Abstract
Case summary
Systemic arterial hypertension is commonly reported in middle-aged-to-older cats. Amlodipine is recommended as the initial antihypertensive drug in cats. In this case report, gingival hyperplasia secondary to the use of amlodipine in a cat is described. Benazepril as a monotherapy was unsuccessful in reducing blood pressure in this cat. After replacement of benazepril by telmisartan, gingival hyperplasia disappeared and blood pressure was well controlled.
Relevance and novel information
This case report describes the first reported case of reversible gingival hyperplasia as a result of the treatment with amlodipine. It also contains the first published data on the effect of telmisartan in a hypertensive cat.
Introduction
In cats, systemic arterial hypertension has been commonly described.1 At the time of treatment of the described cat no antihypertensive drugs were licensed for this species. Therefore, amlodipine was commonly used off label for the reduction of blood pressure. Gingival hyperplasia is a well-known side effect of amlodipine in humans and dogs, but, to our knowledge, this side effect has not been described in cats. Telmisartan is licensed for the treatment of hypertension in humans and a dose-dependent reduction of blood pressure in cats is mentioned in the European Public Assessment Report (EPAR), a review of the registration study of telmisartan performed by the European Medicines Agency (Semintra; Boehringer Ingelheim). In this case report the effects of amlodipine, benazepril and telmisartan on hypertension in a cat with systemic hypertension are described. Gingival hyperplasia induced by amlodipine is also reported in this case.
Case description
A 15-year-old castrated male European Shorthair cat, without a history or clinical signs of hypertension or other disease, was presented for a routine senior check-up.
During the physical examination, a systolic apical murmur grade II/VI and a systolic blood pressure (SBP) of 215 mmHg, measured at the forelimb by a Doppler device (Model 811-B; Parks Medical Electronics), was established. Fundoscopy did not reveal any abnormalities. The results of the haematology and plasma biochemistry examination were all within the reference interval (RI; Table 1). A urine sample was collected by cystocentesis. The urine was minimally concentrated (specific gravity of 1.015); urinalysis, including urine protein: creatinine ratio and bacterial culture, revealed no abnormalities. The rest of the physical examination was unremarkable and body condition score was 5/9. Dental examination revealed no calculus or gingivitis.
Table 1.
Laboratory results of the initial examination
| Test | Result | RI |
|---|---|---|
| TT4 (nmol/l) | 24.5 | 10–60 |
| Urea (mmol/l) | 11.7 | 5.7–13.5 |
| Creatinine (µmol/l) | 130 | <168 |
| Sodium (mmol/l) | 151 | 147–159 |
| Potassium (mmol/l) | 3.5 | 3.3–5.8 |
| Inorganic phosphate (mmol/l) | 1.0 | 0.8–2.2 |
| Bilirubin (µmol/l) | 3.6 | <6.8 |
| ALT (U/l) | 120 | <175 |
| ALP (U/l) | 36 | <73 |
| GGT (U/l) | <1 | <5 |
| AST (U/l) | 104 | <71 |
| GLDH (U/l) | 1 | <11 |
| Total protein (g/l) | 81 | 59–87 |
| Albumin (g/l) | 34 | 27–44 |
| Globulin (g/l) | 47 | 29–54 |
| Albumin:globulin ratio | 0.71 | >0.57 |
| Glucose (mmol/l) | 7.3 | 3.5–7.8 |
| Cholesterol (mmol/l) | 6.9 | <8.5 |
| Fructosamine (µmol/l) | 328 | 190–365 |
| CK (U/l) | 306 | <542 |
| LDH (U/l) | 178 | <182 |
| Calcium (mmol/l) | 2.5 | 2.2–2.9 |
| Magnesium (mmol/l) | 0.8 | 0.6–1.1 |
| Triglycerides (mmol/l) | 0.3 | 0.2–4.9 |
| Leukocytes (g/l) | 12.5 | 6–11 |
| Erythrocytes (t/l) | 8.5 | 5–10 |
| Haemoglobin (g/dl) | 9.9 | 9–15 |
| PCV (%) | 33 | 28–45 |
| MCV (fl) | 39 | 40–55 |
