ABSTRACT
Following damage by pore forming toxins (PFTs) host cells engage repair processes and display profound cytoskeletal remodeling and concomitant plasma membrane (PM) blebbing. We have recently demonstrated that host cells utilize similar mechanisms to control cytoskeletal dynamics in response to PFTs and during cell migration. This involves assembly of cortical actomyosin bundles, reorganisation of the endoplasmic reticulum (ER) network, and the interaction between the ER chaperone Gp96 and the molecular motor Non-muscle Myosin Heavy Chain IIA (NMHCIIA). Consequently, Gp96 regulates actomyosin activity, PM blebbing and cell migration, and protects PM integrity against PFTs. In addition, we observed that PFTs increase association of Gp96 and ER vacuoles with the cell surface or within PM blebs loosely attached to the cell body. Similarly, gut epithelial cells damaged by PFTs in vivo were shown to release microvilli structures or directly purge cytoplasmic content. Cytoplasmic purging involves profound cytoskeletal remodeling and ER vacuolation, suggesting that our observations recapitulate recovery processes in vivo. Here, we discuss our findings in light of the current understanding of PM repair mechanisms and in vivo recovery responses to PFTs.
KEYWORDS: actomyosin, blebbing, endoplasmic reticulum chaperone, listeriolysin O, plasma membrane repair, pore-forming toxin
Introduction
Evolutionary conserved mechanisms allow eukaryotic cells to sustain mechanical and chemical stress that injure the PM.1-3 The changes in the intracellular concentration of calcium and potassium caused by PM rupture initiate recovery processes which depend on the size of the damage, the cell types involved and the nature of the inflicted stress (e.g. mechanical injuries or insertion of stable protein pores such as those created by bacterial PFTs).1-3 In general, cells engage PM repair pathways, rearrange the cytoskeleton, control their metabolic state and activate stress-associated signaling.2,3
PM damage promotes calcium influx, which enhances exocytosis, predominantly of lysosomes. These vesicles patch large mechanical wounds (> 100 nm),4 and promote acid-sphingomyelinase (ASM) release, which generates PM-ceramide domains that engulf PM damage in caveolae-derived endosomes.2,5 Stable protein pores cannot be patched and are removed by endocytosis or shedding within small PM vesicles (nm size).6 PM shedding may actually constitute an intrinsic repair mechanism that senses PFT oligomerisation and is potentiated upon damage and calcium influx.7 Shedding depends on endosomal sorting complexes required for transport (ESCRT) and is similar to the budding of viral particles.6-8 In vivo, recovery from PFT-mediated damage appears to involve the cooperation between different mechanisms. Host survival requires regulators of both endocytic and exocytic trafficking and epithelial cells display increased rates of endocytosis, shedding of PM material9 and/or direct purging of cytoplasmic content.10 In addition, epithelia compact its cytoskeletal network and display alterations of cellular organelles while preserving coherence and functionality.10
The fine control of the cytoskeletal dynamics is therefore necessary to promote PM recovery.11 Indeed, following mechanically-induced PM damage, microtubules allow recruitment of distal vesicles while local actin rearrangements and myosin activity relief tension facilitate vesicle delivery and provide force to re-establish PM integrity.12-16 The importance of cytoskeletal dynamics in cells targeted by PFTs remains poorly defined.
Novel regulators of cytoskeletal dynamics protect against PFTs
We recently identified the ER chaperone Gp96 and NMHCIIA as regulators of cytoskeletal dynamics following PFT-mediated PM damage.17 Gp96 and NMHCIIA interact upon PFT intoxication and accumulate into distinct bundles at sites of PM blebbing (Fig. 1 and Supp Mov 1).17 These processes require calcium influx generated by PM damage and occur during Listeria monocytogenes (Lm) infection, which depends on the PFT listeriolysin O (LLO). The reorganisation of the actomyosin network is mediated by Gp96, which modulates myosin II activity and coordinates PM blebbing during PFT intoxication. Both Gp96 and NMHCIIA promote cell survival upon LLO intoxication.17
Figure 1.
