Abstract
The objective of this integrative review was to describe current US trends for health technology-enabled adherence interventions among behaviorally HIV-infected youth (ages 13–29 years), and present the feasibility and efficacy of identified interventions. A comprehensive search was executed across five electronic databases (January 2005–March 2016). Of the 1911 identified studies, nine met the inclusion criteria of quantitative or mixed methods design, technology-enabled adherence and or retention intervention for US HIV-infected youth. The majority were small pilots. Intervention dose varied between studies applying similar technology platforms with more than half not informed by a theoretical framework. Retention in care was not a reported outcome, and operationalization of adherence was heterogeneous across studies. Despite these limitations, synthesized findings from this review demonstrate feasibility of computer-based interventions, and initial efficacy of SMS texting for adherence support among HIV-infected youth. Moving forward, there is a pressing need for the expansion of this evidence base.
Keywords: Patient compliance, Smartphone, Text messaging, Cell phones, HIV, Adherence, Retention in HIV care, Technology
Introduction
An estimated 955,081 persons were living with human immunodeficiency virus (HIV)-infection in the US, at the end of 2014 [1]. HIV-infected ethnic minority adolescents and young adults, bear a disproportionate burden of disease [2] with young black men who have sex with men (MSM) ages 15–29 years of age, accounting for (16.3%) of the 39,515 new HIV diagnoses in 2015 [1]. Staggering disparities along the HIV care cascade exist for HIV-infected youth living in the US including lack of sustained HIV RNA viral suppression, adherence to antiretroviral therapy (ART), and retention in care [3, 4]. Antiretroviral therapy is recommended for all HIV-infected individuals irrespective of HIV biomarkers [5], and optimal HIV disease management is contingent upon high levels of adherence, greater than >95% to prescribed unboosted protease inhibitor (PI) containing ART regimens, and 80% adherence for boosted PI ART regimens [6, 7]. However, despite the availability of effective ART, estimates of HIV RNA viral suppression range from 30.5% [4] to less than six percent among HIV-infected youth [3], and uncontrolled HIV replication imposes an increased risk of morbidity and mortality at all CD4-T lymphocyte strata [8].
Recently, conceptualization of ART adherence has been broadened to include early engagement and sustained retention in HIV care [5]. Yet nearly 60% of youth initiating ART are not retained in care [3], thereby impairing access to HIV medications and increasing risk for ART resistance [9], treatment failure [9, 10], patient mortality [11, 12], and sexual transmission to seronegative partners [13, 14]. To date, technology-enabled interventions have shown promise for a range of chronic conditions [15], including HIV-in-fected adolescent, young adult, and adult cohorts from domestic and international settings [16–21]. However, much less is known about the impact of health technology-enabled interventions on the HIV care cascade [22] with significant knowledge gaps in research targeting ART adherence and retention outcomes among HIV-infected youth [23].
Mobile and or electronic health technologies represent a viable option to deliver behavioral interventions targeting ART adherence [17, 24] and also to reach, engage and retain young adults in HIV prevention and care [25]. Reported benefits include a more efficient use of provider resources, increased reach for hard to access populations [26], and potential to mitigate barriers, including travel to healthcare settings [27]. Increased technology use has been reported in HIV-infected youth [28, 29], with the cell phone described as the preferred route for communication with a healthcare provider and the internet being the first choice for obtaining health related information [28, 29]. These findings are consistent with current US trends of expanded technology use and ownership, including cell phone and internet use among adolescents and young adults [30–32] with increased patterns of smartphone use among US ethnic minority youth [33].
Widespread belief exists that technology has the potential to transform healthcare management, yet large-scale implementation is not possible without an adequate evidence base [34]. Recent literature reviews including cohorts of HIV-in-fected youth provide evidence on interventions for ART adherence [35], service delivery for linkage, retention and ART adherence [36], and trends in technology use [25]. However, individual studies within each of these reviews represent a broad range of outcomes and populations [25], domestic and international settings [25, 35, 36], and a range of delivery modes with and without technological platforms [35]. Therefore, critical appraisal and synthesis of data extracted from a more homogenous body of evidence is a vital first step to inform the development of effective health technology-enabled interventions for support of HIV-infected youth progressing through the HIV care cascade. Hence, the objectives of this integrative review are to: (1) describe current US trends in the development and testing of health technology-enabled interventions for support of ART adherence and or retention in HIV care among behaviorally HIV-infected adolescents and young adults (ages 13–29 years), and (2) present the feasibility and efficacy of identified interventions, including recruitment settings and strategies.
Methods
The methodological development for this integrative review was guided using criteria from Whittemore and Knafl’s framework [53] and the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [37]. The integrative review framework of Whittemore and Knafl [53] provides a systematic, method for combining diverse data sources using a narrative analysis. A primary goal of this methodology is for a synthesis of the evidence, through application of strategies for data evaluation, and analysis (data reduction, data display, data comparison, conclusion drawing, and verification).
Literature Search
Development of the Search Strategy and Search Results
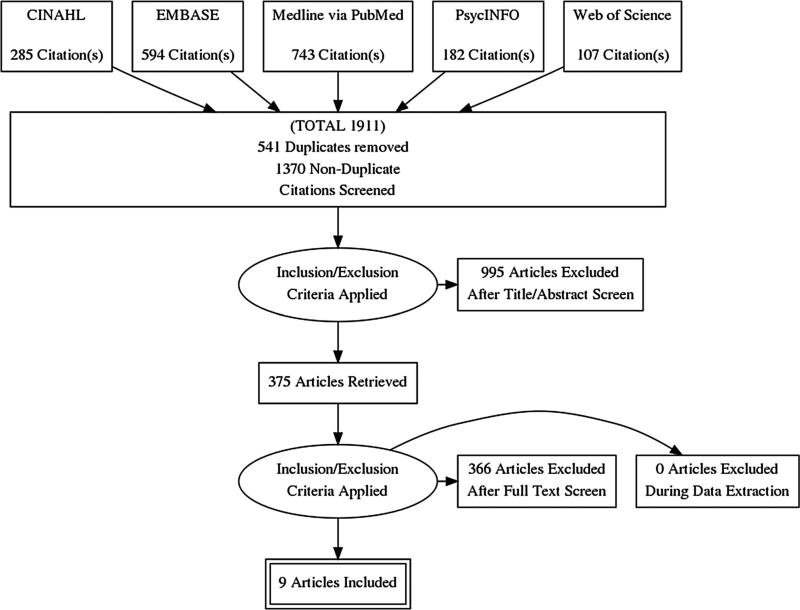
We conducted a comprehensive search of five electronic library databases, including PubMed, CINAHL, EMBASE, PsycINFO, and Web of Science to identify relevant studies published between January 1, 2005–March 18, 2016. The final search strategy with search terms shown in Appendix reflects results of an iterative process generated during a four-month period, and distinct for each of the five electronic article databases. Subject headings and Medical Subject Headings (MeSH) terms unique to each database were extracted from citations retrieved during the initial search phase, and relevant terms were continually added to build the search strategy. Manual reference searches of citations within identified systematic reviews were also conducted. Retrieved citations (N = 1911) were downloaded to EndNote X7, and then copied to Covidence software program for management of systematic reviews (https://www.covidence.org/). A total of 541 duplicates (defined as studies with exact author, publication year and title) were identified and removed using Covidence software (n = 450) and manual searching of the data set (n = 91), resulting in 1370 potentially relevant studies.
Inclusion and Exclusion Criteria
Published studies were eligible for inclusion if they were primarily quantitative or mixed methods, domestic, intervention studies using a technology platform for support of ART adherence and or retention in care among behaviorally HIV-infected adolescents and young adults (ages 16–29 years). Qualitative studies were excluded because this integrative review was specific to research studies with published results of primary health technology-enabled, HIV adherence interventions.
More specifically, we defined adolescence and young adulthood as representing the age groups of 13–18, and 19–24 years of age, respectively (http://www.ncbi.nlm.nih.gov/mesh/68000293) (http://www.ncbi.nlm.nih.gov/mesh/68055815), as per PubMed’s categorical limits. Extension of the upper age limits from 24 to 29 years to define young adulthood was performed for consistency with the present US epidemiologic trends of HIV-infection [1].
Operational Definitions of Primary Search Terms
Technology platforms entailed any electronic device applied for health communication. Broad search terms specific for each article database were used to identify the full scope of delivery platforms (Appendix).