| MCH (pg) | 12 | 13–17 |
| MCHC (g/dl) | 30 | 31–35 |
| Thrombocytes (g/l) | 535 | 150–550 |
| Reticulocytes (relative) (%) | 0.39 | |
| Reticulocytes (absolute) (/µl) | 33,000 | |
| Basophils (relative) (%) | 0 | 0–1 |
| Eosinophils (relative) (%) | 4 | 0–6 |
| Segmented neutrophils (relative) (%) | 81 | 50–75 |
| Lymphocytes (relative) (%) | 13 | 15–50 |
| Monocytes (relative) (%) | 1 | 0–4 |
| Basophils (absolute) (/µl) | 0 | |
| Eosinophils (absolute) (/µl) | 511 | 0–600 |
| Segmented neutrophils (absolute) (/µl) | 10,142 | 3000–11,000 |
| Lymphocytes (absolute) (/µl) | 1620 | 1000–6000 |
| Monocytes (absolute) (/µl) | 162 | 0–500 |
RI = reference interval; TT4 = total thyroxine; ALT = alanine transaminase; ALP = alkaline phosphatase; GGT = gamma glutamyl transpeptidase; AST = aspartate aminotransferase; GLDH = glutamate dehydrogenase; CK = creatine kinase; LDH = lactate dehydrogenase; PCV = packed cell volume; MCV = mean cell volume; MCH = mean cell haemoglobin; MCHC = mean cell haemoglobin concentration
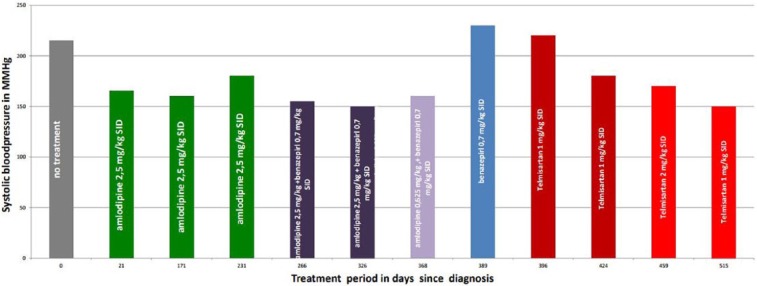
Treatment for hypertension with amlodipine (Norvasc; Pfizer) was started at 2.5 mg q24h. This dose was higher than the 1.25 mg/kg normally used; why the attending veterinarian made this decision remains unknown. Three weeks later SBP was 165 mmHg (Table 2, Figure 1). Treatment with amlodipine was continued. Recheck blood pressure measurements were advised every 3 months.
Table 2.
Type and duration of treatment and systolic blood pressure (SBP)
| Treatment | SBP | Treatment period since last BP measurement in days | Total treatment period in days |
|---|---|---|---|
| None | 215 | 0 | |
| Amlodipine 2.5 mg q24h | 165 | 21 | 21 |
| Amlodipine 2.5 mg q24h | 160 | 150 | 171 |
| Amlodipine 2.5 mg q24h | 180 | 60 | 231 |
| Amlodipine 2.5 mg/kg and benazepril 0.7 mg/kg q24h | 155 | 35 | 266 |
| Amlodipine 2.5 mg/kg and benazepril 0.7 mg/kg q24h | 150 | 60 | 326 |
| Amlodipine 0.625 mg and benazepril 0.7 mg/kg q24h | 160 | 42 | 368 |
| Benazepril 0.7 mg/kg q24h | 230 | 21 | 389 |
| Telmisartan 1 mg/kg | 220 | 7 | 396 |
| Telmisartan 1 mg/kg | 180 | 28 | 424 |
| Telmisartan 2 mg/kg | 170 | 35 | 459 |
| Telmisartan 2 mg/kg | 150 | 56 | 515 |
BP = blood pressure
Figure 1.
Blood pressure over time with different treatments
Five months later a SBP of 160 mmHg was measured (Table 2, Figure 1). Mild gingival hyperplasia was identified; no treatment was initiated. The cat also showed signs of bilateral conjunctivitis, including bilateral dried-up ocular discharge. The Schirmer tear test (STT) was 7 mm/min for the left eye and 6 mm/min for the right eye (reference >10 mm/min).2 Based on these findings a diagnosis of keratoconjunctivitis sicca (KCS) was made. The cat was treated with Optimmune (ciclosporin A 0.2%; MSD Animal Health) twice daily in both eyes. After 1 month of treatment the STT for the left eye was 7 mm/min and the STT for the right eye was 13 mm/min. The conjunctivitis had healed. Treatment with Optimmune was continued in the left eye twice daily and in the right eye once daily. No clinical abnormalities other than the development of mild gingival hyperplasia and KCS were seen or reported.
Two months later blood pressure was 180 mmHg and gingival hyperplasia had worsened. The KCS was well controlled: STT of the left and right eye were both 14 mm/min; treatment with Optimmune once daily in both eyes was continued.
To achieve better control of systemic hypertension benazepril (0.7mg/kg q24h [Benakor; AST Farma]), was added to amlodipine therapy. Five weeks later SBP decreased to 155 mmHg; therefore, therapy with amlodipine and benazepril was continued, and tear production was unchanged.