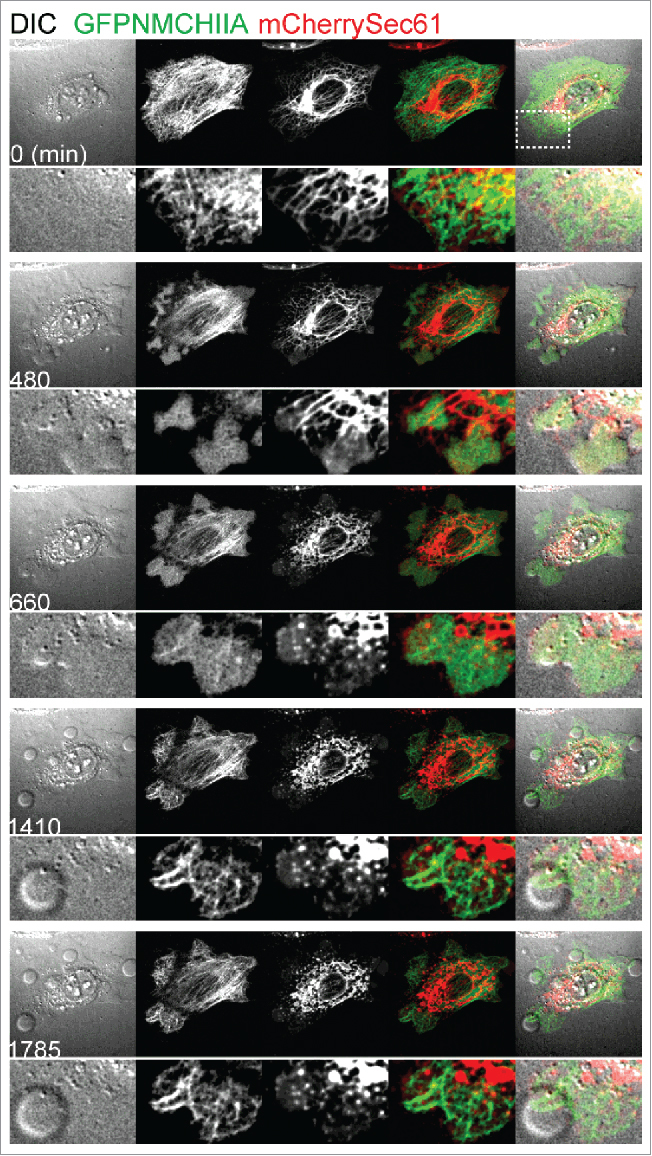
Redistribution of NMHCIIA and ER network upon LLO treatment. Sequential frames of time-lapse confocal microscopy sequence of LLO-treated HeLa cells expressing simultaneously GFPNMHCIIA and mCherrySec61. LLO was added to culture medium 10 seconds before t0. DIC – differential interference contrast. Highlighted inset depicts ER structures within NMHCIIA bundles and PM blebs.
We characterized further the formation of NMHCIIA bundles during PFT intoxication and found that host cells utilize similar mechanisms to regulate cytoskeletal dynamics during recovery of PM integrity and cell migration.17 (i) PFT-induced actomyosin bundles accumulate proteins found at the trailing edge of migrating cells;18,19 (ii) upon PFT intoxication, Gp96 interacts with Filamin-A, an actin cross-linker that regulates cell migration;20 and (iii) stimulation of cell migration with Wnt5a, which promotes assembly of rear-end ER-actomyosin structures, also enhances NMHCIIA-Gp96 interaction. In line with these observations, we showed that Gp96 regulates general cytoskeletal organization and therefore modulates cell shape and cell motility.17
Recent independent studies have also proposed a role for Gp96 in cytoskeletal organization, cell polarity and cell migration. This may occur through the control of vesicular trafficking and/or interaction with different cytoskeletal proteins such as F-actin-capping protein 1, Actin, Radixin and ROCK2.21,22 Of note, Gp96 is predominantly expressed at early stages of development and contributes to the establishment of epithelial gut morphology and apical specification.23 Polarized lysosome secretion and establishment of cell polarity are regulated by NMHCIIA.14,24 Therefore, it is possible that Gp96 and NMHCIIA interact to coordinate vesicular trafficking and cytoskeletal dynamics necessary for the definition of cell polarity and for efficient PM repair. Whether NMHCIIA and Gp96 directly interact remains unknown. Yet, Gp96 is the ER paralogue of the cytosolic chaperone HSP90, which binds myosin head domains and is necessary to coordinate assembly and folding of myosin thick filaments.25
Few additional molecules were associated with the cytoskeletal reorganisation following PFT-mediated PM damage. RhoA and Rac1 GTPases promote actin remodelling26 and Src-family kinases mediate microtubule bundling and stabilization.27 The importance of such processes for cell recovery from PFT-mediated wounding is uncertain. Nevertheless, GTPases (RhoA, Rac and Cdc42) coordinate the assembly and dynamics of actomyosin rings, which promote closure of laser-induced wounds in Xenopus oocytes,28 and Src, together with myosin light chain kinase (MLCK), regulate PM expansion during osmotic stress.29
