Abstract
Alopecia is common among captive populations of nonhuman primates. There are many potential causes of alopecia, including physiological conditions such as hormonal imbalance and infection, features of the captive environment such as housing type, ground substrate, and group density, as well as behavioral abnormalities such as self-plucking. A potential behavioral cause of alopecia in group-housed primates is social hair pulling, where one animal pulls hair from a conspecific. While social hair pulling has been conflated with overgrooming in some of the alopecia literature, other authors have categorized it as a form of aggression rather than a form of excessive grooming. In this study we examined social hair pulling, grooming, and aggression within 7 groups of rhesus macaques (Macaca mulatta) (N=319). We took weekly 30-minute behavioral observations on each group for one year to assess the patterns of hair pulling and grooming, which monkeys were receiving and initiating these behaviors, as well as aggression and other behaviors indicating dominance. We also assessed the amount of alopecia on each individual monthly. While grooming tended to be directed “up” the hierarchy (i.e., monkeys were more likely to groom animals of a higher rank than lower rank), most hair pulling was directed “down” the hierarchy. Further, hair pulling seldom co-occurred with aggressive behaviors, suggesting that it was not a form of aggression. Hair pulling also usually resulted in ingestion of the pulled hair. Hair pulling was correlated with alopecia; monkeys who were frequent recipients of hair pulling scored higher on monthly alopecia ratings than those who were less often observed having hair pulled. Our results suggest that social hair pulling is a behavior distinct from either grooming or aggressive behavior, and that it may contribute to alopecia in socially housed macaques.
Keywords: Hair pulling, alopecia, overgrooming, trichophagia
INTRODUCTION
Alopecia, or hair loss, is a common condition among captive macaque (Macaca spp.) colonies, and is exhibited across a range of environments, from individual indoor housing to outdoor social housing [Kroeker, Bellanca, Lee, Thom & Worlein, 2014; Novak & Meyer, 2009; Steinmetz et al., 2006]. A recent survey of indoor singly-housed rhesus macaques (M. mulatta) at four primate facilities in the United States found that 34.3% to 86.5% of animals showed some degree of alopecia [Lutz, Coleman, Worlein & Novak, 2013]. Similar amounts of alopecia have been found in group-housed macaques [e.g., Beisner & Isbell, 2009; Steinmetz et al., 2006]. In a study of 183 rhesus macaques group-housed in either indoor, outdoor, or indoor-outdoor enclosures at the German Primate Center in Goettingen, Germany, 69% of animals showed some amount of alopecia [Steinmetz et al., 2006]. Further, in a year-long survey of outdoor, group-housed animals at the Oregon National Primate Research Center (ONPRC), 85% of animals evaluated showed some degree of alopecia, with 49.4% showing alopecia on more than 10% of their body [unpublished data]. Whether or not alopecia is indicative of poor welfare is unclear, however, its prevalence in captivity is of concern to regulatory agencies and to managers of nonhuman primate colonies.
Nonhuman primate (NHP) alopecia has been associated with, and attributed to, multiple factors [Novak & Meyer, 2009], including dermatological conditions such as inflammation [Bernstein & Didier, 2009; Kramer et al., 2010] or infection [Newcomer, Fox, Taylor & Smith, 1984], hypothalamic-pituitary-adrenal (HPA) axis activity [Novak et al., 2014; Steinmetz et al., 2006], and dietary deficiencies [protein: Coward & Whitehead, 1972; zinc: Swenerton & Hurley, 1980]. To date, the vast majority of studies on alopecia in macaques have focused on demographic (e.g., age, sex) or environmental (e.g., housing) factors. Several studies found that females generally had higher rates of alopecia than males [Beisner & Isbell, 2009; Lutz & Sharp, 2015; but see Luchins et al., 2011], and that alopecia increased with age [Beisner & Isbell, 2009; Huneke, Foltz, VandeWoude, Mandrell & Garman, 1996; Steinmetz et al., 2006; but see Lutz & Sharp, 2015]. Alopecia has been found to vary with a variety of management practices. In one study, alopecia was positively correlated with the number of housing relocations and sedations an individual experienced [Lutz et al., 2016]. Indoor and single housing have also been identified as risk factors for the development of alopecia; singly-housed macaques or those group-housed indoors tended to have more alopecia than those group-housed outdoors [Kroeker et al., 2014; Steinmetz et al., 2006]. The amount of time an individual spent singly-housed was also positively correlated with alopecia [Lutz et al., 2016]. Further, indoor-housed animals with a history of continual indoor-housing were found to be more alopecic than those that had lived part of their lives outside [Kramer et al., 2010]. Environmental factors can also influence alopecia in outdoor housed animals. In group-housed settings, alopecia has been positively correlated with group density [Beisner & Isbell, 2009; Steinmetz et al., 2006] and negatively associated with environmental complexity [Beisner & Isbell, 2008], and has been associated with cyclic changes, including pregnancy and seasonal weather patterns [Beisner & Isbell, 2009; Davis & Suomi, 2006; Dettmer et al., 2015; Lutz & Sharp, 2015; Steinmetz et al., 2006; Vessey & Morrison, 1970].
In addition to environmental or demographic variables, alopecia has been associated with behavioral factors, specifically hair pulling and overgrooming. Hair pulling, also known as hair plucking or barbering, occurs when an animal pulls out hair from itself or others. Often, hair pulling is exhibited as the meticulous removal of individual hairs with the fingers or teeth [Kramer, Mansfield, Simmons & Bernstein, 2011; Lutz et al., 2013], but it may involve pulling out larger clumps of hair at a time. Hair pulling is frequently associated with overgrooming (i.e., grooming oneself or another to excess) [Honess, Gimpel, Wolfensohn & Mason, 2005; Lutz et al., 2013; Novak et al., 2014], and some authors have used the terms interchangeably [e.g., Beisner & Isbell, 2008; Kroeker et al., 2014; Reinhardt, 2005]; however, there are important distinctions between the two behaviors. Whereas overgrooming is presumed to lead to hair loss through excessive manipulation of hair follicles [Steinmetz, Kaumanns, Dix, Neimeier & Kaup, 2005], hair pulling is the deliberate removal of hair [Kramer et al., 2011; Luchins et al., 2011; Lutz et al., 2013]. Macaques and other species often ingest hair that they pull from themselves or others, something not regularly seen with grooming [Luchins et al., 2011; Lutz et al., 2013; Reinhardt, Reinhardt & Houser, 1986; Sarna, Dyck & Whishaw, 2000]. Grooming, and presumably overgrooming, typically follows a subordinate-to-dominant direction, especially when between non-kin (i.e., animals are more likely to groom those who are dominant, as opposed to subordinate, to themselves [Sade, 1972; Seyfarth, 1977]), while hair pulling in rhesus macaques is thought to follow a pattern in which dominant animals tend to pull hair from subordinate animals, and often in an aggressive manner [Reinhardt et al., 1986]. Further, while overgrooming is considered to be a normal and adaptive behavior that has been exhibited to a maladaptive degree, hair pulling is typically viewed as an abnormal behavior, particularly when it is self-directed [Lutz et al., 2013; Novak, Kelly, Bayne & Meyer, 2012].
