Abstract
Chemical calcium indicators have been commonly used to monitor calcium (Ca2+) activity in cell bodies, i.e., somata, of isolated dorsal root ganglion neurons. Recent studies have shown that dorsal root ganglion somata play an essential role in soma–glia interactions and actively participate in the transmission of nociceptive signals. It is therefore desirable to develop methods to study Ca2+ activity in neurons and glia in intact dorsal root ganglia. In our previous studies, we found that incubation of intact dorsal root ganglia with acetoxymethyl dye resulted in efficient Ca2+ dye loading into glial cells but limited dye loading into neurons. Here, we introduce a useful method to load Ca2+ dyes in intact dorsal root ganglion neurons through electroporation. We found that electroporation greatly facilitated loading of Fluo-4 acetoxymethyl, Oregon green bapta-1-488 acetoxymethyl, and Fluo-4 pentapotassium salt into dorsal root ganglion neurons. In contrast, electroporation did not further facilitate dye loading into glia. Using electroporation followed by incubation of acetoxymethyl form Ca2+ dye, we can load acetoxymethyl Ca2+ dye well in both neurons and glia. With this approach, we found that inflammation induced by complete Freund’s adjuvant significantly increased the incidence of neuron–glia interactions in dorsal root ganglia. We also confirmed the actions of capsaicin and morphine on Ca2+ responses in dorsal root ganglion neurons. Thus, by promoting the loading of Ca2+ dye in neurons and glia through electroporation and incubation, Ca2+ activities in neurons and neuron–glia interactions can be well studied in intact dorsal root ganglia.
Keywords: Electroporation, dorsal root ganglion, calcium, calcium dye, inflammation, pain, neuron–glia interaction
Introduction
Isolated and round-shaped cell bodies (somata) of dorsal root ganglion (DRG) neurons have been used traditionally as a model cell preparation to study electric properties of central and peripheral sensory terminals based on the assumption that the somata and terminals have similar electrical characteristics1 and somata are not actively participating in sensory signaling.2 This view has recently been challenged.3–7 We and others have shown that DRG somata are essential in soma–glial interactions and actively participate in the transmission of nociceptive signals.5,6,8–12
To better understand the involvement of neuronal somata and the role of somata–glia interactions in pain signaling, some recorded the activity of DRG somata using sharp electrodes and/or patch electrodes and studied changes in Ca2+signaling through the injection of a salt form of fluorescent Ca2+ dye into a small number of neurons in intact DRGs.8,9,13–17 We bulk loaded Fluo-4 acetoxymethyl Ca2+ dye in L4 or L5 ganglia with sciatic nerves attached.5,6 The approach allowed us to examine neuron–glia interactions in DRGs under different experimental conditions. This dye loading technique resulted in efficient Ca2+ dye loading into glial cells. However, only a limited number of neurons were loaded. We therefore tested various Ca2+ dye loading techniques and found that Ca2+ dyes could be efficiently loaded in intact DRG neurons through electroporation. Conditions to optimize the study of neuron–glia interactions were explored further.
Materials and methods
Animals
Male adult (100–250 g) Sprague Dawley rats were used in this study. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and were in accordance with the guidelines of the National Institutes of Health and of the International Association for the Study of Pain.
Complete Freund’s adjuvant induced inflammation
To induce inflammation, animals were anesthetized with isoflurane (5%), and complete Freund’s adjuvant (CFA; 50 μl) was injected into the plantar surface of the left hindpaw. Solution of CFA was prepared by mixing Mycobacterium butyricum (10 mg/ml; Difco, Detroit, MI) in a peanut oil–saline (1:1) emulsion. Signs of localized inflammation in left hindpaw, such as redness and swelling, were seen several hours later. Ca2+ imaging experiments were performed three to nine days after the CFA injection.
Retrograde loading of dextran form of Ca2+ dye
In some rats, under isoflurane anesthesia (initialed at 5% and maintained at 2%), the left sciatic nerve was transected in midthigh, and the distal cut end of the nerve was tightly ligated with 5-0 silk. The proximal cut end was soaked in ∼10 µl, 1% calcium green-1 dextran 3000 (Ca green dextran) solution for 30 min. The incisions were closed in layers. Animals were returned to their cages under standard colony conditions, with food and water available ad libitum. On day 5 after the Ca2+ dye loading, the L4 and L5 DRGs were taken out to prepare intact DRG preparations for Ca2+ imaging experiments as described below.
Intact DRG preparation
Under pentobarbital (50 mg/kg, i.p.) anesthesia, L4 or L5 DRGs were removed from normal (including those that had undergone retrograde Ca2+ dye loading) and CFA rats, in some cases, with the sciatic nerve attached. After carefully removing the capsule, DRGs were recovered in an extracellular solution for 30 min at room temperature. The extracellular solution contained (in mM) 115 NaCl, 5.6 KCl, 1 NaH2PO4, 2.0 CaCl2, 1 MgCl2, 11 glucose, and 25 NaHCO3 and was bubbled with O2.
