Abstract
Purpose
To report novel optical coherence tomography findings in a case of anti-α-enolase cancer associated retinopathy.
Observations
An elderly female presented with bilateral decreased vision and a recent diagnosis of ovarian carcinoma. Optical coherence tomography demonstrated bilateral loss of outer retinal structures and macular edema. Serum testing found antibodies against α-enolase and 82–84 kDa proteins. Outer retinal structures showed recovery, macular edema resolved and repeat anti-retinal antibody testing became negative following cancer therapy and topical difluprednate treatment.
Conclusions and importance
Cancer associated retinopathy is a paraneoplastic disease that results in damage to retinal structures through an autoimmune response. The damage is generally considered to be irreversible; however, in rare cases, such as observed here, retinal structures may demonstrate recovery after treatment.
Keywords: Cancer associated retinopathy, Optical coherence tomography
1. Introduction
Cancer associated retinopathy (CAR) is a paraneoplastic disease in which retinal degeneration occurs as an immune response to cancer antigens sharing homology with endogenous retinal proteins.1 Past studies have found various retinal proteins to be antigenic, which include recoverin, α-enolase, arrestin, and transducin.2 The inhibition of enolase, a glycolytic enzyme, results in metabolic disruption of retinal cells and the induction of apoptosis.3 Anti-α-enolase autoantibodies are capable of accessing tissue and targeting ganglion cells, Muller cells, and photoreceptors.2 It is believed that death of retinal cells is an irreversible process.3 We report a patient with gynecological-CAR who experienced objective improvement in photoreceptor architecture following treatment of her underlying malignancy.
2. Case report
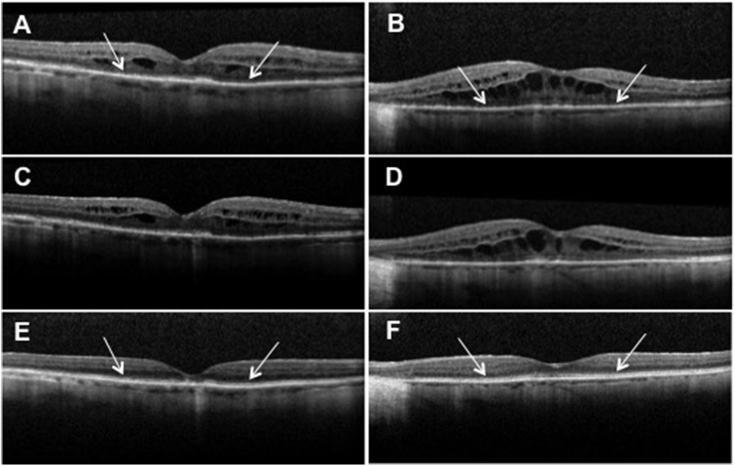
An 80-year Hispanic female with a history of chronic, bilateral Vogt-Koyanagi-Harada associated uveitis presented to the Casey Eye Institute Uveitis Clinic for a routine follow up visit. At that time, she reported a new diagnosis of ovarian carcinoma and had started her first chemotherapy session consisting of carboplatin and paclitaxel. Due to severe aortic stenosis, the patient was not a candidate for surgical intervention. Her vision was 20/30 bilaterally without evidence of active uveitis. Four months later she returned with a bilateral decrease in vision to 20/50. The patient underwent imaging with macular volume scans centered on the fovea (Heidelberg Spectralis spectral domain ocular coherence tomography (OCT) with eye tracking software, Heidelberg, Germany) that demonstrated a disrupted inner segment/outer segment junction (IS/OS) and cystoid macular edema (CME) bilaterally (Fig. 1A-B). Clinical and OCT findings were suspicious for CAR and anti-retinal antibody testing was pursued. The patient declined local or systemic immunosuppression and continued to undergo planned chemotherapy. One month later, her vision had dropped to 20/50 OD and 20/100 OS. Repeat OCT mapped to the original images continued to demonstrate loss of the ISOS junction and CME in both eyes (Fig. 1C-D). Serum tested for the presence of anti-retinal autoantibodies showed antibodies against α-enolase and 82–84 kDa proteins. Immunohistochemistry of the patient's serum showed positive staining of the photoreceptor cell layer in human retina. The patient continued to decline periocular injection or systemic immunosuppression and was prescribed difluprednate drops three times daily. Two months later, there was partial return to normal reflectivity of the IS/OS junction on OCT, and the CME had improved. Six months later, there was resolved CME on OCT, and the IS/OS reflectivity returned to near normal in the subfoveal region. At this time, the vision was 20/40 bilaterally. The patient was instructed to stop difluprednate drops. Over the following six months, the patient's visual acuity stabilized at 20/60 OD and 20/50 OS. There was no recurrence of CME and the OCT showed normalized IS/OS reflectivity except in the fovea where there was a stable elevated outer retinal lesion OD and near-normalized ISOS reflectivity in the left macula except in the fovea (Fig. 1E-F). Serum was negative for anti-retinal autoantibodies on repeat testing.