While hair pulling and overgrooming are often cited as potential causes for alopecia [e.g., Beisner & Isbell, 2008; Beisner & Isbell, 2009; Kramer et al., 2010; Lutz et al., 2013; Runeson, Lee, Crockett & Bellanca, 2011; Steinmetz et al., 2006], there are surprisingly few studies that explicitly examine their roles in hair loss for socially-housed macaques [Beisner & Isbell, 2008; Kramer et al., 2010; Kroeker, Lee, Bellanca, Thom & Worlein, 2017; Luchins et al., 2011; Lutz et al., 2013]. Further, although grooming patterns in group housed macaques are well established, we know of only one study that specifically investigates social hair pulling [Reinhardt et al., 1986]. In the present study, we examined hair pulling behavior in socially-housed rhesus macaques. We began by assessing factors associated with the frequency of initiating and receiving this behavior, including age, sex, and two dominance rank variables (rank and average dominance probability). These factors were chosen because they underlie social roles in hierarchical, sexually dimorphic primates such as rhesus macaques [Bernstein & Sharpe, 1966]. As detailed above, alopecia is more prevalent in females than males [Beisner & Isbell, 2009; Lutz & Sharp, 2015], and increases with age [Beisner & Isbell, 2009; Huneke et al., 1996; Steinmetz et al., 2006]. Thus, if hair pulling is a significant contributor to alopecia, adult females should be the most frequent recipients of the behavior. Additionally, since adult females primarily interact with other adult females [Bernstein & Sharpe, 1966], we expected that they would also be the most frequent initiators of hair pulling. Further, we predicted that dominance would influence hair pulling interactions such that dominant individuals would pull hair more often than subordinate animals, as was reported in Reinhardt et al. [1986]. In addition to rank, we also examined average dominance probability (ADP), a social network measure that quantifies the certainty of individual’s dominance position [Fushing, McAssey & McCowan, 2011]. ADP has been linked to stress and health outcomes [Vandeleest et al., 2016], and may be complementary to absolute rank as a measure of the social stress experienced by animals in a hierarchical social system. In a review of hair pulling in captive mammals, Reinhardt [2005] concluded that hair pulling is a result of chronic stress in the captive environment. If, as Vandeleest et al. [2016] suggest, animals with low ADP experience more social stress than animals with high ADP due to relatively insecure rank positions, then animals with low ADP should initiate hair pulling more than animals with high ADP (i.e., secure rank positions).
Because hair pulling has been associated with grooming in the alopecia literature, but also classified as aggressive [Reinhardt et al., 1986], we next examined the relationship between hair pulling and these other behaviors. Based on Reinhardt et al.’s [1986] accounts of hair pulling patterns, we expected to find important differences between social hair pulling and grooming interactions, specifically that whereas grooming is more often directed “up” the hierarchy (from subordinate to dominant), hair pulling would more often be directed “down” the hierarchy (from dominant to subordinate). In contrast to Reinhardt et al.’s [1986] description of social hair pulling as an aggressive behavior, when observing aggression in our colony, we have not commonly seen social hair pulling occur in that context [A. Heagerty, personal observation]. Therefore we did not expect our data to show an association between hair pulling and aggressive interactions. Finally, we quantified the impact that hair pulling had on alopecia in our colony.
METHODS
Subjects and housing
Subjects were 319 rhesus macaques (Macaca mulatta) living in 7 social groups at the Oregon National Primate Research Center (ONPRC). Animal ages varied, and included: 125 adult females (aged 5–20 years); 7 adult males (aged 11–16 years); 58 subadult females (aged 3–4 years); 27 subadult males (aged 3–5 years); and 102 infants/juveniles (aged 6 months to 2 years). The mean age of adult females was 8.8 +/− SD 3.10 years, and the mean age of adult males was 12.9 +/− SD 1.05 years. Groups ranged in size from 42 to 68 animals each, and had been formed between 2.5 and 9.0 years prior to the start of the study (mean=6.6 +/− SD 2.27 years). Groups were comprised of one adult male and small matrilineal units, the majority of which had only two generations (i.e., a female and her offspring). Female offspring were kept in their natal groups, whereas male offspring were usually removed before 5 years of age. Data were collected from January through December 2015. During the course of the study, 69 infants were born and 91 animals were removed from study groups either for clinical reasons or because of social incompatibility. Subjects were individually identifiable by tattooed ID numbers and facial features, and juveniles were marked with black Nyanzol-D dye to aid in identification when needed.
Groups were housed in environmentally controlled outdoor “sheltered housing” units. Sheltered enclosures were approximately 130m2, on a cement floor with a translucent plastic roof. The external sides of the enclosures were low cement walls with wire mesh extending to a mesh ceiling. Enclosures were subdivided into three equally sized rooms with solid interior walls. Adjacent rooms were connected by two approximately 0.5 × 0.6 meter openings through which the animals could pass freely. During cold weather, supplemental heat was provided via overhead heaters and heated floors, and misters provided cooling during warmer months. Enclosures were equipped with climbing structures, perches, swings, and chew toys, which were rotated. Fiber Balance Monkey Jumbo, Lab Diet-R, old world primate chow (Purina 5000 diet, Animal Specialties, Woodburn, OR), was fed twice daily ad libitum and water was continuously accessible from lixit fixtures. Foraging enrichment, such as fresh produce or a grain mixture, was distributed once daily. The ONPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC, International) and this study was approved by the ONPRC Institutional Animal Care and Use Committee. Additionally, this research adhered to the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
Behavioral observations
A single observer (AH) conducted 30-minute behavioral observations of each group once per week using all occurrences sampling [Altmann, 1974]. Groups were observed for a minimum of 43 sessions and a maximum of 47 sessions. The identities of the initiator and recipient were recorded in each of the following types of interactions: hair pulling, grooming, aggression, and subordination (see Table 1 for ethogram).
Table 1.
Ethogram of social interactions recorded during weekly 30 minute behavioral observations.
| Behavior | Description |
|---|---|
| Groom | Inspecting and manipulating the hair of self or another individual with fingers or mouth. |
| Hair pull | Forcible removal of either individual hairs or clumps of hair from self or another animal. Whether or not the initiator put the hair in their mouth was also recorded. |
| Aggression | Facial or vocal threat, chase, lunge, or contact aggression such as slap or bite. |
| Subordination | Signals of submission such as move/run away, avoid, grimace, or rump present unprovoked by dominant animal. |
Hair pulling was differentiated from grooming by the swift jerking movements used to remove the hair(s) from the follicles. Hair pulling was typically done with one hand, and less frequently with the teeth. Most often, the initiator pulled out clumps of hair with each motion, but some animals pulled out single hairs at a time. In contrast, groomers usually used both hands to inspect the hair or skin with more meticulous attention. If hairs or other material were pulled from the coat during grooming, the motion was more smooth and gentle than during hair pulling bouts. Animals were typically clearly engaged in only one activity at a time, and were never observed to alternate between hair pulling and grooming within the same bout of activity.
