Abstract
Short-term bed rest in older adults is characterized by significant loss in leg lean mass and strength posing significant health consequences. The purpose of this study was to determine in healthy older adults if the daily combination of neuromuscular electrical stimulation and protein supplementation (NMES+PRO) would protect muscle mass and function after 5 days of bed rest. Twenty healthy older adults (∼70 years) were subjected to 5 days of continuous bed rest and were randomized into one of two groups: NMES+PRO (n = 10) or control (CON) (n = 10). The NMES+PRO group received bilateral NMES to quadriceps (40 minutes/session, 3 × /day; morning, afternoon, and evening) followed by an interventional protein supplement (17 g). The CON group received an isocaloric equivalent beverage. Before and after bed rest, vastus lateralis biopsies occurred before and after acute essential amino acid (EAA) ingestion for purposes of acutely stimulating mechanistic target of rapamycin (mTORC1) signaling, a major regulator of muscle protein synthesis, in response to bed rest and NMES+PRO. Baseline (pre and post bed rest) muscle samples were also used to assess myofiber characteristics and gene expression of muscle atrophy markers. Thigh lean mass and muscle function were measured before and after bed rest. Five days of bed rest reduced thigh lean mass, muscle function, myofiber cross-sectional area, satellite cell content, blunted EAA-induced mTORC1 signaling, and increased myostatin and MAFbx mRNA expression. Interestingly, NMES+PRO during bed rest maintained thigh lean mass, but not muscle function. Thigh muscle preservation during bed rest with NMES+PRO may partly be explained by attenuation of myostatin and MAFbx mRNA expression rather than restoration of nutrient-induced mTORC1 signaling. We conclude that the combination of NMES and protein supplementation thrice a day may be an effective therapeutic tool to use to preserve thigh muscle mass during periods of short-term hospitalization in older adults. However this combined intervention was not effective to prevent the loss in muscle function.
Keywords: : disuse, aging, mTORC1, NMES, leucine, atrophy
Introduction
The hallmark sign of disuse in older adults as a result of hospitalization, injury, or illness is the rapid deterioration of skeletal muscle mass and strength. Repeated cycles of disuse-induced atrophy followed by incomplete muscle recovery1,2 has been suggested to be a contributor to the development of age-related sarcopenia.3,4 Therefore, interventional strategies are needed to offset deficits in muscle function with disuse in older adults, thereby halting the cascade of deleterious physical and health consequences associated with sarcopenia.
A primary mechanism of muscle atrophy during short-term disuse (e.g., bed rest, limb immobilization, reduced activity) in healthy young and older adults is the reduced acute amino acid stimulation of muscle protein synthesis5–7 regulated by the mechanistic target of rapamycin (mTORC1) signaling pathway.8,9 It is well known that muscle contraction and essential amino acids (EAAs) are powerful anabolic stimuli to activate mTORC1 signaling and fundamental to maintain skeletal muscle mass and possibly strength.10 Therefore, practical therapeutic strategies capitalizing on these core principles of maintaining muscle mass and strength may be effective in preserving muscle health in older adults during short-term disuse periods in which ambulation may not be feasible or possible such as hospitalization, the most common form of muscle disuse in aging.
Leucine or the leucine metabolite, β-hydroxyl-β-methylbutyrate, provided daily to middle age and older adults for 10–14 days of bed rest has been shown to partially preserve muscle mass and function.11,12 However, preventative strategies to preserve muscle mass and function during shorter periods of disuse (e.g., 5 days) akin to what occurs during acute hospitalization,13 have been largely unexplored in older adults especially during bed rest. In a recent study, Dirks et al. found that a provision of protein (∼20 g) twice daily was not effective to limit leg muscle atrophy in older adults during 5 days of cast immobilization14 suggesting that more potent interventions are needed during the early periods of disuse.
Neuromuscular electrical stimulation (NMES) has a long history of being a safe and effective intervention to enhance muscle size and strength during disuse and rehabilitation.15–19 More recently, NMES has been used as an inpatient intervention to offset muscle, strength, and physical function associated with muscle deconditioning in critically ill patients.20–22 In healthy young adults, the positive effects of NMES on muscle may occur by activation of mTORC1 signaling,23 stimulation of muscle protein synthesis,24 and/or enhancement of protein synthesis before pre-sleep protein feeding.25 In addition, NMES may attenuate molecular regulators associated with muscle atrophy. For example, Dirks et al. showed that daily NMES delivered twice daily during 5 days of cast immobilization protected against disuse-induced leg muscle losses, but not strength, and this corresponded with the attenuation of mRNA markers of muscle atrophy, namely myostatin and MAFbx.26 Unfortunately, practical and robust strategies to moderate muscle loss and strength in older adults during short-term bed rest (e.g., 5 days) have not been investigated.
The purpose of this study was to test if the combination of daily NMES and protein supplementation would be a potent two-pronged approach to preserve the loss of muscle and strength in older adults confined to 5 days of bed rest. We hypothesized that daily NMES and protein supplementation during 5 days of bed rest in older adults would preserve lower extremity muscle mass and strength. Second, we hypothesized that molecular readouts of muscle protein synthesis (nutrient-induced mTORC1 signaling) and protein breakdown would relate to changes in muscle mass and strength with and without NMES+PRO after 5 days of bed rest.
Methods
Participants
Twenty healthy older adults (60–80 years; BMI <30 kg/m2) (Table 1) were recruited from around the Salt Lake City area utilizing radio advertisement and word of mouth. Participants were excluded during screening if they had: uncontrolled hypertension, diabetes, HIV, hepatitis B and/or C, hyper/hypothyroidism, cardiovascular, kidney, respiratory, vascular and liver disease, history of DVT, neurological disorders, recent cancer treatment (within 1 year of enrolling), osteopenia, depression, and alcohol or other substance abuse. Enrolled participants were modestly physically active as defined as weekly walks, hikes, bike rides, and/or participation in approximately 2 sessions of aerobic or resistive-type activities/week. All subjects read and signed the informed consent form. The study took place at the University of Utah (November 2014–May 2016) and was approved by the University of Utah Institutional Review Board, which is in agreement with the Declaration of Helsinki. This study is registered at www.clinicaltrials.org (NCT02566590).
Table 1.
Participant Characteristics and Body Composition Before and After 5 Days Bed Rest in CON and NMES+PRO Groups
| CON | NMES+PRO | |||||
|---|---|---|---|---|---|---|
| PreBR | PostBR | Δ (%) | PreBR | PostBR | Δ (%) | |
| Age (years) | 69.0 ± 2.0 | — | — | 70.0 ± 2.0 | — | — |
| M/F | 9/1 | — | — | 8/2 | — | — |
| Activity level (steps/day) | 6588 ± 241 | — | — | 6299 ± 265 | — | — |
| Height (m) | 1.75 ± 0.02 | — | — | 1.72 ± 0.04 | — | — |
| Weight (kg) | 77.3 ± 2.7 | 76.3 ± 2.7* | −1.5 ± 0.5 | 76.5 ± 3.6 | 75.5 ± 3.5* | −1.3 ± 0.4 |
| BMI (kg/m2) | 25.3 ± 1.0 | 24.9 ± 1.0* | −1.4 ± 0.4 | 25.7 ± 0.8 | 25.4 ± 0.8* | −1.3 ± 0.4 |
| Lean mass (kg) | 52.2 ± 1.9 | 51.0 ± 1.9* | −2.3 ± 0.7 | 49.9 ± 2.9 | 49.1 ± 2.9* | −1.6 ± 0.7 |
| Trunk lean mass (kg) | 26.4 ± 1.1 | 25.6 ± 1.1* | −2.9 ± 0.8 | 25.5 ± 1.5 | 24.9 ± 1.4* | −2.3 ± 1.1 |
| Leg lean mass (kg) | 16.8 ± 0.6 | 16.2 ± 0.5* | −2.9 ± 1.1 | 15.6 ± 11.1 | 15.3 ± 10.8 | −1.8 ± 1.0 |
| Fat mass (kg) | 22.3 ± 2.0 | 22.4 ± 2.1 | 0.6 ± 0.8 | 23.9 ± 1.1 | 23.7 ± 1.1 | −0.8 ± 1.1 |
| Leg MQ (Nm/kg) | 11.7 ± 0.3 | 10.1 ± 0.2* | −10.6 ± 1.7 | 10.1 ± 0.5 | 9.3 ± 0.4* | −14.2 ± 1.9 |
Values are mean ± SEM. Leg MQ was determined as the ratio of leg isometric strength (Nm) relative to thigh lean mass (kg). One subject was unable to complete post bed rest strength tests; therefore leg strength and muscle quality were n = 9 for the NMES+PRO group.