HIV-infected adolescents and young adults. Although the focus of this search was on youth with behaviorally acquired HIV-infection, studies with samples of both perinatal (mother to child HIV transmission) and behaviorally HIV-infected youth were included if ≥ 50% of the study sample were described as behaviorally HIV-infected. Pre-exposure prophylaxis (PrEP) intervention studies were excluded because samples include individuals at risk for, and or exposed to HIV-virus, and not HIV-infected individuals.
Adherence to ART was operationalized using indirect and direct outcomes with indirect measures such as self-report [38, 39], pharmacy refill [40] and HIV biomarkers being commonly applied in clinical research studies, and eligible for inclusion. Although less frequently applied, studies using direct adherence measures were also eligible for inclusion, such as therapeutic drug monitoring [39].
Retention in care. To date, there is no gold standard for measuring retention in care, and therefore selection of a retention measure may be tailored to context [41] with broad outcome criteria accepted in published studies. For example, retention outcomes have included proportion of kept to scheduled visits (range 0–100%) [41, 42] or achieving a minimum number of visits during a specific time period, as proposed by both the Institute of Medicine (IOM) and US Department of Health and Human Services (HHS). More specifically, retention indicators used by the HHS in 2014 entail one or more medical visits every six months during a 24-month period [43], in comparison to the recommendations by the IOM expert committee defining retention as completing two or more routine HIV care visits, at least 90 days apart during a 12-month period [44]. Strong agreement between these two retention indicators exists, irrespective of distinctions [45]. When available, biomarkers have also been applied as a retention proxy with being retained in care defined as two or more CD4-T lymphocyte counts or HIV viral loads measured at least three months apart [46].
Screening for Initial Inclusion: Title and Abstract Evaluation
Titles and abstracts of the data set (n = 1370) were screened for relevance and initial inclusion by the primary reviewer. Studies meeting the broad list of inclusion criteria were coded as ‘yes’. A code of ‘no’ was assigned for any one of the following reasons: non-experimental design, conducted outside of the US, and or with outcomes not specific to adherence and or retention in care. Titles and or abstracts explicitly stating that the sample population was primarily perinatally HIV-infected or HIV-exposed, and or the adherence intervention included PrEP were also assigned to a ‘no’ category. A code of ‘maybe’ was assigned if eligibility was questionable based on the title and or abstract review. Because it was not possible to determine age of the sample population from the title and or abstract, age limits were not imposed during this stage. Results from the initial screening reduced potentially relevant sources from 1370 to 375 (coded as yes and maybe) by focusing the review and excluding 995 irrelevant studies.
Screening for Full Inclusion: Full Text Review
During this stage, the primary reviewer was assisted by two trained, graduate educated reviewers to independently evaluate the 375 potentially relevant studies for final inclusion or exclusion by applying the full set of priori inclusion/exclusion criteria. Additionally, systematic reviews meeting inclusion criteria were hand searched to determine if included studies met criteria for eligibility. Justification for exclusion of all studies were recorded and discussed. A total of eleven studies met the full set of priori inclusion criteria. However, two of these studies were excluded because they were abstracts [47, 48] representing preliminary work of two intervention studies meeting full inclusion criteria [49, 50], thereby resulting in a final sample of nine relevant studies (See Flow Diagram of Study Selection—Fig. 1).
Fig. 1.
Flow diagram of study selection
Data Evaluation
Quality appraisal of each study was independently performed by two content experts specializing in HIV care and health technology, respectively, using the National Institutes of Health (NIH) Quality Assessment Measures [51] (http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/). Selection of the NIH assessment measure was specific for the study design of the data source and in this review included Quality Assessment Tools for: pre-post no control group, controlled intervention, and observational cohort studies. Assigned quality ratings within this appraisal system are classified as good, fair or poor. In general, studies with a good rating have the least risk of bias, and results are considered to be valid. Studies deemed as fair are susceptible to some bias, yet not sufficient to invalidate its results and studies with a poor rating indicating significant risk of bias. (http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduc tion/tools/background). If the independent ratings differed, then reviewers discussed the article in an effort to reach consensus. If consensus was not achieved, then the article was forwarded to an arbitrator, a senior methodologist, for quality adjudication [52]. We did not exclude any papers based on assigned quality rating and instead quality scores were included as a variable in the data analysis stage. Reports with poor and fair quality ratings contributed less to the analytic process [53].
Data Analysis
A comprehensive and objective interpretation of the studies was conducted for synthesis of the identified evidence, and achieved by data reduction, extraction and display [53]. Beginning with the development of a classification system specific to HIV technology intervention studies for adherence support/retention in care, similar data were extracted from each individual source and displayed using matrices, allowing for systematic comparison of select variables such as methodological design, content, and study results, characteristics of the sample populations/settings, recruitment, operationalization of adherence, and components of the HIV technology interventions (i.e., delivery platform, theoretical framework, dose, frequency). In addition to making contrasts and comparisons, other elements of data analysis entailed clustering and counting, subsuming particulars into general and building a logical chain of evidence [53].
Presentation of Results
Overview of Study Sample
The final sample yielded a total of nine citations (Table 1). Identified studies were published between 2006 and 2016 with eight of these nine studies published during the years of 2012–2016. Among the sample, 77.8% (n = 7) represent pilot work [49, 50, 54–58] with sample sizes ranging from eight [56] to 105 participants [59]. More specifically, five of these seven pilots included total study samples of ≤25 participants with a shared sample in two of the pilots [49, 50]. Randomized control study designs were implemented in 33.3% (n = 3) [24, 54, 59] with the RCT conducted by Garofalo et al. [59] representing the largest sample of the cohort (n = 105).
Table 1.
Overview of identified studies
| Author (Ref. #) |
Study design | Objective | Setting(s) | Sample size |
Mean age in years |
Route of HIV- infection |
Description of the intervention |
Technology used |
Follow up |
Primary results |
|---|---|---|---|---|---|---|---|---|---|---|
| Belzer et al. [24] | Randomized Control Trial (RCT) with control group | To assess if cell phone support would improve ART adherence | Five NIH-funded sites in the Adolescent Trials Network (ATN) for HIV/AIDS consortium | N = 37 | 20.43 | BI = 54.1% | Daily cell phone conversation (Monday–Friday) with Adherence Facilitators | Cell phone | 48 weeks | Improved 30-day self-reported adherence at 24 and 48 weeks (p = 0.007) |
| PI = 45.9% | Log 10 HIV viral load significantly lower at 24 (p = 0.002) & 48 weeks (p = 0.043) | |||||||||
| Dowshen et al. [49] | Prospective pilot study using a pre-post design | To evaluate the feasibility/ acceptability and preliminary efficacy of SMS text messaging reminders for improved ART adherence | Transgender focused health center | N = 25 | 23.00 | BI = 84% | Personalized, interactive, daily SMS reminders with participant text back feature one hour later for assessment of adherence | SMS text messaging | 24 weeks | SMS text reminders were feasible and acceptable |
| PI = 12% | No significant changes in HIV biomarkers from baseline | |||||||||
| Unsure = 4.0% | Improved mean VAS scores (d = 1.13; p < 0.001) and 4 day adherence recall (d = 0.73; p = 0.005) from baseline to 24 weeks | |||||||||
| Dowshen et al. [50] | Pilot study | To determine the feasibility/ acceptability of interactive text message response (ITR) as a measure of ART adherence | Transgender focused health center | N = 25 | 23.00 | BI = 84% | Personalized, interactive, daily SMS reminders with participant text back feature one hour later for assessment of adherence | SMS text messaging | 24 weeks | ITR and self-reported adherence using the VAS were moderately, positively correlated (r = 0.52, p < 0.05) during first 6 weeks of study |
| PI = 12% | ||||||||||
| Unsure = 4.0% | ||||||||||
| Garofalo et al. [59] | RCT with control group | To pilot test the feasibility, acceptability and initial efficacy of daily two-way, personalized SMS text messaging for ART adherence support | Community based health settings | N= 105 | 24.1 | BI = 81.9% | Daily, two-way, personalized text messaging intervention | SMS text messaging | 12 months | Intervention feasible with high participant satisfaction scores |
| Block random assignment (blocks of 2 at 1:1 ratio) | PI = 9.5% Other = 8.6% |
Initial message followed with second message 15 min later to assess ART adherence | Average effect for the percentage with ≥90% self-reported adherence increased from 28% (baseline) to 61% during the 6 month intervention period (Odds Ratio = 2.12, 95th CI 1.01–4.45) | |||||||
