Abstract
Gap Junction (GJ) channels, including the most common Connexin 43 (Cx43), have fundamental roles in excitable tissues by facilitating rapid transmission of action potentials between adjacent cells. For instance, synchronization during each heartbeat is regulated by these ion channels at the cardiomyocyte cell-cell border. Cx43 protein has a short half-life, and rapid synthesis and timely delivery of those proteins to particular subdomains are crucial for the cellular organization of gap junctions and maintenance of intracellular coupling. Impairment in gap junction trafficking contributes to dangerous complications in diseased hearts such as the arrhythmias of sudden cardiac death. Of recent interest are the protein-protein interactions with the Cx43 carboxy-terminus. These interactions have significant impact on the full length Cx43 lifecycle and also contribute to trafficking of Cx43 as well as possibly other functions. We are learning that many of the known non-canonical roles of Cx43 can be attributed to the recently identified six endogenous Cx43 truncated isoforms which are produced by internal translation. In general, alternative translation is a new leading edge for proteome expansion and therapeutic drug development. This review highlights recent mechanisms identified in the trafficking of gap junction channels, involvement of other proteins contributing to the delivery of channels to the cell-cell border, and understanding of possible roles of the newly discovered alternatively translated isoforms in Cx43 biology.
Graphical Abstract
1. Introduction
Cell-cell communication is critical for all organ systems, and assured by transmembrane channels forming conductive pores in the plasma membranes between adjacent cells [1]. These channels located in so-called gap junctions (GJ) which consist of connexin (Cx) proteins [2, 3]. All human connexins have a common organization: four transmembrane domains separating two extracellular and one intracellular loop. Both the amino (NT) and carboxyl (CT) termini are intracellular [4]. Each transmembrane channel (hemichannel or connexon) is a hexamer of connexins, and connexons from one cell dock to hemichannels from an adjacent cell to form GJ channels [5]. GJs allow the diffusion of small (typically less than 1 kDa) hydrophilic substances molecules and ions without exposure to the extracellular environment [6, 7]. GJs are the only channels which mediate direct cytoplasmic exchange - a process called gap junctional intercellular communication (GJIC) [8–12]. Interestingly, mutations in connexin genes, and therefore, in gap junctional communication, are associated with a large variety of pathologies and inherited connexin-associated disorders affect almost every major organ system [13, 14].
Of all 20 murine and 21 human connexins, Connexin 43 (Cx43, from the gene GJA1) is the most commonly expressed GJ protein [15, 16]. Cx43 has a fundamental role in excitable tissues to facilitate low resistance communication and thus rapid action potential transmission between adjacent cardiac cells. Such rapid communication synchronizes the cardiac heartbeat, and propagates electrical signals in the brain [17–19]. Cx43 is particularly enriched in ventricular cardiomyocytes where it is localized to the cardiomyocyte gap junction as part of the intercalated disc (ID) to facilitate action potential propagation [20].
In recent years, it has been reported that Cx43 can be involved in non-canonical events such as cell cycle regulation and cancer progression, wound healing, muscle differentiation, gene regulation and development [21–24]. Cx43 protein, and in particular its C-terminus, has also been implicated in the trafficking of cardiac ion channels such as Nav1.5 and junctional protein N-cadherin [25–28]. These studies identify Cx43 by immunohistochemistry with antibody epitopes at the C-terminus [25], or with a C-terminus truncation of the distal 5 amino acid residues [26, 27], or with co-immunoprecipitation with N-Cadherin [28]. Therefore, it is not a surprise that mutations or any deficiency in Cx43 expression or gap junction formation are associated with diverse pathologies, including heart disease [29–35], connective tissue disease [36], and cancer [21, 24]. Cardiac pathologies are frequently associated with connexin redistribution which is a form of gap junction remodeling [32, 37]. Moreover, impaired Cx43 trafficking contributes to the arrhythmias of sudden cardiac death [17, 30, 32, 38–49]. Decrease in expression and distribution of Cx43 in the cardiomyocytes has been described in the patients with hypertrophic cardiomyopathies [50–52], dilated cardiomyopathies [53, 54], ischemic cardiomyopathies [50, 52] and clinical congestive heart failure [33], and hereditary disorders [55]. Cx43 hemichannels and gap junctions have been implicated in mediating ischemia/reperfusion injury, cardioprotection and neuroprotection [56].
As many of the above studies highlight, several decades of research on Cx43 has revealed that many of these functional roles are attributed to the C-terminal domain (CT) of this protein which can not be explained by the formation and existence of intercellular channels. Recent findings on the existence of endogenous internally translated C-terminal isoforms from Cx43 mRNA [57–60], which can explain general cytoplasmic activity of the proteins, are opening a new understanding of the regulation of gap junction trafficking and function. Roles previously assigned to full length Cx43 may in fact be due to the smaller isoforms instead.
This review is focused on current understandings of the mechanisms of Cx43 trafficking and related regulation of gap junction organization. Suggestions are also provided regarding the directions of future research.
2. The Cx43 trafficking life cycle
2.1 Anterograde trafficking
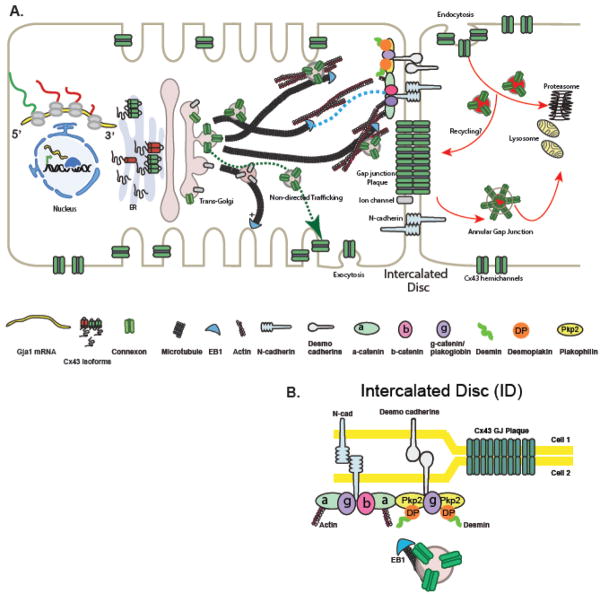
Like all connexin proteins, Cx43 follows a traditional secretory pathway (Fig. 1A). It is inserted into the membrane of the endoplasmic reticulum (ER) after transcription in the nuclei and translation in ER, where folding and post-translational modifications occur. Checkpoints control exit of hemichannels and if proper channel folding fails, those proteins are transferred to ER-associated degradation by quality control mechanisms [18, 61]. Interestingly, some chemicals such as 4-phenylbutyrate and glycerol can potentially rescue the misfolding of Cx43 and normalize trafficking of retained protein [13]. Following checkpoint regulation, ion channels exit the ER and are packaged into vesicles coated with coat protein complex II (COPII) and are translocated to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) [62]. Further sorting continues within the ERGIC and posttranslational modifications occur while ion channels progress from cis-Golgi network to trans-Golgi (TGN) to generate functional channels ready for delivery [63].