Different than respective PreBR (p < 0.05).
BR, bed rest; MQ, muscle quality; NMES+PRO, neuromuscular electrical stimulation and protein supplementation.
Experimental design
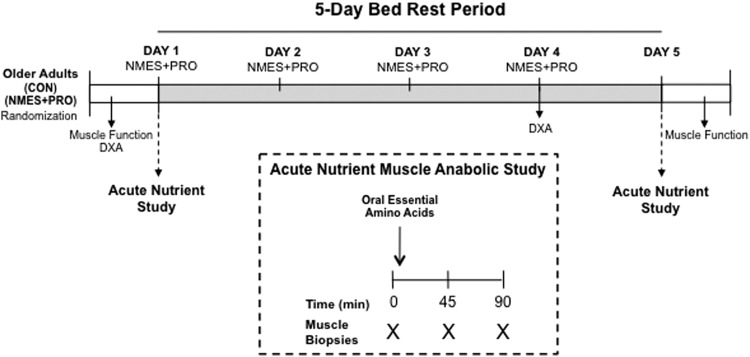
Enrolled participants were randomized (blocked and balanced by sex) into one of two groups: neuromuscular electrical stimulation in addition to protein supplementation (NMES+PRO) or control (CON) and underwent a 5-day bed rest study (Fig. 1). Tissue composition (through dual energy X-ray absorptiometry [DXA]) and muscle function (leg strength and power) were determined before and immediately after bed rest. Participants in the intervention group (NMES+PRO) received 40 minutes of NMES (3 × /day) followed by protein supplementation. A nutrient anabolic sensitivity study was conducted before and after bed rest in which thigh muscle biopsies were sampled in the fasted condition (for purposes of measuring fiber characteristics) and after ingestion of EAA for purposes of capturing EAA induced muscle mTORC1 signaling.
FIG. 1.
Experimental design. Participants underwent 5 days of bed rest and were randomized into a CON or NMES+PRO group. The NMES+PRO treatment group was given NMES and a protein supplement thrice a day (morning, afternoon, and evening) for a total of 12 sessions. The first NMES+PRO treatment session was provided immediately after the first acute nutrient study experiment. Muscle strength and power were measured before and after bed rest. Tissue composition using DXA scan was determined before and at day 4 of bed rest. CON, control; DXA, dual energy X-ray absorptiometry; NMES+PRO, neuromuscular electrical stimulation and protein supplementation.
Tissue composition and muscle and physical function
Lean mass (whole body and isolated thigh region), fat mass, knee extensor isometric strength, and leg power occurred within 1 week before bed rest and repeated the next day following completion of the 5-day bed rest period. However, to be consistent with our previous 5-day bed rest study,8 lean mass (through DXA) was determined at day 4 of bed rest. The DXA (Hologic) scan was measured in the morning after an overnight fast and immediately before a 60-minute supine rest period.27 DXA encompassed whole body analysis and isolated thigh. The isolated thigh region was defined as the end plate of the femur and top of the iliac crest. Isometric strength was assessed with a maximal voluntary isometric contraction effort developed by the knee extensors (at a 60° knee angle) on a HUMAC NORM (CSMi) dynamometer system. Both legs were assessed and averaged for final analysis for DXA and strength testing. DXA measurement and strength testing conditions were similar for all participants before and after each of their bed rest periods.
Physical function was determined following the strength and power tests using three tests: (1) distance covered in the 6-minute walk (6 MW) test, (2) timed up and go (TUG), and (3) gait speed (GS). These performance tests were chosen to represent mobility function and have been shown to be both valid and reliable in older adults.28–30 The 6 MW test, a measure of the distance the subject was able to walk without running in 6 minutes, was recorded in meters.29 The TUG test required participants to rise from a seated position, walk out 3 m, turn around, and return to the seated position as quickly and safely as possible. Self-selected GS (m/s) was measured over a 10 m course. Individuals were instructed to walk at a comfortable pace over a 10 m path and timed with a stop watch. The TUG and GS were conducted three consecutive times, and the average was recorded.
Nutritional and physical activity assessment
Before bed rest, participants were given specific instruction to record food intake over 5 days (including 1 weekend day). Caloric intake was later calculated using the Food Processor Nutrition Analysis software and reported in Table 2. At the same time, participants were provided a step activity monitor (Orthocare Innovations) to measure daily habitual physical activity (Table 1). Participants were given a prepackaged dinner to consume the night before the bed rest protocol. The meal consisted of a standard caloric and macronutrient density based on the individuals' body weight as we have done previously for bed rest studies.8,9 All participants reported eating the standardized meal.
Table 2.
Caloric and Macronutrient Intake Before (Habitual) and During Bed Rest
| CON | NMES+PRO | |||
|---|---|---|---|---|
| Habitual diet | Bed rest diet | Habitual diet | Bed rest diet | |
| Calories (kcal) | 2619 ± 75.2 | 1631 ± 19.7 | 2500 ± 83.9 | 1707 ± 28.4 |
| Fat (% of energy) | 37 ± 0.6 | 36 ± 0.3 | 31 ± 0.3 | 31 ± 0.1 |
| CHO (% of energy) | 49 ± 0.6 | 53 ± 0.5 | 57 ± 0.5 | 56 ± 0.1 |
| Protein (% of energy) | 16 ± 0.3 | 14 ± 0.3 | 16 ± 0.2 | 16 ± 0.1 |
| Fat (g) | 107 ± 2.2 | 57 ± 0.5* | 100 ± 3.1 | 58 ± 1.0* |
| CHO (g) | 327 ± 13.2 | 232 ± 1.7* | 331 ± 12.8 | 236 ± 4.1* |
| Protein (g) | 104 ± 2.8 | 65 ± 0.5* | 85 ± 2.7 | 66 ± 1.1* |
| Protein breakfast (g) | 25 ± 0.8 | 24 ± 0.3 | 13 ± 0.7# | 23 ± 0.5* |
| Protein lunch (g) | 62 ± 3.2 | 24 ± 0.2* | 32 ± 1.3# | 23 ± 0.3* |
| Protein dinner (g) | 77 ± 0.8 | 23 ± 0.1* | 40 ± 1.6# | 25 ± 0.4* |
Data are mean ± SE.
Different than habitual diet.
Different than CON.
CHO, carbohydrate.
5-day bed rest
Total caloric intake during bed rest was predetermined by the research dietician using the Harris–Benedict equation adjusted for no physical activity (physical activity level: 1.2). Daily caloric intake was evenly distributed over three meals (at hours 0800, 1300, and 1800) and composed of a macronutrient distribution of 15% protein, 55% carbohydrate, and 30% fat (Table 2). Participants were also provided water or noncaloric flavored beverages ad libitum throughout the 5 days of bed rest. All enrolled participants took part in a short-term (5 days) supervised bed rest study at the University of Utah Center for Clinical and Translational Science (CCTS) using an identical protocol and safety guidelines as we have described previously.8 The bed rest protocol was designed so that the participants spent a majority of their time in bed for 5 straight days and consistent with other recent bed rest studies, including studies conducted in our laboratory.8,9,31–35 We did not incorporate a head-down tilt approach as this study was not designed to simulate microgravity, but instead was used as a model to study acute periods of whole body disuse and nonweight bearing typically as a result of hospitalization. Specifically, during bed rest, the participant was allowed to adjust the hospital bed head height for reading, eating, browsing the internet, and watching television, but otherwise spent a majority of their time in bed with the bed flat for sleeping and napping. Bathing and hygiene activities were performed at the sink in a wheel chair. Participants also accessed the bathroom using a wheel chair assisted by nursing staff. Adherence to bed rest was monitored by nursing staff 24 hours a day. As part of standard of care for hospitalized patients, participants were treated with intermittent serial compression devices and were required to wear compression stockings on their lower limbs. In addition, participants were provided with daily (1 × /day; 20 minutes/visit) passive range of motion (by a physical therapist or physical therapy student) to the major lower extremity joints.
