Abstract
Background
Influenza viruses gradually accumulate point mutations, reducing the effectiveness of prior immune protection.
Methods
Children aged 9–14 years received 2010–2011 trivalent inactivated influenza vaccine (TIV). Vaccination history, hemagglutination-inhibition (HI) titers, and cell-mediated immune responses were assessed to investigate the cross-reactivity with past and future influenza virus strains.
Results
2010–2011 TIV induced significant T-cell responses and HI titers of ≥160, with a fold-rise of ≥4 and titers of ≥100 maintained for >7 months in the majority of children. Pre-existing memory B cells in these children differentiated quickly to antibody-secreting cells to the new vaccine antigens. Children vaccinated in the previous year maintained high HI titers well into 2010, demonstrating elevated HI titers against A/Perth/16/2009, the future (in 2010–2011) H3N2 component. Prior vaccination enhanced CD8+ T-cell responses to A/Perth/16/2009. Children vaccinated with the prior 2009–2010 seasonal vaccine also demonstrated higher preexisting levels of interferon γ–secreting CD4+CD69+ T cells to 2009 pandemic influenza A(H1N1). Children previously vaccinated with 2009–2010 seasonal influenza vaccine also showed greater expansion of tumor necrosis factor α–secreting CD8+CD69+ T cells to 2009 pandemic influenza A(H1N1) upon vaccination in the 2010–2011 season than those who were not previously vaccinated.
Conclusions
Seasonal influenza viruses continuously drift, which allows them to circumvent protective immunity, but conserved epitopes provide immunological cross-reactivity in children through either vaccination directly or through prime/boost in the prior influenza season.
Keywords: B cell, children, cross-reactivity, hemagglutination inhibition, immune response, influenza, T cell, vaccine
Influenza viruses continuously evolve through antigenic drift and shift. On rare occasions, unique strains arise when an influenza strain acquires genetic segments from another strain. These antigenically shifted strains are serologically distinct with no cross-protection in the human population, resulting in influenza pandemics approximately once every 36 years [1]. More commonly, influenza viruses evolve gradually, accumulating point mutations in hemagglutinin (HA) antibody-binding sites, enhancing their ability to escape preexisting immunity and reinfect previously protected individuals [2].This antigenic drift prompts a yearly race to accurately predict next season’s circulating strains and manufacture a sufficient quantity of vaccine prior to the influenza season. This occasionally results in mismatch between vaccines and circulating strains that have significantly drifted early in the influenza season, as occurred during the 2014–2015 influenza season [3]. Despite continuous drift, emerging strains maintain some degree of antigenic similarity with their immediate predecessors [4, 5], and vaccines usually provide at least partial protection to drifted strains [3, 6, 7].
Annual influenza epidemics exert effects worldwide; however, children are disproportionately affected. The US Advisory Committee on Immunization Practices recommends that all children aged 6 months to 18 years receive influenza vaccine annually [8]. Nevertheless, it is estimated only half of American children are vaccinated yearly [9]. Despite waning immunity throughout the year, children vaccinated in the prior influenza season likely receive some additional benefit in subsequent seasons due to retained antigenic similarity. We examined immune responses in children vaccinated with trivalent inactivated influenza vaccine (TIV), assessing parameters likely to play a role in protection, including development of hemagglutination-inhibition (HI) titers, T-cell and B-cell responses, and influences of prior vaccination.
METHODS
Methods are summarized briefly here and in detail in Supplementary Figure 1.
Study Design
Fifty children aged 9–14 years (Table 1) were vaccinated on 9–16 October 2010 with unadjuvanted 2010–2011 TIV composed of A/California/07/09 (2009 pandemic influenza A[H1N1]; hereafter, A[H1N1]pdm09), A/Perth/16/2009(H3N2) (hereafter, A/Perth/16), and B/Brisbane/60/2008 (hereafter, B/Bris/60) strains. Serum was collected at 0 days, 28 days, and 7 months after vaccination, and peripheral blood mononuclear cells (PBMCs) were collected on day 0 from all subjects and at only 1 time point after vaccination, either 7 days, 14 days, or 28 days, because of the subjects’ age. One child was unavailable for the 7-month serum collection. Informed consent was obtained from subjects, and human experimentation guidelines of the Department of Health and Human Services were followed. Procedures, informed consent documents, and data collection forms were approved by participating institutional review boards.
Table 1.
Subject Characteristics, by Postvaccination Time of Peripheral Blood Mononuclear Cell Collection After Vaccination
| Group | Overall (n = 50) |
7 d (n = 16) |
14d (n = 17) |
28 d (n = 17) |
|---|---|---|---|---|
| Age, y | ||||
| Range | 9–14 | 9–14 | 10–14 | 9–14 |
| Average | 11.8 | 12.0 | 11.8 | 11.5 |
| Sex | ||||
| Male | 68.0 | 68.8 | 58.8 | 76.5 |
| Female | 32.0 | 31.2 | 41.2 | 23.5 |
| Race | ||||
| White | 96.0 | 87.5 | 100.0 | 100.0 |
| Other | 4.0 | 12.5 | 0.0 | 0.0 |
| Prior vaccine receipt | ||||
| 2009–2010 TIV | 28.0 | 25.0 | 35.3 | 23.5 |
| 2009–2010 LAIV | 10.0 | 6.3 | 17.6 | 5.9 |
| A(H1N1)pdm09 monovalent | 32.0 | 18.8 | 23.5 | 52.9 |
Data are percentage of subjects, unless otherwise indicated.