Besides actomyosin reorganisation and simultaneous PM blebbing, cells modify the entire ER network following PFT intoxication,17 as depicted by the alteration of the characteristic ER reticular pattern and formation of vacuoles containing mCherry-Sec61β (a subunit of the ER membrane translocon complex Sec61) (Fig. 1 and Supp Mov 1). Vacuolation of the ER and other cellular organelles has been reported in response to different PFTs in various cell types and in vivo.1,10 The relevance of such morphological alteration is not understood and has been mainly associated with organelle damage and cell death.1 However, following toxin wash-out, cells recover normal actomyosin and ER distribution with equivalent kinetics.17
Lysosomes and the ER are major intracellular calcium stores and their dynamics are crucial for functioning. In particular, the transient distribution of ER and lysosomes to the trailing edge of migrating cells directs calcium signaling and assembly of cytoskeletal complexes that mediate tail retraction.18 Of note, stimulation of such process enhances Gp96-NMHCIIA interaction.17 However, the role of the ER during recovery from PFT-induced PM damage remains unclear. ER proteins have been detected at PM wounds of mechanically injured cells30 and inhibition of ER stress pathways or calcium sequestration compromises survival after PFT intoxication.31,32 Whether lysosomes or the ER control calcium signaling and actomyosin dynamics during PM repair is still speculation.30 Nevertheless, Gp96 regulates calcium homeostasis at the ER.33
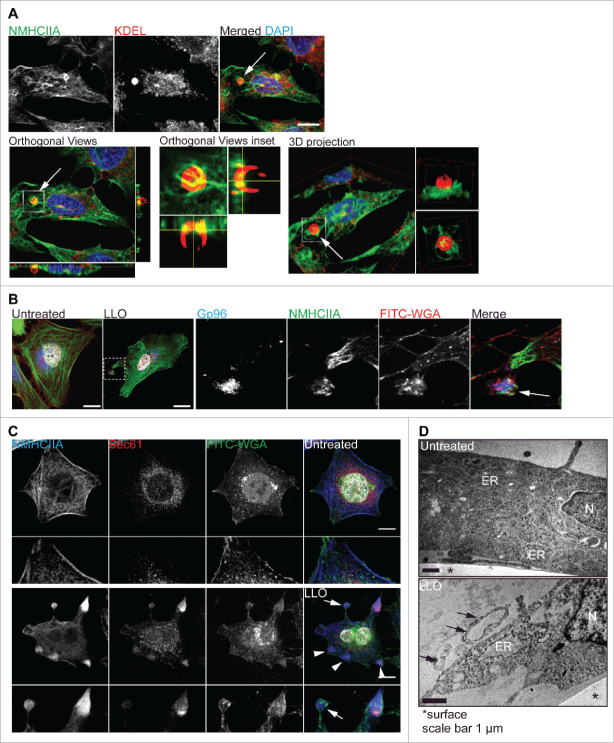
We observed that certain LLO-intoxicated cells appear to expose ER compartments containing ER-retention sequence KDEL, Gp96 and Sec61α at the cell surface or within large PM blebs loosely attached to the cell body (Fig. 2A-C).17 Transmission electron microscopy (TEM) of intoxicated HeLa cells confirmed that such vacuoles are detected within large bleb-like structures at the proximity of the PM and apparently detached from the cell body (Fig. 2D). Thus, upon damage, cells can release ER-derived compartments to the extracellular environment. Whether the release of ER vacuoles only occurs in dying cells or upon organelle damage is still unclear. Yet these processes may constitute a common feature of cellular responses to PFTs, since targeting of gut epithelial cells by PFTs in vivo induces release of microvilli structures, cytosolic purging, ER vacuolation and rearrangement of the cellular cytoskeleton.9,10
Figure 2.