Chemical agents
All Ca2+ dyes were purchased from Thermo Fisher Scientific (Waltham, MA). Morphine was obtained from Hospira (Lake Forest, IL) and capasaicin was from Sigma (St. Louis, MO).
Calcium imaging
Calcium imaging studies were conducted on intact DRG preparations at room temperature. Ganglia were electroporated with the Amaxa Neucleofector II. Briefly, ganglia with or without attached sciatic nerves were put into a cuvette which contained 100 µl of extracellular solution with Ca2+ dye for electroporation. The protocols used in the study were A003, C002, or C003. Since these three electroporation protocols did not yield significant differences in Ca2+ dye loadings, results were combined in our data analyses. For acetoxymethyl (AM)-form Ca2+ dyes, ganglia were incubated with the same Ca2+ dye solution for 1 h immediately after electroporation unless stated otherwise.
The concentrations of Ca2+ dyes used were as follows: Fluo-4 AM (0.92 mM) and Oregon Green™ 488 BAPTA-1 AM (OGB 488, 0.79 mM). To improve membrane perforation of the dyes, a final concentration of 0.4% of Pluronic F127 (dissolved in 20% in DMSO) was added into AM dye solutions. In addition, Fluo-4 pentapotassium salt (1.08 mM) and Ca green dextran (1%) were also tested.
After dye loading, a DRG was moved to a custom-built recording chamber or a MatTek culture dish, held down with a nylon mesh, and superfused with the oxygenated extracellular solution. Ca2+ imaging experiments were performed under a Nikon confocal microscope with a 20× objective or a custom-built two-photon laser-scanning microscope based on a Coherent Laser System with a water immersion 60× objective. For the confocal microscope, the excitation wavelength was 488 nm. For the two-photon microscope, the laser system was operated at 800 nm.
The Ca2+ images were recorded before, during, and after an electrical sciatic nerve stimulation or a bath application of chemicals, e.g., high KCl (80 mM or 55 mM) or capsaicin (10 µM). For electrical stimulation, the cut end of the sciatic nerve was inserted into a suction electrode and connected to a Master-8 stimulator through an isolator. The stimuli were 1 ms square pulses, ≤7 mA, delivered at 20 Hz. Images were analyzed off-line with a NIH ImageJ software or Nikon NIS Elements software. Data are presented as the relative change in fluorescence (ΔF/F0), where F0 is basal fluorescence and ΔF = F − F0.
Data analyses
Data were expressed as means ± SE or as percentages. Student’s t and chi square tests were used to access the significance of changes. The Mann–Whitney Rank Sum Test was used when the normality test failed for the Student’s t test. Comparisons between multiple means were done with one-way analysis of variance followed by the Holm-Sidak post hoc test. A value of P < 0.05 was considered significant.
Results
Electroporation significantly improves the loading of Fluo-4 AM Ca2+ indicator into DRG neurons
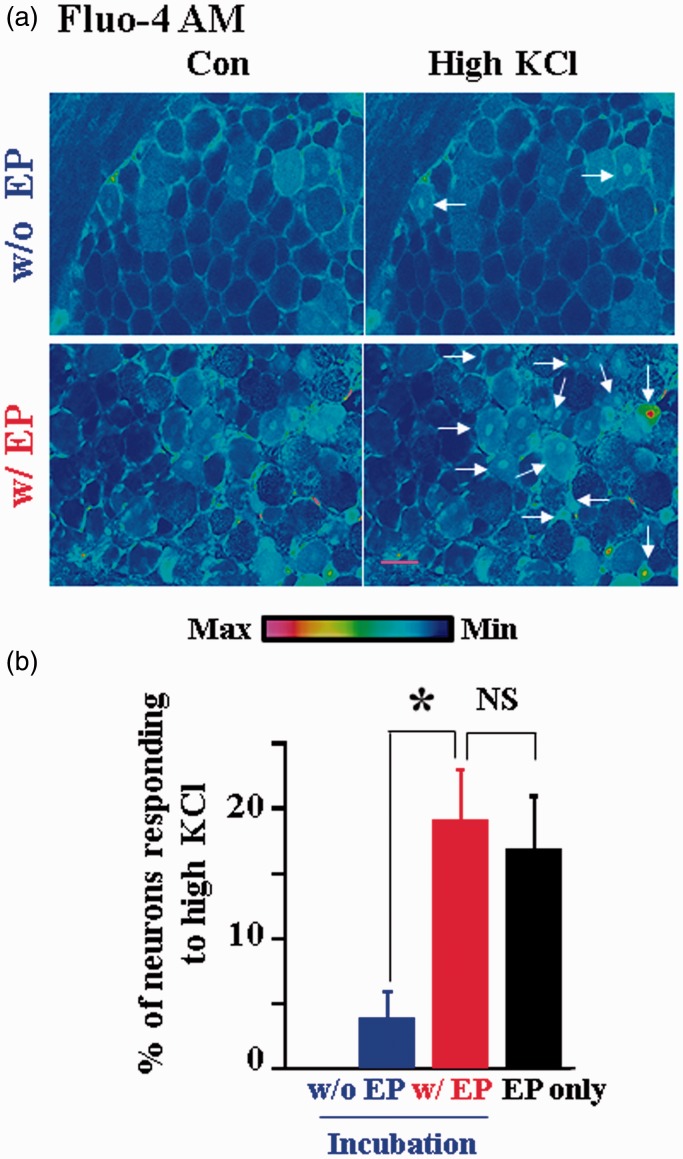
In our previous studies of neuron–glia interactions,5,6 we loaded Fluo-4 AM into neurons and glial cells in whole DRGs through 1 h incubation period. The method allowed us to load Fluo-4 AM into glia. In contrast, only a limited number of neurons were loaded. To improve the Ca2+ dye loading in neurons, DRGs first underwent electroporation, followed by 1 h dye incubation. We found that electroporation greatly increased dye loading in neurons but had little effect on loading in glial cells (Figure 1). To further confirm that the electroporation-treated labeled neurons are functional, we studied the effect of electroporation on the number of labeled neurons in response to a high concentration (80 mM) of KCl treatment. KCl is known to reliably depolarize neurons causing Ca2+ influx into functional neurons.18
Figure 1.
