Abstract
Different subclasses of γ-aminobutyric acid (GABA) cortical neurons can be distinguished by their content of neuropeptides such as somatostatin (SST), or calcium-binding proteins such as calretinin (CR). SST, but not CR, neurons have been reported to be altered in the prefrontal cortex (PFC) of subjects with schizophrenia. Understanding the functional significance of the SST neuron disturbances in schizophrenia requires knowledge of the specialized synaptic circuitry of these neurons relative to that of CR neurons. Consequently, we used immuno-electron microscopy to examine the synaptic type and postsynaptic targets of SST-immunoreactive (IR) axon terminals in monkey PFC and compared these findings with similar data for CR-IR axon terminals. SST-IR axon terminals formed exclusively symmetric synapses and contacted only dendritic shafts (86%) and dendritic spines (14%), whereas CR-IR terminals also formed synapses with cell bodies. The postsynaptic targets of SST-IR axon terminals also differed across layers with synapses onto dendritic spines more frequent in the superficial (20%) than in the deep (8%) layers. Dual-label immunoelectron microscopy revealed that CR-IR axon terminals targeted GABA-IR dendritic shafts with a greater frequency (60%) than did SST-IR axon terminals (21.5%). Conversely, SST-IR axon terminals contacted unlabeled dendritic shafts, presumably belonging to pyramidal neurons, more frequently than did CR-IR axon terminals (57% vs. 19%, respectively). This specialized synaptic circuitry of SST neurons in the primate PFC suggests that the alterations of these neurons in schizophrenia is likely to have distinct functional consequences.
Keywords: interneuron, pyramidal neurons, electron microscopy, schizophrenia
INTRODUCTION
Local circuit neurons in the cerebral cortex that utilize the neurotransmitter γ-aminobutyric acid (GABA) can be divided into different subpopulations based on their content of neuropeptides, such as somatostatin (SST), or calcium-binding proteins, such as calretinin (CR) and parvalbumin (PV). Subpopulations of GABA neurons can also be distinguished by the preferred synaptic targets of their axon terminals (e.g., pyramidal cells vs. other GABA neurons; the perisomatic region vs. distal dendrites). For example, the axons of PV-containing basket and chandelier cells primarily synapse on the perisomatic region of pyramidal neurons (Kawaguchi and Kubota, 1997), the axons of SST-containing Martinotti cells target the distal apical dendrites of pyramidal neurons (Gonchar et al., 2002), and the axons of CR-containing neurons are more likely to synapse with other GABA neurons (Gonchar and Burkhalter, 1999).
These differences in the synaptic targets of subpopulations of GABA neurons have been primarily based on observations in the sensory cortices or hippocampus of rodents, with relatively few electron microscopic studies conducted in the highly expanded and differentiated primate prefrontal cortex (PFC). Understanding the synaptology of GABA neurons in this brain region of macaque monkeys is important for several reasons. First, the proportions of chemically identified populations of GABA neurons differ across species. For example, in monkey PFC the relative density of CR-containing neurons is twice that of PV- or SST/calbindin-containing neurons (Condé et al., 1994; Gabbott and Bacon, 1996), whereas in rodent PFC the PV-containing GABA neurons are the most numerous group (Gabbott et al., 1997). Second, the proportions of different subpopulations of GABA neurons differ by cortical region and layer. For example, in monkeys the largest proportion of GABA neurons in the primary visual cortex contains PV (Glezer et al., 1998), whereas in the PFC, CR-containing neurons are the predominant subgroup (Condé et al., 1994; Gabbott and Bacon, 1996). Furthermore, these subpopulations of GABA neurons have different laminar patterns of distribution. For example, in monkey PFC, layers 2, superficial 3 and 5 contain the greatest density of SST-containing neurons (Lewis et al., 1986), layer 2 has the highest density of CR-containing cells (Condé et al., 1994; Gabbott and Bacon, 1996), and layers deep 3 and 4 contain the majority of PV-containing neurons(Condé et al., 1994; Gabbott and Bacon, 1996). Finally, markers of GABA neurotransmission appear to be altered in specific subsets of GABA neurons in the PFC of subjects with schizophrenia. For example, the expression of PV and SST mRNA, but not of CR mRNA, was reported to be altered in the PFC of subjects with schizophrenia (Hashimoto et al., 2003, 2007; Morris et al., 2008). Given the critical role of prefrontal GABA neurotransmission in working memory processes (Rao et al., 2000; Sawaguchi et al., 1989; Wilson et al., 1994), these alterations in specific subpopulations of GABA neurons may contribute to well-documented deficits in working memory functioning exhibited by subjects with schizophrenia (Weinberger et al., 1986).
