Abstract
Serum testosterone (T) levels decrease with aging in both humans and rodents. Using the rat as a model system, it was found that age-related reductions in serum T were not due to loss of Leydig cells, but rather to the reduced ability of the Leydig cells to produce T in response to luteinizing hormone (LH). Detailed analyses of the steroidogenic pathway have suggested that two defects along the pathway, LH stimulated cAMP production and cholesterol transport to and into the mitochondria, are of particular importance in age-related reductions in T production. Although the mechanisms involved in these defects are far from certain, increasing oxidative stress appears to play a particularly important role. Interestingly, increased oxidative stress also appears to be involved in the suppressive effects of endocrine disruptors on Leydig cell T production.
Keywords: Leydig cell, aging, endocrine disruptors, steroidogenesis, oxidative stress
1. Introduction
Steroid hormones regulate critical phases of development and are essential for homeostasis of key physiological functions. In the male, the Leydig cells of the testis are responsible for producing testosterone (T), and T is essential for spermatogenesis. T also is important for the maintenance of secondary sexual functions. A number of longitudinal studies have shown that in most men there is a slow decline in serum levels of total and bioactive T with aging, beginning at about age 30, even in the absence of disease (Harman et al. 2001). Decline of serum T usually is accompanied by increased or unchanging levels of LH and increased serum levels of FSH (Zwart et al. 1996), suggesting that the relative unresponsiveness of the Leydig cells to LH, rather than, or in addition to, defects in the hypothalamic-pituitary axis, plays a primary role in age-related reductions in serum T (Mulligan et al. 2001, Keenan et al. 2006). This is discussed further below. T deficiency in the adult has consequences for the general health of individuals, including increased body fat and fatigue, and decreased muscle mass, bone density, cognitive function and immune response (Huhtaniemi 2014).
In Brown Norway rats, as in aging men, serum T levels were shown to decrease with age (Zirkin et al. 1993). However, unlike the many rat strains in which age-related decreases in serum T levels are a consequence of reduced LH levels, LH levels do not decrease in Brown Norway rats. This suggests that in this strain, as in men, there is primary hypogonadism, with age-related changes in Leydig cell steroidogenesis occurring at the gonadal level rather than secondary to hypothalamic-pituitary changes.
T production can be affected not only by aging but also by endocrine disruptors. There now is evidence suggesting that there may be common mechanisms that explain both age-related and endocrine disruptor-induced reduced T production. This review will emphasize research on Leydig cell steroidogenesis, focusing on the effects of aging on Leydig cell T production. We also will discuss mechanisms in common with aging by which some endocrine disruptors also affect steroidogenesis.
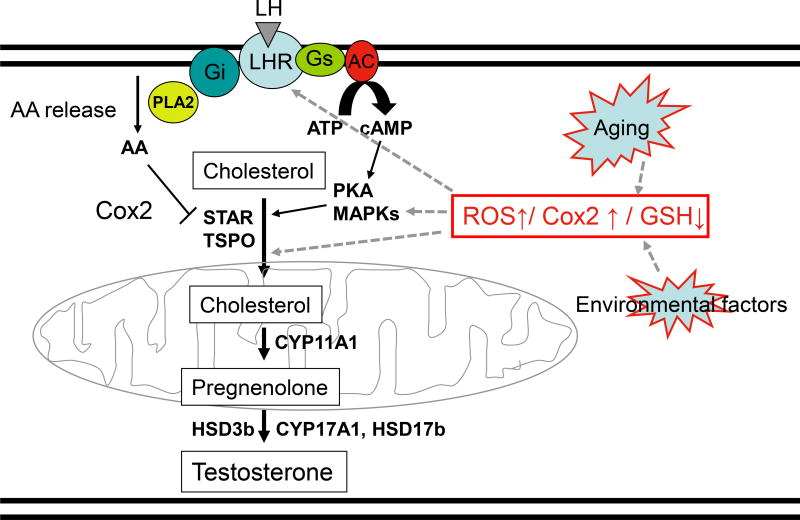
2. Testosterone synthesis in Leydig cells
Steroidogenesis is a multi-step process that converts cholesterol into final steroid hormone products. As reviewed in Aghazadeh et al (2015), steroidogenesis consists of cholesterol mobilization from lipid droplets and/or the plasma membrane, cholesterol transport into mitochondria, pregnenolone formation in the mitochondria, and subsequent conversion of pregnenolone into the final steroid products by enzymes of the smooth endoplasmic reticulum (Fig. 1). In the adult testis, Leydig cell T production depends upon the pulsatile secretion of LH by the pituitary gland into the peripheral circulation. LH plays two essential roles in Leydig cell steroidogenesis: 1) maintenance of optimal levels of steroidogenic enzymes (trophic regulation), and 2) mobilization and transport of cholesterol into the inner mitochondrial membrane (acute regulation).
Figure 1. Leydig cell steroidogenesis.
LH binds its receptor on the Leydig cell membrane. LH receptor/G protein coupling results in increased cAMP and arachidonic acid (AA) production. cAMP stimulates the mobilization and transport of cholesterol to and into the mitochondria in part by activating PKA and MAPK signaling. At the same time, AA can be converted into prostaglandin by Cox2 to negatively regulate the transport of cholesterol across the mitochondrial membranes. At the inner mitochondrial membrane, cholesterol is converted to pregnenolone by CYP11A1, and pregnenolone is converted into testosterone by enzymes in the smooth endoplasmic reticulum (HSD3b, CYP17A1 and HSD17b). Aging and environmental factors may impact steroidogenesis by affecting the intracellular redox balance in part through increased ROS production. This has significant effects on cAMP formation and/or cholesterol transport into the mitochondria, and thus on steroid formation. Steroidogenesis also might by affected by ROS-induced increases in Cox2 production and redox sensitive MAPK synthesis.
Both the trophic and acute effects of LH are mediated by signaling pathways that begin with cAMP production (Fig 1). LH binds to and activates G protein-coupled receptors, resulting in the activation of adenylyl cyclase, increased intracellular cAMP formation, and cAMP-dependent phosphorylation of proteins through protein kinase A (PKA). The acute stimulation of Leydig cells by LH results in cholesterol transfer into the mitochondria in part through the actions of steroidogenic acute regulatory protein (STAR), translocator protein (18kDa; TSPO), and other proteins of the transduceosome (Midzak et al. 2011). Cholesterol transport into the mitochondria, the rate-limiting step in steroid biosynthesis, is followed by the conversion of cholesterol to pregnenolone by the C27 cholesterol side-chain cleavage cytochrome P450 enzyme (CYP11A1) located on the matrix side of the inner mitochondrial membrane. Pregnenolone then is metabolized into T by 3β-hydroxysteroid dehydrogenase (3b-HSD; HSD3B), 17α-hydroxylase/17,20 lyase (CYP17A1) and type 3 17β-hydroxysteroid dehydrogenase (17b-HSD3, HSD17B) in the smooth endoplasmic reticulum (Payne & Hales 2004, Miller & Bose 2011, Aghazadeh et al. 2015, Beattie et al. 2015).
3. Effects of aging on steroidogenesis
3.1. Aging and declining testosterone production by Leydig cells
With aging, serum T levels decrease in the males of a number of species, including human and rodent (Harman et al. 2001, Mulligan et al. 2001, Keenan et al. 2006). Studies in men have shown a reduced ability of Leydig cells to produce T in response to LH stimulation (Veldhuis et al., 2005, Veldhuis et al., 2012; Surampudi et al., 2012). In many rat strains, including Sprague-Dawley, decreased LH levels that result from hypothalamic- pituitary changes are responsible for decreased T formation by aging Leydig cells, and thus for decreased serum T (Zirkin et al. 1993). This is referred to as secondary hypogonadism. In Brown Norway rats, however, as in men, T levels decrease with age despite unchanging LH and increasing FSH levels (Zirkin et al. 1993, Chen et al. 1994, Chen et al. 1996, Chen et al. 2015a, Beattie et al. 2015). Some years ago, we showed that when Brown Norway rat testes were perfused with maximally stimulating LH, testes from old (18–24 month-old) rats produced significantly less T than testes from young (3–6 month-old) rats (Zirkin et al. 1993). Consistent with this, Leydig cells isolated from aged rats produced less T than cells from young rats in response to maximally stimulating LH (Chen et al. 1994). Leydig cells in the adult testis rarely turn over, and their numbers do not change or increase with age (Wang et al. 1993, Chen et al. 1994). Consequently, the reduced serum T levels in old rats results from the relative unresponsiveness of the Leydig cells to LH (Chen et al. 2002).
