Abstract
Objective:
This study aimed to assess the effect of new generation oral, direct factor Xa inhibitor rivaroxaban on intimal hyperplasia and smooth muscle cell proliferation at the carotid artery anastomosis site of rabbits.
Methods:
In total, 14 New Zealand male rabbits weighing 3–3.5 kg were randomized into two groups. Group A (7 rabbits) served as the control group and received no medication. Rivaroxaban was perorally administered to group B (7 rabbits) mg/kg/day for 28 days. Following anesthesia induction, carotid arteries were dissected through a right neck incision. following heparinization at 100 IU/kg, vertical full thickness arteriotomy was performed, then was repaired continuously with 8-0 polypropylene. At day 28, all rabbits were sacrificed and the anastomosed carotid artery segments were analyzed using light microcopy. Hematoxylin–eosin and Masson’s trichrome stained images were analyzed using a digital image analysis program, and lumen diameter, lumen area, intimal and medial thickness, and media areas were measured and results were compared.
Results:
In the serial sections, the average lumen diameter of group B was higher than that of group A (p=0.001). The lumen areas of group B were also higher than those of group A (p=0.004). The intimal thickness of group B was lower than that of group A (p=0.001). When the section series were evaluated for media thickness, the thickness of group B was lesser than that of group A; the difference was statistically significant (p=0.002).
Conclusion:
This study may imply a potential midterm benefit of rivaroxaban following arterial anastomosis by reducing intimal proliferation and restenosis.
Keywords: rivaroxaban, carotid artery, intimal hyperplasia, smooth muscle cell proliferation, rabbit
Introduction
Intimal hyperplasia is characterized by vascular smooth muscle cell (VSMC) proliferation, inflammatory cell infiltration, endothelial cell injury, and augmented position of extracellular matrix. Intimal hyperplasia development is a vasoactive process, which begins with endothelial injury and ends up with partial or total restenosis in the long period. These mechanisms produce vascular lumen renarrowing or restenosis, leading to the failure of bypass or angioplasty, which is commonly performed to treat cardiovascular disease (1). Intimal hyperplasia is not only an adaptive process against the hemodynamic stress but also a characteristic of arterial injury healing. Reconstructive operations are frequently performed for treating obstructive arterial diseases, and operative success is frequently undermined by spontaneous thrombosis and restenosis during follow-up. While coronary arterial stent implantation without preventive intervention the incidence of restenosis can reach approximately 30%–50% within 6 months (2).
Rivaroxaban is a novel oral anticoagulant that inhibits direct-acting factor Xa, which is central in the coagulation cascade and is involved in the initiation, amplification, and propagation phases of clot formation (3). Exposure to tissue factor following plaque rupture or erosion activates factor Xa, which catalyzes the conversion of prothrombin to active thrombin. It also plays an important role in both cellular proliferation and inflammatory pathway (4,5). Although rivaroxaban is commonly used in patients with atrial fibrillation and venous thrombosis, only a few trials have evaluated its role in arterial diseases, such as acute coronary syndrome or carotid artery dissection (6, 7). We believe it might also have a beneficial effect in this setting through its effects on intimal hyperplasia associated with arterial healing via avoiding biochemicals’ effects which participating coagulation cascade and induces the expression of cell adhesion molecules such as vascular endothelial growth factor (8).
To evaluate the effect of rivaroxaban on vascular proliferation following arterial surgery, we assessed the effect of rivaroxaban on intimal hyperplasia and smooth muscle cell proliferation in a rabbit carotid artery anastomosis model.
Methods
Grouping stage in experimental animals
In total, 14 New Zealand male rabbits averagely weighing 3.35 (± 0.264 kg) and aged 2 years (range, 20–26 months) were selected for the experiment. All animals were housed separately and kept at an ambient temperature of 25°C and 7% humidity. They were randomly divided into two groups of seven animals. Group A rabbits served as the control group and received no medication. Rivaroxaban (Xarelto, Bayer HealthCare AG, Wuppertal, Germany) dispersed in 2 mL water was perorally administered to group B 3 mg/kg/day for 28 days. The nutrition was maintained with standard rabbit chow, with single cage feeding and free drinking water, and the total daily food intake was about 120 g.