Electroporation increases Fluo-4 AM loading in intact DRG neurons. (a) Examples of pseudocolor intracellular Ca2+ images in cells responding to bath application of 80 mM KCl in intact DRGs with or without electroporation (EP) of Fluo-4 AM, followed by 1 h dye incubation. Arrows indicate the neurons responding to high KCl treatment. Scale bar: 50 µm. Color-coded intensity calibration bar is shown below. (b) Electroporation increased the number of DRG neurons responding to high KCl stimulation (3.90 ± 2.10%, w/o EP, n = 3 DRGs; 19.10 ± 3.86%, w/EP; n = 4; *P < 0.05). In the absence of postincubation, electroporation of Fluo-4 AM yielded a similar percentage of neurons responding to KCl (16.90 ± 0.04% EP only, n = 4 DRGs; P > 0.05 compared with EP + incubation). Note that glia were labeled well with 1 h Fluo-4 AM incubation. Electroporation of Fluo-4 AM had minimal effect on glia labeling.
Electroporation followed by incubation was found to increase Fluo-4 AM labeled neurons responding to high KCl by 4.9 fold (Figure 1(b)). In addition, we found that Fluo-4 AM can be efficiently loaded into neurons through electroporation without postincubation (Figure 1(b)). These results suggest that electroporation significantly improves Fluo-4 AM loading into DRG neurons in intact DRGs. On the other hand, AM dye incubation can label glia well without electroporation.
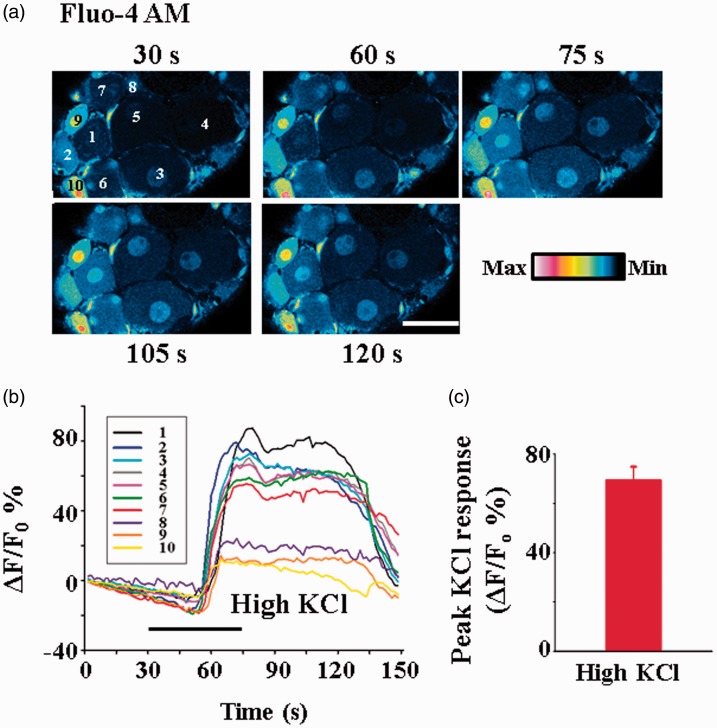
The improvement of dye loading also allowed us to study the time-dependent changes in intracellular Ca2+ in different neurons in intact DRGs. Following electroporation and incubation of Fluo-4 AM, we determined the dynamic changes in fluorescence in response to high KCl treatment (Figure 2). The fluorescence intensity peaked ∼35 s after high KCl application. The average peak fluorescence Ca2+ response (ΔF/F0) was increased by ∼69% (Figure 2).
Figure 2.
Dynamic changes in intracellular Ca2+ in response to high KCl in intact DRG neurons loaded with Fluo-4 AM. (a) Example of Ca2+ changes in the neurons in response to bath application of 80 mM KCl. The time in each frame indicates the time at which the image was taken. Scale bar: 40 µm. (b) Time-dependent changes of intracellular Ca2+ in DRG neurons in response to 80 mM KCl application. The line numbers correspond to the cell numbers labeled in (a). (c) The average peak fluorescence Ca2+ response (ΔF/F0) induced by 80 mM KCl in all the responding DRG neurons (n = 76) in this experiment is 69.00 ± 5.00%. F0 is the basal fluorescence before KCl stimulation.