After 2 months blood pressure and presumed KCS were well controlled (SBP 150 mmHg) (STT left eye 10 mm/min; STT right eye 25 mm/min). Gradual progression of gingival hyperplasia continued and it was even visible with closed mouth. Because of this side effect the owner wanted to reduce treatment with amlodipine, owing to concerns that the gingival hyperplasia would cause discomfort. The dose of amlodipine was gradually reduced by 50% every 3 weeks while continuing benazepril. While decreasing the dose of amlodipine to 0.625 mg q24h, blood pressure was monitored and remained stable at a SBP of 160 mmHg (Table 2, Figure 1).
At the time when the dose of amlodipine was reduced to 0.625 mg the owner discontinued treatment with Optimmune. Despite discontinuing the Optimmmune, clinical signs of conjunctivitis did not recur and tear production remained adequate.
Upon the owner’s request, the amlodipine was discontinued and treatment with benazepril was continued. The possibility of an increase in blood pressure when stopping amlodipine was discussed with the owner.
Three weeks later SBP measured 230 mmHg (Table 2, Figure 1). The owner refused to restart amlodipine. Treatment with telmisartan (an angiotensin II receptor blocker) once daily at 1 mg/kg body weight was started (Semintra; Boehringer Ingelheim). Benazepril was discontinued 24 h before starting treatment with telmisartan.
One week and 4 weeks after starting therapy with telmisartan, SBP was 220 mmHg and 180 mmHg, respectively (Table 2, Figure 1). Since the antihypertensive effect of telmisartan is dose related (EPAR), the dose given was doubled after 4 weeks of treatment (2 mg/kg body weight) to try to achieve more pronounced control of hypertension. Five weeks later SBP was 170 mmHg (Table 2, Figure 1). At this point blood and urinalysis were repeated. The urine was minimally concentrated (1.017) and creatinine was mildly elevated (155 µmol/l). During the treatment with telmisartan, no other abnormalities were noticed and the gingival hyperplasia gradually decreased. Three months later gingival hyperplasia was almost completely dissolved. SBP had decreased further to 150 mmHg (Table 2, Figure 1). At the last check-up, 808 days after the first treatment with amlodipine, the gingival hyperplasia completely disappeared and SBP was 160 mmHg (Table 2 ).
Discussion
In this case amlodipine therapy was started at a dose of 2.5 mg q24h, which is higher than the recommended dose (0.625–1.25 mg/cat q24h). Gingival hyperplasia associated with calcium channel blockers is known to be a dose-dependent adverse event in humans.3 In cats, dosage may also be a contributing factor to gingival hyperplasia. Even though a reduction of amlodipine dose did not resolve the gingival hyperplasia in this case, it is possible that at a lower dose the side effect would not have appeared. The side effect only disappeared when the amlodipine was discontinued. Blood pressure monitoring was performed during a total period of 26 months.
Systemic hypertension in cats is most commonly secondary to an underlying cause. The differential diagnosis for systemic arterial hypertension in cats includes renal disease, hyperthyroidism, diabetes mellitus, hyperaldosteronism, hyperadrenocorticism, phaeochromocytoma, liver disease, chronic anaemia and primary or idiopathic hypertension. As the results of the haematology and biochemistry examination (including thyroxine, glucose, fructosamine, liver enzymes and haematocrit) were normal, it was possible to exclude diabetes mellitus, liver disease and anaemia. Occult hyperthyroidism is unlikely as total thyroxine was twice within the RI (24.5 nmol/l and 25.7 nmol/l, respectively [RI 10–60 nmol/l]).
While white coat hypertension also occurs in cats, in this case this was highly unlikely. The cat was persistently hypertensive prior to treatment and normotensive while receiving antihypertensive medications, making anxiety an unlikely cause of the hypertension.
The main differentials for the hypertension in this case are idiopathic or underlying chronic kidney disease (CKD).4 The presence of minimally concentrated urine could be a result of underlying CKD or pressure diuresis from the hypertension. The progressive increase in creatinine similarly maybe due to underlying CKD or evidence of target organ damage due to the hypertension or an effect of the antihypertensive medication.
Both benazepril and telmisartan reduce glomerular capillary pressure, which can give a mild increase in creatinine concentration. The use of an angiotensin-converting enzyme (ACE) inhibitor is rarely sufficient to significantly reduce blood pressure in cats with systemic hypertension.5 However, an earlier study reported blood pressure returning to the normal range in 69% of cats treated with ramipril alone,6 although this study lacked a placebo control group. In this case benazepril as a monotherapy was unsuccessful in reducing blood pressure.