Surgical procedure
In both groups the surgical protocol was the same: for anesthesia, 50 mg/kg ketamine (Ketalar, Pfizer, İstanbul, Turkey) and 5 mg/kg xylazine (Alfazyne, Bayer, Istanbul, Turkey) was administered to the gluteus muscle. Animals were first placed in supine position. After shaving and disinfection, a right vertical neck incision was made. Right carotid arteries were identified near the trachea and excised approximately 1 cm for the operation. Next, with a silk suture to its vertical axis, a 1-mm incision was made in the blocked interval of the artery using an iris blade for coronary surgery after giving 100 IU/kg heparin by ear vein. The right carotid artery segment was sutured with continuous suture technique using 8-0 polypropylene (Ethicon, Somerville, NJ, 6.5 mm 3-8 circle, US). Maintenance of arterial blood flow during the procedure was achieved using this technique. Skin closure was performed with 2-0 polypropylene (Johnson & Johnson Medical, Belgium). All procedures were performed with sterile instruments and surgical asepsis by a single operator using 3.5X magnification. Every animal survived the procedure and was followed-up for 28 days without any complications. While study animals (group B) received rivaroxaban daily by gavages, control animals received normal food and water.
At day 28, all animals were anesthetized using the same protocol. The right carotid arteries were removed. The arterial segments were kept in 10% neutral formalin and sent to histology laboratory for analysis. All rabbits were sacrificed using 150 mg/kg pentothal at the end of this study.
Laboratory methods
The arterial segments were stained using hematoxylin–eosin and Masson’s trichrome; then, xylene was utilized for pellucidity, and they were embedded in paraffin. Next, 5 µm thickness slides were gained using rotary microtome (RM 2255-Leica, Leica Biosystems Nussloch, Germany) and examined under light microscope by a histologist who was blinded to the groups.
Statistical analysis
Statistical Package for the Social Sciences for windows version 15.0 for windows (SPSS, Chicago, IL, USA) was used for acquired data analysis. Measured data were presented as mean±standard deviation. Each group had only seven subjects; Mann–Whitney U test was used for comparison between the two groups.
A p value of <0.05 was considered statistically significant within 95% confidence range. Posteriori power analysis applied to each researched parameter.
Results
Arterial anastomosis was patent in all animals in both the groups at the end of the study at 28 days. After preparation, histological slides at the level of the suture lines were compared for the luminal diameter, luminal area, intimal thickness, and medial thickness. Slides were stained using both hematoxylin–eosin (Fig. 1a–d) and Masson’s trichrome (Fig. 2a–d) to clarify the measurements. Group A images were shown in 10X (Fig. 1a, 2a) and 50X (Fig. 1b, 2b) magnifications. Group B images were also shown with the same magnification (Fig. 1c, 1d, 2c, 2d). Tunica intima was marked red in all images for comparison. Mean and standard deviation of the measured parameters are summarized with minimum and maximum values in Table 1.
Figure 1.
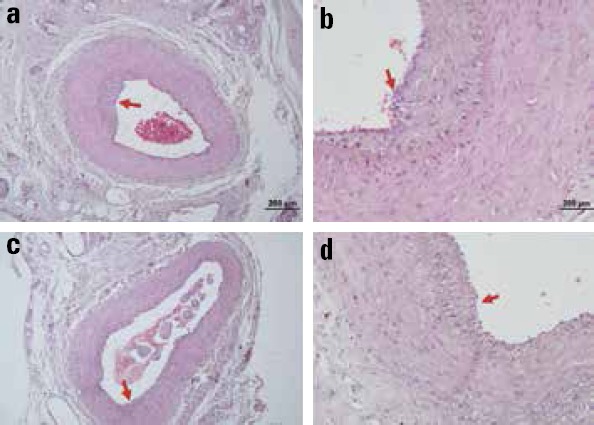
10X and 50X images stained using hematoxylin–eosin under light microscope. Group A (a, b); Group B (c, d). Tunica intima marked red in all images for comparison
Figure 2.
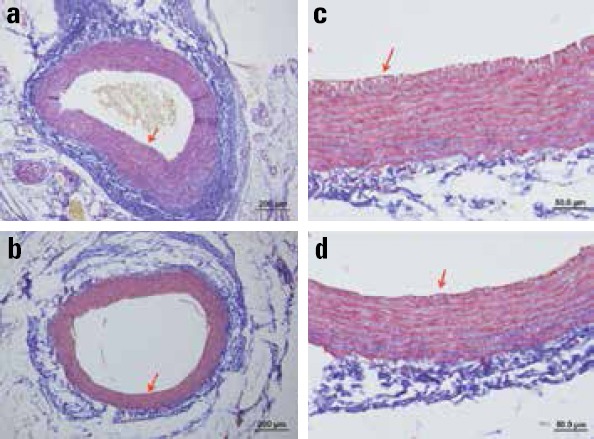
10X and 50X images stained using Masson’s trichrome under light microscope. Group A (a, b); Group B (c, d). Tunica intima marked red in all images for comparison
Table 1.