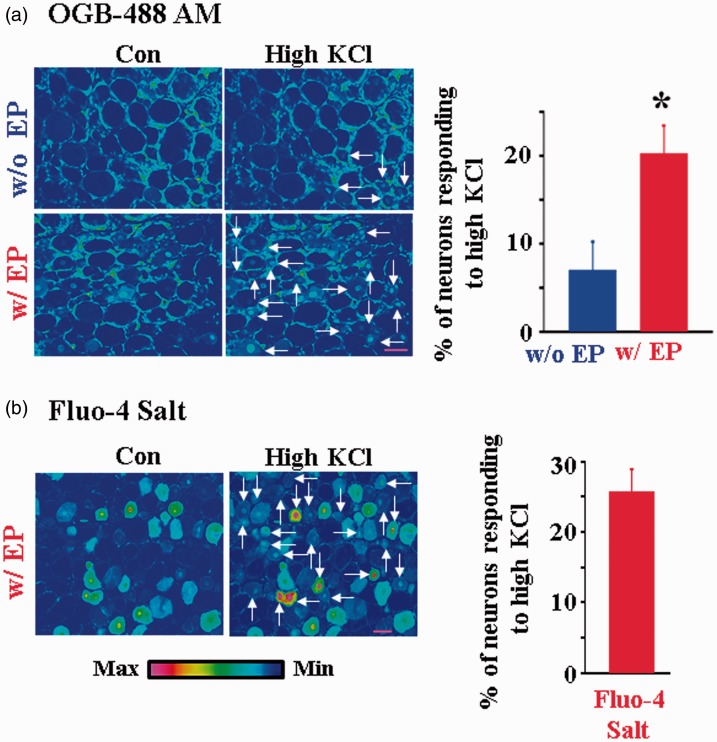
Electroporation also improves loading other AM and salt forms of Ca2+ dye in DRG neurons
To determine whether neuronal dye loading by electroporation is dye dependent, a number of other Ca2+ dyes were tested. We found that electroporation of OGB-488 AM was equally efficient in producing functional dye loaded DRG neurons (Figure 3(a)). In previous studies, a salt form Ca2+ indicator, e.g., Fluo-4 pentapotassium, was loaded into a small number of neurons by direct intracellular injection.19 About 26% of neurons responded to high KCl following electroporation (Figure 3(b)). In contrast to Ca2+ AM dyes, the loading of Fluo-4 salt into glia was rather limited.
Figure 3.
Electroporation improves loadings of OGB-488 AM and Fluo-4 salt in intact DRG neurons. (a) Electroporation of OGB-488 AM significantly increased the number of DRG neurons in response to 80 mM KCl (7.01 ± 3.19% w/o EP, n = 4 DRGs; 20.30 ± 3.13%, w/EP; n = 10; P < 0.05). Note that many glia were labeled with the dye. Scale bar: 50 µm. (b) Electroporation also promoted the loading of Fluo-4 salt in DRG neurons. High KCl evoked an increase in fluorescence intensity in many DRG neurons (25.80 ± 3.13%; n = 4 DRGs). In contrast, the number of glia labeled by Fluo-4 salt was limited. Scale bar: 50 µm.
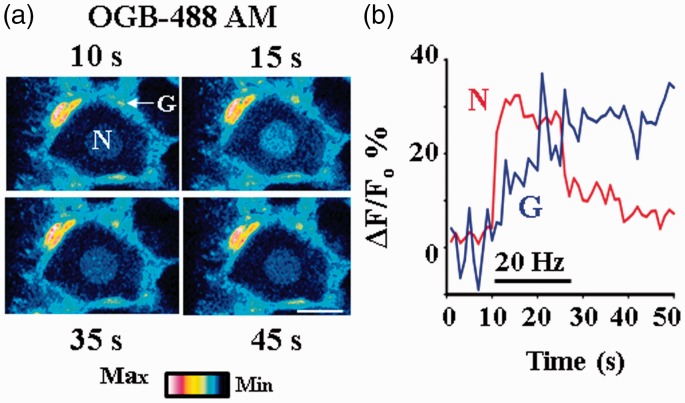
We also determined changes in intracellular Ca2+ fluorescence in L4 or L5 DRG neurons in response to electrical stimulation of sciatic nerves. The DRG preparation has been electroporated and then incubated with OGB488 AM. Following electric stimulation, the Ca2+ fluorescence in neurons robustly increased and reached to its peak within 1–2 s. The fluorescence quickly dropped when the stimulation was stopped and then gradually returned to the baseline (Figure 4). We found that the average peak increase was 35.00 ± 5.00% (ΔF/F0; n = 5). These results suggest that both axons and somata of DRG neurons are functional following electroporation. Using Fluro-4 AM, we previously showed that neurons and glia interact.5,6 To determine whether neuron–glia interactions are maintained when DRGs undergo electroporation, Ca2+ changes in glia were also determined. Glial Ca2+ was increased with a delay (Figure 4), similar to our studies without electroporation.5 Thus, neuron–glia interactions are indeed preserved.