| Comparable changes from baseline to 6month follow-up were also reported in the control group (24% vs. 51%). | ||||||||||
| No significant difference in log viral load or viral suppression between groups at 3 or 6 months | ||||||||||
| La Grange et al. [58] | Pilot-feasibility study | To examine feasibility of two-way SMS text messaging for ART adherence | Two urban HIV clinics | Not reported | Mean age not reported (16–22 year old youth in study) | Not reported | Daily, automated, and tailored SMS text messaging | SMS text messaging [CareSpeak adherence platform] | 30 days | Two-way text messaging was feasible to support adherence to ART |
| Naar-King et al. [54] | Pilot study, randomized two group design | To pilot test a two session computer delivered motivational intervention for ART adherence support | Eight NIH-funded sites in the ATN for HIV/ AIDS consortium | N = 76 | 20.32 | BI = 100% | Two session computer-delivered motivational intervention - Motivational Enhancement System for Adherence (MESA) | Computer [Computer intervention authoring software (CIAS)] | 6 months | MESA intervention was feasible and acceptable with high participant satisfaction ratings |
| Intervention group with overall greater decrease in HIV viral load from baseline to three (Cohen’s d = 0.39) and 6 months (Cohen’s d = 0.19) | ||||||||||
| Mean adherence greater in intervention group for weekend (p < 0.01) and 7-day (p < 0.05) adherence | ||||||||||
| Outlaw et al. [55] | Pilot study | To assess initial feasibility of a computer based intervention; current study to precede a RCT | Two NIH-funded sites in the ATN for HIV/ AIDS consortium | N= 10 | 20.6 | BI = 100% | Individually tailored two-session computer-based interactive motivational interviewing (MI) | Computer | —— | Participants very/mostly satisfied with sessions |
| Intervention was feasible with 100% retention to both sessions | ||||||||||
| Puccio et al. [56] | Pilot study, one group design | To examine practicality and acceptability of cell phone reminders for improved ART adherence | Health facility (type not specified) | N = 8 | 20.63 | BI = 62.5% | Tapered cell phone reminders with decreasing frequency during 12 week intervention period | Cell phone | 24 weeks | Cell phone reminders were acceptable and feasible |
| PI = 25.0% | 2/8 with undetectable viral load at week 24 | |||||||||
| Blood = 12.5% | ||||||||||
| Saberi et al. [57] | Pilot intervention study | To examine the feasibility and acceptability of telehealth in a controlled setting for ART medication counseling | HIV health center | N= 14 | 23.9 | BI (MSM) = 100% | One-time telehealth session for medication counseling, led by pharmacist to participants within a controlled clinical setting | Computer [Movi software application] | One time session | Telehealth was feasible and acceptable when conducted within a controlled clinical setting |
ART antiretroviral treatment; BI behaviorally infected; PI perinatally infected; MSM men who have sex with men; SMS short messaging service; VAS visual analogue scale
Quality of Included Studies
Quality assessment assignments based on the NIH Assessments Measures ranged from fair [49, 50, 54, 55, 57, 59] to poor [24, 56], with insufficient data precluding appraisal of one study [58]. Threats to internal validity for non-randomized control pilots with a fair quality rating included no justification for sample size [49, 50, 55, 57], measurement of pre-test outcome measures at one single data point [49], and no objective adherence outcome measures [50]. Among the three identified randomized control trials, examples of threats to internal validity were attrition bias with no intent to treat analysis [24, 54], and lack of allocation concealment and/or blinding [59]. Other limitations included statistically significant baseline differences in total number of HIV medications [59]. Additionally in one RCT, while not significant due to low statistical power, there were substantial baseline differences between intervention and control conditions on participant route of HIV transmission [24]. A quality rating of poor was assigned to two of the nine studies with increased risk of bias due to attrition of greater than 50% of the sample population [56] and analysis of only completers [24].
Patient Characteristics & Settings
The nine studies represented 275 HIV-infected youth with a mean age of 21.85 years (SD = 1.74), identified as predominantly African American (71.27%) and Hispanic (16.36%), and male (80.0%) gender. Of these 275 HIV-infected youth, route of HIV transmission was behavioral (84.4%), perinatal (11.6%), and 4.0% with an unknown/other route of transmission. Sample size, race, gender and transmission route were not described in one study [58]. Sample populations exclusive to behaviorally acquired HIV-infection were observed in three of the nine studies [54, 55, 57] with total sample populations of 14 and 10, in two of these pilots [57] and [55].
Sexual orientation was reported in only 22.2% (n = 2) of the studies [54, 55] with each describing more than half of the sample populations as homosexual. Highest level of education ranged from elementary school to some college as per three studies [54, 55, 59]. Substance use/abuse screening results were reported in two studies [56, 59] with a psychometrically valid and reliable tool (ASSIST) used in one study [59]. Of note in Garofalo [59], more than half of the sample reported moderate to high-risk marijuana usage during the past three months, and high depressive symptomology was observed in 41% of study participants as measured using the Brief Symptom inventory (BSI). None of the nine studies reported on health insurance coverage, and employment status was described in only one study [55] with six of the ten participants working ten or more hours per week. Barriers to ART adherence were solely described in one study [57] with pharmacy (i.e., difficulty picking up refills) and lifestyle issues (i.e., homelessness, substance use), and physical or emotional side effects of ART treatment being among some of the challenges described by study participants.
Recruitment Sites and Strategies
Health care settings were the recruitment sites for all nine studies. Three studies recruited from National Institute of Health (NIH) Adolescent Trials Network (ATN) for HIV/AIDS Interventions centers including locations in New York, Pennsylvania, Louisiana, Florida, Washington DC, Tennessee, Illinois, and California [24, 54, 55]. Among the three studies recruiting from ATN sites, each used one or more ATN site with patient recruitment from eight ATN sites in one study [54]. Recruitment strategies were described in two of the nine studies [57, 59] and entailed posting/flyers and or palm cards [57, 59], and referral from local HIV centers [57]; details of recruitment methods were not described in the remaining seven studies. Additionally, recruitment efficacy assessed with recruitment rates or the ratio of recruited to eligible participants [60] was not provided in any of the nine studies.
Characteristics of the Technology Interventions
The cell phone was the most common delivery platform for adherence interventions with SMS text-message reminders representing the most frequently applied delivery mode [49, 50, 58, 59], followed by live telephone support [24, 56]. In the remaining three studies, computer delivered interventions were tested [54, 55, 57].
Frequency of Contact
Diversity in cell phone contact was observed ranging from weekday cell phone conversations occurring once or twice daily for 24 weeks [24] to calls delivered daily during the first four-week study period, followed by an incremental tapering during the remaining eight weeks of the intervention period [56]. SMS text messaging adherence interventions were consistent with messaging delivered daily, typically interactive [49, 50, 58] and personalized [49, 50, 58, 59], despite variability in the total number of text messages sent. Among the computer-based interventions, Naar-King et al. [54] and Outlaw et al. [55] used two interactive sessions with the telehealth intervention conducted by Saberi et al. [57] implementing one virtual session.
Theoretical Frameworks
Interventions, including two-way text messaging [59] and daily phone calls with an adherence facilitator [24] were guided by social cognitive, and social support theories, respectively. Motivational interviewing approaches were implemented using computer technology in two studies within this sample [54, 55]. Theoretical frameworks were not reported in five studies [49, 50, 56–58].
Feasibility and Preliminary Efficacy
Feasibility studies are conducted to establish if an intervention is suitable for further evaluation [61]. Respective study authors reported the feasibility of SMS text messaging [49, 50, 58, 59], cell phone reminders [24, 56], and computer delivered interventions [54, 55, 57, 59]. However, supporting criteria for the establishment of intervention feasibility varied, including four studies designed to assess changes in post-intervention adherence outcomes [24, 49, 54, 59].
Texting
Feasibility of two-way texting for adherence support was shown in LaGrange [58], as participants described no interruption of lifestyle and or daily activities. However, details of the total number of study participants and or response rates were not available. In two studies from the same author, interactive text messaging was also described as feasible based on participant satisfaction and/or retention in the study [49, 50]. However, participant response rates to texts were 57.4% [50] and 48% [49], respectively. Findings from Garofalo et al. [59] also demonstrated participant acceptability, and satisfaction with a two-way, personalized texting intervention, and response rates were similar to Dowshen et al. [50] with a 58% average response rate to text messages.
Preliminary efficacy of SMS texting was demonstrated in two studies [49, 59] with statistically significant post-intervention improvements in self-reported adherence at 24 weeks in both studies (Table 1).