Luminal diameter, lumen area, intimal and medial thickness measurements with mean and median values. Group A and Group B
| Valid N | Mean | Standard deviation | Median | Minimum | Maximum | P | |||
|---|---|---|---|---|---|---|---|---|---|
| GROUP | A | Luminal diameter, µm | 7 | 527.45 | 106.14 | 513.04 | 476.49 | 779.62 | P=0.001 |
| B | Luminal diameter, µm | 7 | 770.55 | 88.33 | 783.89 | 725.947 | 860.82 | ||
| GROUP | A | Luminal area, µm2 | 7 | 258,616.93 | 164.53 | 231,363.07 | 171,504 | 447,037.55 | P=0.004 |
| B | Luminal area, µm2 | 7 | 443,505.33 | 580.22 | 513,384.90 | 402,420.90 | 726,744.20 | ||
| GROUP | A | Intimal thickness, µm | 7 | 33.286 | 5.14 | 37.626 | 22.133 | 69.946 | P=0.001 |
| B | Intimal thickness, µm | 7 | 12.415 | 3.25 | 13.016 | 10.54 | 15.236 | ||
| GROUP | A | Media thickness, µm | 7 | 142.653 | 32.14 | 133.806 | 108.956 | 161.503 | P=0.002 |
| B | Media thickness, µm | 7 | 104.965 | 29.766 | 101.586 | 86.99 | 115.976 |
Luminal diameters
The mean luminal diameter was 527.45±106.14 µm (476.49–779.62 µm) in group A and 770.55±88.33 µm (725.947–860.82 µm) in group B. It was significantly larger in group B (p=0.001) (Fig. 3a). Posteriori power analysis calculated was 0.96 (a=0.05).
Figure 3.
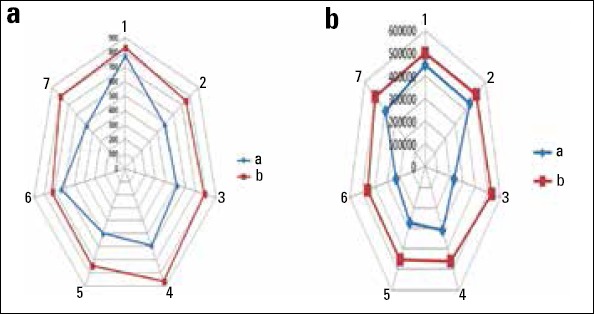
(a) Matching luminal diameters. Group A and Group B. (b) Matching luminal areas. Group A and Group B
Luminal areas
The mean luminal area was 258,616.93±164.53 µm2 (171,504–447,037 µm2) in group A and 443,505.33±580.22 µm2 (402,420.9–726,744.2 µm2) in Group B. It was significantly lesser in group A. The results were statistically significant (p=0.004) (Fig. 3b). Post-hoc power analysis resulted as 0.12 (a=0.05).
Intimal thickness
The mean intimal thickness was 33.28±5.14 µm (22.61–69.94 µm) in group A and 12.41±3.25 µm (10.54–15.23 µm) in group B. Group B had significantly less intimal width compared with group A (p=0.001) (Fig. 4a). Outcomes were applied to post-hoc power analysis as 1.00 (a=0.05).
Figure 4.
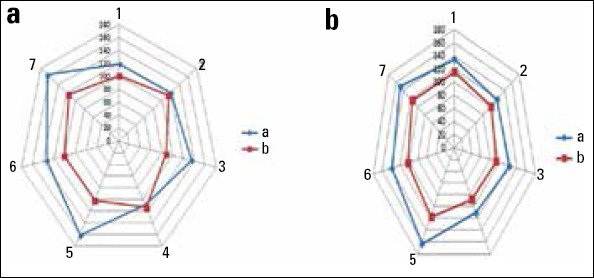
(a) Matching intimal thickness. Group A and Group B. (b) Matching medial thickness. Group A and Group B
Medial thickness
The mean medial thickness was 142.6±32.16 µm (108.9–161.5 µm) in group A and 104.29±29.76 µm (101.59–115.98 µm) in group B. Group B had significantly less medial and intimal proliferation compared with group A (p=0.002) (Fig. 4b). Posteriori power analysis calculated as 0.62 (a=0.05).