Among the important changes in the steroidogenic pathway that have been implicated in the reduced T production that characterizes aging Leydig cells are reductions in cAMP production and protein kinase A (PKA) activities (Chen et al. 2002, Liao et al. 1993). LH binding to LH receptors initiates a cascade of events that include receptor coupling to Gs proteins, activation of adenylyl cyclase, and ultimately increased cAMP production and activation of cAMP- dependent PKA (Fig 1). Signaling through cAMP/PKA is essential for the expression of the downstream steroidogenic proteins and enzymes of the mitochondria and smooth endoplasmic reticulum (Payne & Hales 2004). Reduced numbers of LH receptors or their reduced coupling efficiency to G-protein affects cAMP production, and also affects other signaling cascades including arachidonic acid/cyclooxygenase-2 (COX-2) (Wang et al. 2005, Castillo et al. 2006) and mitogen-activated protein kinase (MAPK) (Tai & Ascoli 2011). cAMP, in response to LH, stimulates the transport of cholesterol into the mitochondria (Aghazadeh et al. 2015). Whereas the culture of old cells with LH was found to result in T production significantly below that of LH-stimulated young cells, culture with dibutyryl cAMP (dbcAMP) for 3 days resulted in increased T production by old cells to the level of LH-stimulated young cells (Chen et al. 2004a). This suggests that relative insensitivity of old Leydig cells to LH, and consequent deficient signal transduction, contributes to, or may cause, the reduced T that characterizes aging Leydig cells.
What is the molecular mechanism by which cAMP production is reduced with aging? Stimulation of adenylyl cyclase in old Leydig cells with forskolin, which activates adenylyl cyclase directly via by-passing the ligand and G proteins, was found to result in equivalent production of cAMP by young and old cells (Chen et al. 2002). Likewise, in the absence of LH, direct activation of Gs protein by cholera toxin stimulated cAMP synthesis in old cells to young levels, and inhibition of the inhibitory G protein (Gi) by pertussis toxin did not restore the ability of the aged Leydig cells to produce cAMP at high levels in response to LH (Chen et al. 2002). These observations suggest that G protein and adenylyl cyclase deficiencies are unlikely to cause aged cells to produce less cAMP than young cells. The number of LH binding sites was shown to decrease significantly with age, indicating reduced plasma membrane LH receptor numbers (Chen et al. 2002). However, it has been known for some time that only approximately 10% of LH receptor occupancy is required to elicit a biological response, indicative of reserve or “spare” receptors (Hsueh et al. 1977). In fact, Leydig cells from young, LH-suppressed rats, which have even lower numbers of LH receptors than aged cells, nonetheless were found to have an unaltered ability to synthesize cAMP in response to LH (Chen et al. 2002). These results, taken together, lead to the conclusion that LH receptor-G protein coupling deficiency is likely to be responsible, at least in part, for reduced cAMP production by aged Leydig cells. It also has been suggested that increased cAMP degradation may play a role (Sokanovic et al. 2014). In rodents, Leydig cell cAMP is metabolized by phosphodiesterases (PDE4a, PDE4b, and PDE2a), found to be increased in aging Leydig cells (Sokanovic et al. 2014). However, the extent to which these increases might reduce cAMP levels in aged cells is unclear.
In addition to its effects on cAMP production, LH stimulation also results in arachidonic acid release in Leydig cells (Fig 1) (Wang et al. 2005, Castillo et al. 2006). The released arachidonic acid is metabolized by cellular lipoxygenases, epoxygenases or cyclooxygenases (COX). The metabolites have been shown to be capable of modulating steroidogenesis in part by affecting STAR protein expression (Wang et al. 2005). In MA-10 Leydig cells and rat primary Leydig cells, inhibition of COX-2 activity resulted in significantly increased T production, suggesting that COX-2 can negatively affect steroidogenesis (Wang et al. 2005, Chen et al. 2007). In aging Leydig cells, COX-2 message and protein levels have been shown to be elevated relative to their levels in cells from young rats (Wang et al. 2005, Chen et al. 2007), suggesting the possible involvement of COX-2 in age-related reductions in T production (Wang et al. 2005, Chen et al. 2007). This is supported by the observation that long-term treatment of aged rats with COX-2 antagonist can partially reverse reduction in serum T levels (Wang et al. 2005). These findings suggest the possibility that COX2 may also be involved in the age-related decline in T production.
Members of the MAPK signaling family, including ERK and p38, also have been implicated in age-related reductions in steroidogenesis (Fig 1) (Abidi et al. 2008a, Sokanovic et al. 2014). LH has been shown to increase ERK1/2 phosphorylation (Tai & Ascoli 2011). Inhibition of ERK1/2 phosphorylation can significantly block the effect of LH stimulation, suggesting that ERK1/2 in part mediates LH function. Age-related modulation of the p38 MAPK signaling pathway has been shown in adrenal and Leydig cells to be associated with reductions in steroidogenesis elicited by prooxidants (Abidi et al. 2008a, Abidi et al. 2008b), and reductions in steroidogenesis can be partially prevented by inhibition of p38 (Chen et al. 2010). In a recent study, it was found that the oxidant-sensitive p38 MAPK can inhibit cAMP-induced steroidogenesis in part by repressing STAR (Zaidi et al. 2014), suggesting that age-related Leydig cell dysfunction may be related to changes in both MAPK and cAMP signaling (Sokanovic et al. 2014).
3.2. Cholesterol synthesis, mobilization and mitochondrial transport
In addition to the reductions in LH-stimulated cAMP in aged Brown Norway rat Leydig cells, there are reductions in STAR, TSPO, the mitochondrial enzyme CYP11A1, and downstream steroidogenic enzymes of the smooth endoplasmic reticulum (Luo et al. 1996, Luo et al. 2001, Culty et al. 2002, Luo et al. 2005). Although the mitochondrial and smooth endoplasmic reticulum steroidogenic enzymes are reduced in aging Leydig cells, their levels nonetheless are sufficient to support high levels of steroid production if enough cholesterol is translocated into the mitochondria and thus is available to CYP11A1 of the inner mitochondrial membrane (Culty et al. 2002). This suggests that defects in cholesterol import into the mitochondria might underlie the differential steroidogenic abilities of young versus old Leydig cells, and may ultimately be responsible for the reduced T formation in response to LH that characterizes the old cells. Cholesterol translocation involves mobilization of cholesterol and its transport to the outer and then the inner mitochondrial membrane where CYP11A1 resides (Aghazadeh et al. 2015, Venugopal et al. 2016).
The precise location of the intracellular cholesterol utilized in steroid formation is uncertain. Studies have shown that cholesterol utilized in steroidogenic cells derives in part from lipoprotein in the circulation that is imported by membrane-bound scavenger receptor class B type I (SR-B1) lipoprotein receptor (Azhar & Reaven 2002), or is synthesized de novo within the cells from acetyl-CoA. Cholesterol may be stored in cytoplasmic lipid droplets in the form of cholesteryl esters. A recent study reported that the plasma membrane is a major source of cholesterol for steroidogenesis (Venugopal et al. 2016). In response to hormonal stimulation, cholesteryl esters are converted into free cholesterol by cholesterol esterases and then transferred to the mitochondrial outer membrane (Shen et al. 2003, Miller & Boss 2011). Important components in this process, including SR-B1, carboxyesterase (ES-10) and hormone sensitive lipase (HSL) are down-regulated in aged Leydig cells (Chen et al. 2004b), suggesting that cholesterol import, synthesis, and mobilization are all affected by aging. This is consistent with the observations that cholesterol synthesis and mobilization are reduced in Leydig cells of aged as compared to young rats (Liao et al. 1993).