Telmisartan is an angiotensin II receptor blocker (ARB) that is licensed for the reduction of proteinuria associated with CKD in cats. In humans, telmisartan is licensed to treat essential hypertension. The EPAR of Semintra describes a dose-dependent reduction of blood pressure. Theoretically, an ARB can reduce blood pressure in cats because it inhibits the renin–angiotensin–aldosterone system (RAAS), therefore reducing blood pressure. It is not known if all forms of feline hypertension are caused by RAAS activation.7,8 In this case benazepril, which is also a RAAS-suppressing agent, was not able to reduce BP as a monotherapy. However, telmisartan did result in a dose-dependent reduction in blood pressure. The reason for this difference is unclear; it may be a result of the mode of action of the two drugs. Telmisartan selectively binds to the angiotensin II type 1 receptor and blocks the activity of angiotensin II (ANG II). Benazepril is an enzyme inhibitor that blocks the formation of ANG II by blocking ACE. One of the possible explanations for the apparent difference in efficacy between telmisartan and benazepril in this case is the ACE escape phenomenon that has been described in several species, including cats.9–11 The formation of ANG II from angiotensin I is catalysed by ACE. There are different pathways/enzymes (eg, chymase) that can also catalyse the formation of ANG II. If this happens ACE is still reduced by the ACE inhibitor, but ANG II reverts to previous levels.
There were some limitations in this case. There was no diagnostic investigation for hyperaldosteronism. Hyperaldosteronism is an established cause of feline hypertension and should be ruled out before a diagnosis of idiopathic hypertension can be made. A grade II/VI systolic heart murmur was heard, but the owner declined echocardiographic examination. Because of the possible adverse consequences of hypertension, the owner elected to treat with antihypertensive medications and declined further diagnostic tests. The dose of amlodipine used in this case was higher than recommended; this might have contributed to the occurrence of gingival hyperplasia.
The cause of the KCS remains unknown. It may have been caused by a herpes infection, even though the cat had a good vaccination status, or it could have been a side effect of amlodipine. The work-up on the cause of this clinical sign was insufficient to make a clear diagnosis.
Conclusions
In this cat, telmisartan was an effective and safe alternative to amlodipine. It gave a sufficient reduction of SBP (230 mmHg before telmisartan, 150–160 mmHg on 2 mg/kg telmisartan) and during treatment the side effects of the treatment with amlodipine completely disappeared.
Based on this case report telmisartan might be a good alternative for amlodipine for the treatment of feline hypertension. Replacement of amlodipine with benazepril did not give sufficient blood pressure control. Further research of the use of telmisartan in feline hypertension is necessary to make further conclusions.
Footnotes
Conflict of interest: Jeroen van der Meer is currently employed at Boehringer-Ingelheim, the registration holder of Semintra (telmisartan).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 18 October 2016
References
- 1. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 2. Sebbag L, Kass PH, Maggs DJ. Reference values, intertest correlations, and test-retest repeatability of selected tear film tests in healthy cats. J Am Vet Med Assoc 2015; 15: 426–435. [DOI] [PubMed] [Google Scholar]
- 3. Kaur G, Verhamme KMC, Dieleman JP, et al. Association between calcium channel blockers and gingival hyperplasia. J Clin Periodontol 2010; 37: 625–630. [DOI] [PubMed] [Google Scholar]
- 4. Taylor SS, Sparkes AH, Briscoe K, et al. ISFM consensus guidelines on the diagnosis and management of hypertension in cats. J Feline Med Surg 2017; 19: 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen J, Henik RA, Brownfield M, Armstrong J. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res 1997; 58: 535–540. [PubMed] [Google Scholar]
- 6. Van Israel N, Desmoulins PO, Huyghe B, et al. Ramipril as monotherapy for the treatment of feline hypertension. ECVIM Proceedings; 2009. Sept 10–12; Porto. [Google Scholar]
- 7. Syme HM, Markwell PJ, Elliott J. Aldosterone and plasma renin activity in cats with hypertension and/or chronic renal failure. J Vet Intern Med 2002; 16: 354 [Google Scholar]
- 8. Jensen J, Henik RA, Brownfield M, Armstrong J. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res 1997; 58: 535–540. [PubMed] [Google Scholar]
- 9. Aramaki Y, Uechi M, Takase K. Angiotensin converting enzyme and chymase activity in the feline heart and serum. J Vet Med Sci 2003; 65: 1115–1118. [DOI] [PubMed] [Google Scholar]
- 10. Côté E, Macdonald KA, Meurs KM, et al. Hypertrophic cardiomyopathy. In: Côté E, Macdonald KA, Meurs KM, et al. (eds). Feline cardiology. Chichester: Wiley-Blackwell, 2011, p 158. [Google Scholar]
- 11. Macdonald KA, Kittleson MD, Larson RF, et al. The effect of ramipril on left ventricular mass, myocardial fibrosis, diastolic function, and plasma neurohormones in Maine Coon cats with familial hypertrophic cardiomyopathy without heart failure. J Vet Intern Med 2006; 20: 1093–1105. [DOI] [PubMed] [Google Scholar]