Figure 4.
Neuron–satellite glial cell interactions triggered by electrical nerve stimulation. Sciatic nerve stimulation at 20 Hz increased intracellular Ca2+ in DRG neurons and glial cells. The DRG with attached sciatic nerves was electroporated and incubated with OGB 488-AM. (a) Pseudocolor images of fluorescence changes in a neuron (N) and a glial cell (G). The number at the top or bottom in each frame indicates the time at which the image was taken. Scale bar: 20 µm. (b) Time courses of relative fluorescence changes, i.e., ΔF/F0, in the neuron and glial cell. F0 is the basal fluorescence in either the neuron or the glial cell before nerve stimulation. The horizontal line indicates the period of nerve stimulation. Nerve stimulation evoked intracellular Ca2+ increase in the neuron first and then in the glial cell with a delay of ∼2.0 s.
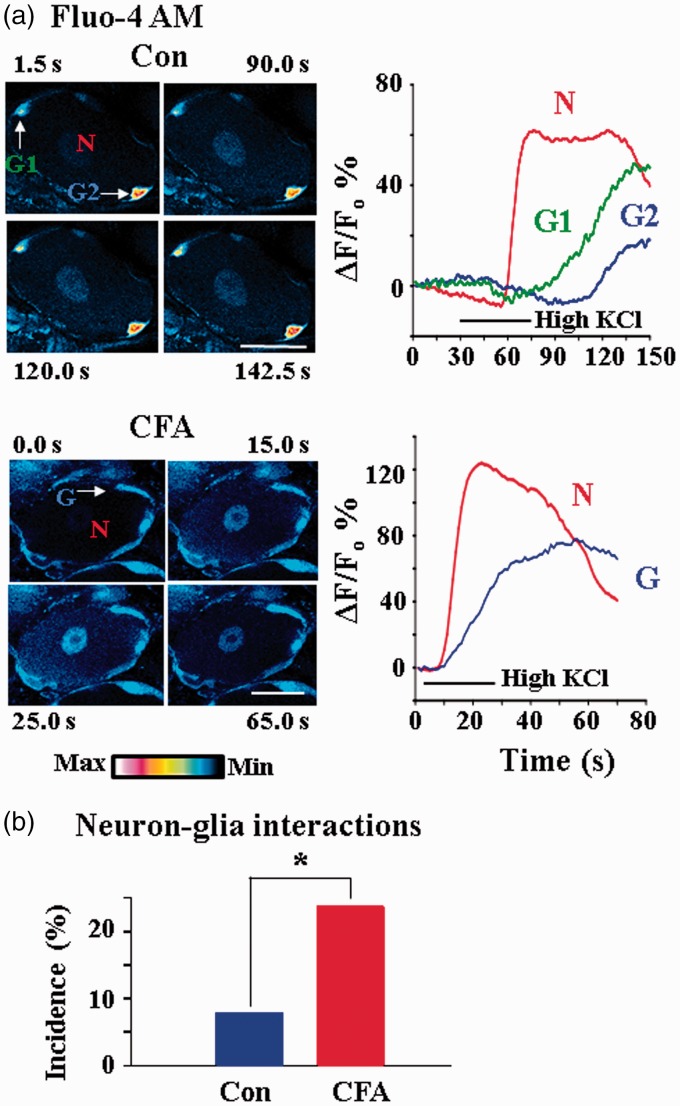
We repeated the experiments using high KCl stimulation (Figure 5). As expected, the rates of Ca2+ fluorescence increase in neurons and glia following chemical, e.g., KCl, stimulation are slower than those following electrical stimulation. The delay in Ca2+ fluorescence increase between neurons and glia persisted. Following CFA, neuron–glia interactions were preserved, and the interactions occurred in more neuron–glia cell pairs. Six neuron–glia interactions were found in 76 KCl responsive neurons (7.9%) in normal rats, and 11 interactions were seen in 46 KCl responsive neurons (23.9%) in CFA rats.
Figure 5.
Inflammation increases the incidence of neuron–glia interactions. Inflammation was induced by injecting CFA into the left hindpaw of a rat. DRGs from control and CFA rats were taken out and electroporated and incubated with Fluo-4 AM. (a) Example pseudocolor images of intracellular Ca2+ changes in DRG neurons from a control (upper) and a CFA (lower) rat in response to bath application of 80 mM KCl. High KCl increased the intracellular Ca2+ first in the neuron (N) and then in glial cells (G1 and G2) with a delay. The number at the top or bottom in each frame indicates the time the image was taken. Time courses of relative fluorescence changes, i.e., ΔF/F0, in the neuron and in the glial cells are shown on the right. The horizontal lines indicate the period of high KCl application. For the images of the CFA rat, high KCl induced similar responses with those in the control rat both in the neuron and in the glial cell. Scale bars: 30 µm. Time courses of relative fluorescence changes are also shown on the right. (b) Neuron–glia interactions were significantly increased in DRGs of CFA rats. In response to high KCl stimulation, 7.9% KCl responsive neurons interacted with glia in control and 23.9% in CFA rats interacted.