Cholesterol translocation into mitochondria is mediated by cAMP through the proteins of the transduceosome (Papadopoulos et al. 2015). Translocator protein (TSPO), one such protein, comprises 2% of the outer mitochondrial membrane proteins of young Leydig cells. TSPO is reduced significantly in old Leydig cells (Culty et al. 2002). Incubating old Leydig cells with LH plus a specific TSPO drug ligand resulted in the restoration of T production to the high levels of LH-stimulated young cells (Chung et al. 2013). In complementary in vivo studies, administering TSPO ligand to aged rats restored serum T to the level of young rats. (Chung et al. 2013). A domain in the C-terminus of TSPO was characterized as a cholesterol recognition/interaction amino acid consensus (CRAC). These studies, among those conducted over many years, strongly implicate TSPO as a mediator of cholesterol translocation and thus steroidogenesis (Papadopoulos et al. 2015). However, recent studies of the effects of the genetic deletion of Tspo in mice have reported that TSPO knockout has little or no effect on steroidogenesis, thus calling into question the role of TSPO in cholesterol translocation (Tu et al. 2014). The latter results are disputed, however (Fan et al. 2015; Papadopoulos et al. 2015), and yet to be confirmed.
Several additional cytosolic proteins, including PKA, acyl-CoA binding domain-containing protein 3 (aka PAP7), and STAR may be involved in the import of cholesterol to the inner mitochondrial membrane (Papadopoulos et al. 2007, Miller 2013). STAR, for one, plays a significant role in steroidogenesis (Kallen et al. 1998, Clark 2016). When Leydig cells are stimulated by LH, STAR levels increase rapidly (Clark et al. 1994). In rodents, deletion of the Star gene, or knockdown of the STAR protein, has been shown to suppress steroidogenesis (Lin et al. 1995). Impairment in the synthesis of adrenal and gonadal steroids has been shown to be associated with congenital lipoid adrenal hyperplasia in the human, a disease characterized in part by mutations of the Star gene (Bose et al. 1997). Although the importance of STAR in mitochondrial cholesterol transfer is clear, the mechanism by which it functions remains uncertain. Early in vitro studies showed that STAR contains a domain (StAR-related lipid-transfer domain, or START) that binds cholesterol and may be involved in cholesterol transfer (Ponting & Aravind 1999) and thus in steroid production (Arakane et al. 1996). In vivo, the expression of a full-length STAR transgene in Star knockout mice restored adrenal and gonadal steroidogenesis (Sasaki et al. 2008). These observations, and the localization of STAR at the outer mitochondrial membrane, suggest a critical role for STAR in cholesterol translocation (Clark 2016, Stocco et al. 2017). Moreover, recent reports of functional interaction between TSPO and STAR have led to the proposal that such interaction may be an integral part of cholesterol translocation to and into the mitochondria in steroidogenic cells (Liu et al. 2006, Miller, 2013, Papadopoulos et al. 2015).
There are other potential players in steroid formation that are part of the transduceosome. These include the 14-3-3 proteins. It has been shown that 14-3-3γ is increased 4-fold upon hCG stimulation, and that it binds to STAR and acts as a negative regulator of steroid formation (Midzak et al. 2015). Each of TSPO, STAR, 14-3-3γ, and perhaps other transduceosome proteins, might serve as targets to regulate and thus enhance T formation by old Leydig cells in a more physiological fashion than providing T exogenously.
4. Mechanism(s) responsible for age-related changes in testosterone formation
4.1. Extrinsic factors
Cellular aging can result from changes in factors within and/or outside the target cells. Intrinsic factors, including reactive oxygen species (ROS), are discussed below. Extrinsic factors might include hormones, growth factors, oxidants, and antioxidants produced by local cells or transported to the target cells via the circulation. Extrinsic factors have been shown to contribute to the aging of cells of the immune system (Badowski et al. 2014), skin (Lephart 2016) and muscle (Cannon 1998), among others, and to affect Leydig cells as well. For example, after the elimination of Leydig cells from the testes of young (3 month-old) and aged (18 month-old) Brown Norway rats by administering ethane dimethanesulfonate (EDS), a new generation of Leydig cells was restored to the aged testes by 10 weeks that produced as much T as cells restored to the young testes (Chen et al. 1996). However, T production by the newly formed Leydig in the testes of old rats was reduced to “old” levels 20 weeks later. This was in striking contrast to the new cells formed in young testes, the T production of which did not change in that period of time (Chen et al. 2015a). These observations suggested that factors outside the newly formed cells themselves may play important roles in their aging.
Leydig cell steroidogenic function has been shown to be affected by factors produced locally, and also at a distance via the circulatory system. Within the testis, Sertoli cell and macrophage products have been shown to have stimulatory effects on Leydig cell steroidogenesis (Haider 2004). Negative effects also have been shown. For example, hydrogen peroxide produced by macrophages may negatively impact neighboring Leydig cells over time (Hales 2002). Factors produced by the pituitary (gonadotropins), liver (IGF-1), thyroid (T3), pancreas β-cells (insulin), bone marrow (osteocalcin), and immune system reach the Leydig cells via the circulatory system. It remains to be determined how changes in these extrinsic factors affect the steroidogenic function of aging Leydig cells.
4.2. Intrinsic factors: oxidants and antioxidant molecules
Imbalance between prooxidants and antioxidants often occurs as cells age (Rebrin et al. 2003). Such imbalance can result in an altered redox state and an accumulation of oxidative damage to intracellular macromolecules, thus contributing to age-related functional deficits (Finkel & Holbrook 2000). Leydig cells produce ROS from several sources, including the mitochondrial electron transport chain and mitochondrial and microsomal cytochrome P450 enzyme reactions (Hanukoglu 2006). Recent studies indicate ROS production might be in response to LH; incubating Leydig cells with LH resulted in increased ROS levels as well as in DNA damage (Beattie et al. 2013). Leydig cells from aged rats produce significantly more reactive oxygen than cells from young rats, and this occurs despite reduced mitochondrial volume (Chen et al. 2001). Aging of Leydig cells also is accompanied by the reduced expressions of key enzymatic and non-enzymatic antioxidants, including Cu- Zn-SOD, Mn-SOD, glutathione peroxidase (GPX-1), microsomal glutathione S-transferase (MGST1), glutathione S-transferase (GSTM2), and glutathione (GSH), leading to increased oxidative stress and oxidative damage (e.g. lipid peroxidation) (Chen et al. 2001, Cao et al. 2004). Age-related decreases in Leydig cell antioxidant activities, gene expression, and protein levels are consistent with the hypothesis that the loss of steroidogenic function that accompanies Leydig cell aging may result in part from an altered antioxidant defense system (Cao et al. 2004, Luo et al. 2006).
Experimental studies designed to go beyond correlation to establish cause-effect relationships have been reported. The long-term administration of the antioxidant vitamin E delayed age-related decreases in steroidogenesis, while long-term vitamin E deficiency had the opposite effect (Abidi et al. 2004, Chen et al. 2005). GSH is among the most significant antioxidants reduced in aging Leydig cells (Cao et al. 2004, Luo et al. 2006). To examine whether loss of GSH would affect Leydig cell T production in vivo, young and old rats were treated with buthionione sulfoximine (BSO) for a week to reduce Leydig cell intracellular GSH levels. Significantly decreased T production by both young and old Leydig cells was seen following experimental reduction of GSH, suggesting that such reductions might lead to alterations in the redox environment of Leydig cells and ultimately to decreases in T production (Chen et al. 2008). Depletion of GSH in vitro also reduced Leydig cell steroidogenic function, while the antioxidants vitamin E, N-tert-butyl-α-phenylnitrone and Trolox suppressed the effect of loss GSH (Chen et al. 2008). A critical role of GSH in regulation of Leydig cell steroidogenesis was also suggested by a recent study reporting that the expression of γ-glutamyl transferase 5 (GGT5), an enzyme involved in GSH metabolism, was negatively correlated with Leydig cell steroidogenesis (Li et al. 2016).