Abbreviations: A(H1N1)pdm09, 2009 pandemic influenza A(H1N1); LAIV, live-attenuated vaccine; TIV, trivalent inactivated influenza vaccine.
Serological Assays
HI assays were performed by Focus Diagnostics (Cypress, California) as previously described [10]. HI titers were determined against the 2010–2011 vaccine strains A(H1N1)pdm09, A/Wisconsin/15/2009(H3N2) (an A/Perth/16-like strain), and B/Bris/60; the 2009–2010 vaccine strains A/Brisbane/59/2007(H1N1) (hereafter, A/Bris/59) and A/Uruguay/716/2007(H3N2) (an A/Brisbane/10/2007-like strain; A/Bris/10); and the 2008–2009 influenza B vaccine strain, B/Florida/4/2006 (B/Flor/4).
T-Cell Responses
PBMCs were stimulated overnight with live A(H1N1)pdm09 (A/California/08/09) or A/Perth/16 as previously described [11] or with recombinant HA (rHA; Influenza Reagent Resource, Manassas, VA). PBMCs were stained with Live/Dead Stain (Life Technologies, Grand Island, New York) and then stained for surface CD56, CD69, CD4, and CD8 and intracellular tumor necrosis factor α (TNF-α), interleukin 2, and interferon γ (IFN-γ). T-cell activation determined within the CD56− population was expressed as CD4+CD69+ or CD8+CD69+ cells in total CD4+ or CD8+ T cells. Cytokine-producing cells were expressed within total CD4+CD69+ or CD8+CD69+ cells.
Antibody-Secreting Cells (ASCs)
Antigen (Ag)–specific memory B cells were assessed in PBMCs collected 28 days after vaccination, and plasmablasts were derived from preexisting memory B cells assessed in samples obtained 7 days after vaccination. PBMCs stimulated to induce polyclonal activation (Supplementary Figure 1) were added to enzyme-linked immunospot (ELISPOT) plates coated with anti-human immunoglobulin G (IgG), immunoglobulin M (IgM; Southern Biotech, Birmingham, Alabama), or monovalent influenza vaccines (kindly provided by Sanofi Pasteur, Swiftwater, Pennsylvania). Spot-forming units were assessed by ImmunoSpot ELISPOT reader (Cellular Technology, Cleve-land, Ohio) and expressed as the percentage of Ag-specific IgG-or IgM-secreting B cells out of the total number of IgG- or IgM-secreting B cells.
Statistical Analysis
Log2-transformed HI titers were used as dependent variables, summarized as geometric mean titers (GMTs). Means and differences in means were estimated using repeated measures linear mixed models as previously reported [11]. Model-estimated means yielded GMTs and differences between GMTs (28 days and 0 days, 7 months and 0 days, and 7 months and 28 days) yielded GMT ratios (fold rise). B-cell responses were analyzed as previously reported [11]; percentages were log10 transformed and summarized as geometric mean percentages (GMPs) and GMP ratios. T-cell analyses used a generalized linear mixed model with binomial error distribution to directly estimate the percentage of responding cells. Final results were back-transformed to a linear scale. Analyses were performed using SAS software (SAS Institute, Cary, North Carolina). Children grouped by prior vaccination (both seasonal and monovalent A[H1N1]pdm09 vaccines) were assessed for effects of prior vaccination on 2010–2011 TIV responses. For this analysis, post-vaccination T-cell responses (on days 7, 14, and 28) were grouped and differences in cellular responses and HI titers determined using unpaired t tests. The Fisher exact test was used to compare the proportions of children reaching HI titers of ≥40, 80, and 160.
RESULTS
Serological Responses
Children had increased HI titers to all 2010–2011 TIV strains (Figure 1), with the majority achieving a fold-rise of ≥4 (Table 2). Titers declined over 7 months but remained well above prevaccination levels (Figure 1). Vaccination also induced a fold-rise of ≥4 to the previous year’s A(H3N2) strain in 68% of children (Table 2).
Figure 1.