Acute nutrient muscle anabolic sensitivity experiment
After an overnight fast (10 hour fast), a muscle biopsy was sampled from the vastus lateralis using a modified Bergstrom needle approach with manual suction36 after application of 1% lidocaine (Fig. 1). Afterward, participants consumed an EAA drink (2.4 g leucine, 1.6 g histidine, 1.0 g isoleucine, 3.1 g lysine, 0.8 g methionine, 1.0 g phenylalanine, 1.2 g threonine, and 1.5 g valine) mixed in a low-calorie flavored water (5 kcal; Crystal Light). An additional muscle biopsy was sampled at 45 and 90 minutes after EAA ingestion. The second and third muscle biopsies were taken from a separate incision (∼7 cm from first incision) on the same leg. The biopsy needle for the second and third biopsies was angled such that the biopsies were sampled ∼5 cm from each other.
The follow-up nutrient anabolic sensitivity experiment was repeated after bed rest (day 5) under identical circumstances as the experiment on day 1 except muscle biopsies occurred on the opposite leg. Muscle tissue used for western and RNA analysis was immediately washed with cold saline and dissected of visible nonmuscle tissue, flash-frozen in liquid nitrogen, and stored at −80°C for later analysis. For histochemical analysis, a portion of the baseline muscle biopsy (not washed with saline) was carefully mounted on cork in OCT then frozen in liquid nitrogen cooled in isopentane. Samples were placed in dry ice then transported to a −80°C freezer for later sectioning and immunofluorescence.
Daily inpatient intervention
The NMES+PRO group underwent a dual contraction and nutrition intervention thrice a day (after each meal at 10AM, 2PM, and 7PM) on days 1–4 of bed rest for a total of 12 sessions. However, two subjects at the initiation of the study received NMES+PRO twice a day during bed rest, but no difference was found between these two subjects and the remaining subjects in this group so we pooled them together.
For the NMES protocol, neoprene sleeves were tightly wrapped around each thigh of the participant, while a towel roll was placed under the knees to set the knee angle at ∼30°. Three large (7.6 × 12.7 cm) self-adhesive surface electrodes, assisted by a trained nurse or investigator, were embedded on the sleeve and carefully placed on the vastus lateralis, vastus medialis, and rectus femoris of both legs.
The NMES protocol (EMPI Phoenix biphasic rectangular waveform stimulator) began with a 2 minute warm-up (6 Hz, 300 microseconds) in which the muscle twitched comfortably but did not contract. Immediately after, the protocol moved to the “work phase” for 20 minutes (75 Hz, 300 microseconds). The work phase consisted of an intermittent contraction (4 seconds) and rest period (10 seconds). The work phase was followed by a 3-minute cool down phase (3 Hz, 300 microseconds). The NMES protocol was repeated twice in a row for a total work phase time per session of 40 minutes. The intensity of the stimulus during the work phase was gaged by the investigators or CCTS nursing staff and defined as a visual muscle contraction yet set at a maximal intensity that the participant was able to tolerate. To be pragmatic with a goal of implementing NMES with hospitalized patients and for the participant to tolerate a higher NMES intensity with minimal discomfort,37 the participant was instructed to isometrically contract their quadriceps during each contraction phase since evidence suggests that NMES intensity is correlated with strength gains.38 To maintain the intensity level of the stimulus with each session, the participant was encouraged to manually increase the intensity during and with each subsequent session to the highest tolerable level. This NMES protocol was modified based upon previous NMES protocols used to acutely stimulate muscle protein synthesis rates in older type 2 diabetic patients,24 preserve muscle mass in young healthy26 and intensive care patients,20–22 and increase strength in older adults recovering from total knee replacement.18,19
Daily leucine-enriched whey protein supplementation (PRO) (BCAA PepForm; Glanbia Nutritionals) was provided thrice a day to the NMES+PRO group. The whey protein beverage/serving (80 kcal, 1 g fat, 3 g carbohydrate [CHO], 1 mg iron, and 160 mg sodium) was made up of 17 g of protein and enriched in leucine (4.5 g Leu). The protein supplement was dissolved in water by CCTS staff and ingested by the participant within 1 hour following each NMES session. To account for extra calories consumed by the NMES+PRO group, the CON group also received a beverage mixed in water thrice a day consumed at the same time of day and was similar in calories (80 kcal, <1 g fat, 19 g CHO, <1 g protein, 1 mg iron, 40 mg sodium) as the whey protein supplement, but nearly absent of protein. CON group did not receive the NMES intervention.
Immunohistochemistry
Skeletal muscle satellite cell content was quantified using traditional immunohistochemical methods adapted from Arentson-Lantz et al.39 Only n = 9 was used in each group due to methodological and tissue constraints. Day 1: Slides were fixed in 4% paraformaldehyde for 7 minutes then subsequently underwent epitope retrieval protocol using sodium citrate (10 mM, pH 6.5) at 92°C. Slides were washed in phosphate-buffered saline (PBS) and incubated overnight at 4°C in primary antibodies directed against myosin heavy chain 1 (BA.D5C, 1:75; Developmental Studies Hybridoma Bank) and laminin (No. L9393, 1:200; Sigma-Aldrich). Day 2: Following a wash in PBS, endogenous peroxidase activity was blocked with a 7-minute wash in 3% H2O2. Slides were then washed in PBS and incubated for 1 hour with appropriate secondary antibodies (MyHC I: goat anti-mouse IgG2b, Alexa Fluor 647, 1:250, Invitrogen A21242; Laminin: Goat anti-rabbit IgG1, Alexa Fluor 555, 1:500, Invitrogen A21429). Next, slides were washed in PBS and subsequently blocked in 2.5% normal horse serum (NHS) (Vector, S-2012) for 1 hour at room temperature, followed by an overnight incubation (4°C) with Pax7 primary antibody (Pax7-concentrate, 1:100; Developmental Studies Hybridoma Bank). Day 3: Slides were then incubated for 1 hour at room temperature in goat anti-mouse biotinylated secondary antibody (Cat No. 115-065205, 1:1000 in 2.5% NHS; Jackson Immuno Research). Utilizing a Tyramide Signal Amplification (TSA) Kit (T20932; Life Technologies), slides were incubated for 1 hour in streptavidin-horseradish peroxidase (1:100) and then for 20 minutes in AF488 tyramide (1:200). Finally, slides were counterstained with DAPI (No. D35471, 1:10,000; Invitrogen), washed in PBS, and mounted with fluorescent mounting media (H-1000, Vector).
Slides were analyzed using an upright fluorescent microscope (Nikon Eclipse Ti-E inverted microscope equipped with an automated stage). Images were captured at 20 × magnification and subsequently merged to seamlessly visualize the cross section in its entirety. Objects that were Pax7+/DAPI+ within a laminin border were counted as satellite cells. Myonuclei were assessed by counting objects stained DAPI+ that were also under the laminin border. Myonuclei were manually counted using ImageJ software to determine the number of myonuclei per fiber. Objects that were Pax7+/DAPI+ were not included in the myonuclear count. Captured images in each filter block were overlaid using NIS Elements software (Nikon). MyHC I, Pax7+/DAPI+ cell counting was performed using ImageJ software (U.S. National Institutes of Health), while myofiber cross-sectional area (CSA) analysis was performed using SMASH.40 For each image, the number of muscle fibers for pure MHC type I (purple converted to blue in FUJI for analysis in SMASH) and MHC II (negative stain) was counted, and CSA for MHC type I and II fibers was measured. Approximately 350 ± 140 fibers were analyzed for SC counting/fiber-type distribution and ∼158 ± 99 suitable muscle fibers were included for myofiber CSA analysis.