The synaptic targets of PV- and CR-positive neurons in monkey PFC have been evaluated in previous studies (Melchitzky et al., 1999, 2005; Williams et al., 1992), but those of SST-containing neurons have not. Consequently, in this study we used immunocytochemistry and electron microscopy to identify the postsynaptic targets of SST-immunoreactive (IR) axon terminals in the superficial and deep layers of monkey PFC. To inform the potential consequences of the marked alterations in SST-containing, but not of the CR-containing neurons, in the PFC of subjects with schizophrenia, we also compared the postsynaptic targets of SST-IR axon terminals to those of CR-IR axon terminals.
METHODS
Immuno electron microscopy
Tissue preparation
Five young adult, male cynomolgus monkeys (Macaca fascicularis) were used in this study. All animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and all procedures were approved by the University of Pittsburgh’s Animal Care and Use Committee. All monkeys were deeply anesthetized with ketamine hydrochloride (25 mg/kg) and pentobarbital sodium (30 mg/kg), intubated, and mechanically ventilated with oxygen delivered at a flow rate of 2 l/min. The chest was opened, and 1.5 ml of 1% aqueous sodium nitrite was injected into the left ventricle of the heart. The descending aorta was clamped, and the animal was perfused transcardially with 1% paraformaldehyde and 0.05% glutaraldehyde in 0.1M phosphate buffer (PB), pH 7.4, for 5–30 s, followed by 4% paraformaldehyde and 0.2% glutaraldehyde in the same buffer for 9 min at a flow rate of 350 ml/min. Both of these solutions were maintained at 29°C. The brain was then removed, and coronal blocks (5–6 mm thick) containing the PFC were immersed in cold 4% paraformaldehyde for 2 h. Tissue blocks were then washed in 0.1M PB, pH 7.4, and sectioned coronally on a Vibratome at 50 µm.
Immunocytochemistry
Sections from PFC area 9 from monkeys CM292 and CM293 were processed for SST single label immunocytochemistry. Sections were initially treated with 1% sodium borohydride in 0.1M PB for 30 min followed by several washes in 0.1M PB. Sections were then incubated in a blocking solution containing 0.2% bovine serum albumin (BSA), 0.04% Triton X-100, 3% normal donkey serum and 3% normal human serum in phosphate-buffered saline (PBS), pH 7.4, for 30 min. Next, sections were incubated overnight in blocking solution containing a rabbit polyclonal anti-somatostatin-28 (S309 antibody; diluted 1:3000) and a rabbit polyclonal anti-SST-281–12 antibody (S320 antibody; diluted 1:4000). These antibodies were kindly provided by Dr. Robert Benoit (McGill University, Montreal, Canada). The following day sections were incubated in blocking solution containing a donkey anti-rabbit biotinylated IgG (1:200, Vector Laboratories, Burlingame, CA) followed by incubation in PBS containing Vectastain ABC reagents (Vector Laboratories). The bound peroxidase was visualized by treating sections with 0.05% 3,3′-diaminobenzidine (DAB) and 0.003% H2O2 in 0.03M PB.
Sections from PFC area 9 of monkeys CM292, CM293, and CM300 were processed for dual-label immunocytochemistry for SST and GABA. Sections were incubated overnight in a blocking solution containing both the S309 (1:1500) and S320 (1:2000) antibodies as well as a mouse anti-GABA antibody (1:1000; Sigma-Aldrich, St. Louis, MO). The SST antibodies were visualized with peroxidase-DAB as described earlier. Tissue sections were then rinsed in 0.01M PBS, followed by an overnight incubation at room temperature in 0.01 M PBS containing 0.8% BSA, 0.1% fish gelatin (provided with the gold-conjugated secondary), and a goat anti-mouse IgG conjugated to 1 nm gold particles (1:50, Amersham, Arlington Heights, IL). The next day, after rinsing in 0.01M PBS, tissue sections were incubated in a solution of 2% glutaraldehyde in 0.01M PBS for 10 min, rinsed in 0.01M PBS, and then incubated in a silver enhancement solution (Amersham) for 5–10 min.
Sections from PFC area 9 of monkeys CM237, CM240, and CM300 were processed by dual-label immunocytochemistry for CR and GABA as described earlier except that they were incubated overnight in blocking solution containing a mouse monoclonal CR antibody (1:2000, Swant, Bellinzona, Switzerland) and a rabbit polyclonal GABA antibody (1:750, ImmunoStar, Hudson, WI). The CR antibody was visualized with DAB-peroxidase and the GABA antibody was visualized with immunogold, as described earlier.