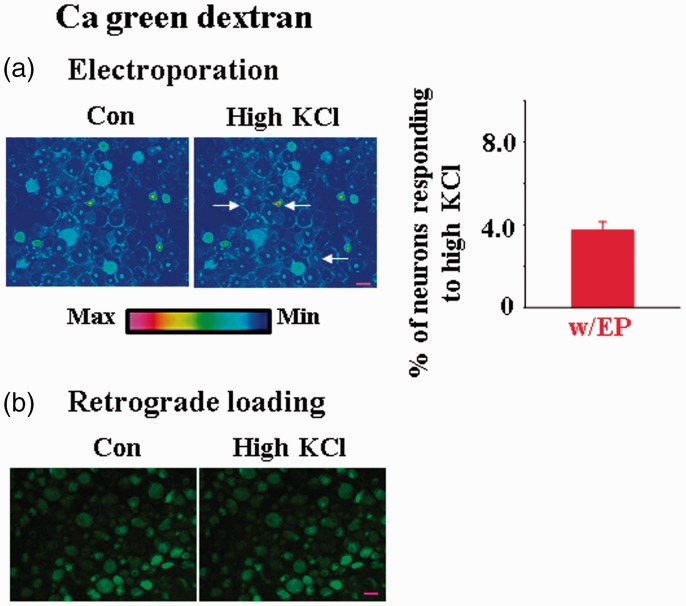
Dextran Ca2+ indicators respond to high KCl stimulation in very few rat DRG neurons
One exception to the success in functional preservation using electroporation was the use of dextran form Ca2+ indicator, i.e., Ca green dextran. Although we were able to load the dextran dye in intact DRGs, only a very low percentage of neurons (3.72 ± 0.42%; n = 5) responded to high KCl treatment (Figure 6(a)). We also used retrograde transport instead of electroporation to load Ca green dextran in DRGs. Neurons with retrograde loaded dye did not respond to high KCl treatment (Figure 6(b)). Thus, the failed KCl experiments are not dye loading method dependent. Others have reported that in adult rats none of the DRG neurons that underwent retrograde loading of Ca green dextran responded to sciatic nerve electric stimuli or bath application of high KCl.20 Since peripheral or CNS neurons loaded with dextran Ca2+ dyes do respond to manipulations such as KCl or electrical nerve stimulation,21–23 the observations suggest that dextran Ca2+ dyes are not suitable for studying Ca2+ functions in DRG neurons. Reasons underlying the failure are unclear.
Figure 6.
Few DRG neurons loaded with the dextran form of Ca2+ dyes are functional. (a) Bath application of 80 mM KCl evoked intracellular Ca2+ changes in a limited number of neurons in DRGs that underwent electroporation of Ca green dextran (3.72 ± 0.42%, n = 5 DRGs). Arrows indicate the responding neurons. Scale bar: 50 µm. (b) In another rat, true color cell images indicated that none of the DRG neurons retrogradely loaded Ca green dextran responded to high KCl (n = 3 DRGs). Scale bar: 50 µm.
Electroporation facilitates studies of pain signaling processing in intact DRG neurons
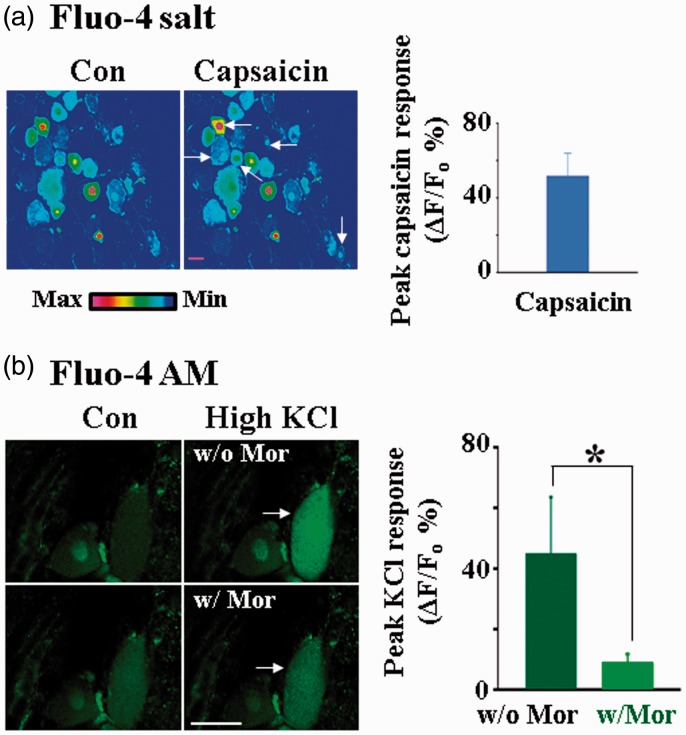
To determine whether the electroporation technique facilitates the study of pain signaling processing in intact DRG neurons, we studied the effect of capsaicin in DRGs treated with electroporation of Fluo-4 salt. Capsaicin was found to increase Ca2+ concentrations in many small and medium DRG neurons (Figure 7(a)), consistent with the studies obtained from the distribution of capsaicin receptors in DRGs.24–27 Thus, the increase in Ca2+ dye loading with electroporation is likely to help us characterize functions of capsaicin receptor activation in intact DRG neurons.