Immunoblotting
Proteins related to the mTORC1 signaling pathway were determined in fasted (0 minute) and EAA-stimulated (45 and 90 minutes) muscle biopsy samples using standard western blotting procedures as we have done previously.8,31 Frozen muscle biopsy samples (∼30 mg) were homogenized 1:10 (w/v) using a glass tube and mechanically driven pestle grinder in an ice-cold buffer containing 50 mM Tris (pH 7.5), 250 mM mannitol, 40 mM NaF, 5 mM pyrophosphate, 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100 with a protease inhibitor cocktail. Homogenates were centrifuged for 10 minutes at 4°C. After centrifugation, the upper phase (supernatant) was collected and the protein concentration determined using a modified Bradford protein assay and measured by a spectrophotometer (EPOCH; BioTek). Proteins from the supernatant fraction were separated by polyacrylamide gel electrophoresis (7.5% or 12%), transferred onto a polyvinylidene difluoride membrane, and incubated with primary and secondary antibodies directed against the proteins of interest.
Chemiluminescence reagent (ECL Plus; Thermo Scientific) was applied to each blot for 5 minutes. Membranes were exposed on a ChemiDoc XRS (Bio-Rad) and quantified with Image lab software (Bio-Rad). Membranes containing phospho-detected proteins were stripped (25 mM glycine, pH 2.0, and 1% SDS) of primary and secondary antibodies then reprobed for total protein. Pre and post bed rest samples from a single subject (six samples in replicate) were loaded on a single gel. We also included the same loading CON on each gel (loaded in duplicate) to correct for gel-to-gel variability. In the case for phospho-specific proteins, data were normalized to total protein levels of the target protein (i.e., S6K1 [Thr389]/total S6K1). REDD1 and REDD2 were normalized to GAPDH. Figures are reported as fold change from baseline levels (0 minute), and therefore, baseline was set to 1.
Antibodies
The specific antibodies (Cell Signaling Technologies), diluted in 5% bovine serum albumin or 2% non-fat dry milk, used to detect target proteins were: phospho-S6K1 (Thr389, 1:500; No. 9205), total S6K1 (1:1000, No. 9202), phospho-4EBP1 (Thr37/46, 1:1000; No. 9459), total 4EBP1 (1:1000; No. 9452), phospho-rpS6 (Ser240/244, 1:500; No. 2215), total rpS6 (1:1000; No. 2217), and GAPDH (1:10,000). REDD1 was purchased from Proteintech Group (1:1000; 10638-1-AP), and REDD2 was purchased from Abcam (1:2000; ab67431). Secondary antibodies (1:6000) were purchased from Santa Cruz Biotechnology.
Real-time PCR
Step-by-step methods for RNA isolation, cDNA synthesis, and real-time PCR can be found in a previous publication.41 Total RNA was isolated by homogenizing ∼15 mg tissue with a handheld homogenizer in a solution containing TRI Reagent RT (RT111; Molecular Research Center). The RNA was separated by Bromoanisole then precipitated using isopropanol. Extracted RNA was washed with ethanol then suspended in a known amount of nuclease-free water with EDTA. RNA concentration was determined using the EPOCH (Take3; BioTek) spectrophotometer. The average 260/280 ratio was 1.95 ± 0.003. RNA (1 μg) was synthesized into cDNA using a commercially available kit (iScript; Bio-Rad). All isolated RNA and cDNA samples were stored at −80°C until analyzed.
Real-time qPCR was carried out with a CFX Connect real-time PCR cycler (Bio-Rad) in combination with SYBR green fluorescence (iQ SYBR Green Supermix; Bio-Rad). Cycle threshold values of target genes were normalized to the geometric mean of beta 2-microglobulin, GAPDH, and β-actin, then fold change values were calculated (2−ΔΔCt). Forward and reverse primer sequences for human MURF-1, MAFbx, myostatin, and MyoD have been published previously.42,43 Independently and as geometric means, the housekeeping remained stable with bed rest and between groups. One subject had poor quality RNA and was removed from this analysis in the NMES+PRO group.
Statistical analysis
Data were analyzed with an unpaired t-test (subject characteristics), a two-way ANOVA (BED REST, GROUP) for body composition, muscle function, fiber characteristics, and proteolytic markers or three-way ANOVA (BED REST, GROUP, EAA) for nutrient-induced mTORC1 signaling. Analysis was followed up with Tukey post hoc multiple comparison adjustment. Spearman correlations were used to determine relationships between Pax7+ cells and fiber CSA and between the change in protein intake (from habitual to bed rest) and change in thigh lean mass after bed rest. Data are presented as mean ± SE, and p ≤ 0.05 was considered to be statistically significant. Analysis was carried out by GraphPad Prism version 7.0.
Results
Subject characteristics, tissue composition, nutritional information, and NMES
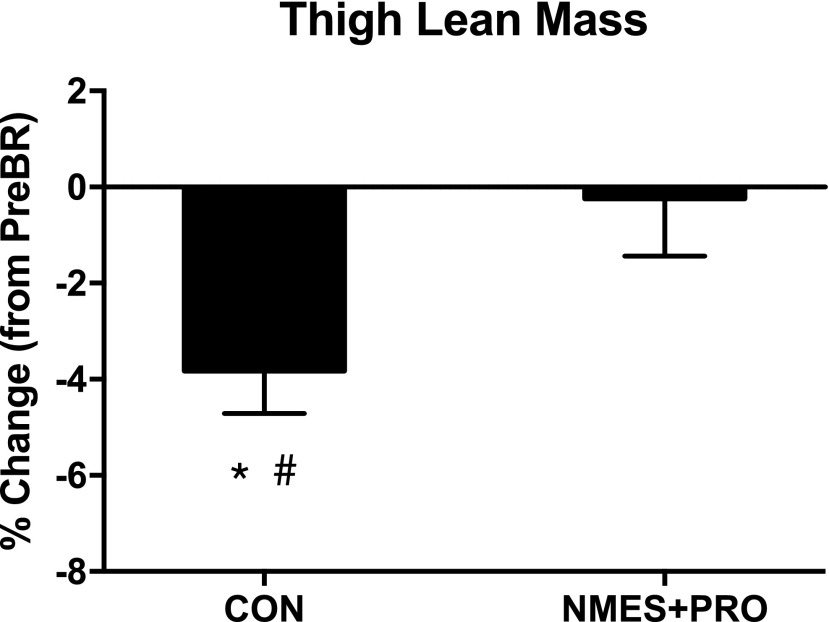
There were no baseline differences in demographics between the CON and NMES+PRO groups (Table 1). In response to bed rest, body weight (p < 0.001), BMI (p < 0.001), whole body (p < 0.001) and trunk (p < 0.01) lean mass, and leg muscle quality (p < 0.01) decreased in both groups with no group differences. Furthermore, leg lean mass decreased in the CON group after bed rest, but there were no group differences. Interestingly, thigh lean mass only decreased in the CON group after bed rest (p < 0.05) and this was different than NMES+PRO (p < 0.05; Fig. 2). There were no differences in fat mass as a result of bed rest or by group.
FIG. 2.
NMES+PRO effects on thigh lean mass. Figure denotes the change in thigh lean muscle after 5 days of bed rest in the CON (n = 10) and NMES+PRO (n = 10) groups and are reported as mean ± SEM. Data are reported as the percent change in thigh lean mass from pre-bed rest levels. *Different from PreBR (p < 0.05). #Different from NMES+PRO (p < 0.05). BR, bed rest.
As designed, caloric and macronutrient intake was less during 5 days of bed rest (vs. habitual diet) (Table 2). However, the decrease in protein intake from habitual to the bed rest period was greater in the CON versus the NMES+PRO group, but this was not associated with loss in thigh lean mass (r = 0.11, p = 0.64). With regards to NMES intensity during bed rest, NMES intensity averaged 15.3 mA on the first day and by the last day of bed rest (day 4) increased ∼14% to 18.5 mA.