For each of the above conditions, some sections were mounted on gel-coated slides for light microscopy. All other sections were postfixed in 2% osmium tetroxide for 1 h, dehydrated in ascending alcohols and embedded in Epon 812 (Melchitzky et al., 1998).
The prosomatostatin-derived peptides include SST-14, SST-28, and SST281–12, with SST-28 being an N-terminal extension form of SST14 and SST281–12 being the N-terminal 12 amino acids of SST28.The anti-SST antibodies used in this study, S320 and S309, are directed against the C-terminus of SS281–12 and the 11 N-terminal amino acids of SS28, respectively (Benoit et al., 1982). The S320 antibody does not recognize SS28 and the S309 antibody primarily recognizes SS28 with 10% cross-reactivity with SS281–12 (Benoit et al., 1982, 1985). It has previously been demonstrated that SS28 is located primarily in the perinuclear region of cell bodies and that SS281–12 is located in distal processes (Lewis et al., 1986; Morrison et al., 1983). The anti-CR antibody (SWant) is produced in mice by immunization with recombinant human CR (22 Kd) and has been shown to selectively label CR without cross-reactivity for calbindin (Schwaller et al., 1993). The immunogen for the mouse anti-GABA antibody (Sigma) was GABA conjugated to BSA, and the antibody does not exhibit any cross-reactivity with BSA, l-glutamic acid, l-aspartic acid, glycine or l-glutamine. This antibody has been shown to specifically label GABA neurons (Storm-Mathisen et al., 1983). The rabbit anti-GABA antibody (ImmunoStar, formerly DiaSorin) was produced using GABA coupled to BSA with glutaraldehyde. This antibody has previously been utilized to specifically label GABA-containing structures in rodent and primate brain (Kubota and Kawaguchi, 2000; Melchitzky and Lewis, 2003; Melchitzky et al., 2001; Super et al., 1998).
Specificity of the secondary antibodies was tested by using mismatched secondary antibodies with the primary antibodies (i.e., the anti-mouse biotinylated IgG with the rabbit primary antibodies and the anti-rabbit biotinylated IgG with the mouse primary antibody). These tests showed no specific labeling of neuronal, axonal, or dendritic structures.
Sampling procedures and analyses for SST single label
For each animal, separate trapezoid blocks were cut from layers 2–3a (superficial layers) and layers 5- superficial 6 (deep layers) in PFC area 9 (Fig. 1) and then sectioned on a Reichert ultramicrotome (Nussloch, Germany) at 80 nm. Serial ultrathin sections (2–4) were collected on 200-mesh copper grids and counterstained using uranyl acetate and lead citrate. For each block, 2 grids (separated by at least 5 grids) were examined. One section per grid was arbitrarily chosen as the starting point for analysis. Tissue was examined on a JEOL 1011 CX electron microscope (JEOL, Peabody, MA), equipped with a digital camera system (Advanced Microscopy Technology, Danvers, MA). Within each selected section, all SST-IR axon terminals were identified and photographed at 40,000×. For those SST-IR terminals with identifiable synaptic specializations, the postsynaptic target was classified. Those terminals without a definite synaptic specialization were classified as appositions and the appositional target was determined. For all axon terminals reported, there was only one appositional or synaptic target in the sections examined.
Fig. 1.
Brightfield photomontage of SST immunoreactivity in area 9 of monkey PFC. Trapezoids illustrate the approximate laminar location of blocks examined for electron microscopy. WM, white matter. Scale bar = 300 µm.
Sampling regions and procedures for SST or CR and GABA dual label
For each animal, trapezoid blocks were cut from the superficial layers in PFC area 9. These blocks were sectioned and grids were collected and counterstained as described earlier. Two grids per block (separated by at least 5 grids) were examined with one section per grid arbitrarily chosen as the starting point for analysis. To reduce false-negative results, sampling was confined to grid squares that contained the interface between the tissue and epon where penetration of the immunoreagents is optimal (Sesack et al., 1998). Within these grid squares, all SST- or CR-IR axon terminals within fields of specific GABA labeling were photographed at 40,000×. SST- or CR-IR terminals were considered to be within a field containing specific GABA labeling if both labeled elements were encompassed in a digital image at 25,000×. For those SST-or CR-IR terminals that had an identifiable synaptic specialization, the postsynaptic target was identified and classified as either GABA-IR or non GABA-IR. Those terminals without a definite postsynaptic specialization were classified as appositions and were not included in further analyses. For all axon terminals reported, there was only one appositional or synaptic target in the sections examined.