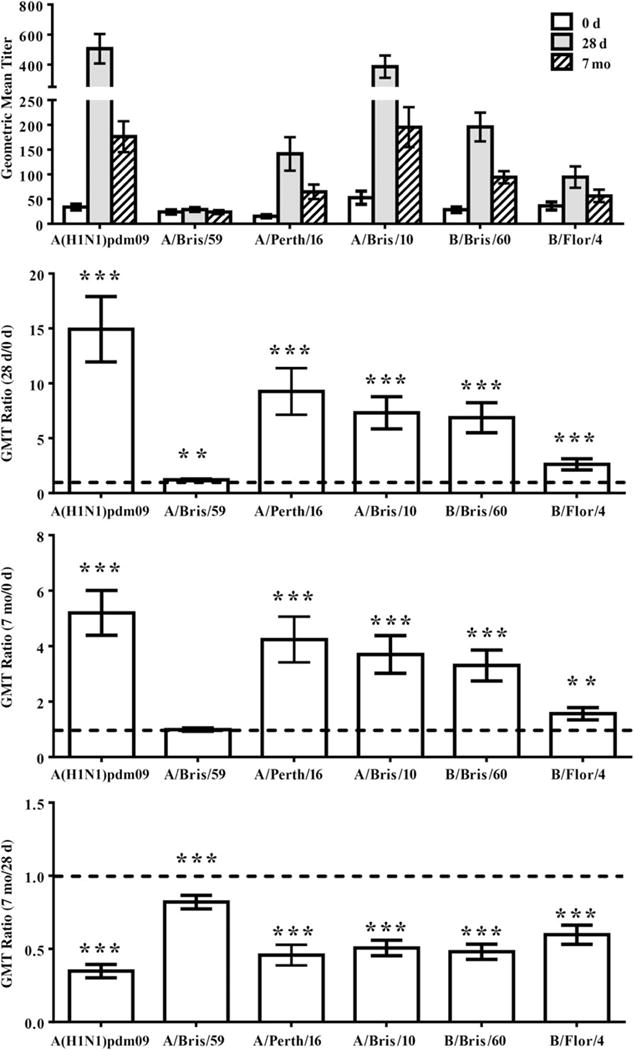
Hemagglutination-inhibition (HI) titers of children vaccinated with 2010–2011 trivalent inactivated influenza vaccine (TIV). HI titers to influenza virus strains included in the 2010–2011 TIV (2009 pandemic influenza A[H1N1] virus [A{H1N1}pdm09], A/Perth/16, and B/Bris/60), the 2009–2010 TIV (A/Bris/59, A/Bris10, and B/Bris/60), and the 2008–2009 TIV (B/Flor/4) were assessed at 0 days, 28 days, and 7 months after vaccination. Geometric mean titer (GMT) ratios (fold rise) were calculated using repeated measures linear mixed models for 28 days vs 0 days, 7 months vs 0 days, and 7 months vs 28 days. A GMT ratio of >1 (line) is indicative of a higher postvaccination response. Error bars represent 1 standard error. *P ≤ .05, **P ≤ .01, and †P ≤ .001.
Table 2.
Influenza Virus Vaccine Strain–Specific Hemagglutination-Inhibition (HI) Titers Among Children Vaccinated With 2010–2011 Trivalent Inactivated Influenza Vaccine, Overall and by Receipt of 2009–2010 Seasonal Influenza Vaccine
| Variable | A(H1N1) Strain, Titer
|
A(H3N2) Strain, Titer
|
B Strain, Titer
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A(H1N1)pdm09
|
A/Bris/59
|
A/Perth/16
|
A/Bris/10
|
B/Bris/60
|
B/Flor/4
|
|||||||||||||
| Overall | No Receipt |
Receipt | Overall | No Receipt |
Receipt | Overall | No Receipt |
Receipt | Overall | No Receipt |
Receipt | Overall | No Receipt |
Receipt | Overall | No Receipt |
Receipt | |
| Fold-rise > 4 | ||||||||||||||||||
|
| ||||||||||||||||||
| 28 d | 86.0 | … | … | 4.0 | … | … | 70.0 | … | … | 68.0 | … | … | 64.0 | … | … | 30.0 | … | … |
|
| ||||||||||||||||||
| HI > 40 | ||||||||||||||||||
|
| ||||||||||||||||||
| 0 d | 68.0 | 66.7 | 68.4 | 42.0 | 13.3a | 84.2 | 30.0 | 6.7a | 63.2 | 64.0 | 40.0a | 100.0 | 50.0 | 30.0a | 78.9 | 60.0 | 53.3 | 68.4 |
|
| ||||||||||||||||||
| 28 d | 96.0 | 96.7 | 94.7 | 44.0 | 20.0a | 78.9 | 84.0 | 76.7 | 94.7 | 94.0 | 90.0 | 100.0 | 96.0 | 100.0 | 89.5 | 80.0 | 83.3 | 73.7 |
|
| ||||||||||||||||||
| 7 mo | 95.9 | 96.7 | 94.4 | 38.8 | 16.7a | 72.2 | 73.5 | 70.0 | 77.8 | 85.7 | 80.0 | 94.4 | 91.8 | 93.3 | 88.9 | 63.3 | 63.3 | 61.1 |
|
| ||||||||||||||||||
| HI > 80 | ||||||||||||||||||
|
| ||||||||||||||||||
| 0 d | 36.0 | 30.0 | 47.7 | 26.0 | 6.7a | 52.6 | 22.0 | 3.3a | 47.4 | 50.0 | 20.0a | 94.7 | 36.0 | 20.0b | 57.9 | 42.0 | 30.0 | 57.9 |
|
| ||||||||||||||||||
| 28 d | 92.0 | 93.3 | 89.5 | 30.0 | 13.3b | 52.6 | 74.0 | 66.7 | 84.2 | 90.0 | 83.3 | 100.0 | 88.0 | 90.0 | 84.2 | 70.0 | 73.3 | 63.2 |
|
| ||||||||||||||||||
| 7 mo | 87.8 | 83.3 | 94.4 | 22.4 | 6.7b | 44.4 | 55.1 | 56.7 | 50.0 | 83.7 | 76.7 | 94.4 | 77.6 | 80.0 | 72.2 | 51.0 | 50.0 | 50.0 |
|