Exposure of ER and Gp96 at blebs from LLO treated cells. (A) Confocal microscopy Z-stack projections of HeLa cells treated with LLO (0.5 nM, 15 min) and immunolabelled for the C-terminal sequence present in ER resident proteins, ER-KDEL (red), NMHCIIA (green) and stained with DAPI (blue). Orthogonal views and 3D projections illustrate exposure of ER vacuoles at the cell surface (arrow). (B-C) Confocal microscopy images of HeLa cells left untreated or treated with LLO and immunolabelled for (B) ER-Gp96 (blue), NMHCIIA (green) and stained with FITCWGA (Plasma membrane, PM-red) and DAPI (white), or (C) Sec61 (red), NMHCIIA (blue) and stained with FITCWGA (green) and DAPI (white). Insets and arrows indicate NMHCIIA-positive PM blebs containing Gp96 or Sec61, loosely attached to the cell body. Arrow-heads show cortical NMHCIIA-Sec61 within the cell body. All scale bars are 10 μm. (D) Longitudinal TEM images of HeLa cells left untreated or treated with 0.5 nM LLO for 15 min. ER - ER cisternae in untreated cells and ER vacuoles in LLO-treated cells; N - nucleus. Arrows show vesicles and bleb-like structures at the proximity of the PM containing ER vacuoles and apparently detached from the cell body.
PFT-induced PM blebbing was considered to be protective and distinct from PM shedding of PFT pores within small vesicles (nm size). Large transient blebs (µm size) presumably promote PM repair by buffering injured sites, preventing excess calcium influx and loss of cytosolic content.2,3,6,34 Blebs can be shed and, during apoptosis, permeabilisation of PM blebs enables the release of cytosolic content.35,36 Thus, it is possible that cytosolic purging and PM blebbing are complementary processes. Finally, increasing evidence supports a role for extruded vesicles during bacterial infections. While some studies have suggested that microvesicle release or cytosolic purging may favor elimination of intracellular bacteria,10,37,38 certain bacteria, such as Lm, were proposed to disseminate within large bleb-like structures.39,40
Conclusion and future perspectives
We have highlighted the importance of NMHCIIA and uncovered an unexpected role for the ER chaperone Gp96 in host cell recovery against PFTs. Future studies are now necessary to understand how cytoskeletal dynamics interfere with polarized secretion and shedding of cellular material, which protect host tissues from PFT attack. Moreover, it will be important to further analyze the physiologic relevance of recovery mechanisms in the context of bacterial infections: What are the consequences of PM blebbing and cytosolic purging in the context of different infections? Are these processes related to the shedding of apoptotic bodies and damaged cells from infected epithelia?
As PM recovery processes display important evolutionary conserved features,2,3,11 the ground-breaking use of amenable models such as zebrafish (Danio rerio) and drosophila (Drosophila melanogaster) to the direct visualization of infectious processes in vivo will continue to be of critical importance.10,17
Materials and methods
Plasmids and antibodies
Plasmid GFPNMHCIIA (#11347) was obtained from Addgene and mCherry-Sec61-N-18 was a gift from M. Davidson through Addgene (# 55130). Rabbit anti-NMHCIIA (Sigma); mouse anti-NMHCIIA (Abcam); rat anti-Gp96 (Enzo); mouse anti-Sec61α G-2 (Santa Cruz) were used at 1/200 for immunofluorescence microscopy (IF). PM was labeled with FITC-conjugated WGA (Sigma) DNA with 4’,6-Diamidino-2-phenylindole dihydrochloride, DAPI (Sigma) and IF fluorescently-conjugated secondary antibodies (Invitrogen) were used at 1/500.