Computers
Feasibility of a computer based motivational intervention was reported by Outlaw et al. [55] with all ten participants completing both sessions, and the majority reporting high satisfaction with each of the two sessions. Saberi et al. [57] and colleagues tested a onetime telehealth medication counseling session to fourteen HIV-infected participants and most youth described the intervention as convenient, comfortable and private [57]. Similarly, a computer delivered motivational intervention using avatars was described as feasible based on participant acceptability and satisfaction [54]. In addition to feasibility, the authors of this study describe preliminary efficacy. Medium to large effect sizes for 7-day self-reported adherence (d = 0.49; p < 0.05) and weekend adherence (d = 0.66; p < 0.01) were observed with smaller effect sizes seen for viral load suppression at three (d = 0.09) and six months (d = 0.28) [54].
Cell Phones
Support for the feasibility of cell phone reminder calls for adherence support in a pilot conducted by Puccio et al. [56] was described as participant perceptions of low-level intrusion, helpfulness, and practicality of calls. Yet only five of the eight participants were able to complete the study intervention through week 24. Post intervention adherence and virologic outcomes were reported and presented as non-aggregate data.
Preliminary efficacy of daily cell phone conversations with non-adherent youth was supported by study findings of statistically significant changes in self-reported adherence (p = 0.007) and HIV RNA viral load (p = 0.04) at 24 and 48 weeks, and medium to large effect sizes in these adherence outcomes [24]. However only 63.1% (12/19) of participants completed the intervention in comparison to 94.4% (17/18) of controls receiving standard care alone.
Operationalization of Adherence
Although conceptualization of adherence has been expanded to include retention in HIV care [5], retention in care was not a reported study outcome in any of the nine studies included in this review. Among the four studies defining adherence to ART, distinctions in conceptualization and operationalization were observed [24, 49, 50, 59]. For instance, in two randomized control trials, dichotomous variables of < 90% or ≥ 90% were used to characterize non-adherence or optimal self-reported adherence, respectively [24, 59]. Indirect measurement was the primary means for assessing ART adherence with self-report using the visual analogue scale (VAS) [24, 49, 50, 54, 59], and self-report of missed doses [54, 56, 57], the most common assessment methods. Specific methodology used to assess adherence was not specified in two of the nine studies [55, 58] (Table 2).
Table 2.
Operationalization of adherence to antiretroviral treatment (ART)
| Author (Ref. #) |
Adherence measure(s) |
Definition(s) | Adherence recall | Adherence data collection time points |
Biomarkers reported |
|---|---|---|---|---|---|
| Belzer et al. [24] | Self-reported adherence—VAS |
Non adherence
|
Past 3 months, 7 days, and last weekend | Baseline, 24 and 48 weeks | HIV RNA viral load |
| Dowshen et al. [49] | Self-reported adherence—VAS |
Poor adherence
|
Past 4 weeks—VAS | Baseline; 6, 12, 18, and 24 weeks | CD4 T-lymphocyte count |
| AND | Past 4 days— ACTG questionnaire | and | |||
| AIDS Clinical Trial Group (ACTG) adherence questionnaire | HIV RNA viral load | ||||
| Dowshen et al. [50] | Self-reported adherence— interactive text response (ITR) |
Adherence difficulty
|
Past ART dose | Daily for 24 weeks | Not reported |
| Garofalo et al. [59] | Self-reported adherence—VAS |
Adherence problems
|
Past 30 days | Three and 6 months | HIV RNA viral load |
| La Grange et al. [58] | Operationalization of adherence not reported in this study | ||||
| Naar-King et al. [54] | Self-report of missed doses— | Not reported | Past 30
days—VAS Past week and weekend— adherence recall |
Three and 6 months | HIV RNA viral load |
| VAS | |||||
| AND adherence recall | |||||
| Outlaw et al. [55] | Not reported | Not reported | Not reported | Not reported | Not reported |
| Puccio et al. [56] | Self-report of missed doses | Not reported | Not reported | Four, 8, 12, and 24 weeks | HIV RNA viral load |
| Saberi et al. [57] | Self-report of missed doses | Not reported | Missed doses during past week and month | One time point (during the telehealth counseling session) | HIV RNA viral load and CD4-T lymphocyte count |
Visual analogue scale (VAS): single item asking participants to consider a specific time-period and estimate along a continuum the percentage of doses taken from 0 to 100%
Recall time frames for assessment of self-reported ART adherence were also incongruous among studies (Table 2). Frequency of adherence assessments varied among these studies with single [57] to multiple data collection time points [24, 49, 50, 54, 56, 59].
HIV biomarkers such as HIV RNA viral load, or both HIV RNA viral load and CD4-T lymphocyte counts were reported in a total of six [24, 49, 54, 56, 57, 59] and two of the nine identified studies, respectively [49, 57]. Length of study follow-up varied considerably among studies ranging from 30 days [58], to 24 [49, 50, 54, 56] and 48 weeks [24], or one year [59].
A formal meta-analysis was considered, but not conducted, since there was too much heterogeneity in interventions, study outcomes, and other methods for the pooling of effect sizes to be useful.
Discussion
The purpose of this review was to describe the state of the science regarding US health technology-enabled interventions for support of ART adherence and or retention in HIV care among behaviorally HIV-infected youth, and our results highlight a body of evidence that is largely in development. Technology-enabled interventions for adherence support have only recently been tested in HIV-infected adolescents and young adults, and although promising, synthesis of the findings from the nine identified primary studies reflects a paucity of published peer-reviewed evidence in the US during the past decade. In fact, the evidence base for adherence interventions and recent trends of intervention research are disproportionate to the needs of this population [23, 35, 36]. Hence, the ability to generate broad recommendations from this review is limited. Nonetheless and unlike other recent reviews of ART adherence, our sample is distinguished by studies with homogenous study outcomes (ART adherence) and intervention delivery modes (i.e., all are technology enabled), and clinical settings within the US health care system. Additionally, our target population is exclusive to HIV-infected adolescents and young adults within a similar age range, thereby allowing for synthesis of shared patterns and characteristics.
Feasibility and Preliminary Efficacy
Synthesis from identified published studies during the past decade highlight preliminary evidence for the feasibility of computer-based and SMS texting interventions, including efficacy of SMS texting for adherence support among HIV-infected youth ages 16–29 years. Of note was a low response texting rate across three studies [49, 50, 59]. Given that text messaging is the primary mode for communication with friends among US adolescents [32], the observed low response rates in study participants may be related to the competing demands of daily, repeated social text messaging exchanges among peers.
There is minimal evidence to support the feasibility and or efficacy of cell phone interventions for adherence support among HIV-infected youth. In the two studies that evaluated cell phone interventions, significant challenges retaining participants were experienced, raising questions about the feasibility for this population and weakened the strength of conclusions that can be drawn about intervention efficacy. It is however important to note that the pilot conducted by Puccio et al. [56] was published over a decade ago, representing an important and initial step to testing a cell phone intervention for HIV-infected youth in the US. Moreover, the sample population in the Belzer et al. [24] study had very low baseline adherence rates with high HIV-RNA viral load levels and non-completion among nearly 40% of HIV-infected youth randomized to the intervention arm [24]. These results are not surprising given that non-adherent, HIV-infected youth are often the most challenging to recruit and retain in a clinical trial, despite being likely to benefit from participation.
Additional considerations for using cell phones in HIV clinical trials are related to the current trends among US teens of reserving phone calls for their closest relationships [32]. Hence, until more data is obtained, synchronous phone calls with providers may not be the most practical health technology delivery model for adolescents and young adults. Careful and deliberate thought must also be given to maintaining privacy as it is more difficult for an adolescent/young adult to be discreet during a phone conversation versus communication with other forms of technology (i.e., text messaging).
Recruitment & Sample Size
None of the included studies described any recruitment challenges and or difficulty identifying patients. Recruitment rates were not provided, nor was the similarity between recruited samples and eligible non-recruits reported. Although the reasons for study refusal are not described in any of these nine HIV studies, recruitment challenges among African Americans date back to nearly two decades ago [60]. Moving forward, it is essential to gain knowledge of the factors leading to non-participation of study eligible, HIV-infected youth in clinical research trials.