| ||||||||||||||||||
| HI > 160 | ||||||||||||||||||
|
| ||||||||||||||||||
| 0 d | 14.0 | 13.3 | 15.8 | 16.0 | 6.7 | 26.3 | 14.0 | 3.3c | 31.6 | 38.0 | 10.0a | 78.9 | 18.0 | 16.7 | 21.1 | 28.0 | 20.0 | 42.1 |
|
| ||||||||||||||||||
| 28 d | 86.0 | 86.7 | 84.2 | 16.0 | 6.7 | 26.3 | 66.0 | 56.7 | 78.9 | 84.0 | 76.7 | 94.7 | 70.0 | 73.3 | 63.2 | 52.0 | 53.3 | 52.6 |
|
| ||||||||||||||||||
| 7 mo | 67.3 | 66.7 | 66.7 | 14.3 | 6.7 | 22.2 | 40.8 | 33.3 | 50.0 | 67.3 | 63.3 | 72.2 | 38.8 | 43.3 | 27.8 | 36.7 | 36.7 | 38.9 |
The influence of prior seasonal vaccination on the percentage of subjects with HI titers of ≥ 40, 80, and 160 were assessed using the Fisher exact test for each virus strain. 2009 pandemic influenza A(H1N1) (A[H1N1]pdm09), A/Perth/16, and B/Bris/60 were included in the 2010–2011 TIV; A/Bris/59, A/Bris10, and B/Bris/60 were included in the 2009–2010 TIV; and B/Flor/4 was included in the 2008–2009 TIV.
Significance is indicated by
P ≤ .001 vs recipients of TIV containing the specified strain.
P ≤ .01 vs recipients of TIV containing the specified strain.
P ≤ .05 vs recipients of TIV containing the specified strain.
HI titers of ≥32 or ≥40 are considered to reduce risk of influenza virus infection by 50% in young, healthy adults [12, 13]; however, recent studies conflict as to what level is applicable to children. Studies by Ng et al confirmed the use of 40 [14], while Black et al suggest that a 50% reduction is associated with HI titers of >100 [15]. We therefore examined HI titers of ≥40, 80, and 160 (Table 2). Before vaccination, 58% of children had HI titers of <40 to A/Bris/59, the previous year’s A(H1N1) component (Table 2). In contrast, 68% exhibited preexisting HI titers of ≥40 to A(H1N1)pdm09, potentially through prior natural infection. 2010–2011 TIV increased A(H1N1)pdm09 titers, with >95% achieving HI titers of ≥40 and titers remaining >40 beyond 7 months; 86% achieved titers of ≥160, with titers in 67.3% remaining >160 for 7 months (Table 2).
Prior to vaccination in 2010, 64% of children had HI titers of ≥40 to A/Bris/10, the 2009–2010 A(H3N2) vaccine component, and 30% had preexisting titers of ≥40 to A/Perth/16, the 2010–2011 A(H3N2) component (Table 2). 2010–2011 TIV induced HI titers to both strains; 66% and 84% achieved HI titers of ≥160 to A/Perth/16 and A/Bris/10, respectively, and maintained these elevated levels beyond 7 months.
The 2010–2011 TIV B component, B/Bris/60, was retained from the previous year. Half of subjects had preexisting HI titers of ≥40 and similar titers to B/Flor/4, the B strain from 2 years prior (Table 2 and Figure 1). 2010–2011 TIV increased HI titers to both strains (Table 2).