Cell lines and toxin
HeLa (ATCC CCL-2) cells were cultivated in DMEM with glucose and L-glutamine, supplemented with 10% FBS. Cells were maintained at 37°C in a 5% CO2 atmosphere. Cell culture media and supplements were from Lonza. LLO was purified as previously17 and treatments and washes were carried in Hank's Balanced Salt Solution (HBSS) as indicated.
Immunofluorescence microscopy
Cells were fixed in 4% paraformaldehyde (15 min), quenched with 20 mM NH4Cl (1 h), permeabilized with 0.1% Triton X-100 (5 min), and blocked with 10% BSA in PBS (30 min). Antibodies were diluted in PBS containing 1% BSA. Coverslips were incubated for 1 h with primary antibodies, washed 3 times in PBS and incubated 45 min with secondary antibodies. DNA was counterstained with DAPI (Sigma). Coverslips were mounted onto microscope slides with Aqua-Poly/Mount (Polysciences). Images were collected with a confocal laser-scanning microscope (Leica SP5II) and processed using ImageJ64 or Adobe Photoshop software.
Live imaging and quantification of PM blebbing of LLO-treated cells
Cells seeded into Ibitreat μ-dishes (Ibidi), simultaneously transfected with GFPNMHCIIA and mcherrySEC61, maintained in HBSS at 37°C with 5% CO2 were imaged using an Andor Revolution XD Spinning-disk confocal system with an EMCCD iXonEM+ camera, 488 nm lase lines, and a Yokogawa CSU-22 unit on an inverted microscope (IX81; Olympus), driven by Andor IQ live-cell imaging software. LLO (0.5 nM) was added 10 min after initial image acquisition. Differential interference contrast (DIC) images and GFP fluorescent data sets with 0.5 μm Z-steps were acquired using a UPLSAPO 100x/1.40 objective lens every 15 sec. ImajeJ64 was used for image sequence analysis and video assembly.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by national funds through FCT - Fundação para a Ciência e a Tecnologia / MEC - Ministério da Educação e Ciência and co-funded by FEDER-COMPETE 2020 and NORTE 2020 within projects POCI-01-0145-FEDER-007274, NORTE-01-0145-FEDER-000012, and Infect ERA/0001/2013 PROANTILIS. F. S. M. and C. B. were supported by FCT fellowships (SFRH/BPD/94458/2013, SFRH/BD/112217/2015). S. S. received supported from FCT Investigator program (COMPETE, POPH, and FCT).
References
- [1].Bischofberger M, Iacovache I, van der Goot FG. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 2012; 12:266-75. PMID:22980324. https://doi.org/ 10.1016/j.chom.2012.08.005. [DOI] [PubMed] [Google Scholar]
- [2].Andrews NW, Almeida PE, Corrotte M. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol 2014; 24:734-42. PMID:25150593. https://doi.org/ 10.1016/j.tcb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cooper ST, McNeil PL. Membrane Repair: Mechanisms and Pathophysiology. Physiol Rev 2015; 95:1205-40. PMID:26336031. https://doi.org/ 10.1152/physrev.00037.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci 2000; 113(Pt 11):1891-902. PMID:10806100. https://doi.org/ 10.1083/jcb.201003053. [DOI] [PubMed] [Google Scholar]
- [5].Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol 2010; 189:1027-38; PMID:20530211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. ESCRT machinery is required for plasma membrane repair. Science 2014; 343:1247136. PMID:24482116. https://doi.org/ 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- [7].Romero M, Keyel M, Shi G, Bhattacharjee P, Roth R, Heuser JE, Keyel PA. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ 2017; 24(5):798-808. PMID:28186501. https://doi.org/ 10.1038/cdd.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Keyel PA, Loultcheva L, Roth R, Salter RD, Watkins SC, Yokoyama WM, Heuser JE. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J Cell Sci 2011; 124:2414-23. PMID:21693578. https://doi.org/ 10.1242/jcs.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Los FC, Kao CY, Smitham J, McDonald KL, Ha C, Peixoto CA, Aroian RV. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe 2011; 9:147-57. PMID:21320697. https://doi.org/ 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee KZ, Lestradet M, Socha C, Schirmeier S, Schmitz A, Spenlé C, Lefebvre O, Keime C, Yamba WM, Bou Aoun, et al.. Enterocyte Purge and rapid recovery is a resilience reaction of the gut epithelium to pore-forming toxin attack. Cell Host Microbe 2016; 20:716-30. PMID:27889464. https://doi.org/ 10.1016/j.chom.2016.10.010. [DOI] [PubMed] [Google Scholar]