Figure 1. The Cx43 trafficking life cycle and Targeted Delivery to the cell-cell border.
(A). The Cx43 lifecycle, comprising anterograde trafficking, lateral diffusion and retrograde trafficking, and highlighting cytoskeletal-based directed targeting of Cx43 to form gap junctions (GJs) at the intercalated disc (ID). Cx43 is targeted to adhesion complexes, containing N-cadherin and catenin-containing regions of the intercalated disc, along EB1-tipped microtubules to form GJ plaques at the ID. Actin is required for GJ localization, but the underlying mechanism is currently unknown.
(B). The Cx43 GJ plaque formation at the ID.
Oligomerization into hemichannels or connexons usually happens during the transition from rough the ER to the Golgi apparatus [5], with modification and sorting to occur later in the TGN (Fig. 1A) [64]. Even though most connexins are oligomerized in the ER, late oligomerization for Cx43 could have been evolutionary established to control heteromeric hemichannel formation with other connexin isoforms [18].
2.1a Targeted Delivery
The mechanism of how newly translated oligomerized protein get to their “working” location is still being explored. After exiting the TGN the folded and oligomerized hemichannels are usually transported to the cell surface where, according to classic theory, they freely diffuse within the lipid bilayer to their final functional destination [65–69]. It is possible that post-Golgi hemichannels take a more direct route to a particular membrane-subdomain such as the cell-cell border and IDs. In the paradigm of a more direct delivery system which we call Targeted Delivery, channels exit the Golgi in vesicles to be trafficked along microtubules via motor proteins to a specific subdomain on the membrane hosting anchor proteins (i.e. adherens junctions) which capture the microtubules, allowing for hemichannel offloading from microtubule to membrane (Fig. 1A,B) [70]. Such Targeted Delivery might be commonly utilized by specialized membrane proteins in polarized cells or by cardiomyocytes for trafficking of Cx43 to the ID which are located primarily at the longitudinal ends of cardiomyocytes, perpendicular to the anchoring cytoskeleton, and support functions of cardiomyocytes both mechanically and electrically [71]. IDs consist of Gap Junction plaque, ion channels, adherens junctions and desmosomes [72–75] (Fig. 1A,B). Several cardiomyopathies are driven by disrupted mechanical junctions associated with altered Cx43 localization and dissociation from desmosomes [51, 76–80].
Targeted Delivery is a model preferred by these authors as it is an efficient model compatible with the short life cycle of Cx43 [39, 70, 81–87] and well substantiated by experimental studies. Biochemical and imaging studies reveal that the half-life of many of the connexin family members are within 2–5 hours, with less than 2 hours at the plasma membrane [70]. This highly dynamic turnover suggests that the entire gap junctional content might be renewed several times on a daily basis at plasma membrane of any cell type [70, 88]. It is a particular interesting phenomena for such an important protein in the heart like Cx43 where one would expect much longer life similar to some other ion channels up to 30–40 hours [89]. Therefore, trafficking of channels needs to be efficient, accurate, and robust.
The Targeted Delivery paradigm is based on the observation that newly synthetized Cx43 hemichannels are targeted directly to adhesion junctions [39, 70, 81–87]. Upon exiting the TGN, vesicles with Cx43 hemichannels must navigate the complex intracellular environment as they are transported along the filamentous microtubule and actin networks [90]. The minus ends of microtubules are nucleated in the microtubule-organizing centers (MTOC) which is co-localized with TGN [91] where the main sorting of cargo-proteins occurs [92]. In the TGN cargo proteins are loaded on molecular motors of microtubules and delivered to the cell periphery [90, 93]. Therefore, in the Targeted Delivery model the dynamic process is dependent upon coordinated action of microtubule-based motors including kinesin [94]. In the context of trafficking, one can consider the Golgi and the TGN to be the “loading dock” and the microtubules dynamic “highways” along which packets of channels are delivered to the plasma membrane. Microtubules-highways pause when they reach and anchor at adherens junction complexes (N-cadherin, β-catenin) in the plasma membrane and deliver cargo to the cell-cell border or IDs.
Microtubule-based vesicle transport has been described for many ion channels and transporters in polarized epithelial cells of kidney and liver and is also capable of delivering vesicles with Cx43 hemichannels to the plasma membrane [66, 69, 70, 95–97]. Capture of microtubules by the appropriate membrane anchor and delivery of cargo to a specific region in the membrane is a critical aspect of Targeted Delivery. For precise Cx43 delivery several members of this paradigm have been identified: a microtubule plus-end binding protein, EB1, is known to be necessary for targeted delivery of connexons to adherens junction [70]. The EB1-tipped microtubule specifically seeks and interacts with β-catenin molecules bound to N-cadherin at the adherens junctions of intercalated discs where vesicles can be unloaded and inserted at the plasma membrane in close proximity to gap junctions (Fig. 1A, B). The presence of p150GLUED is necessary for this process. As details of this paradigm are elucidated, it has been found that the desmosome associated linker protein desmoplakin may also be involved in capturing the EB1-tipped microtubule thereby affecting Cx43 targeted delivery to IDs [98, 99] (Fig. 1B). Interestingly, it was shown that Cx43 and EB1 were diminished at the IDs of patient with ischemic cardiomyopathy and EB1 was displaced from microtubules [39], suggesting that impaired forward trafficking of ion channels contributes to acquired heart failure. Manipulation of EB1 or upstream regulators of EB1 could potentially preserve gap junction during oxidative stress when EB1 is more likely to be displaced from microtubules [39].
In general, Targeted Delivery is an efficient general model of delivery of membrane proteins and channels to the cell-cell border. Regarding Cx43 gap junctions, and taking into account the short half-life of Cx43 protein and the constant need to maintain cellular coupling in the heart, the Targeted Delivery is a fast and efficient way to control GJ coupling at the intercalated discs. Post membrane delivery, free lateral diffusion of connexons within the plasma membrane could also take place, but either for delivery as nonjunctional protein or occurring within a restricted region, for example, within and around the local plaque. There is an urgent need to develop techniques which allow for tracking of a single particle such as connexons in live cells. This technology could greatly assist investigators in understanding the behavior of the highly dynamic Cx43 protein once is already in the plasma membrane.