Of further note, in each of the nine studies, recruitment was performed within a health care setting and in some studies, participants were recruited from more than one clinical setting [24, 54, 55, 58, 59]. Yet the sample size in all but two studies [54, 59] was less than or equal to 37 participants, a trend consistent with other reviews reporting on HIV-infected youth [35] and distinct from adherence interventions conducted with HIV-infected adults [62, 63]. Clearly larger and adequately powered clinical trials are needed, but we will first need to identify improved mechanisms for recruitment outside of clinical settings including peer-led, and community based strategies. Moreover, this data sample included a population that was primarily black, male and with behaviorally-acquired HIV-infection. Although these characteristics are certainly consistent with the epidemiologic trends in the US, research studies inclusive of other hard to reach populations are needed. Transgender youth, young female sex workers and offenders are poorly represented in the HIV-literature and one potential strategy to increase reach is to develop research networks with academic institutions and clinics that provide services to these populations [23]. HIV-testing centers offer another prospective avenue for identification and recruitment of newly infected adolescents and young adults, opening the doors for access to treatment and research interventions to improve adherence and retention upon diagnosis [64]. Internet-based recruitment has been tested among HIV-infected adolescents, however further evaluation is needed before recommendations can be made [65]. Development of multidisciplinary teams with engagement of community stakeholders is key to retaining study participants, and will necessitate removal of institutional barriers to staff and faculty diversity [66]. Hence, an overall reversal in the current patterns for underrepresentation of HIV-infected youth in clinical research trials will be contingent upon a robust research agenda aimed to foster collaboration between academic and clinical settings, and also with HIV-infected youth.
Substance Use and Depression
The prevalence of illegal substance use among HIV-in-fected adolescent and young adults use is approximately 63%, when assessing usage during the past year, and nearly double that of HIV-seronegative counterparts [67]. Depression is another important challenge facing this population with reported estimates of 34.3% [68], and therefore it is not surprising that substance use and depression represent important confounders of ART adherence and retention in HIV. It was, however, remarkable that only two of the nine studies report on psychosocial characteristics of sample populations, including frequency of substance use and depression. Among these studies, moderate to high-risk marijuana usage and depressive symptomatology among HIV-in-fected youth was reported, with the adherence trajectory in the intervention arm moderated by these symptoms during the 12-month follow-period [59]. These findings are consistent with evidence from a large multi-site study, demonstrating that adolescent and young adult participants least likely to be adherent had higher psychological distress and used marijuana weekly. More specifically, a frequency of substance use variable, marijuana use during the past 3 months, was the strongest independent predictor of ART adherence, yielding moderate effect strength sensitivity [6].There is also compelling evidence to show that as the number of psychosocial conditions increases, there is a decreased likelihood of ART adherence [69].
Current and Future Trends of Health Technology-Enabled ART Interventions
In the present data set, text messaging, cell phone calls, and computers, including interactions with a relational agent and videoconferencing, were tested for ART support, with varying levels of success. Surprisingly none of these studies employed or investigated simple standard alarm mechanisms, often used by people to remind themselves of a wide range of events (including the taking of medication), as a form of intervention or control. Although the standard timing of calls or texts, may serve as a form of alarm, in some respect, the autonomy/self-actualization employed in using a standard alarm might have its own merits. Additionally, and of note, many of the studies in this data set combined technology and human interventions, as opposed to sole reliance on the technology. Hence, there is a need for additional research to determine the potential efficacy of technology applications alone, or to guide the type, timing, or duration of such interventions.
As smartphones have advanced and their adoption has become near-ubiquitous among millennials [70] and across most age, gender, and socio-economic groups, each of these modalities (alarms/reminders, voice, text, relational agents, and video conferencing) can now be readily experienced by most individuals, at any time. However, a range of limitations to technological interventions include: power; cell phone plans (bandwidth, minutes, expense); maintenance and effort engaging with service providers; durability/functionality due to breakage/screen; and loss, theft, or confiscation [71]. Some of these limitations may be more prominent among sample populations in HIV-in-fected adolescents and young adults.
Moving forward as technologies continue to evolve, personal tools and strategies (i.e., those used by the quantified self-movement, including gamification) have been used for a wide range of behavioral support. These range from internet of things (IoT) devices to the emerging proliferation of personal robots [72, 73] and relational agents [74, 75]. Irrespective of the health technology used, it important to note that each has unique advantages and disadvantages when used as a delivery platform for adherence interventions. Although some of the included interventions showed preliminary efficacy, questions that remain are the optimal dose including treatment intensity, frequency and duration.
Theoretical Frameworks and Developmental Considerations
Frameworks to inform the technological aspects of interventions are important [25] and yet were not cited in more than half of the identified interventions. These findings are consistent with current trends in published HIV technology intervention studies as few have referenced theoretical or conceptual frameworks, thereby limiting our understanding of key components of successful behavioral interventions [18, 26]. Similarly, the development of theoretical frameworks for mobile messaging is described as a relatively new phenomenon, and evolving from various related fields [76], including social network theories [77].
Mobile health messaging studies also tend to focus mostly on the procedural effect of the intervention, as compared to examining the art of messaging itself and learning about the types of messaging perceived as informative or irritating, and for what specific populations [76]. Moreover, there is a need for technology enabled-inter-ventions tailored to the developmental stage and age of the young person, versus design of a platform for use with a wide-ranging age span [25].
Adherence and Retention Outcomes
Heterogeneity in the operationalization of ART adherence was illustrated by our study findings, demonstrating the range in adherence assessment item content, format, and response options with HIV-infected individuals [38]. Although adherence is a difficult construct to operationalize due to the absence of a gold standard in its measurement [78, 79], greater congruence between adherence definitions in research and clinical evidence is needed. For example, levels of adherence to unboosted PI containing regimens has been established as greater than 95% with more recent evidence to show that 80% ART adherence to boosted PI regimens may be sufficient for optimal virologic control [7]. Yet these adherence levels were not applied in the four studies defining optimal and or suboptimal adherence [24, 49, 50, 59]. Instead, a cut-off of greater than or equal to or less than 90% was applied when dichotomizing optimal or sub-optimal self-reported ART adherence [24, 59]. Definitions of virologic suppression or failure were also inconsistent among primary sources in this data sample and moving forward, parameters applied in research studies should conform to established guidelines from the Department of Health and Human Services (DHHS). Presently virologic suppression is defined as a confirmed HIV RNA level below the level of detection of available assays, and virologic failure as the inability to achieve or maintain suppression of viral replication to an HIV RNA level < 200 copies/mL [5].
Conceptualization of adherence has been broadened to include retention in care [5], yet study outcomes in this data set were exclusive to ART adherence. Our findings are consistent with evidence from recent peer-reviewed publications also demonstrating a weak evidence base for support of adolescents’ linkage, retention and adherence behaviors [36], and effective approaches to retain youth [80]. Findings from a recent retrospective analysis [81] are concerning as more than half of newly infected youth were not retained in care one year after initiation of outpatient HIV health care services. Failure to achieve retention indicators proposed by the DHHS and IOM is associated with all-cause mortality, and missed/no-show clinic visits offer added, independent prognostic value [82].
Mhealth interventions have typically focused on adherence and not retention in care [83], illustrating the need for interventions that target both retention and ART adherence. When comparing barriers between retained and not retained HIV-infected adults, perceived stigma, challenges with transportation and health insurance, and difficulty with clinic staff relationships and appointment scheduling were more frequenty observed among those not retained in care [84]. Further exploration of these obstacles is warranted in the design of effective interventions for support of ART adherence and retention in HIV care.
Limitations
This review has several limitations related to the current state of the science with limited published evidence during the past decade, and application of NIH quality assessment measures that were not exclusive for pilot feasibility studies. The potential for bias exists because the majority of interventions were tested in pilot studies and adherence definitions were incongruent among identified studies. Evidence synthesized from individual data sources represent broad inclusion criteria for age, ranging from early adolescence through young adulthood. Additionally, the impact of developmental stage was not assessed, and a gap of this evidence base. Likewise there is a need to explore feasibility and efficacy by subgroups (age, race, gender, and sexual orientation) in future work. Additionally, this review may not reflect the full portrait of US HIV health technology-enabled interventions such as unpublished evidence from interventions conducted by health care workers in institutions [18] and or staff outreach efforts by community agencies. However irrespective of these limitations, the extensive search strategy allowed for retrieval of nine comparable, primary studies published during the past decade.