- [11].Boucher E, Mandato CA. Plasma membrane and cytoskeleton dynamics during single-cell wound healing. Biochim Biophys Acta 2015; 1853:2649-61. PMID:26209916. https://doi.org/ 10.1016/j.bbamcr.2015.07.012. [DOI] [PubMed] [Google Scholar]
- [12].Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci 2001; 114:3487-94. PMID:11682608. https://doi.org/ 10.1091/mbc.E03-06-0430. [DOI] [PubMed] [Google Scholar]
- [13].Togo T, Steinhardt RA. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol Biol Cell 2004; 15:688-95; PMID:14617807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Encarnacao M, Espada L, Escrevente C, Mateus D, Ramalho J, Michelet X, Santarino I, Hsu VW, Brenner MB, Barral DC, et al.. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J Cell Biol 2016; 213:631-40. PMID:27325790. https://doi.org/ 10.1083/jcb.201511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mandato CA, Bement WM. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr Biol 2003; 13:1096-105. PMID:12842008. https://doi.org/ 10.1016/S0960-9822(03)00420-2. [DOI] [PubMed] [Google Scholar]
- [16].Togo T. Disruption of the plasma membrane stimulates rearrangement of microtubules and lipid traffic toward the wound site. J Cell Sci 2006; 119:2780-6. PMID:16772335. https://doi.org/ 10.1242/jcs.03006. [DOI] [PubMed] [Google Scholar]
- [17].Mesquita FS, Brito C, Mazon Moya MJ, Pinheiro JC, Mostowy S, Cabanes D, Sousa S. Endoplasmic reticulum chaperone Gp96 controls actomyosin dynamics and protects against pore-forming toxins. EMBO Rep 2017; 18:303-18. PMID:28039206. https://doi.org/ 10.15252/embr.201642833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Witze ES, Connacher MK, Houel S, Schwartz MP, Morphew MK, Reid L, Sacks DB, Anseth KS, Ahn NG. Wnt5a directs polarized calcium gradients by recruiting cortical endoplasmic reticulum to the cell trailing edge. Dev Cell 2013; 26:645-57. PMID:24091015. https://doi.org/ 10.1016/j.devcel.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sanchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol 2009; 10:353-9. PMID:19373240. https://doi.org/ 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- [20].Razinia Z, Makela T, Ylanne J, Calderwood DA. Filamins in mechanosensing and signaling. Annu Rev Biophys 2012; 41:227-46. PMID:22404683. https://doi.org/ 10.1146/annurev-biophys-050511-102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hong F, Mohammad Rachidi S, Lundgren D, Han D, Huang X, Zhao H, Kimura Y, Hirano H, Ohara O, et al.. Mapping the interactome of a major Mammalian endoplasmic reticulum heat shock protein 90. PLoS One 2017; 12:e0169260. PMID:28056051. https://doi.org/ 10.1371/journal.pone.0169260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghosh S, Shinogle HE, Galeva NA, Dobrowsky RT, Blagg BSJ. Endoplasmic Reticulum-resident Heat Shock Protein 90 (HSP90) Isoform Glucose-regulated Protein 94 (GRP94) regulates cell polarity and cancer cell migration by affecting intracellular transport. J Biol Chem 2016; 291:8309-23. PMID:26872972. https://doi.org/ 10.1074/jbc.M115.688374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maynard JC, Pham T, Zheng T, Jockheck-Clark A, Rankin HB, Newgard CB, Spana EP, Nicchitta CV. Gp93, the Drosophila GRP94 ortholog, is required for gut epithelial homeostasis and nutrient assimilation-coupled growth control. Dev Biol 2010; 339:295-306. PMID:20044986. https://doi.org/ 10.1016/j.ydbio.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10:778-90. PMID:19851336. https://doi.org/ 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hellerschmied D, Clausen T. Myosin chaperones. Curr Opin Struct Biol 2014; 25:9-15. PMID:24440450. https://doi.org/ 10.1016/j.sbi.