2.1b Role of actin in Targeted Delivery
Non-sarcomeric actin (filamentous or F-actin) is an important participant in Targeted Delivery of Cx43 in additional to microtubules, regulating vesicular transport at early (ER to Golgi) and later (TGN to sarcolemma) stages [90, 100]. There are three forms of actin in cardiomyocytes: α-actin comprising the thin filaments of the sarcomere [101], and β- and γ-actin forming F-actin. F-actin is not associated with generating contractile force [102]. However, it participates in maintenance of membrane subdomains such as intercalated discs, T-tubules, caveolae and in regulating intracellular vesicular transport through motor protein-based trafficking and vesicular fusion with the cell membrane [71, 103–105]. Several studies identified the dependence of GJ formation and maintenance on actin [106–108]. We have found that Cx43 colocalizes with non-sarcomeric actin structures (β-actin) along the vesicular transport pathway at the perinuclear region, as well as intercalated disc region (Fig. 1A). Cx43 cargo transport is slowed down when associated with actin [109]. This finding is consistent with previous studies of Cx32 pausing on actin structures en route to the cell border of hepatocytes [110]. It is not yet clear why actin slows hemichannel transport, or whether there is a benefit to actin’s involvement in trafficking.
We presume that actin can have at least two important roles in forward delivery of Cx43. The first possible role of actin is to provide a pool of already formed hemichannels ready to be delivered to the ID. While paused at the actin and in reserve, the hemichannels could associate with important accessory proteins and undergo post-translational modification. Thus microtubule highways could use actin as a “way station”, in analogy with a highway rest stop. Such rest stops would occur at Z-disc, subcortical locations, or other important cytoskeleton intersections in the cytoplasm. These actin rest stops could also allow the Cx43 containing vesicles to use multiple microtubule highways in their delivery path. Therefore, the second potential role for actin in microtubule based forward delivery is to confer specificity and directionality to delivery. Vesicles transported along microtubules on kinesin motors move rapidly, at a rate of about 1 μm per second [70]. Thus, delivery to most locations at a cell membrane can occur within a minute. Actin can also help stabilize and guide microtubules, redirect moving microtubules to tailor cargo delivery and protein insertion into sarcolemma surface destination. Interestingly, in the context of axonal neuronal growth, there are are examples of actin directing microtubules [111]. Also actin can form the scaffold, like a blueprint for navigation of microtubules in plants and neurites [112, 113] and human cells [114, 115]. In this case actin navigates and plays the role of a road signs for the microtubule highways in myocyte and non-myocyte system. Therefore, in our current hypothesis, non-sarcomeric actin acts upstream of microtubule-based delivery in trafficking.
2.2 Retrograde trafficking (Internalization)
Endocytosis of Cx43 as either hemichannels or whole gap junction channels (with the opposing plasma membrane) takes place right after ubiquitination of Cx43 prior to lysosomal, proteasomal degradation [116, 117] or autophagy (which is involved in degradation of this protein during heart failure) (Fig. 1) [76, 118]. These events are thought to be triggered by posttranslational modifications of Cx43 at the C-terminus in consecutive steps, such as binding of 14-3-3 motif [119], phosphorylation [40, 118], ubiquitination [120, 121], and SUMOylation [122]. Internalization is a very important regulatory step in the Cx43 lifecycle as it is the second determinant of gap junction coupling after forward delivery [123]. Typically, posttranslational modifications occur as a sequential cascade [118, 119, 124, 125] of events resulting in the formation of connexosomes or annular gap junction channels (Fig. 1A) [64, 126]. Connexosomes are destined either for degradation or recycling. Recycling of gap junctions from an “older pool” of Cx43 rather than de novo Cx43 synthesis can occur after the completion of mitosis in cell lines [127, 128]. Whether gap junctions are recycled in cardiomyocytes remains controversial.
In the case of Cx43, phosphorylation at multiple sites are the most well studied post-translational modifications. The importance of phosphorylation has been highlighted by recent findings that casein kinase-dependent phosphorylation alters gap junction remodeling and decreases arrhythmic susceptibility [40]. Many residues on the C-terminus of Cx43, specifically 22 serines, 5 tyrosines, and 4 threonines, are potentially subjected to phosphorylation. To make matters even more complex, it is currently unclear how phosphorylation differs between the individual six connexins of the same connexon.
Our experience with Cx43 protein is that post-translational modification preferentially affects ion channel internalization. Pathological gap junction remodeling is strongly associated with altered phosphorylation of Cx43 [18, 30, 129]. The Cx43 C-terminus contains a phosphorylation-dependent 14-3-3 binding motif at Serine 373 (within 10 amino acids of the end of the protein). 14-3-3 proteins are known to regulate protein transport and have been implicated in facilitating de novo Cx43 transport from ER to Golgi apparatus [130, 131]. Phosphorylation of Ser373 and subsequent 14-3-3 binding provide a gateway to downstream phosphorylation of Ser368, leading to gap junction ubiquitination, internalization and degradation during acute cardiac ischemia [119].
Acute cardiac ischemic injury in isolated rat hearts has also been shown to cause increased ubiquitination of Cx43 at the intercalated discs accompanied by increased interaction between Cx43 and Nedd4 [121]. However, silencing of Nedd4 in HL-1 mouse atrial cells subjected to ischemic conditions did not have any significant effect on Cx43 ubiquitination nor degradation. Only under basal conditions did the knockdown of Nedd4 prevent ubiquitination and degradation of Cx43 [132]. This suggests that other E3 ubiquitin ligases besides Nedd4 may regulate Cx43 ubiquitination and degradation in cardiac injury. Indeed, it has been recently reported in an inducible transgenic mouse model that cardiomyocyte specific overexpression of the ubiquitin ligase Wwp1 caused a significant reduction in Cx43 protein levels in the heart leading to the development of lethal left ventricular arrhythmias [120].
3. Alternative translation initiation sites within Cx43
Alternative translation has not been extensively studied in eukaryotic systems, but seems to be a source of considerable biologic diversity. Less than 20,000 genes encode more than 80,000 protein-coding transcripts and a rough estimation of the number of proteins synthesized from these transcripts is in the range of 250,000 to 1 million in mammalian species. This suggests a substantial regulation at the transcriptional, post-transcriptional, and translational level [133]. In support of this, a recent study confirmed that there is no strong correlation between transcript and protein levels in mammals [134]. Indeed, the use of pre-existing mRNAs through regulation of translation is beneficial in many circumstances, because mRNA biogenesis is time consuming, whereas synthesis of protein is a fast process for many genes (including ion channels). Therefore, translational control plays an important role in a key biological processes. Translation defines not only the amount of protein produced, but ribosomal translation can be initiated from downstream AUG start sites by activation of alternative, internal AUG start codons. As a result distinct proteins can be generated from a single mRNA molecule (Fig. 2) [135].