Figure 7.
Applications of intact DRG Ca2+ imaging in pain research. (a) Bath application of 10 µM capsaicin evoked intracellular Ca2+ fluorescence changes in many small and medium DRG neurons with an average peak response of 51.70 ± 12.2%, (ΔF/F0, n = 14 neurons). DRGs were electroporated with Fluo-4 salt. Scale bar: 25 µm. Arrows indicate the neurons with responses. (b) Bath application of 35 µM morphine inhibited 55 mM KCl-induced Ca2+ increase in DRG neurons. DRGs were electroporated and incubated with Fluo-4 AM. Left: Examples of true color images showing the inhibitory effect of morphine on the intracellular Ca2+ level in a neuron (arrow). Right: Morphine inhibited high KCl-induced Ca2+ increase in DRG neurons (ΔF/F0: 45.00 ± 18.50% w/o morphine, 9.19 ± 2.52% w/morphine, n = 10). Scale bar: 25 µm.
We also studied the morphine effects on DRG neurons underwent electroporation with Fluo-4 AM (Figure 7(b)). The effects of morphine on KCl-induced Ca2+ increases in cells treated with and without morphine were studied. Morphine was found to block the KCl-induced Ca2+ fluorescence in DRG neurons by ∼80%, a result consistent with the observation that morphine blocks voltage-dependent Ca2+ channels in DRG neurons.28
Discussion
In this study, we found that electroporation of intact DRGs greatly facilitates the loading of Ca2+ dyes simultaneously into individual neurons without affecting their physiological functions. By combining electroporation and incubation of AM Ca2+ dye, we were able to study neuron–glia interactions efficiently in intact DRGs. The major advantages of this approach are its simplicity and high efficiency.
Bulk AM Ca2+ dye loading of CNS neurons has been successful in in vitro and in vivo preparations.29–31 It is unclear the reason for poor loading of AM Ca2+ dye in intact DRG neurons in the absence of electroporation. One of the possibilities is that each soma of DRG neuron is tightly surrounded by a layer of SGCs, which form a functional unit enclosed by a connective tissue sheath.32 This results in a physical barrier for Ca2+ dye to gain access to DRG neurons.
The number of neuron–glia interacting pairs induced by high KCl is increased in DRGs in CFA-induced inflamed rats (Figure 5). We have previously shown that CFA-induced inflammation enhances the expression of P2X7 receptors in rats, and the neuron–glia interactions depend on the P2X7 receptors in SGCs in DRGs.5,6 The roles of P2X7R in neuron–glia interactions in CFA rats can now be further determined with the improved Ca2+ dye loading in intact DRGs.
The results that capsaicin causes an increase in Ca2+ signaling in small and medium cells and the µ-opioid receptor agonist, morphine, significantly inhibits high KCl-induced Ca2+ increase in DRG neurons are consistent with published findings.33–36 These observations suggest that the electroporation Ca2+ dye loading method should be useful for studying drug effects in intact DRGs in pain research.
Recently, genetic Ca2+ sensors have become available for studying Ca2+ changes in virus-transfected neurons and/or in mutant mice.37–39 Using these sensors, it is possible to probe the Ca2+ activities in specific subpopulations of sensory neurons.40 However, these approaches usually take weeks or months to produce results. Compared with genetic approaches, Ca2+ dye loading through electroporation and incubation cannot target specific populations of neurons. On the other hand, in combination with specific pharmacological tools, electroporation and incubation of Ca2+ dyes can efficiently provide general ideas about Ca2+ signaling in subpopulations of DRG neurons. We can then use the observations as a prelude to understanding Ca2+ signaling in a specific subpopulation of DRG neurons using genetic Ca2+ sensors.
Acknowledgment
The authors thank Dr. Yanping Gu for valuable comments on the manuscript.
Author Contributions
YC and LMH designed research. YC performed experiments. YC and LMH wrote the paper.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by grants from NINDS NS030045, NIDCR DE017813, National Institutes of Health.