Discussion
Using histomorphological examinations, we demonstrated that the outcomes of rivaroxaban on the cellular and ultrastructural changes focused on intimal hyperplasia and smooth muscle cell proliferation in a rabbit carotid artery model. Hereby, we have revealed that rivaroxaban could significantly inhibit the development of intimal hyperplasia and smooth muscle cell proliferation.
Rivaroxaban is a well-known NOAC and is mostly used in patients with atrial fibrillation or venous thrombosis. Thus, various studies have been previously conducted on the basis of these effects (9, 10). But nowadays, diversified studies have been conducted, for example, Hara et al. (11) demonstrated that rivaroxaban attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Distinctly in our study, rabbit was chosen instead of a rat or a mouse model, based on the close factor Xa patency of rabbits to humans (12).
On the other hand, intimal hyperplasia caused by endothelial dysfunction is associated with higher proinflammatory and procoagulant changes (13). Despite numerous agents which prevent thrombosis occurrence via anticoagulant, antiplatelet or fibrinolytic effects have been studied on this subject so far; however, to our knowledge, this is the first study that hypothesized an association between rivaroxaban and postsurgical restenosis.
Therewithal, some factor Xa inhibitors were recently researched with a similar purpose. Iba et al. (14) have researched low molecule weight heparin (LMWH) effects on endotelial damage in rat model. Three groups each had six rats in trial. LMWH, factor Xa inhibitor, and placebo were administered before mesenteric arterial punction and the slices taken from bleeding area examinated. Even though the bleeding area was larger in anticoagulant group than in the placebo group, leucocyte adhesion and endothelial damage were less at the end. Consequently, it was postulated in this study that both of these drugs, which inhibit factor Xa, also decrease endothelial damage and inflammatory processes.
Hara et al. (11) experimented in mice model and showed that rivaroxaban attenuates neointimal formation via inhibition of both proinflammatory and proliferative activation of VSMCs. Percutaneously, vascular injury was constituted by wire; then, mice were administered 5 mg/kg/day rivaroxaban for 4 weeks, and results were compared with control group. Neointimal area and intima/media ratio was significantly in less in the rivaroxaban group. Furthermore, in vitro experiments determined that rivaroxaban reduced factor Xa and increased mRNA expression of inflammatory and vasoproliferative molecules.
Consequently, their results demonstrated that both proinflammatory activation of macrophages and proliferative activation of VSMCs are prevented in the presence of rivaroxaban. At the end of this study, they pretend that for further studies, factor Xa may serve as a beneficial target for neointima formation after vascular injury and rivaroxaban may be utilized as an efficient drug for that purpose (15).
Additively, Kim et al. (16) have been planning a randomized prospective study, with the aim of evaluating the effects of dabigatran and rivaroxaban on endothelial intima, medial thickness, and atheroma plaques in carotid artery in patients with atrial fibrillation. Warfarin will be administered to control group. The ending date was determined as 2018. Within this study, same processes end-up with restenosis will be assessed in humans (16). Apparently, this trial has a great importance as it is first study in humans assessing rivaroxaban effect on intimal and medial changes.
Taken together, these findings may assert a possible protective effect of rivaroxaban on intimal hyperplasia and smooth muscle cell proliferation progress.
Study limitations
There are some limitations to the present study. First of all, vascular healing is a large and complicated process containing various biochemical steps; thus, the number of animals was limi-ted to determine all this steps. Moreover, due to limited budget we were not able to investigate detailed immunochemical markers participating in the process. Therefore, mostly carotid artery surgery process ends with a patch grafting made by saphenous vein or polytetrafluorethylene. Due to the small vessel size, these methods could not be performed and reflected in the results. On the other hand, new anticoagulants, such as LMWHs, have not been researched in arterial diseases yet. For all these reasons, further human studies should be done on this subject with new oral anticoagulants in larger groups.
Conclusion
Similar to various previous studies with different models, our surgical anastomosis model in rabbits showed that rivaroxaban caused a statistically significant decrease in intimal hyperplasia and medial thickness resulting in greater luminal area and diameter. The results can be attributed to the blocking of inflammatory processes with factor Xa inhibition. Therefore, the lumen diameter and luminal area increment can be regarded as a protective effect against restenosis.