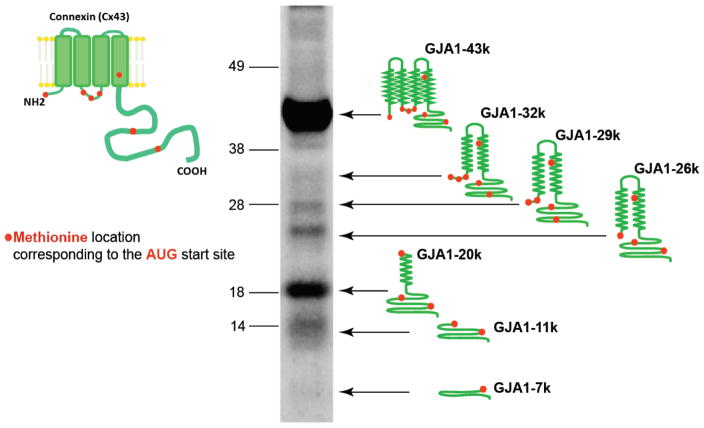
Figure 2. One exon of GJA1 mRNA produces seven proteins.
Western blot of nonfailing heart probed to monoclonal antibodies against the Cx43 C-terminus. (Western blot is taken from Smyth and Shaw, Cell Reports, 2013).
Translation is initiated on most mRNAs by a canonical cap-dependent mechanism which involves binding of 10–13 different eukaryotic initiation factors (eIFs) at the mRNA 5′-UTR. Once recruited to the ribosomes, this initiation complex starts to scan the transcript from the 5′-UTR to locate the AUG start codon with a specific nucleotide context (Kozak sequence) thereby initiating translation [136]. However, several other non-canonical mechanisms were recently described [137]. An internal ribosome entry site (IRES) in viral and some cellular mRNAs was identified in close proximity to AUG start sites. Non-canonical internal intiation is generally observed under stressful physiological conditions (such as starvation, hypoxia, inflammation, or apoptosis) when cap-dependent translation is diminished [137, 138]. Other non-IRES alternative mechanisms of translation have been proposed. They include either direct internal binding of initiation translation factor eIF4E to the coding site of mRNA, or a mechanism called leaky ribosomal scanning [137, 139]. In second case translation can be initiated at the first AUG start site, but it will fail if the Kozak consensus sequence context is not optimal enough to start translation [136, 140, 141]. Translation will then be initiated at the subsequent internal AUG start site. To date, cap-independent translation and leaky ribosomal scanning are thought to represent the two basic mechanisms explaining the existence of alternative translation.
3.1 Alternative translation of Cx43
GJA1 mRNA is the first example of polycistronic molecule and an example of alternative translation observed in human ion channels and in the human heart. After the first AUG (Methionine), there are 6 additional downstream AUG codons within Cx43 mRNA, which means that ribosomal translation can be initiated at multiple internal sites to produce truncated proteins that lack the corresponding non-translated upstream (N-terminal) portions of the protein. A total of 6 additional N-terminally truncated protein isoforms (in addition to a full length Cx43) can be generated from Cx43 mRNA, resulting in a total of 7 different Cx43 proteins [58]. The truncated isoforms are detected as distinct bands on the western blot lysate from the left ventricle of non-failing human heart tissue (Fig. 2). In addition to 43kDa full length isoform, the largest 3 isoforms (32kDa, 29kDa and 26kDa in size) contain 2 transmembrane domains, part of intracellular loop, and the C-terminus. The smallest 3 isoforms are 20kDa, 11kDa, and 7kDa in size and include part of the C-terminal tail with the 20kDa isoform also containing part of the last transmembrane domain. The nomenclature for the smaller protein isoforms has been adopted by using the Cx43 gene name (GJA1), reflecting the origin of the mRNA, in combination with the size of the truncation protein produced. For instance, ribosomal translation beginning at the coding methionine residue 213 of GJA1 produces a 20kDa protein named GJA1-20k (Fig. 2) [58].
3.2 GJA1-20k as a chaperone protein in Targeted delivery
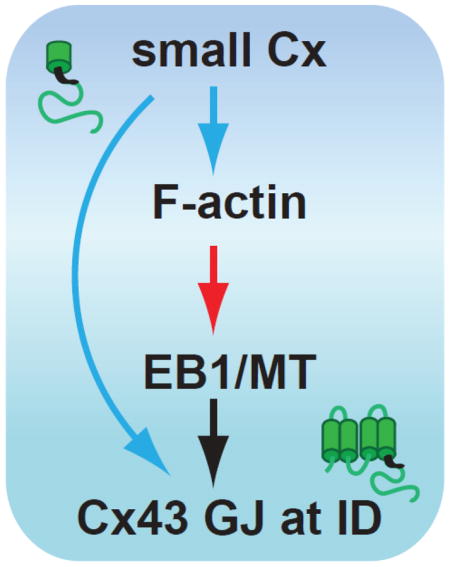
So far, studies mentioned above have also shown that the 20 kDa isoform (GJA1-20k) expression is predominant in human heart tissue, in the heart of zebrafish and in many cancer cell lines (Fig. 2) [58, 59, 142]. Experiments with the mutated 20kDa isoform showed that formation of Cx43 gap junctions was severely reduced, with restoration upon re-introduction of the 20kDa isoform [58]. Co-immunoprecipitation and immunostaining experiments with Brefeldin A (a common inhibitor of transport from ER to Golgi) confirmed interactions between the full length GJA1-43k and GJA1-20k [143]. Therefore, this suggests that GJA1-20k isoform contributes to vesicular trafficking of the 43kDa full length isoform from ER/TGN to the intercalated disc and may function as a protein-chaperone. Furthermore, it was found that the inhibition of the mammalian target of rapamycin (mTOR/PI3K/AKT) pathway increases internal translation and protein levels of the GJA1-20k isoform and the Cx43 gap junction plaque size at the cell-cell borders in cardiomyocytes (Fig. 3) [58]. It is known that the mTOR pathway promotes cap-dependent translation [144]. Therefore, it is possible that Cx43 can be rescued or enhanced with mTOR inhibitors which are common therapeutic immunosuppressants used in organ transplantation. Interestingly, the mTOR pathway is involved in cardiovascular physiology and pathology, and inhibition of this pathway has cardioprotective effect [145].