Nrf2/Keap1 signaling is an important mechanism by which cells respond to oxidative stress (Cho et al. 2005, Li & Kong 2009). Normally, Nrf2 is bound to Keap1 in the cytoplasm and undergoes ubiquination-dependent proteasomal degradation. With exposure to oxidative stress, Nrf2 dissociates from Keap1. When separated from Keap1, Nrf2 binds to the antioxidant response element (ARE) in the promoter of target genes, and thus stimulates the transcription of numerous antioxidant molecules and phase two enzymes (Cho et al. 2005, Li & Kong 2009). With aging, GSH and antioxidant enzymes typically decline despite elevated levels of oxidative stress. This is true of many cell types, including Leydig cells (Rebrin et al. 2003, Cao et al. 2004, Luo et al. 2006), and leads to a pro-oxidant state in aging cells. We reasoned that if Nrf2 and thus the oxidant/antioxidant environment plays a role in age-related reductions in Leydig cell T production, the experimental knockout of Nrf2 should result in increased oxidative stress and therefore, over time, in decreased T production. Using this genetic approach, we found that at 3 months there was no significant difference between wild-type and knockout mice in either serum T concentration or in the ability of Leydig cells to produce T. However, by middle age (8-months), at which time cells from wild-type mice had not yet lost steroidogenic function, T production by Leydig cells from the knockout mice was reduced significantly. By 24 months, both the wild-type and knockout mice had reduced serum T and Leydig cell T production, but with more extensive reductions in the knockouts (Chen et al. 2015b). These observations indicate that without the antioxidant master regulator Nrf2, the age-related loss of Leydig cell steroidogenic function accelerated. Moreover, the antioxidant capacity of the testis was significantly reduced in the knockout as compared to the wild-type mice at each age. With reduced expression of numerous antioxidant molecules and therefore reduced total antioxidant capacity, an increasingly prooxidant environment was seen that was reminiscent of aging, accompanied by reduced T production by Leydig cells and reduced serum T levels. The specific targets of an altered redox environment, and thus the mechanism(s) by which a prooxidant environment affects Leydig cell steroidogenesis, remain unclear.
5. Effects of environmental factors on steroidogenesis
There is growing realization that many of the man-made chemicals released into the atmosphere have significant public health consequences, including perturbation of the endocrine system. Endocrine-disrupting chemicals (EDCs) are defined as “substances in our environment, food, and consumer products that interfere with hormone biosynthesis, metabolism, or action resulting in a deviation from normal homeostatic control or reproduction” (Diamanti-Kandarakis et al. 2009). In this section, we will briefly discuss EDC effects on Leydig cell steroidogenesis, and the striking similarity in mechanisms by which these effects and those that result in age-related reduction in T production are elicited.
5.1. Effects of EDCs on cAMP production and mitochondrial cholesterol metabolism
As indicated above, LH-stimulated cAMP production and mitochondrial cholesterol transport and metabolism have been shown to be critically involved in age-related reductions in T formation (Fig 1). These same elements of the steroidogenic pathway are affected by a number of EDCs, leading to reduced T (summarized in Table 1). Using mouse Leydig tumor cells as a model system, 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47), a polybrominated diphenyl ether, was found to elicit decreases in hCG-induced cAMP levels and in the synthesis of CYP11A1 (Han et al. 2012). Exposure of rat primary Leydig cells to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) also was found to inhibit steroidogenesis through its effects on cAMP and CYP11A1 (Johnson et al. 1992, Fukuzawa et al. 2004). Permethrin, an insecticide, disrupted T formation in mice by decreasing the protein and mRNA levels of STAR and CYP11A1, also indicative of an effect on cholesterol transport and metabolism (Zhang et al. 2007). Atrazine, a widely used herbicide, has similar effects; exposure of rats to atrazine during postnatal days 23–30 caused reduced cAMP levels in Leydig cells and decreased cholesterol transport (Pogrmic et al. 2009). Exposure of Leydig cells to triclosan, a chemical widely used in antimicrobial preparations, resulted in significantly decreased activity of adenylyl cyclase, synthesis of cAMP, and transcriptional and translational activity of P450scc, 3β-HSD, 17β-HSD and STAR (Kumar et al. 2008). Lindane, an insecticide that also is used to treat lice and scabies, also was found to affect cAMP, STAR and steroidogenesis (Ronco et al. 2001, Saradha et al. 2008). Phthalates are plasticizers that are highly abundant, exhibit antiandrogenic properties, and have different effects depending upon when in the lifecycle they are administered. Thus, the exposure of males to phthalates in utero can affect both Leydig cell development and adult steroidogenic function (Foster et al. 2001, Martinez-Arguelles et al. 2013). MEHP was found to inhibit steroidogenesis in MA-10 Leydig cells by interfering with LH-stimulated cAMP production and mitochondrial cholesterol transport/metabolism (Zhou et al. 2013).
Table 1.
Environmental factors affecting LHR-cAMP and mitochondrial cholesterol cascades
| Toxin | Animal Cell |
Age | Dose (/kgBW) Duration |
Effects on (LC or Testis) |
Mechanisms Sensitive Targets |
Reference |
|---|---|---|---|---|---|---|
| BDE-47 | MA10 | 1µM; 24h | P4 production↓ | cAMP↓; Cyp11a1↓ | Han, 2012 | |
| TCDD | mice | Adult | 100µg; once | Testicular T↓ | LHR↓; Cyp11a1↓ | Fukuzawa, 2004 |
| Permethrin | mice | Adult | 35mg; 6w | Serum T↓ Testicular T↓ | TSPO↓; STAR↓; Cyp11a1↓ | Zhang, 2007 |
| Atrazine | rat | Pnd23–50 | 200mg; 4w | T+DHT productions↓ | cAMP↓; LHR↓; SR-B1↓ SF-1↓; STAR↓; Cyp17a1↓; Hsd17b3↓ | Pogrmic, 2009 |
| Triclosan | rat LCs | Adult | 0.01µM; 2h | T production↓ | cAMP↓; STAR↓; Cyp17a1↓ Cyp11a1↓; Hsd17b3↓; Hsd3b1↓ Preventable by forskolin | Kumar, 2008 |
| Lindane | rat LCs | Adult | 10µg/ml; 3h | T production↓ | cAMP↓ | Ronco, 2001 |
| PFDoA | mLTC-1/rat LCs | 10µM; 24h | P4 production ↓ T production↓ | STAR↓ | Shi, 2010 | |
| Salinomycin | mice | Adult | 3mg; 4w | Testicular T↓ Testis W↓ Vesicles W↓ Epididymis W↓ | STAR↓; Cyp11a1↓ | Ojo, 2013 |
| Quinalphos | mice | Adult | 7.5 mg; 7d | Serum T↓ Testis W↓ Vesicles W↓ Epididymis W Prostrate W↓ | STAR↓; Cyp11a1↓ | Kokilavani, 2014 |
| MEHP | MA-10 | 200µM; 24h | P4 production↓ | cAMP↓; STAR↓ | Zhou, 2013 | |
| Cobalt | MA10 | 100µM; 24h | P4 production↓ | Cyp11a1 | Kumar, 2014 | |
| Dimethoate | rat | Adult | 15mg; 5w | Serum T↓ LH↑ FSH↑ | STAR↓ Hsd17b3↓; Hsd3b1↓ | Astiz, 2009 |
| Dimethoate | rat LCs | 1ppm; 24h | T production↓ | STAR↓ Hsd17b3↓; Hsd3b1↓ | Astiz, 2012 |
BDE-47: 2,2',4,4'-Tetrabromodiphenyl Ether;Cyp11a1: Cholesterol side-chain cleavage;Cyp17a1: Cytochrome P450 17a1 (Steroid 17α-hydroxylase/17,20 lyase);DEHP: Diethylhexyl Phthalate;DHT: Dihydrotestosterone;Hsd17b3: Hydroxysteroid 17-Beta Dehydrogenase 3;LHR: Luteinizing Hormone/choriogonadotropin Receptor;MEHP: Monoethylhexyl phthalic acid;P4: Progesterone;PFDoA: Perfluorododecanoic acid;Pnd: Postnatal day;SF-1: Steroidogenic Factor 1 (NR5A1);SR-B1: Scavenger Receptor class B member 1 ;STAR: Steroidogenic Acute Regulatory protein,;T: Testosterone;TCDD: 2,3,7,8-Tetrachlorodibenzodioxin;TSPO: Translocator Protein
5.2 Reduced testosterone formation in response to environmental factors: mechanisms
Although EDCs capable of affecting steroidogenesis are diverse in nature, an altered redox environment that leads to increased oxidative stress and thus reduced steroidogenesis are commonly seen as consequences of exposures, as they are in aging (summarized in Table 2, Mathur & D'Cruz 2011). For example, exposure of mLTC-1 cells to perfluorododecanoic acid (PFDoA) was shown to result in increased levels of ROS and hydrogen peroxide, and in the inhibition of STAR expression and steroidogenesis (Shi et al. 2010). Exposing purified rat Leydig cells to the polychlorinated biphenyl Aroclor 1254 resulted in significant decline in the activities of enzymatic and non-enzymatic antioxidant enzymes, increase in the levels of ROS, and decrease in the mRNA levels of steroidogenic enzymes (Murugesan et al. 2008). These changes were partially prevented by the antioxidants vitamin C and E (Murugesan et al. 2005). Exposure of mice to salinomycin resulted in decreased testicular T in association with depletion of SOD and GSH, and increased lipid peroxidation (Ojo et al. 2013). Exposure of rats to chlorpyrifos resulted in reduced T levels, increased oxidative stress and decreased antioxidant enzymes (Sai et al. 2014). The testicular antioxidant defense system and lipid peroxidation were also significantly affected by quinalphos exposure (Debnath & Mandal 2000, Kokilavani et al. 2014).