Statistical analysis
χ2 analyses were performed to compare the differences in postsynaptic and appositional targets of SST-IR axon terminals across layers and between SST- and CR-IR axon terminals. The CR-IR axon terminals used in these comparisons are a subset (i.e., CR-IR axon terminals forming symmetric synapses identified in single label studies) of those we previously reported (Melchitzky et al., 2005).
RESULTS
General observations
At the light microscopic level, SST immunoreactivity was predominantly localized to axons and varicosities across layers 1–6 of monkey PFC (Fig. 1). The superficial layers contained a high density of SST-IR axons, whereas the deep layers had a moderate density of labeled axons, as previously described (Hayes et al., 1991; Lewis et al., 1986). SST-containing neurons were less common, but SST immunoreactivity was found in the perinuclear region of cell bodies (Hayes et al., 1991; Lewis et al., 1986). At the electron microscopic level, SST immunoreactivity was present in axons, axon terminals and occasionally, somata, consistent with previous findings (de Lima and Morrison, 1989; Hendry et al., 1984).
Postsynaptic targets of SST-IR axon terminals
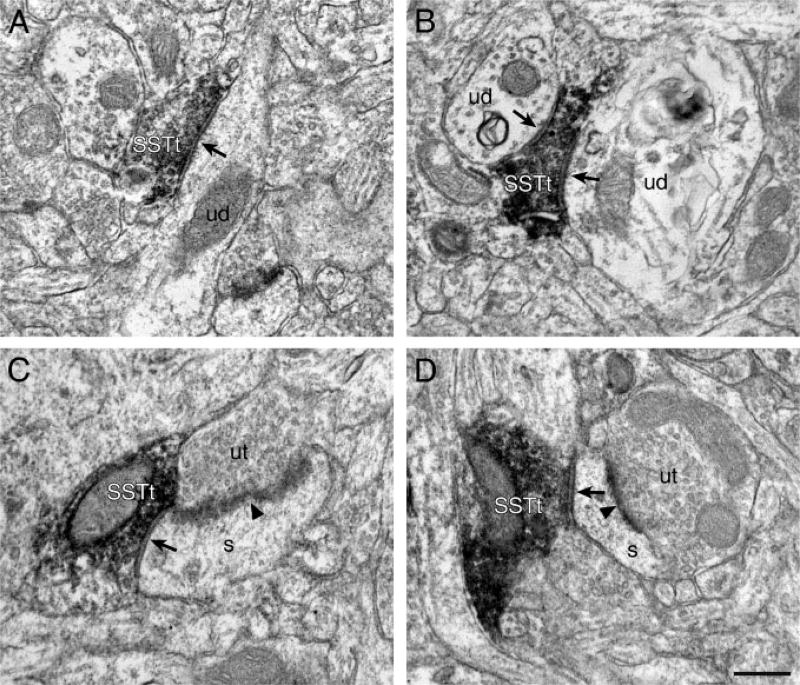
The following data are from tissue single-labeled for SST. All synapses formed by SST-IR axon terminals were symmetric. The majority (86%) of these synapses were onto dendritic shafts (Figs. 2A and 2B). The remainder contacted dendritic spines, approximately half of which also received an asymmetric synapse from an unlabeled axon terminal (Figs. 2C and 2D). SST-IR axon terminals exhibited laminar differences in postsynaptic targets (Table I). In the superficial layers, 80% of the postsynaptic targets were dendritic shafts, whereas dendritic shafts comprised 92% of the postsynaptic targets in the deep layers. Accordingly, SST-IR axon terminals contacted dendritic spines with a greater frequency in the superficial layers (20%) than in the deep layers (8%). These laminar differences in postsynaptic targets were statistically significant (χ2 = 4.90, P = 0.027).
Fig. 2.
Electron micrographs of SST-IR (SSTt) axon terminals in area 9 of monkey PFC. A: A SSTt forms a symmetric synapse (arrow) onto an unlabeled dendritic shaft (ud). B: A SSTt forms two symmetric synapses (arrows) onto two unlabeled dendritic shafts (ud). C and D: SST-IR axon terminals (SSTt) form symmetric synapses (arrows) onto dendritic spines (s). The dendritic spines also receive asymmetric synapses (arrowheads) from unlabeled axon terminals (ut). Scale bar = 0.50 µm for all panels.
TABLE I.