T-Cell Responses
Increased percentages of activated (CD69+) T cells were detectable at most time points after vaccination without in vitro stimulation but were not statistically significant with the exception of IFN-γ–secreting CD4+CD69+ cells 7 days after vaccination (Supplementary Figure 2; P ≤ .05). Activated CD4+ T cells (CD4+CD69+) responded to live-virus stimulation primarily with IFN-γ production, while activated CD8+ T cells (CD8+CD69+) responded primarily with TNF-α secretion. Increased IFN-γ–secreting CD4+CD69+ cells were observed 14 days after vaccination after live A(H1N1)pdm09 stimulation (P ≤ .05) and 14 days (P ≤ .05) and 28 days (P ≤ .01) after vaccination against live A/Perth/16 (Figure 2). A/Perth/16 also stimulated TNF-α production 14 days after vaccination (P ≤ .05). Increased TNF-α–secreting CD8+CD69+ cells were observed 14 days (P ≤ .01) and 28 days (P ≤ .01) after vaccination to A(H1N1)pdm09. T-cell responses primarily target internal viral proteins, but it has been proposed that CD4 HA epitopes may be the most important for antibody generation [16, 17]. Stimulation with rHA revealed responses similar to live-virus stimulation, but responses were generally lower with slightly different kinetics (Supplementary Figure 3).
Figure 2.
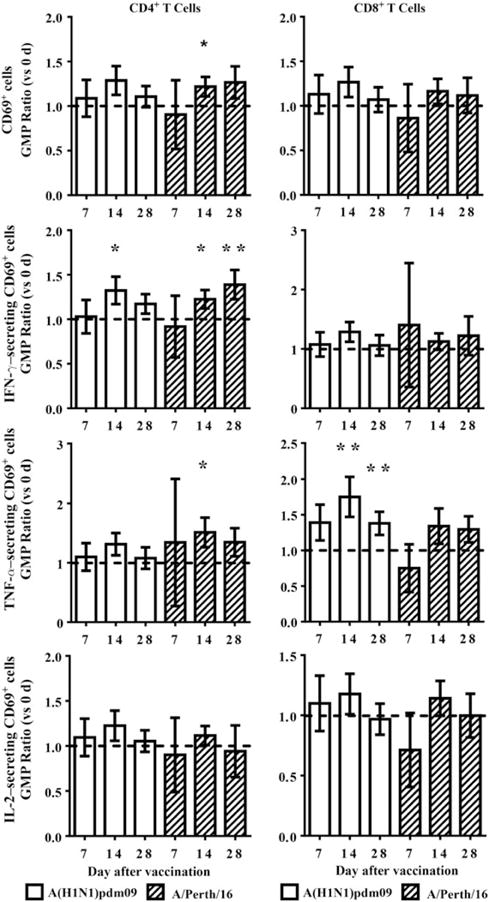
T-cell responses to live-virus stimulation. Cytokine production by activated (CD69+) T cells induced by live-virus stimulation were assessed prior to (0 days) and either 7, 14, or 28 days after vaccination with 2010–2011 trivalent inactivated influenza vaccine. Geometric mean percentage (GMP) ratios vs 0 days were calculated for each time point after vaccination, using repeated measures linear mixed models. A GMP ratio of >1 (line) is indicative of a higher postvaccination response. T-cell activation is expressed as CD4+CD69+ or CD8+CD69+ cells in total CD4+ or CD8+ T cells. Cytokine-producing cells are expressed as percentages of total CD4+CD69+ or CD8+CD69+ T cells. Error bars represent 1 standard error. *P ≤ .05 and **P ≤ .01.
B-Cell Responses
Ag-specific plasmablasts (ASCs) derived from the memory B-cell pool peak in peripheral blood 7 days after vaccination [18, 19]. IgG- and IgM-producing plasmablasts were assessed using 0 days and 7 days PBMCs (Figure 3). Responses were variable, but most individuals responded positively to each vaccine component, with greater responses in IgG+ ASCs. Consistent with 0 days HI titers (Table 2), preexisting A(H1N1)pdm09- and A/Perth/16-specific ASCs were detected for IgG and IgM. Fold-rise (GMP ratio) in Ag-specific plasmablasts was significant in all parameters, with highest fold-rise in A(H1N1)pdm09-specific ASCs (IgG and IgM producing), consistent with the highest GMT ratio in HI titers (28 days to 0 days; Figure 1).
Figure 3.