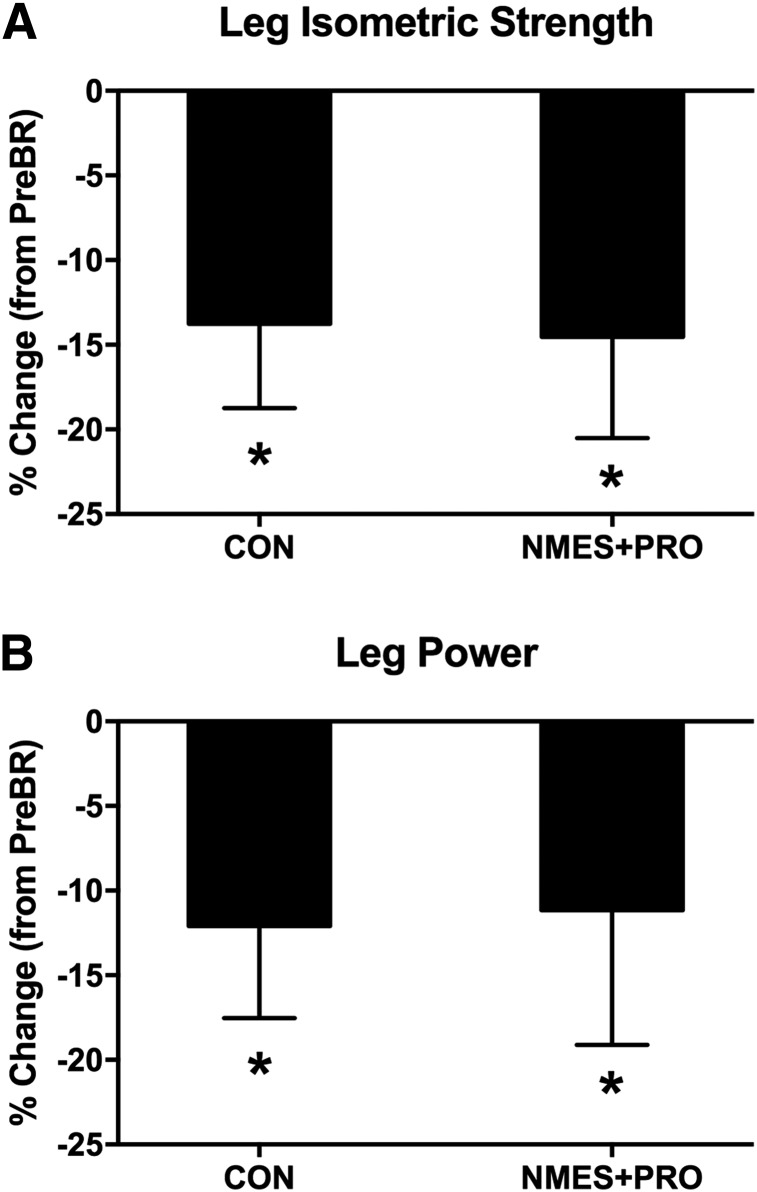
Muscle and physical function
Leg isometric strength (Fig. 3A) and power (Fig. 3B) decreased in both groups after bed rest (p < 0.01). However, there were no differences between the groups. Furthermore, there were no changes in the 6 MW (CON Pre: 677 ± 12 m, CON Post: 668 ± 11 m) (NMES+PRO Pre: 613 ± 6 m, NMES+PRO Post: 634 ± 9 m), TUG (CON Pre: 4.3 ± 0.1 seconds, CON Post: 4.5 ± 0.2 seconds) (NMES+PRO Pre: 4.9 ± 0.1 seconds, NMES+PRO Post: 5.3 ± 0.2 seconds), or GS (CON Pre: 1.14 ± 0.02 m/s, CON Post: 1.14 ± 0.02 m/s) (NMES+PRO Pre: 1.27 ± 0.02 m/s, NMES+PRO Post: 1.25 ± 0.2 m/s) after bed rest or between the groups.
FIG. 3.
NMES+PRO effects on leg muscle function. Figure denotes the change in (A) leg isometric strength and (B) leg power after 5 days of bed rest in the CON (n = 10) and NMES+PRO (n = 10) groups and are reported as mean ± SEM. Data are reported as the percent change from pre-bed rest levels. *Different from PreBR (p < 0.05).
Muscle fiber characteristics
Histological muscle samples collected during the fasted state were used to assess fiber size, fiber type distribution, and satellite cell and myonuclear content after 5 days of bed rest in both groups (Table 3). Driven largely by MyHC I fiber types, CSA and SC content robustly decreased in both groups (p < 0.05 and p < 0.01, respectively), while myonuclear content tended to decrease ∼7% (p = 0.08). No differences were detected between groups. Finally, a positive correlation was detected between fiber CSA and satellite cell content (MyHC I: r = 0.34, p = 0.05; MyHC II: r = 0.34, p = 0.05).
Table 3.
Muscle Fiber Characteristics of Older Adults in Response to 5 Days Bed Rest in CON and NMES+PRO Groups
| CON (n = 9) | NMES+PRO (n = 9) | |||
|---|---|---|---|---|
| MyHC | PreBR | PostBR | PreBR | PostBR |
| Myofiber CSA (μm2) | ||||
| I* | 4449 ± 478 | 3623 ± 247 | 4257 ± 500 | 3689 ± 446 |
| II | 4069 ± 700 | 3279 ± 207 | 3643 ± 406 | 3274 ± 276 |
| Pooled* | 4324 ± 535 | 3411 ± 142 | 3994 ± 401 | 3493 ± 389 |
| % Change | % Change | |||
|---|---|---|---|---|
| Myofiber CSA % change (post-pre) | ||||
| I | −26.3 ± 17.2 | −18.3 ± 10.1 | ||
| II | −19.6 ± 12.0 | −10.9 ± 6.8 | ||
| Pooled | −25.6 ± 16.4 | −16.6 ± 8.3 | ||
| % Fiber (number) | ||||
| I | 52.8 ± 4.1 | 47.4 ± 5.5 | 48.7 ± 5.1 | 47.0 ± 4.3 |
| II | 47.2 ± 4.1 | 52.6 ± 5.5 | 51.3 ± 5.1 | 53.0 ± 4.3 |
| Myonuclei/myofiber | ||||
| I# | 2.7 ± 0.1 | 2.5 ± 0.2 | 2.6 ± 0.1 | 2.4 ± 0.2 |
| II | 2.5 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.4 | 2.3 ± 0.2 |
| Pooled# | 2.6 ± 0.1 | 2.4 ± 0.2 | 2.6 ± 0.3 | 2.4 ± 0.2 |
| Myonuclear domain (μm2/myonuclei) | ||||
| I | 1612 ± 130 | 1540 ± 168 | 1636 ± 195 | 1528 ± 128 |
| II | 1680 ± 287 | 1417 ± 84 | 1442 ± 146 | 1461 ± 129 |
| Pooled | 1661 ± 176 | 1459 ± 95 | 1566 ± 158 | 1496 ± 130 |
| SC content/myofiber | ||||
| I* | 0.092 ± 0.008 | 0.057 ± 0.015 | 0.083 ± 0.019 | 0.057 ± 0.008 |
| II | 0.076 ± 0.007 | 0.060 ± 0.013 | 0.062 ± 0.011 | 0.054 ± 0.008 |
| Pooled* | 0.085 ± 0.005 | 0.06 ± 0.01 | 0.074 ± 0.014 | 0.056 ± 0.007 |
Data are reported as mean ± SE. A histochemical sample for one subject was not obtained while a methodological issue occurred on another histochemical sample. Therefore, data are n = 9 in each group.
Different as a result of bed rest (p < 0.05).
Different as a result of bed rest (p ≤ 0.08).
