014;74:2803–2815. doi: 10.1158/0008-5472.CAN-13-2732. [DOI] [PubMed] [Google Scholar]
- 20.Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 21.Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151:5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gestaut MM, Antoon JW, Burow ME, Beckman BS. Inhibition of sphingosine kinase-2 ablates androgen resistant prostate cancer proliferation and survival. Pharmacol Rep. 2014;66:174–178. doi: 10.1016/j.pharep.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Guo TL, Hait NC, Allegood J, Parikh HI, Xu W, et al. Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2,4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PLoS One. 2013;8:e56471. doi: 10.1371/journal.pone.0056471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, et al. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERalpha expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4:e156. doi: 10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- 26.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, et al. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 29.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, et al. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 30.Ebos JM, Lee CR, Bogdanovic E, Alami J, Van Slyke P, Francia G, et al. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–529. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- 31.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, et al. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 32.Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- 33.Benzekry S, Tracz A, Mastri M, Corbelli R, Barbolosi D, Ebos JM. Modeling Spontaneous Metastasis following Surgery: An In Vivo-In Silico Approach. Cancer Res. 2016;76:535–547. doi: 10.1158/0008-5472.CAN-15-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichtner RB. Estrogen/EGF receptor interactions in breast cancer: rationale for new therapeutic combination strategies. Biomed Pharmacother. 2003;57:447–451. doi: 10.1016/j.biopha.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35:626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- 37.Wahl M, Carpenter G. Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C. J Biol Chem. 1988;263:7581–7590. [PubMed] [Google Scholar]
- 38.Kudlow JE, Cheung CY, Bjorge JD. Epidermal growth factor stimulates the synthesis of its own receptor in a human breast cancer cell line. J Biol Chem. 1986;261:4134–4138. [PubMed] [Google Scholar]
- 39.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 40.Adamson ED, Wiley LM. The EGFR gene family in embryonic cell activities. Curr Top Dev Biol. 1997;35:71–120. doi: 10.1016/s0070-2153(08)60257-4. [DOI] [PubMed] [Google Scholar]
- 41.Dancey JE, Freidlin B. Targeting epidermal growth factor receptor--are we missing the mark? Lancet. 2003;362:62–64. doi: 10.1016/S0140-6736(03)13810-X. [DOI] [PubMed] [Google Scholar]
- 42.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 43.Zou X, Blank M. Targeting p38 MAP kinase signaling in cancer through post-translational modifications. Cancer Lett. 2017;384:19–26. doi: 10.1016/j.canlet.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr Genomics. 2007;8:271–306. doi: 10.2174/138920207782446160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Datta A, Loo SY, Huang B, Wong L, Tan SS, Tan TZ, et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget. 2014;5:5920–5933. doi: 10.18632/oncotarget.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 49.Ohotski J, Rosen H, Bittman R, Pyne S, Pyne NJ. Sphingosine kinase 2 prevents the nuclear translocation of sphingosine 1-phosphate receptor-2 and tyrosine 416 phosphorylated c-Src and increases estrogen receptor negative MDA-MB-231 breast cancer cell growth: The role of sphingosine 1-phosphate receptor-4. Cell Signal. 2014;26:1040–1047. doi: 10.1016/j.cellsig.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Long JS, Edwards J, Watson C, Tovey S, Mair KM, Schiff R, et al. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol Cell Biol. 2010;30:3827–3841. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 52.Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]