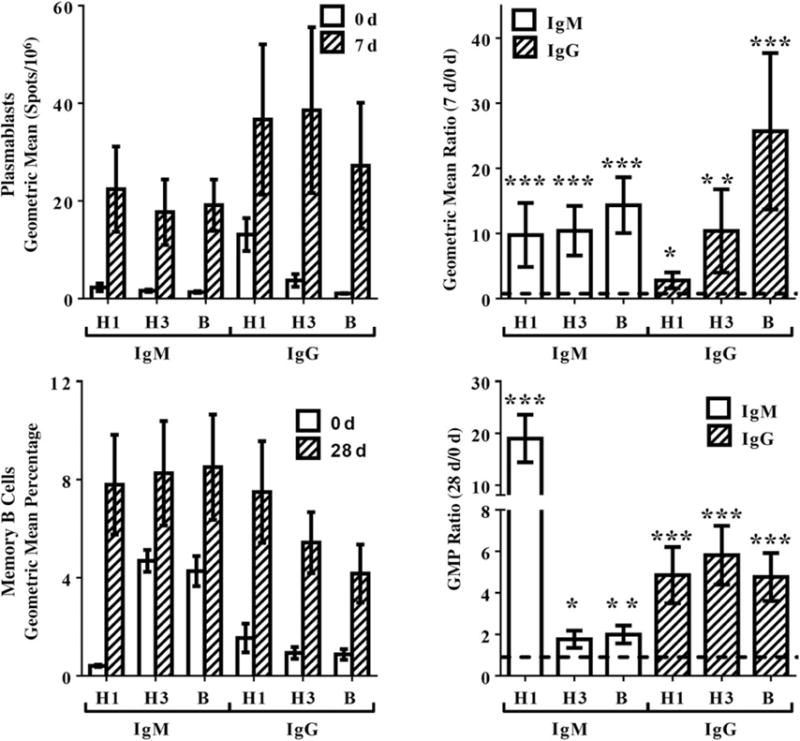
Plasmablast and memory B-cell responses. Immunoglobulin M (IgM)– and immunoglobulin G (IgG)–producing plasmablasts and memory B cells specific for 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) (H1), A/Victoria/2009 (H3), and B/Bris/60 (B) were assessed in children before and after vaccination with 2010–2011 trivalent inactivated influenza vaccine. Geometric mean ratio and geometric mean percentage (GMP) ratio vs 0 days were calculated against the postvaccination time points, using repeated measures linear mixed models. A ratio of >1 (line) is indicative of a higher postvaccination response. Error bars represent 1 standard error. *P ≤ .05, **P ≤ .01, and ***P ≤ .001.
Vaccination-induced memory B-cell frequency was assessed using PBMCs collected at 0 and 28 days (Figure 3). Comparable levels of IgG- and IgM-producing memory B cells specific to all vaccine components were induced by vaccination. While memory B cells were induced to all vaccine components, the greatest induction (GMP ratio for 28 days to 0 days) was detected in IgM-producing A(H1N1)pdm09-specific memory B cells (Figure 3).
Effect of Prior Vaccination on Immune Responses
Thirty-eight percent of children in this study received live-attenuated vaccine (LAIV; 10%; Table 1) or unadjuvanted TIV (28%; Table 1) during the previous influenza season, and 32% received monovalent A(H1N1)pdm09 vaccine (Table 1); 47.4% of children receiving 2009–2010 seasonal vaccine also received A(H1N1)pdm09 vaccine. Children vaccinated with 2009–2010 seasonal vaccine maintained higher HI titers prior to vaccination in 2010 (Table 2). Prior vaccinees did not have higher HI titers to the serologically distinct A(H1N1)pdm09 strain but had higher preexisting HI titers to the 2010–2011 A(H3N2) strain, A/Perth/16 (P ≤ .001; Figure 4). Subjects vaccinated with monovalent A(H1N1)pdm09 vaccine exhibited HI titers to A(H1N1)pdm09 similar to those exhibited by unvaccinated individuals (Figure 4). Of note, subjects receiving 2009–2010 seasonal vaccine elicited slightly lower HI titers to B/Bris/60 upon vaccination with the same strain during the 2010–2011 season (P ≤ .05; Figure 4), although 84.2% still reached HI titers of ≥80 (Table 2).
Figure 4.
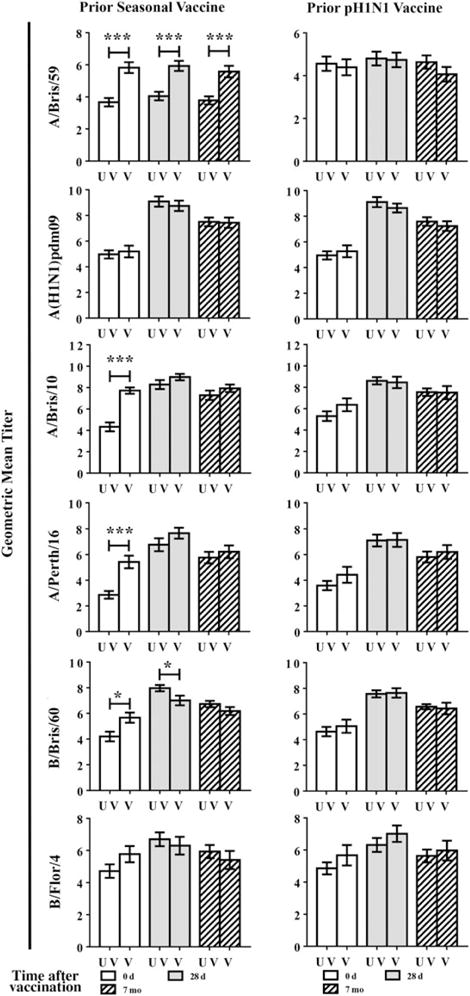
The effect of prior seasonal vaccination on hemagglutination-inhibition (HI) responses. Induction of HI titers by 2010–2011 trivalent inactivated influenza virus (TIV) was assessed in subjects who received prior influenza vaccination during the 2009–2010 influenza season (V) and those who did not (UV). Vaccination with prior seasonal influenza vaccine and monovalent 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) vaccine were assessed separately. HI titers to influenza virus strains included in the 2010–2011 TIV (A[H1N1]pdm09, A/Perth/16, and B/Bris/60), the 2009–2010 TIV (A/Bris/59, A/Bris10, and B/Bris/60), and the 2008–2009 TIV (B/Flor/4) were assessed at 0 days, 28 days, and 1 year after vaccination. Differences between previously vaccinated and unvaccinated subjects were determined using unpaired t tests. Error bars represent 1 standard error. Significance is indicated by *P ≤ .05 and ***P ≤ .001.