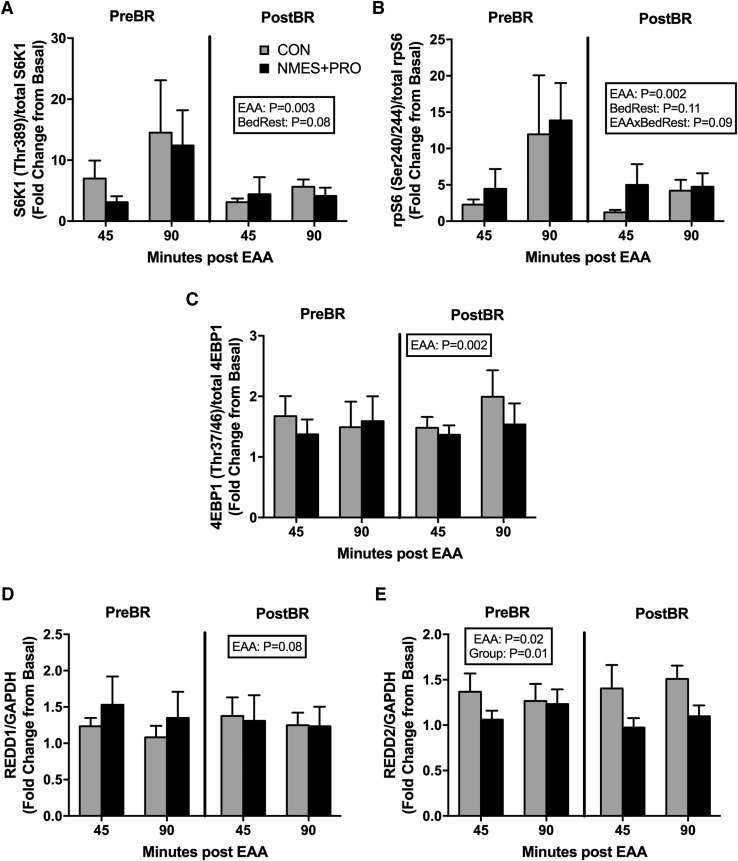
Nutrient-induced muscle mTORC1 signaling readouts
Muscle biopsies sampled in the fasted and after EAA ingestion were used to assess surrogates of muscle mTORC1 signaling since we have previously shown that nutrient-induced mTORC1 signaling was blunted in conjunction with muscle protein synthesis after 5 days of bed rest in older adults (Fig. 4).8,9 As expected there was a main effect of EAA for S6K1 (Thr389; Fig. 4A), rpS6 (Ser240/244; Fig. 4B), and 4EBP1 (Thr37/46; Fig. 4C) (p < 0.01) such that there was an increase in phosphorylation as a result of EAA ingestion. Interestingly, EAA also increased REDD2 (Fig. 4D) protein expression (main effect of EAA; p < 0.05) and a tendency to increase REDD1 (Fig. 4E) protein expression (p = 0.07). Finally, NMES+PRO reduced REDD2 (p < 0.01) expression, and there was tendency for bed rest to reduce S6K1 (Thr389; p = 0.08) and for bed rest to reduce EAA-induced rpS6 phosphorylation (Ser240/244; p = 0.09).
FIG. 4.
NMES+PRO effects on muscle mTORC1 signaling. Figure denotes the skeletal muscle protein expression for (A) S6K1 (Thr389), (B) rpS6 (Ser240/244), (C) 4EBP1 (Thr37/46), (D) REDD1, and (E) REDD2 before and after 5 days of bed rest in the CON (n = 10) and NMES+PRO (n = 10) groups. Participants consumed a bolus of EAA in the fasted state, and protein expression for mTORC1 signaling proteins was determined at 0 (Basal), 45, and 90 minutes after EAA ingestion. Data (mean ± SEM) are reported as fold change from basal. Phosphorylated targets were normalized to total protein levels of the target protein. REDD1 and REDD2 were normalized to GAPDH. EAA, essential amino acid; mTORC1, mechanistic target of rapamycin.
Gene expression
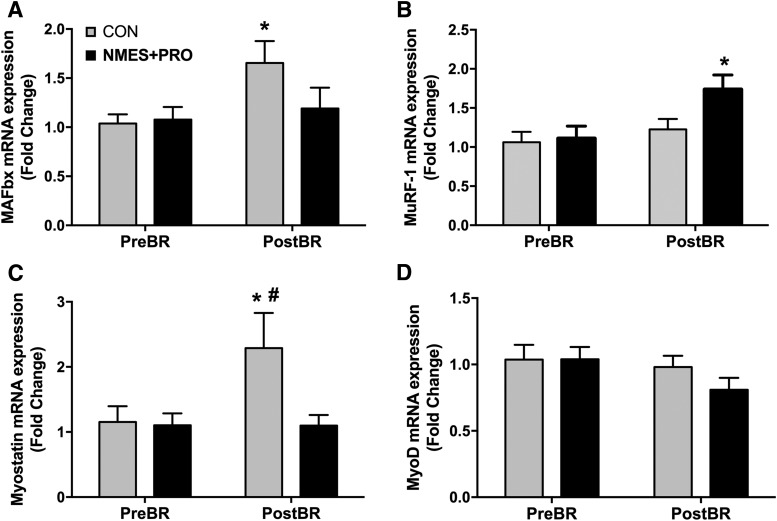
To add insight into possible modulatory effects of NMES+PRO preservation on leg lean mass with bed rest, we evaluated several mRNA markers associated with muscle atrophy and proteolysis (Fig. 5). Interestingly, bed rest only increased MAFbx (Fig. 5A; p < 0.05) and myostatin (Fig. 5C; p = 0.01) mRNA expression in the CON group (p < 0.05). The myostatin mRNA response in the CON group after bed rest was different than the NMES+PRO group (p < 0.05). In addition, NMES+PRO increased MURF-1 (Fig. 5B; p = 0.01) mRNA expression after bed rest, a response that was different than the CON group after bed rest (p = 0.05). There were no changes in MyoD (Fig. 5D) mRNA expression as a result of bed rest or between groups, although there was a tendency (p = 0.08) for an overall decrease with bed rest.
FIG. 5.
NMES+PRO effects on muscle gene expression. Figure denotes the skeletal muscle mRNA expression for (A) MURF-1, (B) MAFbx, (C) myostatin, and (D) MyoD before and after 5 days of bed rest in the CON (n = 10) and NMES+PRO (n = 9) groups and are reported as mean ± SEM. There was insufficient muscle tissue for RNA isolation for one of the NMES+PRO participants; therefore data are only reported in n = 9 for this group. *Different from PreBR (p < 0.05). #Different from NMES+PRO (p < 0.05).
Discussion
The primary finding of this parallel group, randomized clinical trial in healthy older adults, was that daily NMES to the thigh combined with protein supplementation (NMES+PRO) was effective to offset the loss in thigh lean tissue during 5 days of bed rest. Interestingly, the changes observed in thigh lean mass did not translate to the preservation of muscle function and acute stimulation of mTORC1 signaling. However, the increase in myostatin and MAFbx mRNA abundance with bed rest was inhibited with NMES+PRO, suggesting a possible mechanism for protection of whole thigh muscle lean mass. NMES in addition to protein supplementation daily (three sessions/day) may be a feasible option to preserve thigh lean tissue in healthy older adults who undergo muscle disuse for a short-term period. Future investigations are needed to improve muscle function while concurrently maintaining muscle mass during short-term periods of disuse.
Our findings are the first to show an effective, yet practical therapeutic strategy to halt ∼4% thigh muscle loss during 5 days of bed rest in healthy older adults. During bed rest, a total of twelve 40-minute sessions of NMES with each session followed by protein supplementation were an adequate anabolic dose necessary to preserve thigh muscle mass in older adults. Electrical stimulation has demonstrated positive benefits on leg muscle and strength during disuse and on limiting muscle function deficits during orthopedic and controlled circumstances of limb immobilization.44,45 However, until recently, electrical stimulation had not been investigated as a therapeutic strategy during short-term bed rest (<1 week) when muscle atrophy and strength decrements are suggested to be the most rapid3,46 as this has tremendous application to older patients who are acutely hospitalized (<5 days).13 In a previous study in young healthy older adults, Dirks et al. showed that 10 sessions of NMES (two sessions/day) during 5 days of leg immobilization were capable of preventing ∼3.5% loss in quadriceps CSA. Similarly, protein supplementation in middle-age and older adults during longer periods of bed rest (i.e., 10–14 days) has demonstrated protective effects on lean mass,11,12 but this strategy was not effective to maintain muscle size of healthy older adults during 5-days of cast immobilization.14 These later data infer that NMES in our current study may have been the driving intervention altering muscle over short-term bed rest.