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iliev AI, Djannatian JR, Nau R, Mitchell TJ, Wouters FS. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc Natl Acad Sci U S A 2007; 104:2897-902. PMID:17301241. https://doi.org/ 10.1073/pnas.0608213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Iliev AI, Djannatian JR, Opazo F, Gerber J, Nau R, Mitchell TJ, Wouters FS. Rapid microtubule bundling and stabilization by the Streptococcus pneumoniae neurotoxin pneumolysin in a cholesterol-dependent, non-lytic and Src-kinase dependent manner inhibits intracellular trafficking. Mol Microbiol 2009; 71:461-77. PMID:19040644. https://doi.org/ 10.1111/j.1365-2958.2008.06538.x. [DOI] [PubMed] [Google Scholar]
- [28].Abreu-Blanco MT, Verboon JM, Parkhurst SM. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr Biol 2014; 24:144-55. PMID:24388847. https://doi.org/ 10.1016/j.cub.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barfod ET, Moore AL, Van de Graaf BG, Lidofsky SD. Myosin light chain kinase and Src control membrane dynamics in volume recovery from cell swelling. Mol Biol Cell 2011; 22:634-50. PMID:21209319. https://doi.org/ 10.1091/mbc.E10-06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mellgren RL. A plasma membrane wound proteome: reversible externalization of intracellular proteins following reparable mechanical damage. J Biol Chem 2010; 285:36597-607. PMID:20810652. https://doi.org/ 10.1074/jbc.M110.110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wolfmeier H, Schoenauer R, Atanassoff AP, Neill DR, Kadioglu A, Draeger A, Babiychuk EB. Ca+-dependent repair of pneumolysin pores: A new paradigm for host cellular defense against bacterial pore-forming toxins. Biochim Biophys Acta 2015; 1853:2045-54. PMID:25219550. https://doi.org/ 10.1016/j.bbamcr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- [32].Bischof LJ, Kao CY, Los FC, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog 2008; 4:e1000176. PMID:18846208. https://doi.org/ 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ansa-Addo EA, Thaxton J, Hong F, Wu BX, Zhang Y, Fugle CW, Metelli A, Riesenberg B, Williams K, Gewirth DT, Chiosis G, et al.. Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94. Curr Top Med Chem 2016; 16:2765-78. PMID:27072698. https://doi.org/ 10.2174/1568026616666160413141613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Blebbing confers resistance against cell lysis. Cell Death Differ 2011; 18:80-9. PMID:20596076. https://doi.org/ 10.1038/cdd.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Atanassoff AP, Wolfmeier H, Schoenauer R, Hostettler A, Ring A, Draeger A, Babiychuk EB. Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of streptolysin O-induced plasmalemmal damage. PLoS One 2014; 9:e89743. PMID:24587004. https://doi.org/ 10.1371/journal.pone.0089743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, Zander SA, Mleczak A, Sumpton D, Morrice N, Bienvenut WV, et al.. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 2013; 20:1293-305. PMID:23787996. https://doi.org/ 10.1038/cdd.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell 2015; 161:1306-19. PMID:26027738. https://doi.org/ 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Capasso D, Pepe MV, Rossello J, Lepanto P, Arias P, Salzman V, Kierbel A. Elimination of Pseudomonas aeruginosa through Efferocytosis upon Binding to Apoptotic Cells. PLoS Pathog 2016; 12:e1006068. PMID:27977793. https://doi.org/ 10.1371/journal.ppat.1006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Czuczman MA, Fattouh R, van Rijn JM, Canadien V, Osborne S, Muise AM, Kuchroo VK, Higgins DE, Brumell JH. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature 2014; 509:230-4. PMID:24739967. https://doi.org/ 10.1038/nature13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zuck M, Ellis T, Venida A, Hybiske K. Extrusions are phagocytosed and promote Chlamydia survival within macrophages. Cell Microbiol 2017; 19:e12683. PMID:27739160. https://doi.org/ 10.1111/cmi.12683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.