In brief, rivaroxaban as an easy to use oral drug may have a valuable preventive effect on postsurgical arterial restenosis.
Footnotes
Funding The authors received no financial support for the research and/or authorship of this article.
Conflict of interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – G.A.; Design – G.A.; Supervision – Ç.B.; Materials – K.İ.; Data collection &/or processing – P.A.; Analysis &/or interpretation – P.A.; Literature search – T.G..; Writing – G.A.; Critical review – B.U.

Biochemist, MD. Meral Eguz’s collections
References
- 1.Wang B, Zhang M, Takayama T, Shi X, Roenneburg DA, Kent KC, et al. Bromodomain Blockade Mitigates Intimal Hyperplasia in Rat Carotid Arteries. EbioMedicine. 2015;2:1650–61. doi: 10.1016/j.ebiom.2015.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jukema JW, Ahmed TA, Verschuren JJ, Quax PH. Restenosis after PCI. Part 2: prevention and therapy. Nat Rev Cardiol. 2012;9:79–90. doi: 10.1038/nrcardio.2011.148. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65. [PubMed] [Google Scholar]
- 4.Herbert J, Bono F, Herault J, Avril C, Dol F, Mares A, et al. Effector protease receptor 1 mediates the mitogenic activity of factor Xa for vascular smooth muscle cells in vitro and in vivo. J Clin Invest. 1998;101:993–1000. doi: 10.1172/JCI1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirino G, Cicala C, Bucci M, Sorrentino L, Ambrosini G, DeDominicis G, et al. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Invest. 1997;99:2446–51. doi: 10.1172/JCI119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A, Garg A, Borer JS, Krishnamoorthy P, Garg J, Lavie CJ, et al. Role of oral factor Xa inhibitors after acute coronary syndrome. Cardiology. 2014;129:224–32. doi: 10.1159/000368747. [DOI] [PubMed] [Google Scholar]
- 7.Mustanoja S, Metso TM, Putaala J, Heikkinen N, Haapaniemi E, Salonen O, et al. Helsinki experience on nonvitamin K oral anticoagulants for treating cervical artery dissection. Brain Behav. 2015;5:e00349. doi: 10.1002/brb3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seri A, Marta DS, Madalan A, Popescu M, Tiglea AI, Moldoveanu E. Lipoprotein-associated phospholipase A2, myeloperoxidase and vascular endothelial growth factor - predictors of high vascular risk in respiratory bacterial infections. J Med Life. 2016;9:429–33. [PMC free article] [PubMed] [Google Scholar]
- 9.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a mar-ker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN. Arterial compliance to stratify cardiovascular risk: more precision in therapeutic decision making. Am J Hypertens. 2001;14:258–63. doi: 10.1016/s0895-7061(01)02154-9. [DOI] [PubMed] [Google Scholar]
- 11.Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, et al. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis. 2015;242:639–46. doi: 10.1016/j.atherosclerosis.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Wong PC, Crain EJ, Watson CA, Xin B. Favorable therapeutic index of the direct factor Xa inhibitors, apixaban and rivaroxaban, compared with the thrombin inhibitor dabigatran in rabbits. J Thromb Haemost. 2009;7:1313–20. doi: 10.1111/j.1538-7836.2009.03503.x. [DOI] [PubMed] [Google Scholar]
- 13.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, et al. The endothelial cell ecto -ADPase responsible for inhibition of platelet function is CD39. J Clin Inves. 1997;99:1351–60. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iba T, Okamoto K, Ohike T, Tajirika T, Aihara K, Watanabe S, et al. Enoxaparin and fondaparinux attenuates endothelial damage in endotoxemic rats. J Trauma Acute Care Surg. 2012;72:177–82. doi: 10.1097/TA.0b013e31821a83f0. [DOI] [PubMed] [Google Scholar]
- 15.Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Yagi S, et al. Rivaroxaban, a direct factor Xa inhibitor, attenuates-neointima formation after mechanical vascular injury. Circulation. 2014;130:A13438. [Google Scholar]
- 16.Kim JB, Joung HJ, Lee JM, Woo JS, Kim WS, Kim KS, et al. Evaluation of the vascular protective effects of new oral anticoagulants in high-risk patients with atrial fibrillation (PREFER-AF): study protocol for a randomized controlled trial. Trials. 2016;17:422. doi: 10.1186/s13063-016-1541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