Table 2.
Environmental factors affecting redox environment of Leydig cells
| Toxin | Animal Cell | Age | Dose (/kgBW); Duration |
Effects on (LC or Testis) |
Mechanisms Sensitive Targets |
Reference |
|---|---|---|---|---|---|---|
| Lindane | rat | Adult | 5mg; 12h | StAR↓ Hsd17b3↓; Hsd3b1↓ | H2O2↑ | Saradha, 2008 |
| PFDoA | mLTC-1/rat LCs | 10µM; 24h | P4 production ↓ T production↓ | StAR↓; H2O2↑ Preventable by MnTMPyP | Shi, 2010 | |
| Aroclor1254 rat LCs | Adult | 10−8M; 6h | T production ↓ Cyp11a1↓ Hsd17b3↓ Hsd3b1↓ | H2O2↑; LPO↑ Hydroxyl radical↑; SOD↓ CAT↓; GPx↓; γ-GT↓; GR↓ GST↓; Vitamin C and E↓ | Murugesan, 2008 | |
| Salinomycin | mice | Adult | 3mg; 4w | Testicular T↓ StAR↓ Cyp11a1↓ | LPO↑ GSH↓; CAT↓; LDH↓; SOD↓ | Ojo, 2013 |
| Quinalphos | rat | Adult | 250µg; 3d | Serum T↓ Testis W↓ Germ cell loss↑ | LPO↑; SOD↑; GPx ↑ CAT↓; GSH↓ | Debnath, 2000 |
| Quinalphos | mice | Adult | 7.5 mg; 7d | Serum T↓ StAR↓; Cyp11a1↓ Hsd17b3↓ Hsd3b1↓ | SOD↓; CAT↓; GPx↓ Vitamin C↓ Preventable by antioxidant Cissus quadrangularis | Kokilavani, 2014 |
| DEHP | rat | Pnd21 | 1000mg; 10d | Serum T↓ | Serum LH↓; Serum FSH↓ Preventable by selenium diet; | Erkekoglu, 2011 |
| DEHP | rat | Pnd21 | 1000mg; 10d | GSH/GSSG↓ TBARS↑ | Cu,Zn-SOD↓; CAT↑ GSH/GSSG↓; GPx4↓ Preventable by selenium diet | Erkekoglu, 2014 |
| MEHP/ DEHP | MA-10 | 200µM; 24h | P4 production↓ cAMP↓; StAR↓ | ROS↑ GSH depended Vitamin E protective | Zhou, 2013 | |
| MEHP | MA-10 | 1µM; 48h | P4 production↓ T production↓ | ROS↑; Cyp1a1 network↑ | Fan, 2010 | |
| MEHP | MA-10 | 200µM; 24h | P4 production↓ cAMP↓; StAR↓ | ROS↑ GSH depended Vitamin E protective | Zhou, 2013 | |
| Mercury | rat | Adult | 50ppm; 90d | Serum T↓ Sperm count↓ | LPO↑; TBARS↑ SOD↓; CAT↓ | Boujbiha, 2009 |
| Lead | rat | Adult | 25µg; 15d | Serum T↓ Hsd17b3↓ Hsd3b1↓ | LPO↑; GSH↓; GST↓; SOD↓ CAT↓; GPx↓; TBARS↑ Preventable by Vitamin C | Pandya, 2012 |
| Cadmium | rat | Adult | 25µg; 15d | Serum T↓ Hsd17b3↓ Hsd3b1↓ | LPO↑; GSH↓; GST↓; SOD↓ CAT↓; GPx↓; TBARS↑ Preventable by Vitamin C | Pandya, 2012 |
| Cobalt | MA10 | 100µM; 24h | P4 production↓ Cyp11a1↓ | ROS↑ HIF-1a activity↑ | Kumar, 2014 | |
| Arsenite | mice | Adult | 11.5ppm; 36d | Serum T↓ Hsd17b3↓ Hsd3b1↓ | GSH↓ Preventable by Vitamin C | Chang, 2007 |
| BPA | R2C | 0.1nM; 24h | T production↓ Aromatase↑ EP2↑; EP4↑; CREB↑ | COX2↑; PGE2↑ Preventable by inhibitions of PKA/ Akt/ ERK/ JNK/ p38 | Kim, 2010 | |
| Dimethoate | rat | Adult | 15mg; 5w | Serum T↓ StAR↓ LH↑ | LPO↑; PGE2↑; PGF2α↑ COX2↑; α-tocopherol↓ Preventable by TROLOX or rofecoxib | Astiz, 2009 |
| Dimethoate | rat LCs | 1ppm; 24h | T production↓ StAR↓ | LPO↑; COX2↑ Preventable by PUFA | Astiz, 2012 |
BPA: Bisphenol A CAT: Catalase COX2: Cyclooxygenase-2 CREB: cAMP Response Element Binding protein EP2: Prostaglandin E2 receptor 2 EP4: Prostaglandin E2 receptor 4 GPx: Glutathione Peroxidase GR: Glutathione Reductase GST: Glutathione-S-Transferase γ-GT: γ-Glutamyl Transpeptidase HIF-1a: Hypoxia Inducible Factor 1, alpha subunit LDH: Lactate Dehydrogenase LPO: Lipid peroxidation; MnTMPyP: Manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride PGE2: Prostaglandin E2 PUFA: Polyunsaturated Fatty Acids Ppm: Parts per million SOD: Superoxide Dismutase TBARS: Thiobarbituric Acid Reactive Substances TrxR: Thioredoxin Reductase
Phthalates are another group of environmental contaminants that may affect steroidogenesis by increasing oxidative stress (Martinez-Arguelles et al. 2013). One of the well-established consequences of phthalate exposure is activation of peroxisome proliferator-activated receptor (PPAR)-α and -γ nuclear receptors (Hurst & Waxman 2003). Activation of PPARs not only affected lipid metabolism, but also increased oxidative stress in the cells (O'Brien et al. 2005, Zhang et al. 2016). Exposure of rats to DEHP during prepuberty and puberty significantly decreased the GSH/GSSG ratio and increased TBARS levels in the testis, suggesting that phthalate is capable of increasing oxidative stress and affecting the redox environment in vivo (Erkekoglu et al. 2014). In MA-10 cells, MEHP treatment resulted in increased ROS levels, while depletion of GSH by BSO pretreatment greatly exacerbated the MEHP induced-ROS production and resulted in reduced progesterone production (Zhou et al. 2013). These results suggest that a likely mechanism by which MEHP acts is through increased oxidative stress (Zhao et al. 2012).