Alopecia assessments
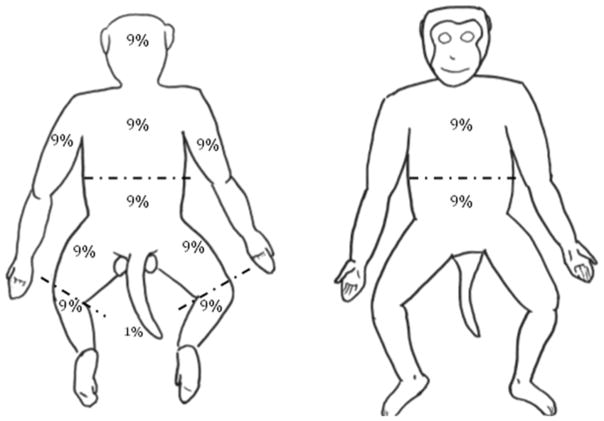
One of two raters (AH and RW) assessed alopecia for each animal once per month. These observers estimated the amount of the subject’s body surface affected by alopecia, using a method similar to the NPRC Behavioral Management Consortium’s scoring system outlined in Baker et al., 2017 and Luchins et al., 2011. Both scoring systems are based on the Wallace “Rule of Nine’s” [e.g., Hettiaratchy & Papini, 2004], a tool developed by the medical community to estimate the percent of body surface affected by burns. The body was broken into 12 segments, each of which is 9% of the body surface area, except for the tail, which is estimated at 1% (Figure 1). The total percent of body affected by alopecia was then categorized on a scale from 0 (0%) to 5 (≥ 75%) (Table 2) [Ellison, Hobbs, Maier & Coleman, 2006]. In four of the twelve study months (April, July, October, December), the raters co-scored alopecia for each individual while the monkeys were sedated for physical exams. In the other eight months of the study, alopecia was scored cage-side while animals were engaged in their normal activities. Cage-side scoring typically took several weeks to complete as not all individuals were clearly visible at any one time and an alopecia score was not recorded until the rater had satisfactorily viewed all body parts of the monkey. Additionally, AH and RW alternated which groups they scored each month to reduce observer bias. If any subjects were particularly difficult to score cage-side, both raters would score the individual and reach a consensus. AH and RW were >85% reliable using percent agreement to assess inter-rater reliability.
Figure 1.
Body segments with percent surface area in Rule of Nine’s alopecia scoring method.
Table 2.
Percent of body affected by alopecia and corresponding alopecia score.
| Percentage | Alopecia score |
|---|---|
| 0 | 0 |
| 1–9 | 1 |
| 10–24 | 2 |
| 25–49 | 3 |
| 50–74 | 4 |
| 75–100 | 5 |
Dominance measures
Using all recorded interactions in which there was a clear “winner” (either the initiator of uncontested aggression against a recipient, or the receiver of unsolicited submission signal(s)) and “loser” (the recipient of uncontested aggression or the sender of unsolicited submission signal(s)), we constructed dominance hierarchies by the simulated annealing method in the Perc package in R version 3.2.1 [Fujii et al., 2016; R Development Core Team, 2011]. This is a social network analysis method that estimates the most likely linear dominance order based on the direct and indirect (i.e., transitive), dominance relationships that can be deduced from the data set [Fushing et al., 2011]. Only the 239 animals who lived in the study groups for the entirety of 2015 were included in these analyses: 90 adult females (aged 5–20 years); 7 adult males (aged 11–16 years); 44 subadult females (aged 3–4 years); 19 subadult males (aged 3–5 years); and 79 yearlings/juveniles (aged 11 months to 2 years). For the purposes of this study, both males and females over 1 year of age were included in a single dominance hierarchy for each social group. Seventeen percent of hair pulling dyads had ambiguous dominance relationships based on the data (i.e., dominance probability was at or close to 0.5). In these dyads, the initiator and/or recipient was a juvenile (<4 years old), in which case the direction of dominance determined by the simulated annealing method was checked against known matrilineal rank relationships (e.g., if two juveniles had a low dominance probability, we confirmed rank using the relative ranks of their mothers). In order to standardize rank values across groups of different sizes, dominance ranks were converted to rank percentiles, reflecting the percentage of animals in the hierarchy with a rank lower than that of the individual. Thus, the most dominant animal in each group had a rank percentile of 1, and the least dominant animal in each group had a rank percentile of 0.
In addition to evaluating dominance rank we used the Perc package to calculate the average dominance probability (ADP) for each animal. Dominance probability is a relatively new social network measure that uses direct and indirect relationships in a dominance network to estimate the level of certainty (ranging from 0.5 to 1) of the dominance relationship between each pair of animals in the group [Fujii et al., 2016; Fushing et al., 2011]. A pair with a predictable dominance relationship (i.e., one individual always “wins” dominance interaction and the other always “loses”) will have a dominance probability close to 1, whereas a pair with an unpredictable relationship (i.e., one individual sometimes “wins” but sometimes “loses” dominance interactions with the other individual) will have a dominance probability closer to 0.5. We calculated the ADP for individuals by taking the average of their dominance probabilities with every other animal in the group. Thus, animals whose dominance interactions are highly consistent will have an ADP close to 1, and animals whose relationships are, in general, less certain will have an ADP closer to 0.5. This metric is separate from hierarchy position in that a high ranking animal can have a low ADP, a low ranking animal can have a high ADP; however ADP is often correlated with dominance rank, such that high ranking animals tend to have predictable dominance interactions [Vandeleest et al., 2016].
Statistical analyses
Individual predictors of hair pulling
All statistical analyses were done using R version 3.2.1 [R Development Core Team 2011]. To determine which demographic and rank variables were associated with the frequency of either initiating or receiving hair pulling, we used generalized linear mixed model (GLMM) regression with a Poisson distribution, regressing the number of 30-minute observation sessions in which individuals initiated hair pulling against the following fixed effects: age, sex, rank percentile, and ADP. We included social group as a random effect. Only the 239 animals who lived in the study groups for the entirety of 2015 were included in these analyses (demographic breakdown included in previous section). To select the regression model that best predicted hair pulling initiated, we compared Akaike’s Information Criteria (AIC) scores from univariate, additive, and interaction models. Lower AIC scores indicate the best fit of the data with the fewest independent variables. An AIC score difference of at least 2 points is considered indicative of a meaningful difference between models [Akaike, 1987]. In the event that the difference in AIC scores between models was less than 2 points, we chose the model with the fewest number of independent variables. We repeated this statistical method with the number of observations in which individuals received hair pulling as the outcome variable.
Hair pulling and other social behaviors
To examine whether hair pulling patterns are similar to grooming patterns, we compared the pathways between the two behaviors in terms of initiator and recipient dominance. Using a Pearson’s chi-squared test, we compared the frequencies of all recorded hair pulling interactions that were from dominant-to-subordinate or from subordinate-to-dominant in each group to the frequencies of dominant-to-subordinate and subordinate-to-dominant grooming interactions. In this and all statistical tests we used a significance threshold of alpha < 0.05.
To determine whether hair pulling was associated with aggression we examined the social context in which hair pulling occurred. For the 2 minutes before and 2 minutes after each record of hair pulling, we looked at whether the initiator and recipient of hair pulling were also engaged in aggression with one another during that time frame.
Hair pulling and alopecia
To quantify the contribution of hair pulling to alopecia we used linear mixed model regression with a Gaussian distribution to regress monthly alopecia scores against the number of weekly observations in which an individual was observed as the recipient of hair pulling. Social group was included as a random effect, as were month and females’ pregnancy status, as these have been found to affect alopecia [Beisner & Isbell, 2009; Dettmer et al., 2015; Novak & Meyer, 2009; Steinmetz et al., 2006].