Fig. 1.
OCT images of initial loss and final improvement of the ISOS junction.
Loss of ISOS hyperreflectivity (white arrows) and concurrent CME at time of initial vision loss OD (A) and OS (B). Persistence of ISOS loss and CME after 1 month of observation OD (C) and OS (D). E: Partial recovery of ISOS hyperreflectivity (white arrows) with residual foveal outer retinal nodularity OD one year after initial findings. F: Recovery of ISOS hyperreflectivity (white arrows) with mild residual foveal disruption one year after initial findings. OCT = optical coherence tomography, ISOS = inner segment/outer segment, CME = cystoid macular edema.
3. Discussion
Anti-retinal autoantibodies can be detected in both retinopathy patients and healthy individuals. Individuals with gynecological CAR have a higher proportion of seropositivity than normal individuals.4, 5 Our patient became symptomatic after diagnosis of ovarian cancer and initiation of chemotherapy treatment. Autoantibodies may be present before the diagnosis of cancer, but it is not until they breach the blood retinal barrier that symptoms become evident.4 Although the presence of anti-retinal autoantibodies can occur in normal patients, high antibody titers are a better indicator of retinopathy.5 Anti-enolase autoantibodies affect the catalytic activity of the enzyme thus depleting glycolytic ATP, increasing levels of intracellular calcium which then induces mitochondrial-mediated apoptosis by the activation of its key elements.3
The loss of outer retinal structures and retinal atrophy observed in autoimmune retinopathy are frequently thought to be irreversible.5, 6 Partial recovery of SD-OCT outer retinal changes in a patient with CAR after treatment with rituximab has been reported, which suggests that therapy targeting B cells and consequently reducing production of anti-retinal antibodies may be beneficial.7 Our patient showed improvement of the reflectivity of the photoreceptor IS/OS junction despite only local therapy with difluprednate, which was started to treat CME and reduce local inflammatory damage, but unlikely to significantly affect autoantibody production. We hypothesize that the prompt initiation of chemotherapy may have contributed to the patient's improvement by possibly decreasing tumor expression of enolase and diminishing the production of anti-retinal autoantibodies or that chemotherapeutic treatment resulted in non-specific suppression of antibody production. The recovery of outer retinal structures in the present case corresponded to anti-retinal antibodies no longer being detected in the patient's serum, supporting a pathologic role for autoantibodies in our patient. Treatments that may limit the production of anti-retinal antibodies such as rituximab should continued to be studied for efficacy in CAR.