There are metals in the environment that also have been shown to affect Leydig cell steroidogenesis, with suggestions that they may do so by increasing oxidative stress. For example, mercury affects Leydig cell steroidogenesis and has effects on the antioxidant defense system (Boujbiha et al. 2009). Vitamin E was shown to protect against mercury-induced toxicity in mice (Rao and Sharma 2001), and sodium selenite and/or vitamin E to result in reduced lipid peroxidation, increased superoxide dismutase, catalase and glutathione peroxidase activities, and reduced histopathological lesions (Kalender et al. 2013). Lead and cadmium, either alone or in combination, were found to disrupt the testicular steroidogenesis and antioxidant defense mechanisms, and the administration antioxidant agents were found to reduce metal-induced oxidative stress and to provide protection against lead and cadmium toxicity (Liu et al. 2009, Ayinde et al. 2012, Pandya et al. 2012, Liu et al. 2013). Exposure of MA-10 Leydig cells to cobalt chloride resulted in an increase in the production of ROS and a decrease in progesterone production (Kumar et al. 2014). The adverse effects of arsenite on the male reproductive system also may be mediated by oxidative stress; the exposure of mice to arsenite reduced testicular GSH levels and increased protein carbonyl content, accompanied by decreases in testicular steroidogenic enzyme activities, and changes induced by arsenite were partially prevented by the antioxidant ascorbic acid (Chang et al. 2007).
In addition to modifying intracellular oxidative stress, some environmental compounds also have been shown to affect COX-2, arachidonate metabolism, and MAPK signaling molecules. For example, bisphenol A (BPA) induced a decrease in T production in rat Leydig R2C cells, associated with increased COX-2 and MAPK signaling (Kim et al. 2010). Dimethoate, a widely used organophosphate, was reported to reduce Leydig cell steroidogenic function in association with decreased arachidonate and increased COX-2 (Astiz et al. 2009, Astiz et al. 2012).
6. Summary and conclusions
Leydig cell T production decreases with aging and exposure to environmental contaminants. Although the exact mechanisms responsible for changes in steroidogenesis remain uncertain, there appear to be common causative features. Both aging and a number of EDC exposures have effects on LH-stimulated cAMP production and cholesterol transport to and metabolism within in mitochondria. Increased oxidative stress appears to be responsible for at least some of the changes in the steroidogenic pathway. COX-2 and MAPK also may be involved. In addition to intrinsic mechanisms, aging and environmental factors also may affect Leydig cell steroidogenesis through endocrine, autocrine and paracrine mechanisms. In light of increased oxidative stress that accompanies aging, it would be of particular interest to determine whether EDC exposures affect aged cells differently than young cells. As yet, this has received little attention.
Acknowledgments
This work was supported by NIH grant R37 AG21092 (B.R.Z), National Natural Science Foundation of China grants NSFC81471411 (H.C.) and NSFC81102150 (L.Y.), and Natural Science Foundation of Zhejiang Province grant Y17H040047 (H.C.)
Footnotes
The authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abidi P, Leers-Sucheta S, Azhar S. Suppression of steroidogenesis and activator protein-1 transcription factor activity in rat adrenals by vitamin E deficiency-induced chronic oxidative stress. J Nutr Biochem. 2004;15:210–219. doi: 10.1016/j.jnutbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Abidi P, Leers-Sucheta S, Cortez Y, Han J, Azhar S. Evidence that age-related changes in p38 MAP kinase contribute to the decreased steroid production by the adrenocortical cells from old rats. Aging Cell. 2008a;7:168–178. doi: 10.1111/j.1474-9726.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Abidi P, Zhang H, Zaidi SM, Shen WJ, Leers-Sucheta S, Cortez Y, Han J, Azhar S. Oxidative stress-induced inhibition of adrenal steroidogenesis requires participation of p38 mitogen-activated protein kinase signaling pathway. J Endocrinol. 2008b;198:193–207. doi: 10.1677/JOE-07-0570. [DOI] [PubMed] [Google Scholar]
- Aghazadeh Y, Zirkin BR, Papadopoulos V. Pharmacological regulation of the cholesterol transport machinery in steroidogenic cells of the testis. Vitam Horm. 2015;98:189–227. doi: 10.1016/bs.vh.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF., 3rd Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci USA. 1996;93:13731–13736. doi: 10.1073/pnas.93.24.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astiz M, Hurtado de Catalfo G, de Alaniz MJ, Marra CA. Exogenous arachidonate restores the dimethoate-induced inhibition of steroidogenesis in rat interstitial cells. Lipids. 2012;47:557–569. doi: 10.1007/s11745-012-3669-y. [DOI] [PubMed] [Google Scholar]
- Astiz M, Hurtado de Catalfo GE, de Alaniz MJ, Marra CA. Involvement of lipids in dimethoate-induced inhibition of testosterone biosynthesis in rat interstitial cells. Lipids. 2009;44:703–718. doi: 10.1007/s11745-009-3323-5. [DOI] [PubMed] [Google Scholar]
- Ayinde OC, Ogunnowo S, Ogedegbe RA. Influence of Vitamin C and Vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. Bmc Pharmacology & Toxicology. 2012;13 doi: 10.1186/2050-6511-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar S, Reaven E. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol Cell Endocrinol. 2002;195:1–26. doi: 10.1016/s0303-7207(02)00222-8. [DOI] [PubMed] [Google Scholar]
- Badowski M, Shultz CL, Eason Y, Ahmad N, Harris DT. The influence of intrinsic and extrinsic factors on immune system aging. Immunobiology. 2014;219:482–485. doi: 10.1016/j.imbio.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MC, Chen H, Fan J, Papadopoulos V, Miller P, Zirkin BR. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol Reprod. 2013;88:100. doi: 10.1095/biolreprod.112.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MC, Adekola L, Papadopoulos V, Chen H, Zirkin BR. Leydig cell aging and hypogonadism. Exp Gerontol. 2015;68:87–91. doi: 10.1016/j.exger.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab. 1997;82:1511–1515. doi: 10.1210/jcem.82.5.3962. [DOI] [PubMed] [Google Scholar]
- Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, El Feki A. Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol. 2009;28:81–89. doi: 10.1016/j.reprotox.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Cannon JG. Intrinsic and extrinsic factors in muscle aging. Ann N Y Acad Sci. 1998;854:72–77. doi: 10.1111/j.1749-6632.1998.tb09893.x. [DOI] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Castillo AF, Maciel FC, Castilla R, Duarte A, Maloberti P, Paz C, Podesta EJ. cAMP increases mitochondrial cholesterol transport through the induction of arachidonic acid release inside this organelle in Leydig cells. Febs Journal. 2006;273:5011–5021. doi: 10.1111/j.1742-4658.2006.05496.x. [DOI] [PubMed] [Google Scholar]
- Chang SI, Jin B, Youn P, Park C, Park JD, Ryu DY. Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicology and Applied Pharmacology. 2007;218:196–203. doi: 10.1016/j.taap.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36:1361–1373. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Guo J, Ge R, Lian Q, Papadopoulos V, Zirkin BR. Steroidogenic fate of the Leydig cells that repopulate the testes of young and aged Brown Norway rats after elimination of the preexisting Leydig cells. Exp Gerontol. 2015a;72:8–15. doi: 10.1016/j.exger.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15:551–557. [PubMed] [Google Scholar]
- Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143:1637–1642. doi: 10.1210/endo.143.5.8802. [DOI] [PubMed] [Google Scholar]
- Chen H, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Zirkin BR. Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology. 2004a;145:4441–4446. doi: 10.1210/en.2004-0639. [DOI] [PubMed] [Google Scholar]