RESULTS
Summary statistics
All summary statistics are presented as mean +/− standard deviation. We collected 198.6 hours of observational data (28.4 +/− 3.7 hours per group), during which 800 hair pulling interactions were recorded. Hair pulling was observed in all seven groups, but the rate of hair pulling varied by group, from 1.5 to 7.3 interactions per hour. Additionally, the number of animals within a group that were observed initiating the behavior ranged from 4 to 21 individuals. In total, 32% (N=104) of animals over 1 year old were observed hair pulling (14 +/− 5.8 individuals per group), and 53% (N=169) were observed having their hair pulled (24 +/− 9.8 individuals per group). Twenty-seven percent (N=86) of subjects were both initiators and recipients of hair pulling, whereas only 16% (N=52) of subjects over 1 year old were never observed in a hair pulling interaction. In 94.6% (N=757) of hair pulling interactions, the initiator was observed ingesting the hair. Most (56%) hair pulling interactions were directed at the recipient’s upper back, with the lower back being the next most frequent body part targeted (18%), followed by the arms (9%), legs (7%), ventrum (5%), head (4%), and tail (1%). The distribution of body segments targeted by hair pullers was significantly different than would be expected based on the surface area represented by each body segment under the Rule of Nine’s method (X2(11)=2506.2, P<0.001).
Among the 239 animals for whom dominance variables were calculated, ADP ranged from 0.510 to 0.999, with a mean of 0.728 +/− 0.128, and was linearly correlated with rank percentile at Pearson’s r=0.65 (P<0.001).
Individual predictors of hair pulling
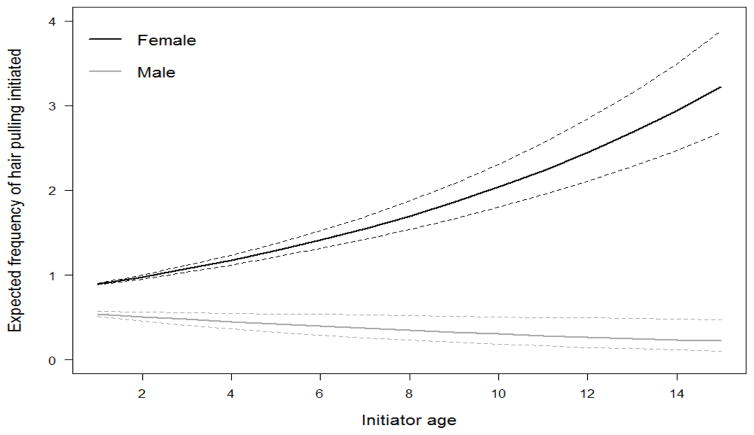
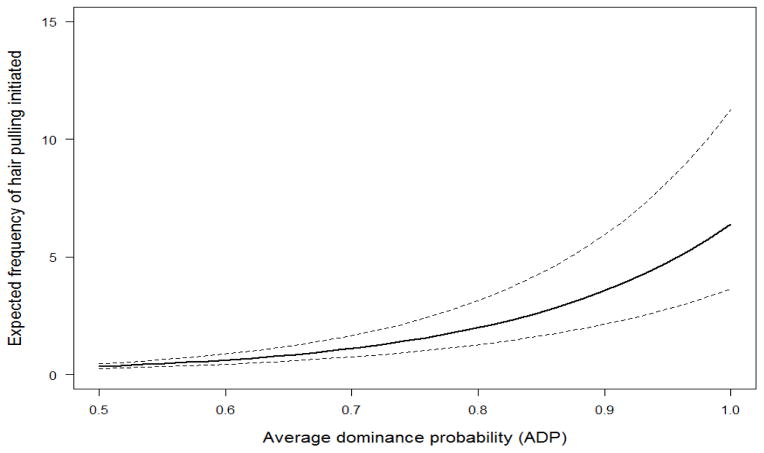
The model that best explained which animals most frequently initiated hair pulling included the fixed effects of sex, age, a sex X age interaction, and ADP (Table 3). This model scored 1.7 AIC points lower than the second best model, which additionally included a non-significant main effect of rank percentile. All other models scored at least 97 AIC points higher than the top two models. The best model indicated that, as predicted, adult females pulled hair more often than adult males. In addition, the frequency of hair pulling increased with age for females, but decreased slightly for males. Juvenile and subadult males occasionally engaged in hair pulling, but adult males rarely did so (Figure 2). Although dominance rank was not predictive of hair pulling as we had initially expected, ADP had a sizeable effect. Contrary to our predictions, animals with a high ADP were more likely to pull hair than those with a lower ADP (Figure 3). In other words, animals whose dominance interactions were more predictable, regardless of whether they were high or low ranking, were more likely to initiate hair pulling than those whose dominance interactions were less certain.
Table 3.
Fixed-effect estimates of regression of hair pulling initiated. Estimates and standard errors are on a log scale.
| Estimate | Std. Error | P value | |
|---|---|---|---|
| Intercept | −4.44 | 0.471 | < 0.001 |
| Male | −0.34 | 0.269 | 0.20 |
| Age | 0.09 | 0.012 | < 0.001 |
| Age X Male | −0.16 | 0.038 | < 0.001 |
| Average dominance probability (ADP) | 5.82 | 0.567 | < 0.001 |
Figure 2.
Estimated amount of hair pulling initiated based on sex and age with 95% confidence intervals. Hair pulling by females increased with age whereas hair pulling by males decreased with age.
Figure 3.
Estimated amount of hair pulling initiated based on ADP with 95% confidence intervals. Initiation of hair pulling increased with higher dominance probability.
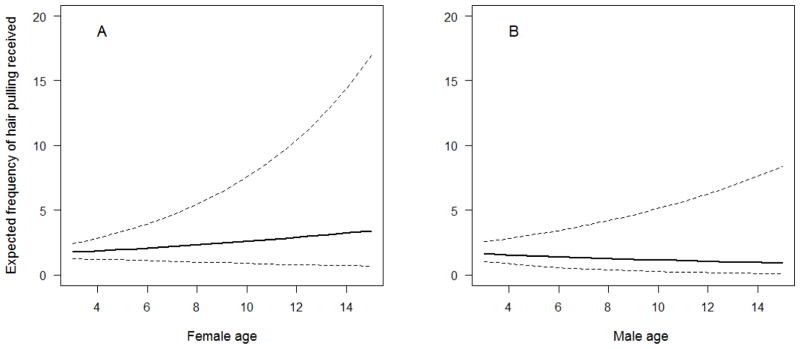
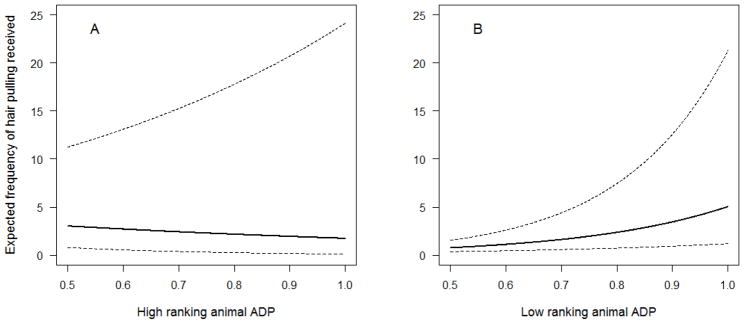
The model that best explained the amount of hair pulling individuals received included the fixed effect variables of sex, age, a sex X age interaction, ADP, rank percentile, and an ADP X rank percentile interaction (Table 4). This model scored 5.3 AIC points lower than the next best model. Among juveniles, there was little difference between the amount of hair pulling that males and females received. However, the amount of hair pulling received increased with age for females, but decreased with age for males. Thus, as adults, females were more likely than males to be recipients of hair pulling (Figure 4), which matches our expectation based on demographic patterns of alopecia in the literature. Rank percentile and ADP showed a more complicated pattern than age and sex. As shown in Figure 5, animals who were either high in both rank percentile and ADP or low in both rank percentile and ADP were the recipients of hair pulling less frequently than animals that were high in one measure but low in the other. However, there were large standard errors for the model estimates for rank percentile, ADP, and the rank percentile X ADP interaction, indicating a large amount of variability in hair pulling received among individuals with similar ranks or ADP.