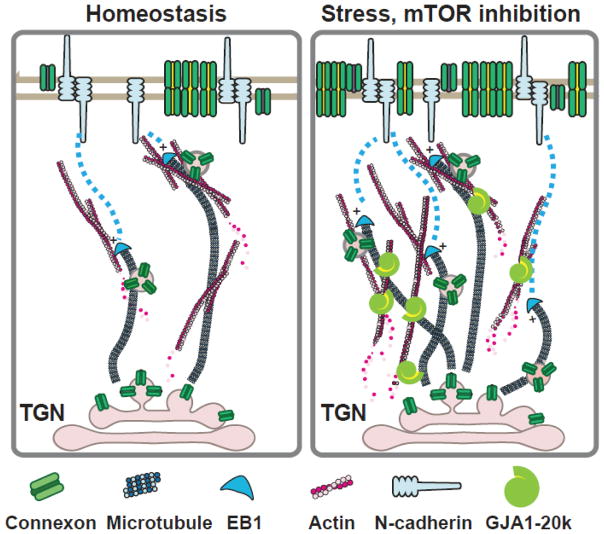
Figure 3. Prospective mechanism of action for GJA1-20k truncated isoform.
The internally translated CJA1-20k isoform is associated with the ER and dynamic vesicles, forming highly organized contacts with F-actin and microtubule networks. GJA1-20k interacts with F-actin, provides stabilization of filamentous actin and microtubules. F-actin disruption by oxidative stress or Latrunculin A impairs growth trajectories of the EB1/microtubule-based trafficking machinery, and decreases GJ localization to the cellular border, which can be rescued by exogenous GJA1-20k.
It remains unclear at what location in the forward pathway that GJA1-20k facilitates Cx43 trafficking. We believe that GJA1-20k might be involved in a trafficking of Cx43 channel-containing vesicles through actin stabilization to guide microtubules for targeted delivery and/or by mediating actin-to-microtubule transfer to assist in the delivery of Cx43 channels as well as multiple ion channels and membrane proteins (Fig. 3). As mentioned earlier, Cx43 or at least portions of the C-terminus of Cx43 have been implicated in the trafficking of other cardiac ion channels (Nav1.5) and junctional proteins (N-cadherin) to the intercalated disc [25–28]. The short half-life of Cx43 may reflect a continuous need for a prepared and sufficient pool of GJA1-20k isoforms. Previous work has shown that F-actin integrity is necessary for de novo Cx43 delivery to cell-cell borders in neonatal ventricular cardiomyocytes. We identified that Cx43 expression at the ID was significantly reduced either in hearts subjected to acute ischemia or F-actin disruption by LatrunculinA. The two effects were not additive, suggesting that a disarray of F-actin was common to both interventions [146]. Therefore, non-contractile F-actin “rest stops” may provide a reservoir of Cx43 channels, which the 20k isoforms could help mobilize during stress, providing a bolus of Cx43 channels in response to stressful conditions (Fig. 3). While we know that GJA1-20k (CT-tail of Cx43) contains a microtubule-binding domain [147, 148], and that CT-tail directly interacts with α- and β-tubulin and co-localize with GJs at intercalated discs [149–152], it is not clear how Cx43 or the smaller isoforms interact with F-actin. We also don’t know whether F-actin and the microtubule machinery interact in directing Cx43 delivery. In future studies, it will be interesting to learn whether or how GJA1-20k regulates actin-to-microtubule movement of Cx43 hemichannel vesicles.
4. Concluding remarks
It is highly likely that the internally translated GJA1-20k isoform is a protein-chaperone or “beta-subunit” that serves and autoregulates the full length Cx43 trafficking to the cell-cell border. Production of isoforms and trafficking are tightly regulated and also directly involved in cytoskeleton based trafficking. Since EB1-tipped microtubules and actin are both impaired in stressed myocardium [39, 109], GJA1-20k could stabilize the cytoskeleton delivery apparatus during the injury or cell stress. Further understanding of the role of GJA1-20k in cytoskeleton-based vesicular transport may lead to introducing new therapeutic drugs such as mTOR inhibitors or others for preserving the abundance of Cx43 and regulating cell coupling at the intercalated disc.
It is clear that Cx43 affects cell proliferation, its trafficking and trafficking of other ion channels through numerous cell signaling and protein-protein interaction pathways, which are demonstrated in many connexin-associated diseases. Cx43 is a dynamic protein and interaction with so many different proteins is necessary to regulate every step of Cx43 life cycle: formation, forward trafficking to the correct subdomain on the plasma membrane, assembly on the plasma membrane, retrograde trafficking, and degradation. Cx43 does not function alone but rather in concert with other proteins and smaller isoforms from the same gene and mRNA. Greater understanding how Cx43 isoforms affect the trafficking and localization of full length Cx43 gap junction channels can provide new targets for regulating gap junction coupling. Furthermore, it is like that the smaller isoforms affects the trafficking of other ion channels, thus broadening the scope of products of Cx43 alternative translation to general regulation of cardiac electrophysiology.
Highlights.
Gap junction channels are essential aspects of cardiomyocyte coupling which provide synchronous cardiac contraction.
The trafficking of gap junction channels to the precise subdomain in the cell known as targeted delivery and its regulation is important for maintenance of cardiac synchrony.
Six endogenous truncated Cx43 isoforms are produced from the same full-length GJA1 mRNA molecule by means of alternative translation.
20kDa alternatively translated isoform of Cx43 is a protein-chaperone and can autoregulate trafficking of the full length protein.
Acknowledgments
We acknowledge our funding sources from the American Heart Association (13EIA4480016) and the National Institute of Health/National Heart, Lung, and Blood Institute (RO1 HL094414). We are very grateful to Dr. Shaohua Xiao and Dr. Shan-Shan Zhang for their significant contributions to figure’s preparation. The authors have no conflicts of interest to declare.