Conclusions
There is emerging and preliminary evidence to support the efficacy of SMS text messaging, and feasibility of computer platforms for health technology-enabled adherence interventions among HIV-infected youth in the US. Nonetheless, the findings from this integrative review highlight a significant gap in the evidence base, as we identified only nine health technology-enabled intervention studies during the past decade. Further expansion of this research area is dependent upon many factors, including active response by the scientific community to the multitude of challenges, when conducting behavioral intervention research with HIV-infected youth. For one, movement away from reliance on fixed interventions with exploration of the utility for new multicomponent and adaptive frameworks such as multiphase optimization strategy (MOST) [85] or sequential multiple assignment randomized trial (SMART) [86] is justified, as the complex psychosocial profiles of HIV-youth coupled with the paucity of efficacious youth-focused adherence interventions demands pursuit of novel, impactful research methodologies.
The proliferation of technology-enabled interventions for improved HIV-disease self-management (i.e., adherence and retention) demands thoughtful integration of theory to gain a better understanding of the modifiable factors linked with behavioral change. As many of the studies in this data set combined technology applications with human interaction, research is needed to determine when to link technology with human delivered interventions, versus exclusive use of technology. Still another significant consideration is to learn what technologies work best for the different adherence challenges, patient characteristics (age groups and populations), and the optimal frequency of contact needed [87]. More specifically, the impact of developmental stage, and other critical factors (gender, sexual orientation) require careful consideration, when designing adherence interventions using technology.
The potential for technology to facilitate success of behavioral interventions, and effect public health has been widely recognized. Yet its commercialization lends to significant gaps in affordability and accessibility for lower socioeconomic groups, calling for creative strategies to increase access among cohorts most likely to benefit from its application [88]. Likewise, researchers must be cognizant of the inherent funding challenges when pursuing grant awards, as the traditional funding cycle is often at odds with the accelerated pace of commercial technology and so allocation of additional funding mechanisms may be needed to support the movement from pilot study to efficacy trial [22, 26].
Finally, much work remains for behavioral interventionists when designing and testing technology-enabled interventions with HIV-infected adolescent and young adult populations. A key starting point for future advancement of this field is the development of effective research collaborations among academic, clinical and community partners with mutual goals for linking evidence to practice along the HIV care cascade. The US National Institutes of Health (NIH)-sponsored Adolescent Medicine Trials Network for HIV/AIDS interventions represents one such highly effective, national model, and similar regional and local paradigms are desperately needed.
Acknowledgments
This study was funded by a National Institute of Nursing Research (NINR) Career Development Award (K233NR015970-02): Adherence Connection Counseling, Education, and Support (ACCESS): A Proof of Concept Study.
Author Contribution We gratefully acknowledge the contributions of Sonia Pathania, DNP, ANP, and Karla Rodriguez, DNP, RN.
Appendix
Table 3.
Search strategy
| Date | Database | Search terms | Number of data sources retrieved |
|---|---|---|---|
| 3-18-16 | Medline via PubMed | (Computer* OR computers OR telemedicine OR telehealth OR mhealth* OR ehealth* OR “text messag*” OR sms OR texting OR video* OR telephone OR smartphone OR “smart phone” OR “Mobile Applications” OR app[all] OR IVR OR “interactive voice response” OR “mobile health” OR “electronic mail” OR “e-mail” OR email OR gamification OR “virtual reality” OR “social media” OR internet OR badge* OR reward* OR reward) | 743 |
| AND | |||
| (“hiv infections” OR hiv OR “acquired immunodeficiency syndrome” OR “acquired immune deficiency” OR “acquired immune-deficiency” OR “anti-retroviral agents” OR “anti-HIV agents”) | |||
| AND | |||
| (“medication adherence” OR “patient compliance” OR memscap OR wisepill OR adherence OR retention OR attrition OR “patient acceptance of healthcare” OR “healthcare utilization” OR “health care utilization” OR “patient engagement” OR “linkage to care” OR “HIV care cascade” OR “reminder packaging” OR “reminder systems” OR EAM OR “electronic adherence monitor*” OR “medication event monitoring system” OR “medication alarm”) | |||
| 3-18-16 | EMBASE via Ovid | (telemedicine OR telehealth OR mhealth* OR ehealth* OR “text messag*” OR sms OR texting OR “remote video conferenc*” OR telephone OR smartphone OR “smart phone” OR “mobile applications” OR IVR OR “interactive voice response” OR “mobile health” OR “electronic mail” OR “e-mail” OR email or gamification OR “virtual reality” OR “social media” OR internet OR badge* OR reward*) | 594 |
| AND | |||
| (exp Human immunodeficiency virus/ OR exp acquired immune deficiency syndrome/ OR “hiv infections” OR hiv OR “acquired immunodeficiency syndrome”) | |||
| AND | |||
| (“medication adherence” OR “patient compliance” OR adherence OR retention OR “patient engagement” OR “linkage to care” OR “HIV care cascade” OR “patient acceptance of healthcare” OR “healthcare utilization” OR “health care utilization” OR “medication event monitoring system” OR wisepill OR memscap OR attrition OR “self-monitoring” OR “health behavior*” OR “reminder packaging” OR “electronic adherence monitor” OR EAM OR “medication alarm” OR “reminder systems”) | |||
| 3-18-16 | PsycINFO via Ovid | exp hiv/ | 182 |
| AND | |||
| (telemedicine/ OR teleconferencing/ OR exp Mobile Devices/ OR exp Cellular Phones/ OR app OR exp telephone systems/ OR sms OR texting OR “remote video conferenc*” OR ehealth* OR mhealth* OR “text messag*” OR smartphone OR “smart phone” OR exp electronic communication/ OR computer assisted therapy/ OR exp telecommunications media/ OR “virtual reality” OR gamification OR computer games/ OR “social media” OR internet OR Badge* OR reward*) | |||
| AND | |||
| (exp Treatment Compliance/ OR exp Compliance/ OR adherence OR retention OR attrition OR wisepill OR memscap OR “medication event monitoring system” OR health behavior/ OR health attitudes/ OR health knowledge/ OR health promotion/ OR exp self help techniques/ OR self monitoring/ OR “electronic adherence monitor” OR EAM OR “reminder packaging” OR “reminder systems” OR “patient acceptance of healthcare” OR “healthcare utilization” OR “health care utilization” OR “patient engagement” OR “linkage to care” OR “HIV care cascade”) | |||
| 3-15-16 | CINAHL | ((MH “Reminder Systems”) OR (MH “Telecommunications + ”) OR (MH “Telehealth + ”) OR “smart phone” OR smartphone OR texting OR text-messag* OR ehealth* OR mhealth* OR “Telemedicine” OR telehealth* OR mHealth* OR SMS OR “remote video conferenc*” OR “mobile applications” OR “interactive voice response” OR “mobile health” OR “electronic mail” OR email OR “e-mail” OR gamification OR “virtual reality” OR reward*) | 285 |
| AND | |||
| ((MH “Patient Compliance + ”) OR “adherence” OR retention OR “Health Resource Utilization” OR “Health Services Accessibility” OR “Health Facilities Utilization” OR “Continuity of patient care” OR “Health care delivery” OR “Health services needs and demands” OR “patient acceptance of health care” OR “health care utilization” OR “patient engagement” OR Attrition OR “linkage” OR “HIV Care Cascade” OR compliance OR (MH “Health Behavior + ”) OR (MH “Adolescent Behavior”) OR (MH “Behavioral Changes”) OR wisepill OR memscap OR “medication event monitoring system”) | |||
| AND | |||
| ((MH “Human Immunodeficiency Virus + ”) OR (MH “HIV Infections + ”) OR antiretroviral OR anti-retroviral OR anti-HIV) | |||
| 3-15-15 | Web of Science | (retention OR adherence OR compliance OR memscap OR “medication event monitoring system” OR wisepill OR “self-monitoring” OR “health behavior*” OR “reminder systems” OR “electronic adherence monitor” OR EAM OR “medication event monitoring system” OR “medication alarm” OR “medication adherence” OR “patient compliance” OR attrition OR “patient acceptance of healthcare” OR “healthcare utilization” OR “patient engagement” OR “linkage to care” OR “HIV care cascade” OR “reminder packaging”) | 107 |
| AND | |||
| (telecommunication* OR telemedicine* OR mobile health OR mhealth* OR ehealth* OR “electronic mail” OR “e-mail” OR email OR “text messag*” OR sms OR internet OR “social media” OR “remote video conferenc*” OR telephone OR “smart phone” OR smartphone* OR “mobile application*” OR computer OR “computer assisted” OR telehealth* OR teleconferencing OR telemedicine OR video* OR IVR OR “interactive voice response” OR gamification OR virtual reality OR badge* OR reward*) | |||
| AND | |||
| (HIV OR AIDS OR “acquired immunodeficiency” OR antiretroviral OR “antiretroviral agents” OR “anti-HIV agents” OR | |||
| HAART OR “acquired-immunodeficiency”) | |||
| AND | |||
| (adolesc* OR “young adult*” OR teen* OR youth) | |||
| Total data sources retrieved = 1911 |
Final search run on March 18, 2016
Search limits (1/1/2005–12/31/2016)
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interests.