A limitation of our study was that we are not able to decipher if the independent effects of NMES and protein supplementation during 5 days of bed rest would have similar effects on muscle compared to the combined therapy herein. However, our goal in designing this study was to take a robust approach on mitigating muscle atrophy and strength loss in healthy older adults since we previously observed that 5 days of bed rest resulted in ∼4% decrease in leg muscle mass and ∼15% decrease in leg isometric strength,8 yet NMES alone during 5 days of cast immobilization was unable to prevent leg muscle weakness.26 Taken together, a combined NMES and PRO intervention was capable of offsetting thigh muscle atrophy during 5 days of bed rest in older adults; however, the independent treatment effects on muscle during short-term bed rest warrant further exploration.
Another major finding was that the dual anabolic approach of NMES combined with protein supplementation did not slow the decline in muscle function (∼15% and 10% decrease in strength and power, respectively) with short-term bed rest. Limited ability of these independent interventions to preserve strength are consistent with short-term leg immobilization studies that have used NMES and PRO independently,14,26 but this is the first study to test the combined intervention on preventing losses in muscle function with bed rest. We have come up with a couple of considerations for the lack of muscle function preservation with our intervention: First, it is possible that these specific interventions (NMES and PRO) may be more effective in preserving muscle function when applied over a longer duration of time, like what has been observed with leucine or HMG supplementation in middle and older aged adults over 10 days11 and 14 days12 of bed rest, respectively. Second, it is worth noting that NMES training is specific to the joint angle in which it was applied47 or NMES training may be more effective on muscle function when targeted at multiple joint angles. In this study, a knee angle of 30° was used during the NMES sessions, while isometric strength was assessed at a knee angle of 60°. Finally, it is possible that the maintained thigh lean mass detected in the NMES+PRO group may not be contractile tissue, but rather noncontractile extracellular tissue or water, as our myofiber CSA data would infer. Although strength was not preserved in the current study, we speculate that the maintenance of thigh lean mass during disuse may serve other beneficial purposes in older adults such as regulating postprandial glucose disposal48 or used as a substrate during injury or disease.49 Investigating optimal nutritional and novel, more aggressive contractile strategies (i.e., assisted walking and/or bed side squats) to combat muscle function deficits following short-term inactivity are needed.
To add a possible mechanism to explain the protective effects of muscle due to our intervention, we targeted key molecular pathways associated with muscle protein synthesis and breakdown. First, we measured EAA-induced mTORC1 signaling after a single dose of EAA ingestion; a dose well known to acutely stimulate muscle protein synthesis.8,9 Interestingly, NMES+PRO did not reverse the nutrient-induced mTORC1 signaling attenuation typically seen after 5–7 days of bed rest.8,24 However, we did not measure muscle protein synthesis (MPS) as this may have limited our current interpretation.
Next, we evaluated mediators of muscle atrophy to add insight to our primary finding since we and others have observed that mRNAs of key E3 ubiquitin ligases involved in proteolysis (MURF-1, MAFbx) are transiently elevated during 2–5 days of leg immobilization or bed rest.8,50,51 In agreement with these prior studies, we found that mRNA abundance of MAFbx was significantly elevated by ∼50% in the CON subjects only. Similarly, myostatin mRNA, a potent negative regulator of muscle growth, was also significantly increased by ∼100% following bed rest in CON, but not in the NMES+PRO group. Strikingly, these data closely resemble muscle molecular outcomes observed by Dirks et al. who showed that 5 days of NMES in young adults prevented leg immobilization-induced increases in skeletal muscle MAFbx and myostatin mRNA.26 This study (in combination with ours) further provides evidence that NMES may be the key intervention (rather than PRO) positively influencing muscle in healthy older adults during short-term bed rest. Together, these data suggest that the muscle protective effects of NMES+PRO during 5 days of bed rest in healthy older adults may target regulators of muscle atrophy (MAFbx, myostatin) rather than restore impaired nutrient-induced protein anabolic signaling (mTORC1).
An interesting finding was that the beneficial effects of NMES+PRO at the myofiber level during bed rest did not fully reflect what we report with the whole muscle. Instead, we observed robust myofiber atrophy and depletion of satellite cells in both groups following bed rest; a relationship that was correlated (r = 0.34, p = 0.05) as others have demonstrated.39,52 Myofiber atrophy is consistent in older adults after 4 days of cast immobilization.51 However, it is important to point out that several short-term immobilization studies (5 days)14,26,50 and even some longer bed rest studies (7 days)53 failed to show atrophy at the fiber level as a result of disuse but rather noticed differences at the whole muscle level using CT and/or DXA.
There are several potential explanations for the inconsistencies between whole muscle and myofiber CSA: (1) small intervention-related differences in myofiber CSA within a single muscle biopsy sample were below the detection limits of this assay, but if added all together may be more consistent with whole muscle size, (2) the inherent variability of biopsy sampling and that pre and post biopsies occurred on opposite legs,54,55 and (3) myofiber atrophy was the same in both whole muscle and at the fiber level, since we cannot exclude the possibility that water or extracellular lean tissue (as opposed to intracellular sarcoplasmic or myofibrillar protein) was altered/maintained due to the intervention.56,57 To reduce variation and increase sensitivity, future investigations should place greater emphasis on assessing as many fibers as possible within each biopsy sample, as well as assessing whole muscle size.
We conclude that daily delivery of NMES and protein supplementation (three times/day) was effective to maintain thigh lean mass but not muscle function in healthy older adults during 5 days of bed rest. Furthermore, our data support that the attenuation of molecular markers associated with protein breakdown may help explain the protective effects of the NMES+PRO on skeletal muscle tissue. Additional research is warranted to study practical and feasible interventions to protect muscle function in older adults during short-term periods of disuse.
Acknowledgments
The authors thank the CCTS nursing, dietary, and medical staff for their assistance with the muscle biopsies, blood sampling, and patient care during the inpatient and outpatient visits. The authors also thank Emma Johnson and Fernando Frajacomo, DPT for providing the standard of care physical therapy to the participants while on bed rest. Finally, we appreciate the generosity of Glanbia Nutritionals (Brent Peterson, Eric Bastian) for providing the premixed EAA's and PepForm BCAA supplements. This study was funded by a University of Utah seed grant and The National Institutes of Health: R03AG047308 (M.D.). Clinical inpatient and outpatient services were supported by an institutional center grant (CTSA) provided by the National Center for Advancing Translational Sciences (UL1-TR001067). The monoclonal antibodies, Pax7 and BA-D5, were deposited by Drs. A. Kawakami and S. Schiaffino, respectively, and were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Authors' Contributions
M.D. and P.L. designed the research proposal; M.D., P.L., P.R., A.M., P.B., D.N., and M.S. conducted the research; M.D., P.R., and A.M. analyzed the data; M.D., P.R., A.M., and P.L. wrote the article; and MD had primary responsibility for the final content. All authors read and approved the final draft of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 2009;107:1172–1180 [DOI] [PubMed] [Google Scholar]
- 2.Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol 2010;109:1628–1634 [DOI] [PubMed] [Google Scholar]
- 3.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: Implications for age-related sarcopenia. Ageing Res Rev 2013;12:898–906 [DOI] [PubMed] [Google Scholar]
- 4.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall BT, Snijders T, Senden JM, Ottenbros CL, Gijsen AP, Verdijk LB, van Loon LJ. Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J Clin Endocrinol Metab 2013;98:4872–4881 [DOI] [PubMed] [Google Scholar]
- 6.Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 2013;98:2604–2612 [DOI] [PubMed] [Google Scholar]