Comparisons of the synaptic targets SST- and CR-IR axon terminals in the superficial and deep layers of monkey PFCa
| SST-IR axon terminals |
CR-IR axon terminals |
Statistical results |
|
|---|---|---|---|
| Superficial layers | |||
| Total number of synapses | 91 | 51 | χ2 = 10.93 |
| Number (%) of synapses onto | P= 0.004 | ||
| Dendritic shafts | 73 (80%) | 41 (80%) | |
| Dendritic spines | 18 (20%) | 5 (10%) | |
| Somata | 0 (0%) | 5 (10%) | |
| Deep layers | |||
| Total number of synapses | 77 | 47 | χ2 = 4.94 |
| Number (%) of Synapses onto | P = 0.085 | ||
| Dendritic shafts | 71 (92%) | 44 (94%) | |
| Dendritic spines | 6 (8%) | 1 (2%) | |
| Somata | 0 (0%) | 2 (4%) |
Terminals examined from tissue single-labeled for either SST or CR.
The SST-IR axon terminals that did not form identifiable synaptic contacts were classified as appositions. Similar to SST-IR axon terminals forming synapses, the majority of SST-IR axon terminals in both the superficial and deep layers were apposed to dendritic shafts [87% (248/285 and 93% (214/231), respectively]. SST-IR axon terminals were significantly (χ2 = 4.31, P = 0.038) more likely to be apposed to dendritic spines in the superficial layers (13%; 37/285) than in the deep layers (7%; 17/231).
Comparison of postsynaptic targets of SST- and CR-IR axon terminals
The following data are from tissue single-labeled for SST or single-labeled for CR. In the superficial layers of monkey PFC area 9, unlabeled dendritic shafts were the principal postsynaptic target of both SST- (80%) and CR-IR (80%) axon terminals. However, these two populations of axon terminals differed in the proportion of synaptic contacts onto dendritic spines and somata (Table I). For example, SST-IR axon terminals formed synapses onto dendritic spines with a greater frequency than did CR-IR axon terminals (20% vs. 10%, respectively). In contrast, 10% of CR-IR axon terminals targeted somata, whereas no SST-IR axon terminals were observed to make axosomatic synapses. These differences in the postsynaptic targets of SST- and CR-IR axon terminals were statistically significant (χ2 = 10.93, P = 0.004). Synaptic contacts between SST-IR axon terminals and SST-IR dendritic shafts were not observed (0/73), whereas 12% (5/41) of the dendritic shafts contacted by CR-IR axon terminals were immunoreactive for CR.
Similar to the superficial layers, the primary targets of both SST- and CR-IR axon terminals in the deep layers were dendritic shafts (92% and 94%, respectively; Table I). In addition, SST-IR axon terminals contacted dendritic spines with a greater frequency than did CR-IR axon terminals (8% vs. 2%, respectively). However, these differences in postsynaptic targets showed only a trend toward statistical significance (Table I). Similar to the superficial layers, a small proportion (4/44; 9%) of CR-IR axon terminals formed synapses onto CR-IR dendritic shafts, whereas no SST-IR axon terminals (0/71) were observed to form synapses onto SST-IR dendrites.
Specificity in the postsynaptic targets of SST- and CR-IR axon terminals was also evident in tissue processed for dual labeling of SST or CR and GABA. A total of 171 SST-IR axon terminals and 263 CR-IR axon terminals were examined. In the superficial layers, some SST-IR axon terminals formed synapses onto GABA-IR dendritic shafts (Fig. 3A). However, most SST-IR axon terminals in fields that contained GABA-labeled dendrites (Fig. 3B) contacted unlabeled (non-GABA-IR) dendritic shafts (Fig. 3C) or dendritic spines. In contrast, CR-IR axon terminals frequently formed synapses onto GABA-IR dendritic shafts (Figs. 4A and 4B). In addition, CR-IR axon terminals targeted other GABA-containing structures, such as GABA-IR somata and CR-IR dendritic shafts (Table II). A smaller proportion of CR-IR axon terminals contacted unlabeled dendritic shafts (Figs. 4C and 4D) and dendritic spines. To determine the relative total input to GABA neurons by SST- and CR-IR axon terminals, we collapsed the data presented in Table II into GABA-containing structures (GABA-IR dendritic shafts, GABA-IR somata and CR- or SST-IR dendritic shafts) and non-GABA-containing structures (unlabeled dendritic shafts and unlabeled spines). A χ2 analysis revealed that a significantly (χ2 = 31.3, P < 0.001) greater proportion of CR-IR axon terminals (73%) targeted GABA-containing structures than did SST-IR axon terminals (21.5%).
Fig. 3.