Ethical Approval This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2015. HIV surveillance report. 2015;27:1–114. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [Google Scholar]
- 2.Agwu AL, Fleishman JA, Korthuis PT, et al. Disparities in antiretroviral treatment: a comparison of behaviorally HIV-In-fected youth and adults in the HIV research network. J Acquir Immune Defic Syndr. 2011;58(1):100–7. doi: 10.1097/QAI.0b013e31822327df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28(3):128–35. doi: 10.1089/apc.2013.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahana SY, Jenkins RA, Bruce D, Fernandez MI, Hightow-Weidman LB, Bauermeister JA, et al. Structural determinants of antiretroviral therapy use, HIV care attendance, and viral suppression among adolescents and young adults living with HIV. PLoS ONE. 2016;11(4):1–19. doi: 10.1371/journal.pone.0151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services (DHHS) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Panel on Antiretroviral Guidelines for Treatment of HIV-infection. 2016 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 6.Gross IM, Hosek S, Richards MH, Fernandez MI. Predictors and profiles of antiretroviral therapy adherence among African American Adolescents and young adult males living with HIV. AIDS Patient Care STDS. 2016;30(7):324–38. doi: 10.1089/apc.2015.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45(3):372–9. doi: 10.1345/aph.1P587. [DOI] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37(8):1112–8. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Giordano TP, Gifford AL, White AC, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 12.Lima VD, Harrigan R, Bangsberg DR, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50(5):529–36. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anglemyer A, Horvath T, Rutherford G. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA. 2013;310(15):1619–20. doi: 10.1001/jama.2013.278328. [DOI] [PubMed] [Google Scholar]
- 14.Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database of Systematic Reviews. 2013;(4):CD009153. doi: 10.1002/14651858.CD009153.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health. 2015;36(1):393–415. doi: 10.1146/annurev-publhealth-031914-122855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath KJ, Danilenko GP, Williams ML, et al. Technology use and reasons to participate in social networking health websites among people living with HIV in the US. AIDS Behav. 2012;16(4):900–10. doi: 10.1007/s10461-012-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellowski JA, Kalichman SC. Recent advances (2011–2012) in technology-delivered interventions for people living with HIV. Curr HIV/AIDS Rep. 2012;9(4):326–34. doi: 10.1007/s11904-012-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catalani C, Philbrick W, Fraser H, Mechael P, Israelski DM. mHealth for HIV treatment & prevention: a systematic review of the literature. Open AIDS J. 2013;7(1):17–41. doi: 10.2174/1874613620130812003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claborn KR, Fernandez A, Wray T, Ramsey S. Computer-based HIV adherence promotion interventions: a systematic review. Transl Behav Med. 2015;5(3):294–306. doi: 10.1007/s13142-015-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS ONE. 2014;9(2):e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherenack EM, Wilson PA, Kreuzman AM, Price GN. The feasibility and acceptability of using technology-based daily diaries with HIV-infected young men who have sex with men: a comparison of internet and voice modalities. AIDS Behav. 2016;20(8):1744–53. doi: 10.1007/s10461-016-1302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muessig KE, Nekkanti M, Bauermeister J, Bull S, Hightow-Weidman LB. A systematic review of recent smartphone, Internet and Web 2.0 interventions to address the HIV continuum of care. Curr HIV/AIDS Rep. 2015;12(1):173–90. doi: 10.1007/s11904-014-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lall P, Lim SH, Khairuddin N, Kamarulzaman A. Review: an urgent need for research on factors impacting adherence to and retention in care among HIV-positive youth and adolescents from key populations. J Int AIDS Soc. 2015;18(Supp 1):19393. doi: 10.7448/IAS.18.2.19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belzer M, Naar-King S, Olson J, et al. The use of cell phone support for non-adherent HIV-infected youth and young adults: an initial randomized and controlled intervention trial. AIDS Behav. 2014;18(4):686–96. doi: 10.1007/s10461-013-0661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hightow-Weidman LB, Muessig KE, Bauermeister J, Zhang C, LeGrand S. Youth, technology, and HIV: recent advances and future directions. Curr HIV/AIDS Rep. 2015;12(4):500–15. doi: 10.1007/s11904-015-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simoni JM, Kutner BA, Horvath KJ. Opportunities and challenges of digital technology for HIV treatment and prevention. Curr HIV/AIDS Rep. 2015;12(4):437–40. doi: 10.1007/s11904-015-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philbin MM, Tanner AE, DuVal A, Ellen J, Kapogiannis B, Fortenberry JD. Linking HIV-positive adolescents to care in 15 different clinics across the United States: creating solutions to address structural barriers for linkage to care. AIDS Care. 2014;26(1):12–9. doi: 10.1080/09540121.2013.808730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarra AM, Neu N, Toussi S, Nelson J, Larson EL. Health literacy and adherence to antiretroviral therapy among HIV-in-fected youth. J Assoc Nurses AIDS Care. 2014;25(3):203–13. doi: 10.1016/j.jana.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn-Navarra AM, Toussi SS, Cohn E, Neu N, Larson EL. Measuring media use in college students with and without human immunodeficiency virus infection. J Pediatr Health Care. 2014;28(4):342–9. doi: 10.1016/j.pedhc.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Anderson A. Technology device ownership: 2015. Pew Research Center. 2015 http://www.pewinternet.org/2015/10/29/technology-device-ownership-2015.
- 31.Lenhart A. Teens, Social Media and Technology Overview 2015. Pew Research Center. 2015 http://www.pewinternet.org/files/2015/04/PI_TeensandTech_Update2015_0409151.pdf.
- 32.Lenhart A, Smith A, Anderson M, Duggan M, Perrin A. Teens, technology and friendships. Pew Research Center. 2015 http://www.pewinternet.org/2015/08/06/teens-technology-and-friendships/
- 33.Pew Research Center. The smartphone difference. 2015 http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/
- 34.Caulfield BM, Donnelly SC. What is Connected Health and why will it change your practice? QJM. 2013;106(8):703–7. doi: 10.1093/qjmed/hct114. [DOI] [PubMed] [Google Scholar]
- 35.Shaw S, Amico KR. Antiretroviral therapy adherence enhancing interventions for adolescents and young adults 13–24 years of age: a review of the evidence base. J Acquir Immune Defic Syndr. 2016;72(4):387. doi: 10.1097/QAI.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacPherson P, Munthali C, Ferguson J, et al. Service delivery interventions to improve adolescents’ linkage, retention and adherence to antiretroviral therapy and HIV care. Trop Med Int Health. 2015;20(8):1015–32. doi: 10.1111/tmi.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–45. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S79–87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMahon JH, Jordan MR, Kelley K, et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis. 2011;52(4):493–506. doi: 10.1093/cid/ciq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med. 2008;16(5):156–61. [PubMed] [Google Scholar]
- 43.Health Resources and Services Administration (HRSA) Ryan White HIV/AIDS Program State Profiles. U.S. Department of Health and Human Services (HHS) Performance Indicators. 2014 https://hab.hrsa.gov/stateprofiles/hhs-indicators.aspx.
- 44.Institute of Medicine (IOM) Monitoring HIV care in the United States: indicators and data systems. Ford MA, Spicer Mason C, editors. National Academy of Sciences Eds. 2012 http://nationalacademies.org/hmd/reports/2012/monitoring-hiv-care-in-the-united-states.aspx. [PubMed]
- 45.Rebeiro PF, Horberg MA, Gange SJ, et al. Strong agreement of nationally recommended retention measures from the Institute of Medicine and Department of Health and Human Services. PLoS ONE. 2014;9(11):e111772. doi: 10.1371/journal.pone.0111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC) Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas. 2014 http://www.cdc.gov/hiv/library/reports/surveillance/
- 47.Dowshen N, Kuhns L, Johnson A, Garofalo R. Evaluation of the feasibility and validity of short message system (SMS) text messaging for assessment of antiretroviral therapy adherence among youth living with HIV/AIDS (YLH) J Int Assoc Physicians AIDS Care (Chic) 2012;11(6):397–8. [Google Scholar]
- 48.Dowshen N, Kuhns L, Johnson A, Holoyda B, Garofalo R. Text message reminders to improve adherence to antiretroviral therapy for HIV-positive youth. J Adolesc Health. 2011;48(2):S64–5. [Google Scholar]
- 49.Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: a pilot study using personalized, interactive, daily text message reminders. J Med Internet Res. 2012;14(2):e51. doi: 10.2196/jmir.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowshen N, Kuhns L, Gray C, Lee S, Garofalo R. Feasibility of interactive text message response (ITR) as a novel, real-time measure of adherence to antiretroviral therapy for HIV+ youth. AIDS Behav. 2013;17(6):2237–43. doi: 10.1007/s10461-013-0464-6. [DOI] [PubMed] [Google Scholar]
- 51.National Institutes of Health (NIH) Study quality assessment tools. 2014 https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/
- 52.Higgins JPT, Green S. The Cochrane handbook for systematic reviews of interventions. Version 5.1.0. 2011 www.handbook.cochrane.org.