References
- 1.Cesare P, McNaughton P. Peripheral pain mechanisms. Curr Opin Neurobiol 1997; 7: 493–499. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman AR. Sensory ganglia. In: Landon D. (ed). The peripheral nerve, London: Chapman and Hall, 1976, pp. 188–278. [Google Scholar]
- 3.Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain 1999Suppl 6, S27–S35. [DOI] [PubMed] [Google Scholar]
- 4.Liu CN, Wall PD, Ben-Dor E, et al. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 2000; 85: 503–521. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Chen Y, Wang C, et al. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A 2007; 104: 9864–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Zhang X, Wang C, et al. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci USA 2008; 105: 16773–16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du X, Hao H, Gigout S, et al. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 2014; 155: 2306–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Mei X, Zhang P, et al. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia 2009; 57: 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang TY, Belzer V, Hanani M. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Euro J Pain 2010; 14: 49.e41–e11. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Li G, Huang LY. P2X7 receptors in satellite glial cells mediate high functional expression of P2X3 receptors in immature dorsal root ganglion neurons. Mol Pain 2012; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang LY, Gu Y, Chen Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013; 61: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Li G, Huang LY. p38 MAPK mediates glial P2X7R-neuronal P2Y1R inhibitory control of P2X3R expression in dorsal root ganglion neurons. Mol Pain 2015; 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JM, Donnelly DF, LaMotte RH. Patch clamp recording from the intact dorsal root ganglion. J Neurosci Methods 1998; 79: 97–103. [DOI] [PubMed] [Google Scholar]
- 14.Ma C, Shu Y, Zheng Z, et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 2003; 89: 1588–1602. [DOI] [PubMed] [Google Scholar]
- 15.Hayar A, Gu C, Al-Chaer ED. An improved method for patch clamp recording and calcium imaging of neurons in the intact dorsal root ganglion in rats. J Neurosci Methods 2008; 173: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemes G, Rigaud M, Koopmeiners AS, et al. Calcium signaling in intact dorsal root ganglia: new observations and the effect of injury. Anesthesiology 2010; 113: 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma C, Donnelly DF, LaMotte RH. In vivo visualization and functional characterization of primary somatic neurons. J Neurosci Methods 2010; 191: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estacion M, Vohra BP, Liu S, et al. Ca2+ toxicity due to reverse Na+/Ca2+ exchange contributes to degeneration of neurites of DRG neurons induced by a neuropathy-associated Nav1.7 mutation. J Neurophysiol 2015; 114: 1554–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong JH, Min CH, Jeong B, et al. Intracellular calcium spikes in rat suprachiasmatic nucleus neurons induced by BAPTA-based calcium dyes. PLoS One 2010; 5: e9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utzschneider DA, Rand MN, Waxman SG, et al. Nuclear and cytoplasmic Ca2+ signals in developing rat dorsal root ganglion neurons studied in excised tissue. Brain Res 1994; 635: 231–237. [DOI] [PubMed] [Google Scholar]
- 21.Kettunen P, Demas J, Lohmann C, et al. Imaging calcium dynamics in the nervous system by means of ballistic delivery of indicators. J Neurosci Methods 2002; 119: 37–43. [DOI] [PubMed] [Google Scholar]
- 22.Nagayama S, Zeng S, Xiong W, et al. In vivo simultaneous tracing and Ca(2+) imaging of local neuronal circuits. Neuron 2007; 53: 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes AR, Chavez-Valdez R, Ezell T, et al. Effect of development on [Ca2+]i transients to ATP in petrosal ganglion neurons: a pharmacological approach using optical recording. J Appl Physiol (1985) 2012; 112: 1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo A, Vulchanova L, Wang J. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 1999; 11: 946–958. [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Samad TA, Jin SX, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36: 57–68. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Yang F, Luo H, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain 2008; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barabas ME, Stucky CL. TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Mol Pain 2013; 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdulla FA, Smith PA. Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J Neurosci 1997; 17: 8721–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regehr WG, Tank DW. Selective fura-2 loading of presynaptic terminals and nerve cell processes by local perfusion in mammalian brain slice. J Neurosci Methods 1991; 37: 111–119. [DOI] [PubMed] [Google Scholar]
- 30.Stosiek C, Garaschuk O, Holthoff K, et al. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A 2003; 100: 7319–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohki K, Chung S, Ch’ng YH, et al. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 2005; 433: 597–603. [DOI] [PubMed] [Google Scholar]
- 32.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev 2005; 48: 457–476. [DOI] [PubMed] [Google Scholar]
- 33.Savidge JR, Ranasinghe SP, Rang HP. Comparison of intracellular calcium signals evoked by heat and capsaicin in cultured rat dorsal root ganglion neurons and in a cell line expressing the rat vanilloid receptor, VR1. Neuroscience 2001; 102: 177–184. [DOI] [PubMed] [Google Scholar]
- 34.Nandi R, Beacham D, Middleton J, et al. The functional expression of mu opioid receptors on sensory neurons is developmentally regulated; morphine analgesia is less selective in the neonate. Pain 2004; 111: 38–50. [DOI] [PubMed] [Google Scholar]
- 35.Schnizler K, Shutov LP, Van Kanegan MJ, et al. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008; 28: 4904–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol 2006; 577: 169–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen TW, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013; 499: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonin RP, Wang F, Desrochers-Couture M, et al. Epidural optogenetics for controlled analgesia. Mol Pain 2016; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YS, Anderson M, Park K, et al. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 2016; 91: 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghitani N, Barik A, Szczot M, et al. Specialized mechanosensory nociceptors mediating rapid responses to hair pull. Neuron 2017; 95: 944–954.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]