Table 4.
Fixed-effect estimates of regression of hair pulling received. Estimates and standard errors are on a log scale.
| Estimate | Std. Error | P value | |
|---|---|---|---|
| Intercept | −3.50 | 0.819 | < 0.001 |
| Male | 0.24 | 0.188 | 0.20 |
| Age | 0.06 | 0.013 | < 0.001 |
| Age X Male | −0.10 | 0.040 | < 0.01 |
| Rank Percentile | 5.13 | 1.248 | < 0.001 |
| Average dominance probability (ADP) | 5.12 | 1.125 | < 0.001 |
| Rank percentile X ADP | −6.55 | 1.594 | < 0.001 |
Figure 4.
Estimated amount of hair pulling received based on age with 95% confidence intervals for (A) females, and (B) males. Frequency of hair pulling received increased with age for females but decreased with age for males.
Figure 5.
Estimated amount of hair pulling received based on ADP for (A) high ranking animals (rank percentile=0.95) and (B) low ranking animals (rank percentile=0.25), with 95% confidence intervals. Animals high in one measure (rank or ADP), but low in the other, were more often recipients of hair pulling than animals that were high or low in both measures.
Hair pulling and other social behaviors
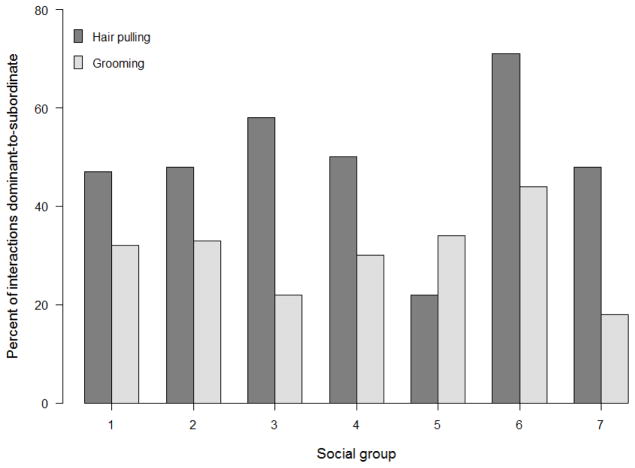
Grooming was much more frequent than social hair pulling. We recorded an average of 422 +/− 165 grooming interactions per group (N=5,854 total), compared to 114 +/− 49.8 hair pulling interactions per group (N=800 total). In six of the seven groups, the majority (63.5% +/− 15.5% per group) of the initiators of hair pulling interactions were dominant to their recipient. However, one group showed a different pattern; in that group, only 31% of hair pulling interactions were initiated by a more dominant individual (Figure 6). In contrast to hair pulling interactions, fewer than half of recorded grooming interactions were initiated by an individual dominant to the recipient (40.7% +/− 7.3% interactions per group). As expected, the distribution of dominant-to-subordinate and subordinate-to-dominant hair pulling interactions among the 7 groups was significantly different from the distribution of grooming interactions (X2(6)=53.04, P<0.001).
Figure 6.
Percent of hair pulling (dark bar) and grooming interactions (light bar) that were initiated by a dominant monkey (relative to the recipient) for each group. Over half of hair pulling interactions were initiated by dominant individuals in all but one group (group 5), whereas dominant animals initiated grooming interactions less than 50% of the time on average.
We did not find an association between hair pulling and aggression in our study. Of the 800 hair pulling bouts, aggression between the initiator and recipient in the 4 minutes surrounding the interaction was only recorded a total 8 times (1%). In 5 of these interactions the hair pulling initiator was the aggressor, and in the other 3 the recipient was the aggressor. The very low occurrence of aggression just prior to, or soon after, hair pulling indicates the behavior was most often exhibited in a nonaggressive context. We did not record detailed responses to hair pulling in this study because of the general tolerance to the behavior by recipients. Fearful or submissive responses to hair pulling, as would be seen with aggression, were very infrequent [A. Heagerty, personal observation].
Hair pulling and alopecia
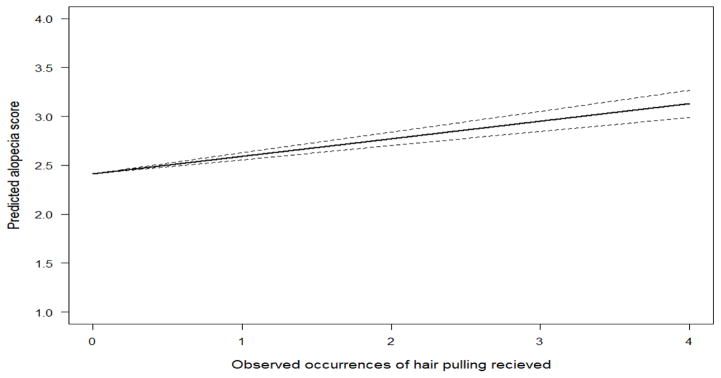
We compared a null regression model of monthly alopecia scores against a model that included the number of weekly observations per month in which individuals were the recipient of hair pulling. The amount of hair pulling received had a small but significant effect on average alopecia scores, and inclusion of the variable improved the model’s AIC score by 20 points. The average alopecia score was 2.2 (P<0.01), which was estimated to increase by B=0.16 (P<0.01) for each observation session in which hair pulling was received (Figure 7).
Figure 7.
Estimated monthly impact of receiving hair pulling on alopecia score with 95% confidence intervals. With each observation of hair pulling received, an individual’s monthly alopecia score increased by an average of 0.16 on a scale of 0 to 5.
DISCUSSION
This study is one of the first to specifically examine factors influencing social hair pulling in group-housed rhesus macaques, and its relationship to grooming, aggression, and alopecia. We found that social hair pulling was prevalent in all 7 study groups, although there was a high degree of individual variation in how frequently animals pulled hair from conspecifics. Adult females pulled hair more often than adult males, and older females pulled hair more than young females. However, subadult and adult females were also more numerous in our study groups than subadult and adult males. There were only seven adult males, and only three were observed pulling hair (on a maximum of 3 occasions); it is possible that these males are not representative of rhesus macaque males in general.