Electron micrographs of SST-IR (SSTt) axon terminals labeled with peroxidase-diaminobenzidine, and GABA-IR dendritic shafts labeled by immuno-gold. A: A SST-IR axon terminal (SSTt) forms a symmetric synapse (arrow) onto a GABA-IR dendritic shaft (Gd), which also receives an asymmetric synapse (arrowhead) from an unlabeled axon terminal (ut). B: Low magnification field showing a SSTt that forms a symmetric synapse (arrow) onto an unlabeled dendrite (ud) in a field containing two GABA-IR dendritic shafts (Gd). C: Higher magnification of the SSTt in panel B. Scale bar = 0.5 µm for all panels.
Fig. 4.
Electron micrographs of CR-IR axon terminals (CRt) labeled with peroxidase-diaminobenzidine, and GABA-IR dendritic shafts labeled by immuno-gold. A and B: A CRt forms a symmetric synapse (arrow) onto a GABA-IR dendritic shaft (Gd). In panel B, an unlabeled axon terminal (ut) forms an asymmetric synapse (arrow-head) onto the Gd. C: A CRt forms a symmetric synapse (arrow) onto an unlabeled dendritic shaft (ud), which also receives an asymmetric synapse (arrowhead) from an unlabeled terminal (ut). D: A lower magnification of the CRt in panel C, showing a GABA-IR dendritic shaft (Gd) in the same microscopic field. Scale bar = 0.50 µm.
TABLE II.
Relative incidence of synaptic contacts between SST- or CR-IR axon terminals and GABA-IR or unlabeled structures in monkey PFC area 9a
| SST-IR axon Terminals |
CR-IR axon terminals |
|
|---|---|---|
| Total number of synapses | 51 | 73 |
| Number (%) of synapses onto | ||
| GABA-IR dendritic shaft | 11 (21.5%) | 44 (60%) |
| GABA-IR somata | 0 (0%) | 5 (7%) |
| SST-IR dendritic shaft | 0 (0%) | Not applicable |
| CR-IR dendritic shaft | Not applicable | 4 (6%) |
| Unlabeled dendritic shaft | 29 (57%) | 14 (19%) |
| Unlabeled dendritic spine | 11 (21.5%) | 6 (8%) |
Terminals examined from tissue dual-labeled for either SST and GABA or CR and GABA.
DISCUSSION
In macaque monkey PFC, SST-IR axon terminals formed exclusively symmetric synapses predominantly onto dendritic shafts with the remainder onto dendritic spines. The proportions of these postsynaptic targets differed by cortical layer, with a greater proportion of synapses onto dendritic spines in the superficial layers than in the deep layers, although these laminar differences might be skewed by the greater number of axospinous synapses in the superficial layers (Bourgeois et al., 1994). Comparison of the postsynaptic targets of SST- and CR-IR axon terminals in the superficial layers revealed that, although both primarily target dendritic shafts, SST-IR axon terminals target dendritic spines with greater frequency than do CR-IR axon terminals. In addition, CR-IR axon terminals appeared to target GABA-containing neurons with a much greater frequency than did SST-IR axon terminals.
Our finding that SST-containing neurons principally target dendritic shafts is consistent with findings in other cortical areas. For example, dendritic shafts were the primary postsynaptic targets of SST-IR axon terminals in monkey temporal (80%; de Lima and Morrison, 1989) and visual (68%; Gonchar et al., 2002) cortices. In addition, and similar to the PFC, dendritic spines were the next most common target in both temporal and visual cortex (17% and 25%, respectively). In contrast to the present study, these studies found that small percentages (0.1–5%) of SST-IR axon terminals contacted somata and axon initial segments (de Lima and Morrison, 1989; Gonchar et al., 2002). The SST-IR axon terminals examined in this study did not contact SST-IR dendritic shafts. This finding is consistent with studies in rodent cortex that demonstrate that SST-containing cells are not connected to each other via chemical synapses, but instead form electrically coupled networks (Amitai et al., 2002; Gibson et al., 1999). However, in our study, SST immunoreactivity was detectable in few postsynaptic structures; thus we cannot exclude the possibility of false negative findings with regards to SST-to-SST connections.