Interestingly, children receiving 2009–2010 seasonal influenza vaccine exhibited higher frequencies of preexisting IFN-γ–secreting CD4+CD69+ cells (P ≤ .05) after stimulation with A(H1N1)pdm09, while the increase in the frequency of TNF-α–secreting CD8+CD69+ cells (P = .07) did not reach significance (Figure 5). These responses further increased upon receipt of 2010–2011 TIV, although only TNF-α–secreting CD8+CD69+ cells reached significance as compared to previously unvaccinated subjects (P ≤ .05). Frequencies of TNF-α–secreting CD4+CD69+ and IFN-γ–secreting CD8+CD69+ cells showed no differences between previously vaccinated and unvaccinated individuals (data not shown). Prior seasonal vaccinees (2009–2010) also developed better A/Perth/16-specific TNF-α–secreting CD8+CD69+ responses after vaccination with 2010–2011 TIV (P ≤ .05). Subjects receiving monovalent A(H1N1)pdm09 vaccine did not exhibit similarly high T-cell responses.
Figure 5.
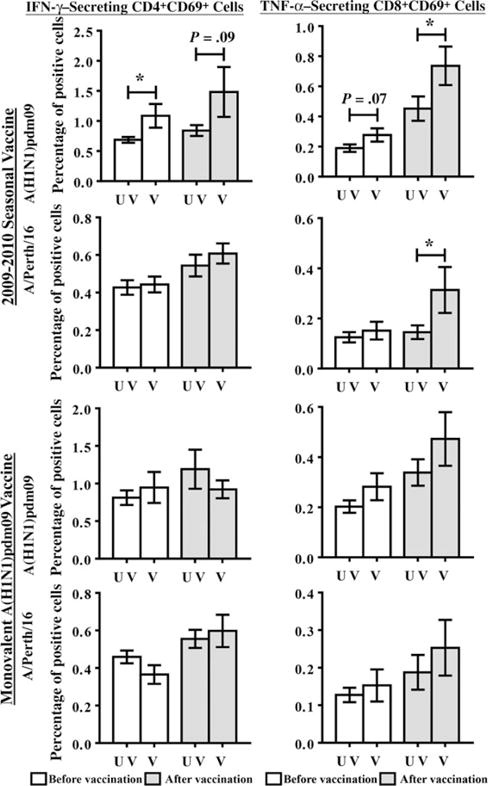
Effect of prior vaccination on T-cell responses. The percentage of interferon γ–secreting CD4+CD69+ and TNF-α–secreting CD8+CD69+ cells responding to stimulation with live 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) or A/Perth/16 virus were determined in subjects vaccinated (V) with seasonal influenza vaccine or the monovalent A(H1N1)pdm09 vaccine during the 2009–2010 influenza season as compared to subjects who were not vaccinated (UV) with these respective vaccines. Differences between previously vaccinated and unvaccinated subjects were determined using unpaired t tests. Error bars represent 1 standard error. *P ≤ .05.
DISCUSSION
Influenza epidemics disproportionately affect children. Nevertheless, challenges associated with performing clinical trials in children have limited our understanding of immunity to influenza virus in this at-risk population. This study constitutes one of the most comprehensive assessments of immunity to seasonal influenza vaccination in children. Our results demonstrate the broader influence of annual vaccination, which affects more than a single influenza season, and provide insight for future protection studies and vaccine development for children.
Recent studies suggest that children may require HI titers greater than the traditional titer of 40—possibly >100—to achieve a 50% reduction in clinical influenza [15]. Children in our study mounted significant serological and cell-mediated responses after immunization with 2010–2011 TIV. While high HI titers are important for effective protection, sustaining prolonged, elevated levels is essential. The majority of children demonstrated HI titers of ≥160, maintaining titers near or greater than 100 for >7 months (Figure 1 and Table 2). 2010–2011 TIV also generated significant IFN-γ–producing CD4+CD69+ cells responsive to A(H1N1)pdm09 and A/Perth/16 (Figure 2). Studies suggest that IFN-γ–producing cells correlate well with protection in young children [20].