- Chen H, Irizarry RA, Luo L, Zirkin BR. Leydig cell gene expression: effects of age and caloric restriction. Exp Gerontol. 2004b;39:31–43. doi: 10.1016/j.exger.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Chen H, Jin S, Guo J, Kombairaju P, Biswal S, Zirkin BR. Knockout of the transcription factor Nrf2: Effects on testosterone production by aging mouse Leydig cells. Mol Cell Endocrinol. 2015b;409:113–120. doi: 10.1016/j.mce.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Baig MU, Kim JM, Zirkin BR. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005;40:728–736. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Luo L, Liu J, Zirkin BR. Cyclooxygenases in rat Leydig cells: effects of luteinizing hormone and aging. Endocrinology. 2007;148:735–742. doi: 10.1210/en.2006-0925. [DOI] [PubMed] [Google Scholar]
- Chen H, Pechenino AS, Liu J, Beattie MC, Brown TR, Zirkin BR. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology. 2008;149:2612–2619. doi: 10.1210/en.2007-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhou L, Lin CY, Beattie MC, Liu J, Zirkin BR. Effect of glutathione redox state on Leydig cell susceptibility to acute oxidative stress. Mol Cell Endocrinol. 2010;323:147–154. doi: 10.1016/j.mce.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med. 2005;38:325–343. doi: 10.1016/j.freeradbiomed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Chung JY, Chen H, Midzak A, Burnett AL, Papadopoulos V, Zirkin BR. Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology. 2013;154:2156–2165. doi: 10.1210/en.2012-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ. ACTH action on StAR biology. Front Neurosci. 2016;10:547. doi: 10.3389/fnins.2016.00547. (1–7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- Culty M, Luo L, Yao ZX, Chen H, Papadopoulos V, Zirkin BR. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl. 2002;23:439–447. [PubMed] [Google Scholar]
- Debnath D, Mandal TK. Study of quinalphos (an environmental oestrogenic insecticide) formulation (Ekalux 25 E.C.)-induced damage of the testicular tissues and antioxidant defence systems in Sprague-Dawley albino rats. J Appl Toxicol. 2000;20:197–204. doi: 10.1002/(sici)1099-1263(200005/06)20:3<197::aid-jat634>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkekoglu P, Giray B, Rachidi W, Hininger-Favier I, Roussel AM, Favier A, Hincal F. Effects of di(2-ethylhexyl)phthalate on testicular oxidant/antioxidant status in selenium-deficient and selenium-supplemented rats. Environ Toxicol. 2014;29:98–107. doi: 10.1002/tox.20776. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P, Rachidi W, Yuzugullu OG, Giray B, Favier A, Ozturk M, Hincal F. Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol Appl Pharmacol. 2010;248:52–62. doi: 10.1016/j.taap.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P, Zeybek ND, Giray B, Asan E, Arnaud J, Hincal F. Reproductive toxicity of di(2-ethylhexyl) phthalate in selenium-supplemented and selenium-deficient rats. Drug Chem Toxicol. 2011;34:379–389. doi: 10.3109/01480545.2010.547499. [DOI] [PubMed] [Google Scholar]
- Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci U S A. 2015;112:7261–7266. doi: 10.1073/pnas.1502670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Traore K, Li W, Amri H, Huang H, Wu C, Chen H, Zirkin B, Papadopoulos V. Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig cells. Endocrinology. 2010;151:3348–3362. doi: 10.1210/en.2010-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Foster PM, Mylchreest E, Gaido KW, Sar M. Effects of phthalate esters on the developing reproductive tract of male rats. Hum Reprod Update. 2001;7:231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- Fukuzawa NH, Ohsako S, Wu Q, Sakaue M, Fujii-Kuriyama Y, Baba T, Tohyama C. Testicular cytochrome P450scc and LHR as possible targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the mouse. Mol Cell Endocrinol. 2004;221:87–96. doi: 10.1016/j.mce.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57:3–18. doi: 10.1016/s0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- Han X, Tang R, Chen X, Xu B, Qin Y, Wu W, Hu Y, Xu B, Song L, Xia Y, Wang X. 2,2',4,4'-Tetrabromodiphenyl ether (BDE-47) decreases progesterone synthesis through cAMP-PKA pathway and P450scc downregulation in mouse Leydig tumor cells. Toxicology. 2012;302:44–50. doi: 10.1016/j.tox.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38:171–196. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Dufau ML, Catt KJ. Gonadotropin-induced regulation of luteinizing hormone receptors and desensitization of testicular 3':5'-cyclic AMP and testosterone responses. Proc Natl Acad Sci U S A. 1977;74:592–595. doi: 10.1073/pnas.74.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi IT. Andropause--lessons from the European Male Ageing Study. Ann Endocrinol (Paris) 2014;75:128–131. doi: 10.1016/j.ando.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Johnson L, Dickerson R, Safe SH, Nyberg CL, Lewis RP, Welsh TH., Jr Reduced Leydig cell volume and function in adult rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin without a significant effect on spermatogenesis. Toxicology. 1992;76:103–118. doi: 10.1016/0300-483x(92)90158-b. [DOI] [PubMed] [Google Scholar]
- Kalender S, Uzun FG, Demir F, Uzunhisarcikli M, Aslanturk A. Mercuric chloride-induced testicular toxicity in rats and the protective role of sodium selenite and vitamin E. Food and Chemical Toxicology. 2013;55:456–462. doi: 10.1016/j.fct.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Kallen CB, Arakane F, Christenson LK, Watari H, Devoto L, Strauss JF., 3rd Unveiling the mechanism of action and regulation of the steroidogenic acute regulatory protein. Mol Cell Endocrinol. 1998;145:39–45. doi: 10.1016/s0303-7207(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology. 2006;147:2817–2828. doi: 10.1210/en.2005-1356. [DOI] [PubMed] [Google Scholar]
- Kim JY, Han EH, Kim HG, Oh KN, Kim SK, Lee KY, Jeong HG. Bisphenol A-induced aromatase activation is mediated by cyclooxygenase-2 up-regulation in rat testicular Leydig cells. Toxicol Lett. 2010;193:200–208. doi: 10.1016/j.toxlet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Kokilavani P, Suriyakalaa U, Elumalai P, Abirami B, Ramachandran R, Sankarganesh A, Achiraman S. Antioxidant mediated ameliorative steroidogenesis by Commelina benghalensis L. and Cissus quadrangularis L. against quinalphos induced male reproductive toxicity. Pestic Biochem Physiol. 2014;109:18–33. doi: 10.1016/j.pestbp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rani L, Dhole B. Role of oxygen in the regulation of Leydig tumor derived MA-10 cell steroid production: the effect of cobalt chloride. Syst Biol Reprod Med. 2014;60:112–118. doi: 10.3109/19396368.2013.861034. [DOI] [PubMed] [Google Scholar]
- Kumar V, Balomajumder C, Roy P. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: probable mechanism of action. Toxicology. 2008;250:124–131. doi: 10.1016/j.tox.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Lephart ED. Skin aging and oxidative stress: Equol's anti-aging effects via biochemical and molecular mechanisms. Ageing Res Rev. 2016;31:36–54. doi: 10.1016/j.arr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu ZQ, Zhang S, Cao R, Zhao J, Sun ZJ, Zou W. Augmented expression of gamma-glutamyl transferase 5 (GGT5) impairs testicular steroidogenesis by deregulating local oxidative stress. Cell Tissue Res. 2016;366:467–481. doi: 10.1007/s00441-016-2458-y. [DOI] [PubMed] [Google Scholar]
- Liao C, Reaven E, Azhar S. Age-related decline in the steroidogenic capacity of isolated rat Leydig cells: a defect in cholesterol mobilization and processing. J Steroid Biochem Mol Biol. 1993;46:39–47. doi: 10.1016/0960-0760(93)90207-d. [DOI] [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- Liu Q, Gu JH, Yuan Y, Liu XZ, Wang YJ, Wang HD, Liu ZP, Wang ZY, Bian JC. Effect of cadmium on rat Leydig cell testosterone production and DNA integrity in vitro. Biomed Environ Sci. 2013;26:769–773. doi: 10.3967/0895-3988.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat leydig cell antioxidant defense system. J Androl. 2006;27:240–247. doi: 10.2164/jandrol.05075. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Are Leydig cell steroidogenic enzymes differentially regulated with aging? J Androl. 1996;17:509–515. [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Leydig cell aging: steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J Androl. 2001;22:149–156. [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J Androl. 2005;26:25–31. [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Campioli E, Culty M, Zirkin BR, Papadopoulos V. Fetal origin of endocrine dysfunction in the adult: the phthalate model. J Steroid Biochem Mol Biol. 2013;137:5–17. doi: 10.1016/j.jsbmb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Mathur PP, D'Cruz SC. The effect of environmental contaminants on testicular function. Asian J Androl. 2011;13:585–591. doi: 10.1038/aja.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Akula N, Lecanu L, Papadopoulos V. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J Biol Chem. 2011;286:9875–9887. doi: 10.1074/jbc.M110.203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Zirkin B, Papadopoulos V. Translocator protein: pharmacology and steroidogenesis. Biochem Soc Trans. 2015;43:572–578. doi: 10.1042/BST20150061. [DOI] [PubMed] [Google Scholar]
- Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379:62–73. doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Veldhuis JD. Pulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging male. J Clin Endocrinol Metab. 2001;86:5547–5553. doi: 10.1210/jcem.86.11.8004. [DOI] [PubMed] [Google Scholar]
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)--induced oxidative damage in Leydig cells. Free Radic Res. 2005;39:1259–1272. doi: 10.1080/10715760500308154. [DOI] [PubMed] [Google Scholar]
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod Toxicol. 2008;25:447–454. doi: 10.1016/j.reprotox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- O'Brien ML, Spear BT, Glauert HP. Role of oxidative stress in peroxisome proliferator-mediated carcinogenesis. Crit Rev Toxicol. 2005;35:61–88. doi: 10.1080/10408440590905957. [DOI] [PubMed] [Google Scholar]
- Ojo OO, Bhadauria S, Rath SK. Dose-dependent adverse effects of salinomycin on male reproductive organs and fertility in mice. PLoS One. 2013;8:e69086. doi: 10.1371/journal.pone.0069086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya C, Pillai P, Nampoothiri LP, Bhatt N, Gupta S, Gupta S. Effect of lead and cadmium co-exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia. 2012;44:813–822. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Aghazadeh Y, Fan J, Campioli E, Zirkin B, Midzak A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol Cell Endocrinol. 2015;408:90–98. doi: 10.1016/j.mce.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol Cell Endocrinol. 2007;265–266:59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- Pogrmic K, Fa S, Dakic V, Kaisarevic S, Kovacevic R. Atrazine oral exposure of peripubertal male rats downregulates steroidogenesis gene expression in Leydig cells. Toxicol Sci. 2009;111:189–197. doi: 10.1093/toxsci/kfp135. [DOI] [PubMed] [Google Scholar]
- Rao MV, Sharma PSN. Protective effect of vitamin E against mercuric chloride reproductive toxicity in male mice. Reprod Toxicol. 2001;15:705–712. doi: 10.1016/s0890-6238(01)00183-6. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco AM, Valdes K, Marcus D, Llanos M. The mechanism for lindane-induced inhibition of steroidogenesis in cultured rat Leydig cells. Toxicology. 2001;159:99–106. doi: 10.1016/s0300-483x(00)00414-5. [DOI] [PubMed] [Google Scholar]
- Sai L, Li X, Liu Y, Guo Q, Xie L, Yu G, Bo C, Zhang Z, Li L. Effects of chlorpyrifos on reproductive toxicology of male rats. Environ Toxicol. 2014;29:1083–1088. doi: 10.1002/tox.21838. [DOI] [PubMed] [Google Scholar]
- Saradha B, Vaithinathan S, Mathur PP. Single exposure to low dose of lindane causes transient decrease in testicular steroidogenesis in adult male Wistar rats. Toxicology. 2008;244:190–197. doi: 10.1016/j.tox.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Sasaki G, Ishii T, Jeyasuria P, Jo Y, Bahat A, Orly J, Hasegawa T, Parker KL. Complex role of the mitochondrial targeting signal in the function of steroidogenic acute regulatory protein revealed by bacterial artificial chromosome transgenesis in vivo. Mol Endocrinol. 2008;22:951–964. doi: 10.1210/me.2007-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WJ, Patel S, Natu V, Hong R, Wang J, Azhar S, Kraemer FB. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: facilitation of cholesterol transfer in adrenal. J Biol Chem. 2003;278:43870–43876. doi: 10.1074/jbc.M303934200. [DOI] [PubMed] [Google Scholar]
- Shi Z, Feng Y, Wang J, Zhang H, Ding L, Dai J. Perfluorododecanoic acid-induced steroidogenic inhibition is associated with steroidogenic acute regulatory protein and reactive oxygen species in cAMP-stimulated Leydig cells. Toxicol Sci. 2010;114:285–294. doi: 10.1093/toxsci/kfq014. [DOI] [PubMed] [Google Scholar]
- Sokanovic SJ, Janjic MM, Stojkov NJ, Baburski AZ, Bjelic MM, Andric SA, Kostic TS. Age related changes of cAMP and MAPK signaling in Leydig cells of Wistar rats. Exp Gerontol. 2014;58:19–29. doi: 10.1016/j.exger.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Zhao AH, Tu LN, Morohaku K, Selvaraj V. A brief history of the search for the protein(s) involved in the acute regulation of steroidogenesis. Mol Cell Endocrinol. 2017;441:7–16. doi: 10.1016/j.mce.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:625434. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P, Ascoli M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol Endocrinol. 2011;25:885–893. doi: 10.1210/me.2010-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem. 2014;289:27444–27454. doi: 10.1074/jbc.M114.578286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Veldhuis NJ, Keenan DM, Iranmanesh A. Age diminishes the testicular steroidogenic response to repeated intravenous pulses of recombinant human LH during acute GnRH-receptor blockade in healthy men. Am J Physiol Endocrinol Metab. 2005;288:E775–781. doi: 10.1152/ajpendo.00410.2004. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Liu PY, Keenan DM, Takahashi PY. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302:E117–122. doi: 10.1152/ajpendo.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal S, Martinez-Arguelles DB, Chebbi S, Hullin-Matsuda F, Kobayashi T, Papadopoulos V. Plasma Membrane Origin of the Steroidogenic Pool of Cholesterol Used in Hormone-induced Acute Steroid Formation in Leydig Cells. J Biol Chem. 2016;291:26109–26125. doi: 10.1074/jbc.M116.740928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP. Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology. 1993;133:2773–2781. doi: 10.1210/endo.133.6.8243304. [DOI] [PubMed] [Google Scholar]
- Wang X, Shen CL, Dyson MT, Eimerl S, Orly J, Hutson JC, Stocco DM. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology. 2005;146:4202–4208. doi: 10.1210/en.2005-0298. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Shen WJ, Bittner S, Bittner A, McLean MP, Han JH, Davis RJ, Kraemer FB, Azhar S. p38 MAPK regulates steroidogenesis through transcriptional repression of STAR gene. Journal of Molecular Endocrinology. 2014;53:1–16. doi: 10.1530/JME-13-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Ito Y, Yamanoshita O, Yanagiba Y, Kobayashi M, Taya K, Li C, Okamura A, Miyata M, Ueyama J, Lee CH, Kamijima M, Nakajima T. Permethrin may disrupt testosterone biosynthesis via mitochondrial membrane damage of Leydig cells in adult male mouse. Endocrinology. 2007;148:3941–3949. doi: 10.1210/en.2006-1497. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shen XY, Zhang WW, Chen H, Xu WP, Wei W. The effects of Di 2-Ethyl Hexyl Phthalate (DEHP) on cellular lipid accumulation in HepG2 cells and its potential mechanisms in the molecular level. Toxicol Mech Methods. 2016:1–24. doi: 10.1080/15376516.2016.1273427. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ao H, Chen L, Sottas CM, Ge RS, Li L, Zhang Y. Mono-(2-ethylhexyl) phthalate affects the steroidogenesis in rat Leydig cells through provoking ROS perturbation. Toxicol In Vitro. 2012;26:950–955. doi: 10.1016/j.tiv.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beattie MC, Lin CY, Liu J, Traore K, Papadopoulos V, Zirkin BR, Chen H. Oxidative stress and phthalate-induced down-regulation of steroidogenesis in MA-10 Leydig cells. Reprod Toxicol. 2013;42:95–101. doi: 10.1016/j.reprotox.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin BR, Santulli R, Strandberg JD, Wright WW, Ewing LL. Testicular steroidogenesis in the aging brown Norway rat. J Androl. 1993;14:118–123. [PubMed] [Google Scholar]
- Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing hormone dose-response relationships for luteinizing hormone, follicle-stimulating hormone and alpha-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol. 1996;135:399–406. doi: 10.1530/eje.0.1350399. [DOI] [PubMed] [Google Scholar]