Abbreviations
- GJs
Gap Junctions
- GJIC
Gap Junctional Intercellular Communication
- CT
Carboxy-tail (C-terminus domain, CT-tail, Cx43-CT)
- Cx43
Connexin 43
- CDS
Coding Domain Sequence
- F-actin
Filamentous actin
- kDa
Kilodalton
- ID
Intercalated Disc
- IRES
Internal Ribosome Entry Site
- ORF
Open Reading Frame
- ER
Endoplasmic Reticulum
- TNG
Trans-Golgi Network
- ERGIC
Endoplasmic Reticulum-Golgi Intermediate Compartment
- eIFs
Eukaryotic Initiation Factors
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 2006;21:103–14. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- 2.Beyer EC. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- 3.Vinken M. Introduction: connexins, pannexins and their channels as gatekeepers of organ physiology. Cell Mol Life Sci. 2015;72(15):2775–8. doi: 10.1007/s00018-015-1958-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skerrett IM, Williams J. A Structural and Functional Comparison of Gap Junction Channels Composed of Connexins and Innexins. Dev Neurobiol. 2016 doi: 10.1002/dneu.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unwin PN, Zampighi G. Structure of the junction between communicating cells. Nature. 1980;283(5747):545–9. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- 6.Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10(19):2045–58. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- 7.Esseltine JL, Laird DW. Next-Generation Connexin and Pannexin Cell Biology. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Eugenin EA, et al. The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system. J Neuroimmune Pharmacol. 2012;7(3):499–518. doi: 10.1007/s11481-012-9352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtenbach S, Kurtenbach S, Zoidl G. Gap junction modulation and its implications for heart function. Front Physiol. 2014;5:82. doi: 10.3389/fphys.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinken M, et al. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology. 2008;47(3):1077–88. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- 11.Saez JC, et al. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83(4):1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kar R, et al. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012;524(1):2–15. doi: 10.1016/j.abb.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly JJ, Simek J, Laird DW. Mechanisms linking connexin mutations to human diseases. Cell Tissue Res. 2014 doi: 10.1007/s00441-014-2024-4. [DOI] [PubMed] [Google Scholar]
- 14.Laird DW. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014;588(8):1339–48. doi: 10.1016/j.febslet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Willecke K, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383(5):725–37. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 16.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62(2):228–32. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81(5):727–41. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 18.Hesketh GG, Van Eyk JE, Tomaselli GF. Mechanisms of gap junction traffic in health and disease. J Cardiovasc Pharmacol. 2009;54(4):263–72. doi: 10.1097/FJC.0b013e3181ba0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62(2):309–22. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105(6 Pt 1):2621–9. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aasen T, et al. Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer. 2016 doi: 10.1038/nrc.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang JX, Penuela S. Connexin and pannexin channels in cancer. BMC Cell Biol. 2016;17(Suppl 1):12. doi: 10.1186/s12860-016-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinken M, et al. Non-channel functions of connexins in cell growth and cell death. Biochim Biophys Acta. 2012;1818(8):2002–8. doi: 10.1016/j.bbamem.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Kardami E, et al. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol. 2007;94(1–2):245–64. doi: 10.1016/j.pbiomolbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Jansen JA, et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9(4):600–7. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubkemeier I, et al. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic Res Cardiol. 2013;108(3):348. doi: 10.1007/s00395-013-0348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agullo-Pascual E, et al. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc Res. 2014;104(2):371–81. doi: 10.1093/cvr/cvu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei CJ, et al. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280(20):19925–36. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 29.Akar FG, et al. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293(2):H1223–30. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 30.Beardslee MA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87(8):656–62. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 31.Smith JH, et al. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139(4):801–21. [PMC free article] [PubMed] [Google Scholar]
- 32.Saffitz JE, Hames KY, Kanno S. Remodeling of gap junctions in ischemic and nonischemic forms of heart disease. J Membr Biol. 2007;218(1–3):65–71. doi: 10.1007/s00232-007-9031-2. [DOI] [PubMed] [Google Scholar]
- 33.Dupont E, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33(2):359–71. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 34.Thibodeau IL, et al. Paradigm of genetic mosaicism and lone atrial fibrillation: physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation. 2010;122(3):236–44. doi: 10.1161/CIRCULATIONAHA.110.961227. [DOI] [PubMed] [Google Scholar]
- 35.Van Norstrand DW, et al. Connexin43 mutation causes heterogeneous gap junction loss and sudden infant death. Circulation. 2012;125(3):474–81. doi: 10.1161/CIRCULATIONAHA.111.057224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paznekas WA, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72(2):408–18. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Severs NJ, et al. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80(1):9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalcheva N, et al. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A. 2007;104(51):20512–6. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth JW, et al. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120(1):266–79. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remo BF, et al. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108(12):1459–66. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96(1):54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- 42.Peters NS, et al. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95(4):988–96. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 43.Asimaki A, et al. Arrhythmogenic Cardiomyopathy - New Insights into Disease Mechanisms and Drug Discovery. Prog Pediatr Cardiol. 2014;37(1–2):3–7. doi: 10.1016/j.ppedcard.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danik SB, et al. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95(10):1035–41. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo CW. Role of gap junctions in cardiac conduction and development: insights from the connexin knockout mice. Circ Res. 2000;87(5):346–8. doi: 10.1161/01.res.87.5.346. [DOI] [PubMed] [Google Scholar]
- 46.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287(4):H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 47.van Rijen HV, et al. Connexins and cardiac arrhythmias. Adv Cardiol. 2006;42:150–60. doi: 10.1159/000092567. [DOI] [PubMed] [Google Scholar]
- 48.Gutstein DE, et al. Heterogeneous expression of Gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104(10):1194–9. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 49.Lerner DL, et al. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000;101(5):547–52. doi: 10.1161/01.cir.101.5.547. [DOI] [PubMed] [Google Scholar]
- 50.Peters NS. New insights into myocardial arrhythmogenesis: distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts. Clin Sci (Lond) 1996;90(6):447–52. doi: 10.1042/cs0900447. [DOI] [PubMed] [Google Scholar]
- 51.Sepp R, Severs NJ, Gourdie RG. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart. 1996;76(5):412–7. doi: 10.1136/hrt.76.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000;86(12):1193–7. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- 53.Kostin S, et al. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242(1–2):135–44. [PubMed] [Google Scholar]