Our findings suggest that the majority of dendritic shafts contacted by SST-containing neurons belong to pyramidal cells. For example, in tissue processed for both SST and GABA labeling, 57% of SST-IR axon terminals formed synapses onto unlabeled dendrites and 21.5% contacted unlabeled dendritic spines, the majority of which originate from pyramidal cells (see Table II). The remaining 21.5% of SST-IR axon terminals formed synapses onto GABA-labeled dendritic shafts. Thus, the predominant targets of SST-containing cells in monkey PFC are most likely pyramidal neurons. In contrast, our data indicate that CR-containing cells preferentially target other GABA neurons. For example, in fields that contained both CR and GABA labels, 60% of CR-IR axon terminals formed synapses onto GABA-IR dendritic shafts and 7% contacted GABA-IR somata (see Table II). In addition, 6% of synapses formed by CR-IR terminals were onto CR-IR dendrites which are also likely to be from GABA neurons. Thus, two-thirds to three-quarters of CR-IR axon terminals contact GABA-containing structures. Because of the restricted labeling of the dendritic arbor of GABA interneurons by the immunogold method, the percentage of GABAergic structures postsynaptic to SST- and CR-IR axon terminals might actually be greater than observed.
The preferential targeting of CR-containing cells to GABA neurons has also been reported in the visual cortex of both monkeys (Meskenaite, 1997) and rodents (Gonchar and Burkhalter, 1999) and in the rodent hippocampus (Gulyas et al., 1996). The synaptic specializations of SST- and CR-IR neurons, which differ from those of PV-IR axon terminals (Melchitzky et al., 1999; Williams et al., 1992), indicate that each of these subtypes of GABA neurons tend to play different roles in shaping the activity of pyramidal neurons in the monkey PFC (Fig. 5). SST-IR neurons are positioned to regulate the impact of excitatory inputs to pyramidal neuron dendrites, the location of PV-IR synapses indicates that they influence the output of pyramidal neurons and CR-IR neurons would be expected to control the disinhibition of pyramidal cells.
Fig. 5.
Schematic illustration of the synaptic contacts of subpopulations of GABA neurons in monkey PFC. Axon thickness represents the relative frequency of the indicated synaptic target. Parvalbumin- (PV) containing chandelier neurons form synapses exclusively onto the axon initial segments of pyramidal cells. Wide-arbor (basket) neurons, which also contain PV, contact the dendrite shafts, cell bodies and dendritic spines of pyramidal neurons. The primary postsynaptic targets of somatostatin- (SST) containing neurons are the dendritic shafts of pyramidal neurons, followed by dendritic spines of pyramidal neurons and dendritic shafts of GABA neurons. In contrast to both the PV- and SST-positive cells, the principal synaptic targets of calretinin-(CR) containing neurons are the dendritic shafts of GABA cells. The dendritic shafts and spines of pyramidal neurons and the somata of GABA cells are secondary targets of CR-containing cells.
Previous findings from other studies support such a specialized role for SST-containing neurons. For example, many SST-containing neurons have the features of Martinotti cells that target the distal and tuft dendrites of pyramidal cells (Kawaguchi and Kubota, 1997; Wang et al., 2004b) and participate in a disynaptic inhibitory pathway between neighboring pyramidal neurons. Specifically, pyramidal cells provide facilitating excitatory input to SST-containing Martinotti cells that, via inhibitory synapses onto the dendrites of neighboring pyramidal neurons, produces disynaptic inhibition (Silberberg and Markram, 2007). In the primate PFC, this disynaptic inhibition may be critical for working memory functioning. For example, computational models of the cortical circuitry involved in working memory predict that inhibitory neurons targeting pyramidal neuron dendrites, such as SST-containing cells, provide resistance to distracting stimuli by inhibiting the dendrites of neighboring pyramidal neurons that are selective for other stimuli (Wang et al., 2004a). Thus, SST-containing, dendritic targeting neurons appear to be necessary for filtering out distracting stimuli during working memory processing. Interestingly, patients with schizophrenia exhibit deficits in working memory tasks, with specific impairments in filtering out distracting and unnecessary information (Silver and Feldman, 2005). In addition, postmortem studies have revealed a robust decrease in SST mRNA expression in the PFC of subjects with schizophrenia that may be associated with a decrease in GABA synthesis in the same neurons (Hashimoto et al., 2008; Morris et al., in press). Based on the synaptic connectivity of SST-positive neurons, these findings suggest that abnormalities in dendritic disynaptic inhibition of pyramidal neurons in the PFC of subjects with schizophrenia could contribute to working memory deficits in affected individuals.
Acknowledgments
The authors thank Mrs. Mary Brady for assistance with the micrographs.
Contract grant sponsor: USPHS; Contract grant number: MH043784.