- 7.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 2008;586:6049–6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, Drummond MJ. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 2015;593:4259–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 2012;302:E1113–E1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 2009;106:1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 2013;32:704–712 [DOI] [PubMed] [Google Scholar]
- 12.English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr 2016;103:465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med 2010;170:1942–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr 2014;144:1196–1203 [DOI] [PubMed] [Google Scholar]
- 15.Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther 1994;74:901–907 [DOI] [PubMed] [Google Scholar]
- 16.Wigerstad-Lossing I, Grimby G, Jonsson T, Morelli B, Peterson L, Renstrom P. Effects of electrical muscle stimulation combined with voluntary contractions after knee ligament surgery. Med Sci Sports Exerc 1988;20:93–98 [DOI] [PubMed] [Google Scholar]
- 17.Eriksson E, Haggmark T. Comparison of isometric muscle training and electrical stimulation supplementing isometric muscle training in the recovery after major knee ligament surgery. A preliminary report. Am J Sports Med 1979;7:169–171 [DOI] [PubMed] [Google Scholar]
- 18.Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: A randomized controlled trial. Phys Ther 2012;92:210–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Schwartz RS, Schenkman M, Kohrt WM. Relationship between intensity of quadriceps muscle neuromuscular electrical stimulation and strength recovery after total knee arthroplasty. Phys Ther 2012;92:1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci (Lond) 2015;128:357–365 [DOI] [PubMed] [Google Scholar]
- 21.Gruther W, Kainberger F, Fialka-Moser V, Paternostro-Sluga T, Quittan M, Spiss C, Crevenna R. Effects of neuromuscular electrical stimulation on muscle layer thickness of knee extensor muscles in intensive care unit patients: A pilot study. J Rehabil Med 2010;42:593–597 [DOI] [PubMed] [Google Scholar]
- 22.Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, Chatzimichail A, Routsi C, Roussos C, Nanas S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: A randomized study. Crit Care 2009;13:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon BS, Williamson DL, Lang CH, Jefferson LS, Kimball SR. Nutrient-induced stimulation of protein synthesis in mouse skeletal muscle is limited by the mTORC1 repressor REDD1. J Nutr 2015;145:708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall BT, Dirks ML, Verdijk LB, Snijders T, Hansen D, Vranckx P, Burd NA, Dendale P, van Loon LJ. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic men. Am J Physiol Endocrinol Metab 2012;303:E614–E623 [DOI] [PubMed] [Google Scholar]
- 25.Dirks ML, Groen BB, Franssen R, van Kranenburg J, van Loon LJ. Neuromuscular electrical stimulation prior to presleep protein feeding stimulates the use of protein-derived amino acids for overnight muscle protein synthesis. J Appl Physiol (1985) 2017;122:20–27 [DOI] [PubMed] [Google Scholar]
- 26.Dirks ML, Wall BT, Snijders T, Ottenbros CL, Verdijk LB, van Loon LJ. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol (Oxf) 2014;210:628–641 [DOI] [PubMed] [Google Scholar]
- 27.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 1993;148:379–385 [DOI] [PubMed] [Google Scholar]
- 28.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009;13:881–889 [DOI] [PubMed] [Google Scholar]
- 29.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, Newman AB, Cardiovascular Health Study. The 6-min walk test: A quick measure of functional status in elderly adults. Chest 2003;123:387–398 [DOI] [PubMed] [Google Scholar]
- 30.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80:896–903 [PubMed] [Google Scholar]
- 31.Drummond MJ, Timmerman KL, Markofski MM, Walker DK, Dickinson JM, Jamaluddin M, Brasier AR, Rasmussen BB, Volpi E. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am J Physiol Regul Integr Comp Physiol 2013;305:R216–R223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dirks ML, Backx EM, Wall BT, Verdijk LB, van Loon LJ. May bed rest cause greater muscle loss than limb immobilization? Acta Physiol (Oxf) 2016;218:10–12 [DOI] [PubMed] [Google Scholar]
- 33.Biolo G, Pisot R, Mazzucco S, Di Girolamo FG, Situlin R, Lazzer S, Grassi B, Reggiani C, Passaro A, Rittweger J, Gasparini M, Simunic B, Narici M. Anabolic resistance assessed by oral stable isotope ingestion following bed rest in young and older adult volunteers: Relationships with changes in muscle mass. Clin Nutr 2016 [DOI] [PubMed] [Google Scholar]
- 34.Pisot R, Marusic U, Biolo G, Mazzucco S, Lazzer S, Grassi B, Reggiani C, Toniolo L, di Prampero PE, Passaro A, Narici MV, Mohammed S, Rittweger J, Gasparini M, Gabrijelcic M, Simunic B. Greater loss in muscle mass and function but smaller metabolic alterations in older compared to younger men following two weeks of bed rest and recovery. J Appl Physiol (1985) 2016;120:922–929 [DOI] [PubMed] [Google Scholar]
- 35.Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJ. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 2016;65:2862–2875 [DOI] [PubMed] [Google Scholar]
- 36.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 1982;14:101–102 [PubMed] [Google Scholar]
- 37.Spector P, Laufer Y, Elboim Gabyzon M, Kittelson A, Stevens Lapsley J, Maffiuletti NA. Neuromuscular electrical stimulation therapy to restore quadriceps muscle function in patients after orthopaedic surgery: A novel structured approach. J Bone Joint Surg Am 2016;98:2017–2024 [DOI] [PubMed] [Google Scholar]
- 38.Parker MG, Bennett MJ, Hieb MA, Hollar AC, Roe AA. Strength response in human femoris muscle during 2 neuromuscular electrical stimulation programs. J Orthop Sports Phys Ther 2003;33:719–726 [DOI] [PubMed] [Google Scholar]
- 39.Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985) 2016;120:965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith LR, Barton ER. SMASH—Semi-automatic muscle analysis using segmentation of histology: A MATLAB application. Skelet Muscle 2014;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E1011–E1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol 2009;106:1403–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 2008;40:691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrissey MC, Brewster CE, Shields CL, Jr., Brown M. The effects of electrical stimulation on the quadriceps during postoperative knee immobilization. Am J Sports Med 1985;13:40–45 [DOI] [PubMed] [Google Scholar]
- 45.Gibson JN, Morrison WL, Scrimgeour CM, Smith K, Stoward PJ, Rennie MJ. Effects of therapeutic percutaneous electrical stimulation of atrophic human quadriceps on muscle composition, protein synthesis and contractile properties. Eur J Clin Invest 1989;19:206–212 [DOI] [PubMed] [Google Scholar]
- 46.Hvid LG, Suetta C, Nielsen JH, Jensen MM, Frandsen U, Ortenblad N, Kjaer M, Aagaard P. Aging impairs the recovery in mechanical muscle function following 4 days of disuse. Exp Gerontol 2014;52:1–8 [DOI] [PubMed] [Google Scholar]
- 47.Requena Sanchez B, Padial Puche P, Gonzalez-Badillo JJ. Percutaneous electrical stimulation in strength training: An update. J Strength Cond Res 2005;19:438–448 [DOI] [PubMed] [Google Scholar]
- 48.Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism 1988;37:802–806 [DOI] [PubMed] [Google Scholar]
- 49.Matthews DE, Battezzati A. Regulation of protein metabolism during stress. Curr Opin Gen Surg 1993:72–77 [PubMed] [Google Scholar]
- 50.Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf) 2014;210:600–611 [DOI] [PubMed] [Google Scholar]
- 51.Suetta C, Frandsen U, Jensen L, Jensen MM, Jespersen JG, Hvid LG, Bayer M, Petersson SJ, Schroder HD, Andersen JL, Heinemeier KM, Aagaard P, Schjerling P, Kjaer M. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One 2012;7:e51238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arentson-Lantz EJ, Paddon-Jones D, Fry CS. The intersection of disuse-induced muscle atrophy and satellite cell content: Reply to Snijders, Nederveen, and Parise. J Appl Physiol (1985) 2016;120:1491. [DOI] [PubMed] [Google Scholar]
- 53.Ringholm S, Bienso RS, Kiilerich K, Guadalupe-Grau A, Aachmann-Andersen NJ, Saltin B, Plomgaard P, Lundby C, Wojtaszewski JF, Calbet JA, Pilegaard H. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am J Physiol Endocrinol Metab 2011;301:E649–E658 [DOI] [PubMed] [Google Scholar]
- 54.Elder GC, Bradbury K, Roberts R. Variability of fiber type distributions within human muscles. J Appl Physiol Respir Environ Exerc Physiol 1982;53:1473–1480 [DOI] [PubMed] [Google Scholar]
- 55.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle Nerve 1983;6:588–595 [DOI] [PubMed] [Google Scholar]
- 56.Trappe T. Influence of aging and long-term unloading on the structure and function of human skeletal muscle. Appl Physiol Nutr Metab 2009;34:459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seene T, Kaasik P, Riso E-M. Review on aging, unloading and reloading: Changes in skeletal muscle quantity and quality. Arch Gerontol Geriatr 2012;54:374–380 [DOI] [PubMed] [Google Scholar]