Unlike previous studies on hair pulling [Reinhardt et al., 1986], absolute dominance rank did not predict how frequently an individual pulled hair from a conspecific. However, we did find an association between average dominance probability (ADP) and hair pulling. If hair pulling is response to chronic stress, as suggested by Reinhardt [2005], we would have expected monkeys with low ADP to show more of this behavior [Vandeleest et al., 2016] than those with high ADP. Instead, we found that animals with higher ADP (i.e., their dominance interactions with others were predictable) initiated more hair pulling than animals with lower ADP. Rather than associating hair pulling behavior with social stress, our results indicate that individuals with secure dominance positions may be more motivated or more able to pull hair from conspecifics, but more work is needed to understand the cause of these results.
Individuals also showed differences with respect to how often they received hair pulling. Similar to initiators of hair pulling, the number of times animals had their hair pulled was explained by sex, age, and ADP. However, rank percentile also played a role in the amount of hair pulling an individual received. Adult females were more often the recipients of hair pulling than males, and this difference increased with age. As with the initiation of hair pulling, it is possible that this finding is due to the small number of adult males in our study, most of whom rarely received hair pulling.
It is reasonable to expect that only low ranking animals would tolerate having hair forcibly removed from their coat, but we did not find this to be the case in our study. Although a majority of hair pulling was directed at an individual with a lower rank than the initiator, rank alone did not explain which animals received the most hair pulling; even top-ranking animals frequently tolerated hair pulling from others. Our results showed that animals who were either high ranking with a low ADP, or low ranking with a high ADP, were more frequently the recipients of hair pulling than animals who were high or low on both measures. However, as mentioned above, there was a large amount of variation in the amount of hair pulling received. It is possible that factors other than social relationships contribute to differences in receipt of hair pulling. Recipients of hair pulling sometimes reacted by flinching, grimacing, or retreating, but many showed no adverse reaction at all [A. Heagerty, personal observation]. Thus, physiological variables, such as differences in skin sensitivity, may explain variation in tolerance of hair pulling.
The terms hair pulling and overgrooming have been used synonymously in several studies of NHP alopecia [Beisner & Isbell, 2008; Kroeker et al., 2014; Reinhardt, 2005], but we did not find evidence that social hair pulling is a form of grooming. As others have noted, unlike grooming behavior, social hair pulling involved the forceful motion required to remove hairs from the follicles [Kramer et al., 2011; Luchins et al., 2011]. We also found that hair pulling differs from grooming behavior in the dominance relationships between initiators and recipients. Although being high in absolute rank did not correlate with hair pulling in our study, hair pullers did generally target animals subordinate to themselves. Fewer than half of grooming interactions were from a dominant initiator to a subordinate recipient, whereas over half of hair pulling interactions were in the dominant to subordinate direction, with the exception of one social group. The outlier group in this case also had the fewest number of hair pulling individuals (8% of the group), and differed from other groups in that its hierarchy underwent a brief period of social instability at the start of the study (January 2015), and a longer period of instability beginning in December 2015, which resulted in the group being disbanded January 2016. This group also had the largest amount of variability in ADP values among individuals compared to the other 6 groups. Unrest in the hierarchy and a relatively small number of hair pullers may explain the irregular pattern of dominance in their hair pulling interactions compared to the other study groups.
Social hair pulling has also been identified as an aggressive behavior [Reinhardt et al., 1986], but our data do not support this classification. Animals involved in hair pulling interactions were unlikely to engage in aggression with each other just prior to or following the hair pulling bout; in fact, the majority of hair pulling occurred when animals were engaging in affiliative behaviors or resting, and recipients frequently permitted the behavior to continue [A. Heagerty, personal observation]. While we did not see submissive or fearful reactions, future studies should examine recipients’ responses to hair pulling in more detail.
While social hair pulling has not been extensively studied in captive primates, it has been documented in several other mammalian species, particularly laboratory rodents [Reinhardt 2005; Sarna et al., 2000]. Hair pulling in primates bears several similarities to what is most commonly called barbering in laboratory rodents; both in the characteristics of the behavior and in how it has been categorized in the literature. Like the hair puller, the barber forcibly removes, and often consumes, fur or whiskers [Sarna et al., 2000]. There are usually multiple barbers in a social group, and recipients typically tolerate the behavior even when there are means of escape [Garner, Dufour, Gregg, Weisker & Mench, 2004]. In the literature, barbering, like hair pulling, was originally conflated with grooming [Militzer & Wecker, 1986], and for many years was thought to be a dominance behavior, and even a positive sign that a hierarchy had been established [Strozik & Festing, 1981]. Sarna and colleagues’ detailed description of mice removing whiskers and fur from cagemates, including how the behavior differed visually from grooming [Sarna et al., 2000], was followed by studies which challenged the assumption that barbering is a normal dominance behavior, and revealed housing and social factors correlated with increased barbering [e.g., Garner et al., 2004]. Similarly, clearly distinguishing social hair pulling in nonhuman primates from other behaviors, such as grooming and aggression, can lead to more focused research and a better understanding of the origin and impacts of this behavior.
The most consistent trait of hair pulling interactions in our study was consumption of the hair (trichophagia) by the initiator. Husbandry staff reported seeing hair in feces, and occasionally animals were seen manipulating bezoar-like clumps of hair in cheek pouches [A. Heagerty, personal observation], indicating that animals were in fact swallowing hair. It is possible that hair consumption is the actual goal of hair pulling, but the motivation for animals to swallow hair is unknown. Several of the proposed causes of alopecia, such as stress or nutrient deficiency, could also apply to trichophagia. For example, in humans, extreme cases of trichophagia and other forms of pica have been linked to iron-deficiency anemia, and show comorbidity with psychiatric symptoms such as anxiety and depression [Flessner et al., 2008; Kettaneh et al., 2005]. As most of the reports about NHP trichophagia has focused on captive animals [Butler & Haines Jr, 1987; Gillin et al., 1990; Gozalo, Montoya & Nolan, 1989; Mejido et al., 2009; Mook, 2002; Nevill & Lutz, 2015; Nolan, Schaffer & Conti, 1987], it is not clear whether wild primates regularly engage in this behavior. However, there are several accounts of consumption of other non-food items, such as soils or clays [Krishnamani & Mahaney, 2000; Mahaney, Hancock & Inoue, 1993; Mahaney et al., 1995; Pebsworth, Bardi & Huffman, 2012; Pebsworth et al., 2013; Wakibara et al., 2001]. More work is needed to understand whether human trichophagia and/or soil consumption by wild primates can shed light on trichophagia in rhesus macaques in captivity.
Regardless of what underlies the motivation for hair pulling behavior, our data show that it can contribute to observable alopecia. Although frequency of receiving hair pulling did not show a large contribution to alopecia in our study, animals that received a great deal of hair pulling showed more alopecia on average than those that were rarely or never observed having their hair pulled. A more detailed recording of hair pulling, including factors such as the duration of hair pulling bouts or estimates of the amount of hair removed may reveal a larger impact on alopecia. Additionally, a correlation between areas of the body typically targeted by hair pullers, which in our colony were the upper and lower back, and areas exhibiting alopecia, would provide further evidence of the contribution of hair pulling to alopecia.