The history of prior uveitis is a potential confounder to our findings; however, the patient did not demonstrate active inflammation during this follow up period. The presence of CME may also confound the ability to image the outer retina; however, we observed patchiness of the ISOS junction outside of regions of retinal edema indicating the outer retinal changes were not a sequel of CME alone. CME is not a common manifestation of CAR, more frequently observed in non-paraneoplastic autoimmune retinopathy,8 but previous case reports of CAR-related CME suggest it is responsive to steroids, as was observed in our patient.9 Unfortunately, the patient declined additional objective testing (visual fields, electroretinography), which would have allowed further clinical correlation. This correlation between objective testing and photoreceptor loss has been demonstrated in prior studies of autoimmune retinopathy.10
We report a patient with CAR who experienced objective improvement in photoreceptor architecture following treatment of her underlying malignancy, a novel observation previously reported only following rituximab therapy. We also note the successful treatment of CAR-associated CME with topical difluprednate, suggesting an alternative therapy to previously reported treatments with periocular or intravitreal steroids.
4. Conclusion
Damage to retinal structures from CAR can be objectively captured by OCT and may demonstrate recovery after treatment in rare cases.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Acknowledgements and disclosures
Funding
P30 EY010572 from the National Institutes of Health (Bethesda, MD), unrestricted departmental funding to the Casey Eye Institute from Research to Prevent Blindness (New York, NY).
Conflict of interest
None relevant to this study, JTR, PL, GA, FJI, LJK, SSS, MS, KB.
The following authors have no relevant financial disclosures for the study: JTR, PL, GA, FJI, LJK, SSS, MS, KB.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Rahimy E., Sarraf D. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: evaluation and management. Surv Ophthalmol. 2013;58(5):430–458. doi: 10.1016/j.survophthal.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Weleber R.G., Watzke R.C., Shults W.T. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with anti-enolase antibodies. Am J Ophthalmol. 2005;139(5):780–794. doi: 10.1016/j.ajo.2004.12.104. [DOI] [PubMed] [Google Scholar]
- 3.Magrys A., Anekonda T., Ren G., Adamus G. The role of anti-alpha-enolase autoantibodies in pathogenicity of autoimmune-mediated retinopathy. J Clin Immunol. 2007;27(2):181–192. doi: 10.1007/s10875-006-9065-8. [DOI] [PubMed] [Google Scholar]
- 4.Adamus G., Choi D., Raghunath A., Schiffman J. Significance of anti-retinal autoantibodies in cancer-associated retinopathy with gynecological cancers. J Clin Exp Ophthalmol. 2013;4(6):307. doi: 10.4172/2155-9570.1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abazari A., Allam S.S., Adamus G., Ghazi N.G. Optical coherence tomography findings in autoimmune retinopathy. Am J Ophthalmol. 2012;153(4):750–756. doi: 10.1016/j.ajo.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepple K.L., Cusick M., Jaffe G.J., Mruthyunjaya P. SD-OCT and autofluorescence characteristics of autoimmune retinopathy. Br J Ophthalmol. 2013;97:139–144. doi: 10.1136/bjophthalmol-2012-302524. [DOI] [PubMed] [Google Scholar]
- 7.Or C., Collins D.R., Merkur A.B., Wang Y., Chan C.C., Forooghian F. Intravenous rituximab for the treatment of cancer-associated retinopathy. Can J Ophthalmol. 2013;48(2):e35–38. doi: 10.1016/j.jcjo.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Ferreyra H.A., Jayasundera T., Khan N.W., He S., Lu Y., Heckenlively J.R. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127(4):390–397. doi: 10.1001/archophthalmol.2009.24. [DOI] [PubMed] [Google Scholar]
- 9.Moyer K., DeWilde A., Law C. Cystoid macular edema from cancer-associated retinopathy. Optom Vis Sci. 2014;91(4S):S66–S70. doi: 10.1097/OPX.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 10.Sepah Y.J., Sadiq M.A., Hassan M. Assessment of retinal structural and functional characteristics in eyes with autoimmune retinopathy. Curr Mol Med. 2015;15(6):578–586. doi: 10.2174/1566524015666150731104626. [DOI] [PubMed] [Google Scholar]