- 53.Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52(5):546–53. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- 54.Naar-King S, Outlaw AY, Sarr M, et al. Motivational enhancement system for adherence (MESA): pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. J Pediatr Psychol. 2013;38(6):638–48. doi: 10.1093/jpepsy/jss132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Outlaw AY, Naar-King S, Tanney M, et al. The initial feasibility of a computer-based motivational intervention for adherence for youth newly recommended to start antiretroviral treatment. AIDS Care. 2014;26(1):130–5. doi: 10.1080/09540121.2013.813624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puccio JA, Belzer M, Olson J, et al. The use of cell phone reminder calls for assisting HIV-infected adolescents and young adults to adhere to highly active antiretroviral therapy: a pilot study. AIDS Patient Care STDS. 2006;20(6):438–44. doi: 10.1089/apc.2006.20.438. [DOI] [PubMed] [Google Scholar]
- 57.Saberi P, Yuan P, John M, Sheon N, Johnson MO. A pilot study to engage and counsel HIV-positive African American youth via telehealth technology. AIDS Patient Care STDS. 2013;27(9):529–32. doi: 10.1089/apc.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LaGrange R, Lewis M. Using mobile technology to support adherence to medications: SMS text reminders and young people living with HIV. J Adolesc Health. 2012;50(50):S94. [Google Scholar]
- 59.Garofalo R, Kuhns LM, Hotton A, Johnson A, Muldoon A, Rice D. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS Behav. 2016;20:1049–59. doi: 10.1007/s10461-015-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holder B, Turner-Musa J, Kimmel PL, et al. Engagement of African American families in research on chronic illness: a multisystem recruitment approach. Fam Process. 1998;37(2):127–51. doi: 10.1111/j.1545-5300.1998.00127.x. [DOI] [PubMed] [Google Scholar]
- 61.Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36(5):452–7. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mbuagbaw L, Sivaramalingam B, Navarro T, et al. Interventions for enhancing adherence to antiretroviral therapy (ART): a systematic review of high quality studies. AIDS Patient Care STDS. 2015;29(5):248–66. doi: 10.1089/apc.2014.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charania M, Marshall K, Lyles C, et al. Identification of evidence-based interventions for promoting HIV medication adherence: findings from a systematic review of US-based studies, 1996–2011. AIDS Behav. 2014;18(4):646–60. doi: 10.1007/s10461-013-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurth AE, Lally MA, Choko AT, Inwani IW, Fortenberry D. HIV testing and linkage to services for youth. J Int AIDS Soc. 2015;18(1):23–8. doi: 10.7448/IAS.18.2.19433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prescott TL, Phillips G, DuBois LZ, et al. Reaching adolescent gay, bisexual, and queer men online: development and refinement of a national recruitment strategy. J Med Internet Res. 2016;18(8):198–210. doi: 10.2196/jmir.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magnus M, Castel A. Breaking down the siloes: developing effective multidisciplinary HIV research teams. AIDS Behav. 2016;20(2):273–80. doi: 10.1007/s10461-016-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shiau S, Arpadi SM, Yin MT, Martins SS. Patterns of drug use and HIV infection among adults in a nationally representative sample. Addict Behav. 2017;2017(68):39–44. doi: 10.1016/j.addbeh.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewis JV, Abramowitz S, Koenig LJ, Chandwani S, Orban L. Negative life events and depression in adolescents with HIV: a stress and coping analysis. AIDS Care. 2015;27(10):1265–74. doi: 10.1080/09540121.2015.1050984. [DOI] [PubMed] [Google Scholar]
- 69.Kuhns LM, Hotton AL, Garofalo R, et al. An index of multiple psychosocial, syndemic conditions is associated with antiretroviral medication adherence among HIV-positive youth. AIDS Patient Care STDS. 2016;30(4):185–92. doi: 10.1089/apc.2015.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poushter J. Smartphone ownership and Internet usage continues to climb in emerging economies. [cited 2016 Dec 20];Pew Research Center. 2016 updated 2016; http://www.diapoimansi.gr/PDF/pew_research%201.pdf.
- 71.Mitchell SG, Schwartz RP, Alvanzo AA, et al. The use of technology in participant tracking and study retention: lessons learned from a clinical trials network study. Subst Abus. 2015;36(4):420–6. doi: 10.1080/08897077.2014.992565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kidd CD, Breazeal C. Robots at home: understanding long-term human-robot interaction. Rep U S. 2008:3230–35. IEEE. [Google Scholar]
- 73.Rane P, Mhatre V, Kurup L. Study of a home robot: JIBO. Int J Eng Res Technol. 2014;3(10):490–3. [Google Scholar]
- 74.Bickmore WT, Utami D, Matsuyama R, Paasche-Orlow KM. Improving access to online health information with conversational agents: a randomized controlled experiment. J Med Internet Res. 2016;18(1):e1. doi: 10.2196/jmir.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aron J. How innovative is Apple’s new voice assistant Siri? New Sci. 2011;212(2836):24. [Google Scholar]
- 76.Kahol K. Mobile phone messaging to improve health. BMJ. 2014;349:g6158. doi: 10.1136/bmj.g6158. [DOI] [PubMed] [Google Scholar]
- 77.Mesch GS, Talmud I, Quan-Haase A. Instant messaging social networks: individual, relational, and cultural characteristics. J Soc Pers Relat. 2012;29(6):736–59. [Google Scholar]
- 78.Wagner JH, Justice AC, Chesney M, Sinclair G, Weissman S, Rodriguez-Barradas M. Patient-and provider-reported adherence: toward a clinically useful approach to measuring antiretroviral adherence. J Clin Epidemiol. 2001;54(12):S91–8. doi: 10.1016/s0895-4356(01)00450-4. [DOI] [PubMed] [Google Scholar]
- 79.Chesney MAP. The elusive gold standard: future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43:S149–55. doi: 10.1097/01.qai.0000243112.91293.26. [DOI] [PubMed] [Google Scholar]
- 80.Lee L, Yehia BR, Gaur AH, et al. The impact of youth-friendly structures of care on retention among HIV-infected youth. AIDS Patient Care STDS. 2016;30(4):170–7. doi: 10.1089/apc.2015.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farmer C, Yehia BR, Fleishman JA, et al. Factors associated with retention among non-perinatally HIV-infected youth in the HIV research network. J Pediatric Infect Dis Soc. 2016;5(1):39–46. doi: 10.1093/jpids/piu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mugavero MJ, Westfall AO, Cole SR, et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 2014;59(10):1471–9. doi: 10.1093/cid/ciu603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rana AI, van den Berg JJ, Lamy E, Beckwith CG. Using a mobile health intervention to support HIV treatment adherence and retention among patients at risk for disengaging with care. AIDS Patient Care STDS. 2016;30(4):178–84. doi: 10.1089/apc.2016.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yehia BR, Stewart L, Momplaisir F, et al. Barriers and facilitators to patient retention in HIV care. BMC Infect Dis. 2015;15(1):246. doi: 10.1186/s12879-015-0990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Collins LM, Kugler KC, Gwadz MV. Optimization of multi-component behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS Behav. 2016;20(1):197–214. doi: 10.1007/s10461-015-1145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A” SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol. 2012;8(21–48):87. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu YP, Hommel KA. Using technology to assess and promote adherence to medical regimens in pediatric chronic illness. J Pediatr. 2014;164(4):922–7. doi: 10.1016/j.jpeds.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Allen LN, Christie GP. The emergence of personalized health technology. J Med Internet Res. 2016;18(5):e99. doi: 10.2196/jmir.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]