Alopecia is a complex and ubiquitous issue among facilities housing rhesus macaques [Beisner & Isbell, 2008; Kroeker et al., 2014; Lutz et al., 2013; Novak & Meyer, 2009]. Although it is often considered a behavioral problem, few studies have specifically examined behaviors that underlie the condition. We found that social hair pulling, a behavior in which an animal intentionally pulls one or more hairs from a conspecific’s coat, can contribute to alopecia in group-housed rhesus macaques. Unlike grooming or overgrooming, the animal initiating the behavior is often dominant to the recipient. Further, hair pulling often results in ingestion of the hair. While hair pulling is relatively prevalent, at least in some populations, it is not widely reported. Understanding that social hair pulling is distinct from grooming and aggression is necessary to generate and test hypotheses on the motivations for this behavior, understand its contribution to alopecia, and most importantly find interventions and remediations.
Acknowledgments
We thank Drs. Kirk Andrews, Anne Lewis, David Erikson, Andrew Haertel, Amanda Vinson, Michael Raboin, Rebecca DuCore, and Andrew Daws for their input and useful discussions, as well as two anonymous reviewers for constructive feedback. We are also indebted to Adriane Maier, Nicola Robertson, Jaclyn Shelton, Greg Johnson, Cara Stull, Eddie Richter and the ONPRC animal care staff for assistance with and facilitation of data collection. This project was supported by NIH grants R24 OD01180-15 and P51 OD011092.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- Altmann J. Observational study of behavior sampling methods. Behaviour. 1974;49(3–4):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Baker K, Bloomsmith M, Coleman K, Crockett C, Worlein J, McCowan B, Pierre P, Weed J. The Behavioral Management Consortium: A partnership for promoting consensus and best practices. In: Schapiro S, editor. The Handbook of Primate Behavioral Management. Boca Raton, FL: CRC Press; 2017. pp. 9–23. [Google Scholar]
- Beisner BA, Isbell LA. Ground substrate affects activity budgets and hair loss in outdoor captive groups of rhesus macaques (Macaca mulatta) American Journal of Primatology. 2008;70(12):1160–1168. doi: 10.1002/ajp.20615. [DOI] [PubMed] [Google Scholar]
- Beisner BA, Isbell LA. Factors influencing hair loss among female captive rhesus macaques (Macaca mulatta) Applied Animal Behaviour Science. 2009;119(1–2):91–100. [Google Scholar]
- Bernstein IS, Sharpe LG. Social roles in a rhesus monkey group. Behaviour. 1966;26(1/2):91–104. doi: 10.1163/156853966x00038. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Didier PJ. Nonhuman primate dermatology: A literature review. Veterinary Dermatology. 2009;20(3):145–156. doi: 10.1111/j.1365-3164.2009.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Haines R., Jr Gastric trichobezoar in a baboon. Laboratory Animal Science. 1987;37(2):232–233. [PubMed] [Google Scholar]
- Coward DG, Whitehead R. Experimental protein-energy malnutrition in baby baboons. British Journal of Nutrition. 1972;28(02):223–237. doi: 10.1079/bjn19720029. [DOI] [PubMed] [Google Scholar]
- Davis E, Suomi S. Hair loss and replacement cycles in socially housed, pregnant, rhesus macaques. American Journal of Primatology. 2006;68:58. doi: 10.1002/ajp.20370. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Rosenberg K, Menard MT, El-Mallah SN, Woodward RA, Suomi SJ, Meyer JS. Differential relationships between chronic hormone profiles in pregnancy and maternal investment in rhesus monkey mothers with hair loss in the neonatal period. American Journal of Physical Anthroplogy. 2017;79:e22489. doi: 10.1002/ajp.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R, Hobbs T, Maier A, Coleman K. An assessment of temperament and behavior in rhesus macaques with alopecia. American Journal of Primatology. 2006;68(S1):107. [Google Scholar]
- Flessner CA, Woods DW, Franklin ME, Keuthen NJ, Piacentini J TLC-SAB. Styles of pulling in youths with trichotillomania: Exploring differences in symptom severity, phenomenology, and comorbid psychiatric symptoms. Behaviour Research and Therapy. 2008;46(9):1055–1061. doi: 10.1016/j.brat.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Fujii K, Jian J, Shev A, Beisner B, McCowan B, Fushing H. R package version 0.1.2 ed. 2016. Perc: Using percolation and conductance to find information flow certainty in a direct network. [Google Scholar]
- Fushing H, McAssey MP, McCowan B. Computing a ranking network with confidence bounds from a graph-based Beta random field. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Science. 2011;467(2136):3590–3612. doi: 10.1098/rspa.2011.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Dufour B, Gregg LE, Weisker SM, Mench JA. Social and husbandry factors affecting the prevalence and severity of barbering (‘whisker trimming’) by laboratory mice. Applied Animal Behaviour Science. 2004;89(3):263–282. [Google Scholar]
- Gillin A, Phippard A, Thompson J, Harewood W, Waugh R, Horvath J. Gastric haemorrhage and perforation caused by a trichobezoar in a baboon (Papio hamadryas) Laboratory Animals. 1990;24(2):180–182. doi: 10.1258/002367790780890176. [DOI] [PubMed] [Google Scholar]
- Gozalo A, Montoya E, Nolan T. Trichobezoars in two saddleback tamarins (Saguinus fuscicollis) Journal of Medical Primatology. 1989;19(2):151–153. [PubMed] [Google Scholar]
- Hettiaratchy S, Papini R. Initial management of a major burn: II-Assessment and resuscitation. BMJ: British Medical Journal. 2004;329(7457):101. doi: 10.1136/bmj.329.7457.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess P, Gimpel J, Wolfensohn S, Mason G. Alopecia scoring: The quantitative assessment of hair loss in captive macaques. Alternatives to Laboratory Animals: ATLA. 2005;33(3):193–206. doi: 10.1177/026119290503300308. [DOI] [PubMed] [Google Scholar]
- Huneke R, Foltz C, VandeWoude S, Mandrell T, Garman R. Characterization of dermatologic changes in geriatric rhesus macaques. Journal of Medical Primatology. 1996;25(6):404–413. doi: 10.1111/j.1600-0684.1996.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Kettaneh A, Eclache V, Fain O, Sontag C, Uzan M, Carbillon L, Stirnemann J, Thomas M. Pica and food craving in patients with iron-deficiency anemia: A case-control study in France. The American Journal of Medicine. 2005;118(2):185–188. doi: 10.1016/j.amjmed.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Kramer J, Fahey M, Santos R, Carville A, Wachtman L, Mansfield K. Alopecia in rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology. Journal of Medical Primatology. 2010;39(2):112–122. doi: 10.1111/j.1600-0684.2010.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JA, Mansfield KG, Simmons JH, Bernstein JA. Psychogenic alopecia in rhesus macaques presenting as focally extensive alopecia of the distal limb. Comparative Medicine. 2011;61(3):263–268. [PMC free article] [PubMed] [Google Scholar]
- Krishnamani R, Mahaney W. Geophagy among primates: Adaptive significance and ecological consequences. Animal Behaviour. 2000;59(5):899–915. doi: 10.1006/anbe.1999.1376. [DOI] [PubMed] [Google Scholar]