- 54.Kitamura H, et al. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13(9):865–70. doi: 10.1046/j.1540-8167.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- 55.Asimaki A, et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6(240):240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz R, et al. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joshi-Mukherjee R, et al. Evidence for the presence of a free C-terminal fragment of cx43 in cultured cells. Cell Commun Adhes. 2007;14(2–3):75–84. doi: 10.1080/15419060701402320. [DOI] [PubMed] [Google Scholar]
- 58.Smyth JWS, RM Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013;5(3):611–8. doi: 10.1016/j.celrep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salat-Canela C, et al. Internal translation of the connexin 43 transcript. Cell Commun Signal. 2014;12:31. doi: 10.1186/1478-811X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ul-Hussain M, et al. Internal Ribosomal Entry Site (IRES) Activity Generates Endogenous Carboxyl-terminal Domains of Cx43 and Is Responsive to Hypoxic Conditions. J Biol Chem. 2014;289(30):20979–20990. doi: 10.1074/jbc.M113.540187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.VanSlyke JK, Musil LS. Dislocation and degradation from the ER are regulated by cytosolic stress. J Cell Biol. 2002;157(3):381–94. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watson P, et al. Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol. 2005;7(1):48–55. doi: 10.1038/ncb1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glick BS, Nakano A. Membrane traffic within the Golgi apparatus. Annu Rev Cell Dev Biol. 2009;25:113–32. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smyth JW, Shaw RM. The gap junction life cycle. Heart Rhythm. 2012;9(1):151–3. doi: 10.1016/j.hrthm.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394(Pt 3):527–43. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lauf U, et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99(16):10446–51. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simek J, et al. Cx43 has distinct mobility within plasma-membrane domains, indicative of progressive formation of gap-junction plaques. J Cell Sci. 2009;122(Pt 4):554–62. doi: 10.1242/jcs.036970. [DOI] [PubMed] [Google Scholar]
- 68.Thomas T, et al. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J Cell Sci. 2005;118(Pt 19):4451–62. doi: 10.1242/jcs.02569. [DOI] [PubMed] [Google Scholar]
- 69.Gaietta G, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296(5567):503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 70.Shaw RM, et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128(3):547–60. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noorman M, et al. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47(1):23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Delmar M. The intercalated disk as a single functional unit. Heart Rhythm. 2004;1(1):12–3. doi: 10.1016/j.hrthm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Palatinus JA, Gourdie RG. Xin and the art of intercalated disk maintenance. Am J Physiol Heart Circ Physiol. 2007;293(5):H2626–8. doi: 10.1152/ajpheart.00954.2007. [DOI] [PubMed] [Google Scholar]
- 74.Severs NJ. Gap junction shape and orientation at the cardiac intercalated disk. Circ Res. 1989;65(5):1458–62. doi: 10.1161/01.res.65.5.1458. [DOI] [PubMed] [Google Scholar]
- 75.Forbes MS, Sperelakis N. Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell. 1985;17(5):605–48. doi: 10.1016/0040-8166(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 76.Hesketh GG, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106(6):1153–63. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kostin S, et al. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res. 2004;62(2):426–36. doi: 10.1016/j.cardiores.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 78.Akar FG, et al. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95(7):717–25. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 79.Fidler LM, et al. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med. 2009;13(10):4219–28. doi: 10.1111/j.1582-4934.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oxford EM, et al. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101(7):703–11. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 81.Hong TT, et al. BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 2012;9(5):812–20. doi: 10.1016/j.hrthm.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong TT, et al. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8(2):e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8(7):808–19. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 84.Levy JR, Holzbaur EL. Special delivery: dynamic targeting via cortical capture of microtubules. Dev Cell. 2007;12(3):320–2. doi: 10.1016/j.devcel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 85.Hendricks AG, et al. Dynein tethers and stabilizes dynamic microtubule plus ends. Curr Biol. 2012;22(7):632–7. doi: 10.1016/j.cub.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chkourko HS, et al. Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart Rhythm. 2012;9(7):1133–1140 e6. doi: 10.1016/j.hrthm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol. 2009;47(2):203–9. doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beardslee MA, et al. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83(6):629–35. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 89.Maltsev VA, et al. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1.5 antisense inhibition. Am J Physiol Heart Circ Physiol. 2008;295(2):H667–76. doi: 10.1152/ajpheart.00111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ross JL, Ali MY, Warshaw DM. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr Opin Cell Biol. 2008;20(1):41–7. doi: 10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. Int J Biochem Cell Biol. 2012;44(2):266–74. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Gu F, Crump CM, Thomas G. Trans-Golgi network sorting. Cell Mol Life Sci. 2001;58(8):1067–84. doi: 10.1007/PL00000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luini A, et al. Morphogenesis of post-Golgi transport carriers. Histochem Cell Biol. 2008;129(2):153–61. doi: 10.1007/s00418-007-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol. 2009;10(11):765–77. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 95.Hamm-Alvarez SF, Sheetz MP. Microtubule-dependent vesicle transport: modulation of channel and transporter activity in liver and kidney. Physiol Rev. 1998;78(4):1109–29. doi: 10.1152/physrev.1998.78.4.1109. [DOI] [PubMed] [Google Scholar]
- 96.Johnson RG, et al. Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules. Exp Cell Res. 2002;275(1):67–80. doi: 10.1006/excr.2002.5480. [DOI] [PubMed] [Google Scholar]
- 97.Duffield A, Caplan MJ, Muth TR. Protein trafficking in polarized cells. Int Rev Cell Mol Biol. 2008;270:145–79. doi: 10.1016/S1937-6448(08)01404-4. [DOI] [PubMed] [Google Scholar]
- 98.Patel DM, et al. Disease mutations in desmoplakin inhibit Cx43 membrane targeting mediated by desmoplakin-EB1 interactions. J Cell Biol. 2014;206(6):779–97. doi: 10.1083/jcb.201312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shaw RM. Desmosomal Hotspots, Microtubule Delivery, and Cardiac Arrhythmogenesis. Developmental Cell. 2014 doi: 10.1016/j.devcel.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19(4):453–8. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 101.Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovasc Res. 2008;77(4):667–75. doi: 10.1093/cvr/cvm048. [DOI] [PubMed] [Google Scholar]
- 102.Hayakawa K, et al. Differential assembly of cytoskeletal and sarcomeric actins in developing skeletal muscle cells in vitro. Zoolog Sci. 1996;13(4):509–17. doi: 10.2108/zsj.13.509. [DOI] [PubMed] [Google Scholar]
- 103.Itoh T, et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9(6):791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Jaiswal JK, V, Rivera M, Simon SM. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137(7):1308–19. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rogers SL, V, Gelfand I. Membrane trafficking, organelle transport, and the cytoskeleton. Curr Opin Cell Biol. 2000;12(1):57–62. doi: 10.1016/s0955-0674(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 106.Theiss C, Meller K. Microinjected anti-actin antibodies decrease gap junctional intercellular commmunication in cultured astrocytes. Exp Cell Res. 2002;281(2):197–204. doi: 10.1006/excr.2002.5652. [DOI] [PubMed] [Google Scholar]