References
- Amitai Y, Gibson JR, Beierlein M, Patrick SL, Ho AM, Connors BW, Golomb D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–4152. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit R, Ling N, Alford B, Guillemin R. Seven peptides derived from pro-somatostatin in rat brain. Biochem Biophys Res Commun. 1982;107:944–950. doi: 10.1016/0006-291x(82)90614-3. [DOI] [PubMed] [Google Scholar]
- Benoit R, Bohlen P, Ling N, Esch F, Baird A, Ying S-Y, Wehrenberg WB, Guillemin R, Morrison JH, Bakhit C, Koda L, Bloom FE. Somatostatin-28(1–12)-like peptides. In: Patel YC, Tannenbaum GS, editors. Proceedings of the 3rd International Symposium on Somatostatin, Montreal. New York: Plenum Press; 1985. pp. 89–107. [DOI] [PubMed] [Google Scholar]
- Bourgeois J-P, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k, or parvalbumin in monkey prefrontal cortex: Distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- de Lima AD, Morrison JH. Ultrastructural analysis of somatostatin-immunoreactive neurons and synapses in the temporal and occipital cortex of the macaque monkey. J Comp Neurol. 1989;283:212–227. doi: 10.1002/cne.902830205. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey. II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Dickie BGM, Vaid RR, Headlam AJM, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: Morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Glezer II, Hof PR, Morgane PJ. Comparative analysis of calcium-binding protein-immunoreactive neuronal populations in the auditory and visual systems of the bottlenose dolphin (Tursiops truncatus) and the macaque monkey (Macaca fascicularis) J Chem Neuroanat. 1998;15:203–237. doi: 10.1016/s0891-0618(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex. Cereb Cortex. 1999;9:683–696. doi: 10.1093/cercor/9.7.683. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Turney S, Price JL, Burkhalter A. Axo-axonic synapses formed by somatostatin-expressing GABAergic neurons in rat and monkey visual cortex. J Comp Neurol. 2002;443:1–14. doi: 10.1002/cne.1425. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Regional survey of gaba-related gene expression in the neocortex of subjects with schizophrenia. Schizophr Bull. 2007;33:261. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Volk DW, Mirnics K, Lewis D. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TL, Cameron JL, Fernstrom JD, Lewis DA. A comparative analysis of the distribution of prosomatostatin-derived peptides in human and monkey neocortex. J Comp Neurol. 1991;303:584–599. doi: 10.1002/cne.903030406. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4:2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Morrison JH. An immunohistochemical characterization of somatostatin-28 and somatostatin-28 (1–12) in monkey prefrontal cortex. J Comp Neurol. 1986;248:1–18. doi: 10.1002/cne.902480102. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Pyramidal neuron local axon terminals in monkey prefrontal cortex: Differential targeting of subclasses of GABA neurons. Cereb Cortex. 2003;13:452–460. doi: 10.1093/cercor/13.5.452. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol. 1998;390:211–224. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Lewis DA. Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: Laminar, regional and target specificity of Type I and Type II synapses. J Comp Neurol. 1999;408:11–22. [PubMed] [Google Scholar]
- Melchitzky DS, González-Burgos G, Barrionuevo G, Lewis DA. Synaptic targets of the intrinsic axon collaterals of supra-granular pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2001;430:209–221. doi: 10.1002/1096-9861(20010205)430:2<209::aid-cne1026>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Eggan SM, Lewis DA. Synaptic targets of calretinin-containing axon terminals in macaque monkey prefrontal cortex. Neuroscience. 2005;130:185–195. doi: 10.1016/j.neuroscience.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Meskenaite V. Calretinin-immunoreactive local circuit neurons in area 17 of the cynomolgus monkey, Macaca fascicularis. J Comp Neurol. 1997;379:113–132. [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia. Cereb Cortex. doi: 10.1093/cercor/bhm186. (e-pub, 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Benoit R, Magistretti PJ, Bloom FE. Immunohistochemical distribution of pro-somatostatin-related peptides in cerebral cortex. Brain Res. 1983;262:344–351. doi: 10.1016/0006-8993(83)91031-4. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Buchwald P, Blümcke I, Celio MR, Hunziker W. Characterization of a polyclonal antiserum against the purified human recombinant calcium-binding protein calretinin. Cell Calcium. 1993;14:601–610. doi: 10.1016/0143-4160(93)90089-o. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA. Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: Ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex. 1998;8:614–622. doi: 10.1093/cercor/8.7.614. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 2005;17:391–398. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Super H, Martinez A, del Rio JA, Soriano E. Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. J Neurosci. 1998;18:4616–4626. doi: 10.1523/JNEUROSCI.18-12-04616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA. 2004a;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004b;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in primate prefrontal cortex. J Comp Neurol. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci USA. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]