- Kroeker R, Bellanca R, Lee G, Thom J, Worlein J. Alopecia in three macaque species housed in a laboratory environment. American Journal of Primatology. 2014;76(4):325–334. doi: 10.1002/ajp.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker R, Lee GH, Bellanca RU, Thom JP, Worlein JM. Prior facility affects alopecia in adulthood for rhesus macaques. American Journal of Primatology. 2017;79(1):1–9. doi: 10.1002/ajp.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchins KR, Baker KC, Gilbert MH, Blanchard JL, Liu DX, Myers L, Bohm RP. Application of the diagnostic evaluation for alopecia in traditional veterinary species to laboratory rhesus macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2011;50(6):926–938. [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Coleman K, Worlein J, Novak M. Hair loss and hair-pulling in rhesus macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2013;52(4):454–457. [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60(1):1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Coleman K, Worlein JM, Kroeker R, Menard MT, Rosenberg K, Meyer JS, Novak MA. Factors influencing alopecia and hair cortisol in rhesus macaques (Macaca mulatta) Journal of Medical Primatology. 2016;45(4):180–188. doi: 10.1111/jmp.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CK, Sharp RM. Alopecia in outdoor group-and corral-housed baboons (Papio hamadryas spp.) Journal of the American Association for Laboratory Animal Science. 2015;54(4):384–388. [PMC free article] [PubMed] [Google Scholar]
- Mahaney W, Hancock R, Inoue M. Geochemistry and clay mineralogy of soils eaten by Japanese macaques. Primates. 1993;34(1):85–91. [Google Scholar]
- Mahaney W, Stambolic A, Knezevich M, Hancock R, Aufreiter S, Sanmugadas K, Kessler M, Grynpas M. Geophagy amongst rhesus macaques on Cayo Santiago, Puerto Rico. Primates. 1995;36(3):323–333. [Google Scholar]
- Mejido DC, Dick EJ, Jr, Williams PC, Sharp RM, Andrade MC, DiCarlo C, Hubbard GB. Trichobezoars in baboons. Journal of Medical Primatology. 2009;38(5):302–309. doi: 10.1111/j.0047-2565.2009.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militzer K, Wecker E. Behaviour-associated alopecia areata in mice. Laboratory Animals. 1986;20(1):9–13. doi: 10.1258/002367786781062061. [DOI] [PubMed] [Google Scholar]
- Mook DM. Gastric trichobezoars in a rhesus macaque (Macaca mulatta) Comparative Medicine. 2002;52(6):560–562. [PubMed] [Google Scholar]
- Nevill CH, Lutz CK. The effect of a feeding schedule change and the provision of forage material on hair eating in a group of captive baboons (Papio hamadryas sp.) Journal of Applied Animal Welfare Science. 2015;18(4):319–331. doi: 10.1080/10888705.2014.980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer C, Fox J, Taylor R, Smith D. Seborrheic dermatitis in a rhesus monkey (Macaca mulatta) Laboratory Animal Science. 1984;34(2):185–187. [PubMed] [Google Scholar]
- Nolan T, Schaffer L, Conti P. A gastric trichobezoar in a chimpanzee. Journal of Medical Primatology. 1987;17(1):63–65. [PubMed] [Google Scholar]
- Novak M, Meyer J. Alopecia: Possible causes and treatments, particularly in captive nonhuman primates. Comparative Medicine. 2009;59(1):18–26. [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Hamel AF, Coleman K, Lutz CK, Worlein J, Menard M, Ryan A, Rosenberg K, Meyer JS. Hair loss and hypothalamic–pituitary–adrenocortical axis activity in captive rhesus macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2014;53(3):261–266. [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Kelly BJ, Bayne K, Meyer JS. Behavioral Disorders of Nonhuman Primates. In: Mansfield K, Tardif S, Morris T, editors. Nonhuman Primates in Biomedical Research. 2. Boston: Academic Press; 2012. pp. 177–196. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austira: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Pebsworth P, Bardi M, Huffman M. Geophagy in chacma baboons: Patterns of soil consumption by age class, sex, and reproductive state. American Journal of Primatology. 2012;74(1):48–57. doi: 10.1002/ajp.21008. [DOI] [PubMed] [Google Scholar]
- Pebsworth PA, Seim GL, Huffman MA, Glahn RP, Tako E, Young SL. Soil consumed by chacma baboons is low in bioavailable iron and high in clay. Journal of Chemical Ecology. 2013;39(3):447–449. doi: 10.1007/s10886-013-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt V. Hair pulling: A review. Laboratory Animals. 2005;39(4):361–369. doi: 10.1258/002367705774286448. [DOI] [PubMed] [Google Scholar]
- Reinhardt V, Reinhardt A, Houser D. Hair pulling and eating in captive rhesus monkey troops. Folia Primatologica. 1986;47(2–3):158–164. doi: 10.1159/000156272. [DOI] [PubMed] [Google Scholar]
- Runeson EP, Lee GH, Crockett CM, Bellanca RU. Evaluating paint rollers as an intervention for alopecia in monkeys in the laboratory (Macaca nemestrina) Journal of Applied Animal Welfare Science. 2011;14(2):138–149. doi: 10.1080/10888705.2011.551626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade DS. Sociometrics of Macaca mulatta I. Linkages and cliques in grooming matrices. Folia Primatologica. 1972;18(3–4):196–223. doi: 10.1159/000155480. [DOI] [PubMed] [Google Scholar]
- Sarna JR, Dyck RH, Whishaw IQ. The Dalila effect: C57BL6 mice barber whiskers by plucking. Behavioural Brain Research. 2000;108(1):39–45. doi: 10.1016/s0166-4328(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. A model of social grooming among adult female monkeys. Journal of Theoretical Biology. 1977;65(4):671–698. doi: 10.1016/0022-5193(77)90015-7. [DOI] [PubMed] [Google Scholar]
- Steinmetz HW, Kaumanns W, Dix I, Heistermann M, Fox M, Kaup FJ. Coat condition, housing condition and measurement of faecal cortisol metabolites–a non-invasive study about alopecia in captive rhesus macaques (Macaca mulatta) Journal of Medical Primatology. 2006;35(1):3–11. doi: 10.1111/j.1600-0684.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Steinmetz HW, Kaumanns W, Dix I, Neimeier K-A, Kaup F-J. Dermatologic investigation of alopecia in rhesus macaques (Macaca mulatta) Journal of Zoo and Wildlife Medicine. 2005;36(2):229–238. doi: 10.1638/04-054.1. [DOI] [PubMed] [Google Scholar]
- Strozik E, Festing M. Whisker trimming in mice. Laboratory Animals. 1981;15(4):309–312. doi: 10.1258/002367781780953040. [DOI] [PubMed] [Google Scholar]
- Swenerton H, Hurley LS. Zinc deficiency in rhesus and bonnet monkeys, including effects on reproduction. The Journal of Nutrition. 1980;110(3):575–583. doi: 10.1093/jn/110.3.575. [DOI] [PubMed] [Google Scholar]
- Vandeleest JJ, Beisner BA, Hannibal DL, Nathman AC, Capitanio JP, Hsieh F, Atwill ER, McCowan B. Decoupling social status and status certainty effects on health in macaques: A network approach. PeerJ. 2016;4:e2394. doi: 10.7717/peerj.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey S, Morrison J. Molt in free-ranging rhesus monkeys, Macaca mulatta. Journal of Mammalogy. 1970;51(1):89–93. [Google Scholar]
- Wakibara J, Huffman M, Wink M, Reich S, Aufreiter S, Hancock R, Sodhi R, Mahaney W, Russel S. The adaptive significance of geophagy for Japanese macaques (Macaca fuscata) at Arashiyama, Japan. International Journal of Primatology. 2001;22(3):495–520. [Google Scholar]