- 107.Qu C, Gardner P, Schrijver I. The role of the cytoskeleton in the formation of gap junctions by Connexin 30. Exp Cell Res. 2009;315(10):1683–92. doi: 10.1016/j.yexcr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 108.Thomas T, Jordan K, Laird DW. Role of cytoskeletal elements in the recruitment of Cx43-GFP and Cx26-YFP into gap junctions. Cell Commun Adhes. 2001;8(4–6):231–6. doi: 10.3109/15419060109080729. [DOI] [PubMed] [Google Scholar]
- 109.Smyth JW, et al. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res. 2012;110(7):978–89. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fort AG, et al. In vitro motility of liver connexin vesicles along microtubules utilizes kinesin motors. J Biol Chem. 2011;286(26):22875–85. doi: 10.1074/jbc.M111.219709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hasaka TP, Myers KA, Baas PW. Role of actin filaments in the axonal transport of microtubules. J Neurosci. 2004;24(50):11291–301. doi: 10.1523/JNEUROSCI.3443-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schaefer AW, et al. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev Cell. 2008;15(1):146–62. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sainsbury F, et al. Developmental reorientation of transverse cortical microtubules to longitudinal directions: a role for actomyosin-based streaming and partial microtubule-membrane detachment. Plant J. 2008;56(1):116–31. doi: 10.1111/j.1365-313X.2008.03574.x. [DOI] [PubMed] [Google Scholar]
- 114.Bartolini F, Ramalingam N, Gundersen GG. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell. 2012;23(20):4032–40. doi: 10.1091/mbc.E12-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J Cell Biol. 2003;161(5):845–51. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qin H, et al. Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem. 2003;278(32):30005–14. doi: 10.1074/jbc.M300614200. [DOI] [PubMed] [Google Scholar]
- 117.Falk MM, et al. Gap junction turnover is achieved by the internalization of small endocytic double-membrane vesicles. Mol Biol Cell. 2009;20(14):3342–52. doi: 10.1091/mbc.E09-04-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leithe E, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem. 2004;279(48):50089–96. doi: 10.1074/jbc.M402006200. [DOI] [PubMed] [Google Scholar]
- 119.Smyth JW, et al. A 14-3-3 mode-1 binding motif initiates gap junction internalization during acute cardiac ischemia. Traffic. 2014;15(6):684–99. doi: 10.1111/tra.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Basheer WA, et al. Cardiomyocyte-specific overexpression of the ubiquitin ligase Wwp1 contributes to reduction in Connexin 43 and arrhythmogenesis. J Mol Cell Cardiol. 2015;88:1–13. doi: 10.1016/j.yjmcc.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martins-Marques T, et al. Heart ischemia results in connexin43 ubiquitination localized at the intercalated discs. Biochimie. 2015;112:196–201. doi: 10.1016/j.biochi.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 122.Kjenseth A, et al. The gap junction channel protein connexin 43 is covalently modified and regulated by SUMOylation. J Biol Chem. 2012;287(19):15851–61. doi: 10.1074/jbc.M111.281832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leithe E, et al. Endocytosis and post-endocytic sorting of connexins. Biochim Biophys Acta. 2012;1818(8):1870–9. doi: 10.1016/j.bbamem.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 124.Fong JT, Nimlamool W, Falk MM. EGF induces efficient Cx43 gap junction endocytosis in mouse embryonic stem cell colonies via phosphorylation of Ser262, Ser279/282, and Ser368. FEBS Lett. 2014;588(5):836–44. doi: 10.1016/j.febslet.2014.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leykauf K, et al. Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J Cell Sci. 2006;119(Pt 17):3634–42. doi: 10.1242/jcs.03149. [DOI] [PubMed] [Google Scholar]
- 126.Jordan K, et al. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci. 2001;114(Pt 4):763–73. doi: 10.1242/jcs.114.4.763. [DOI] [PubMed] [Google Scholar]
- 127.Boassa D, et al. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic. 2010;11(11):1471–86. doi: 10.1111/j.1600-0854.2010.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vanderpuye OA, Bell CL, Murray SA. Redistribution of Connexin 43 during Cell Division. Cell Biol Int. 2016 doi: 10.1002/cbin.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marquez-Rosado L, et al. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818(8):1985–92. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Majoul IV, et al. Limiting transport steps and novel interactions of Connexin-43 along the secretory pathway. Histochem Cell Biol. 2009;132(3):263–80. doi: 10.1007/s00418-009-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Batra N, et al. 14-3-3theta facilitates plasma membrane delivery and function of mechanosensitive connexin 43 hemichannels. J Cell Sci. 2014;127(Pt 1):137–46. doi: 10.1242/jcs.133553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martins-Marques T, et al. Ischaemia-induced autophagy leads to degradation of gap junction protein connexin43 in cardiomyocytes. Biochem J. 2015;467(2):231–45. doi: 10.1042/BJ20141370. [DOI] [PubMed] [Google Scholar]
- 133.de Klerk E, C‘t Hoen PA. Alternative mRNA transcription, processing, and translation: insights from RNA sequencing. Trends in Genetics. 31(3):128–139. doi: 10.1016/j.tig.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Ghazalpour A, et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011;7(6):e1001393. doi: 10.1371/journal.pgen.1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kochetov AV. Alternative translation start sites and hidden coding potential of eukaryotic mRNAs. Bioessays. 2008;30(7):683–91. doi: 10.1002/bies.20771. [DOI] [PubMed] [Google Scholar]
- 136.Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234(2):187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 137.Shatsky IN, et al. Transcriptome-wide studies uncover the diversity of modes of mRNA recruitment to eukaryotic ribosomes. Crit Rev Biochem Mol Biol. 2014;49(2):164–77. doi: 10.3109/10409238.2014.887051. [DOI] [PubMed] [Google Scholar]
- 138.Martinez-Salas E, et al. RNA-binding proteins impacting on internal initiation of translation. Int J Mol Sci. 2013;14(11):21705–26. doi: 10.3390/ijms141121705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev. 2011;75(3):434–67. doi: 10.1128/MMBR.00008-11. first page of table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299(1–2):1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Somers J, Poyry T, Willis AE. A perspective on mammalian upstream open reading frame function. Int J Biochem Cell Biol. 2013;45(8):1690–700. doi: 10.1016/j.biocel.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chatterjee B, et al. Developmental regulation and expression of the zebrafish connexin43 gene. Dev Dyn. 2005;233(3):890–906. doi: 10.1002/dvdy.20426. [DOI] [PubMed] [Google Scholar]
- 143.Smyth JW, Shaw RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013;5(3):611–8. doi: 10.1016/j.celrep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mathieu Laplante1 aDMS. mTOR signaling at a glance. Science. 2009 doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Basheer W, Shaw R. The “tail” of Connexin43: An unexpected journey from alternative translation to trafficking. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamcr.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang SS, Shaw RM. Trafficking highways to the intercalated disc: new insights unlocking the specificity of connexin 43 localization. Cell Commun Adhes. 2014;21(1):43–54. doi: 10.3109/15419061.2013.876014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta. 2012;1818(8):1831–43. doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herve JC, et al. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta. 2012;1818(8):1844–65. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 149.Kang EY, et al. Identification of binding partners for the cytoplasmic loop of connexin43: a novel interaction with beta-tubulin. Cell Commun Adhes. 2009;15(5–6):397–406. doi: 10.1080/15419060902783833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Giepmans BN, et al. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol. 2001;11(17):1364–8. doi: 10.1016/s0960-9822(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 151.Giepmans BN, Verlaan I, Moolenaar WH. Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Commun Adhes. 2001;8(4–6):219–23. doi: 10.3109/15419060109080727. [DOI] [PubMed] [Google Scholar]
- 152.Saidi Brikci-Nigassa A, et al. Phosphorylation controls the interaction of the connexin43 C-terminal domain with tubulin and microtubules. Biochemistry. 2012;51(21):4331–42. doi: 10.1021/bi201806j. [DOI] [PubMed] [Google Scholar]