Children exhibited moderately elevated HI titers prior to 2010–2011 vaccination. Elevated A(H3N2)- and B-specific titers are consistent with prior vaccination (discussed below). However, A(H1N1)pdm09 is serologically distinct from A/Bris/59, the prior A(H1N1) component, yet 68% had A(H1N1)pdm09 HI titers of ≥40 prior to vaccination (Table 2). As neither seasonal nor monovalent A(H1N1)pdm09 vaccinees had significant serological differences from unvaccinated individuals, this likely reflects prior infection (Figure 4). Studies examining longitudinal immunogenicity demonstrated rapid decline in HI titers invoked by A(H1N1)pdm09 vaccine [21, 22]. In contrast to monovalent A(H1N1)pdm09 vaccine, 67.3% of children vaccinated with 2010–2011 TIV maintained A(H1N1)pdm09 HI titers of ≥160 for >7 months (Table 2). Differences in longevity could be explained as a booster effect, with children maintaining higher HI titers over a longer period because of a secondary response stimulated by primary exposure to monovalent A(H1N1)pdm09 vaccine and/or natural infection. Alternatively, enhanced T-cell responses induced by 2010–2011 TIV may have aided in the longevity of the response (Figure 2 and Supplementary Figure 3).
Plasmablast responses at 7 days are indicative of an accelerated secondary response [18, 19]. Although subjects were relatively young, their robust IgG- and IgM-producing ASC formation at 7 days suggest development from an already established memory B-cell pool, readily differentiating to plasmablasts in response to strains in the new seasonal vaccine. IgG-producing plasmablasts were predominant, but significant IgM-producing plasmablasts were produced, albeit at reduced levels to IgG. Additionally, induction of long-lived IgM memory B cells was comparable to IgG counterparts (Figure 3). Although studies show that IgM memory B cells participate in secondary responses to bacterial or inert Ag primarily by entering germinal centers, rather than by directly secreting antibodies [23–27], their role in repeated influenza vaccine responses remains unknown. Considering that secondary antibody responses are typically dominated by highly specific IgG and little, if any, IgM, our current findings may indicate a continual maturation of the B-cell compartment in this age group. Studies examining plasmablast responses in adults who received A(H1N1)pdm09 vaccine showed a predominance of IgG-over IgM-producing plasmablasts in adults [18].
Influenza vaccination of children resulted in broader immunity than to strains included in the vaccine and influenced responses the following year. Children receiving 2009–2010 seasonal vaccine maintained high HI titers into 2010 and stimulated responses to strains not present in the vaccine (Figure 4 and Table 2). Children vaccinated with A/Bris/10, the 2009–2010 A(H3N2) vaccine component, demonstrated elevated A/Perth/16 HI titers, the future (2010–2011) A(H3N2) component, prior to vaccination in 2010 (Table 2). Conversely, vaccination with 2010–2011 TIV boosted HI titers to all 2009–2010 components. B/Bris/60, an influenza B Victoria lineage strain, also boosted HI titers to B/Flor/4, a Yamagata lineage strain from the 2008–2009 season (Figure 1). Studies in adults vaccinated during the 2006–2007 influenza season demonstrated the induction of cross-reactive antibodies to drifted influenza virus strains that emerged the following year [28]. Those studies further showed that adults were less able to induce cross-reactive antibodies with increasing age.
Relatively few studies have examined cross-protection against drifted seasonal influenza virus in children, which is afforded by TIV. Vesikari et al [29, 30] addressed cross-reactive HI titers in children vaccinated with MF59-adjuvanted versus unadjuvanted influenza vaccines. Children in those studies receiving 2 doses of unadjuvanted vaccine exhibited variable cross-reactivity, with moderate cross-reactivity to drifted A(H3N2) strains and little to none with B strains (also mismatches between Yamagata and Victoria lineages). Our study demonstrated much higher cross-reactivity to drifted strains, especially the B strain; however, our study was performed in older children, who respond better to vaccination. Vesikari et al examined children ages 6–72 months [30] and 6–35 months [29].
Influenza virus continuously evolves through antigenic drift, the gradual accumulation of point mutations allowing it to reinfect previously protected hosts [2]. However, this gradual evolution retains enough conserved epitopes with the previous strain to provide at least partial protection. Recent studies on human antibody responses against A(H3N2) strains showed substantially increased HI titers against historic strains, depending on antigenic distance from the infecting virus, an effect termed “back-boost” [5]. Boosting of related strains observed in our study, while consistent with cross-reactive epitopes, may also be explained by the back-boost effect.
Preexisting T-cell responses to A(H1N1)pdm09, presumably derived from cross-reactive epitopes from prior seasonal infection or vaccination, have been demonstrated in humans [16,31]. Children in our study who received 2009–2010 seasonal influenza vaccine exhibited higher frequencies of preexisting T cells to A(H1N1)pdm09 (Figure 5). Similarly, adults vaccinated with 2007–2008 seasonal influenza exhibited enhanced IFN-γ–producing cells to A(H1N1)pdm09 according to ELISPOT analysis [32]. Although subjects receiving monovalent A(H1N1)pdm09 vaccine exhibited T-cell responses to A(H1N1)pdm09, the level was similar to that in unvaccinated individuals (Figure 5). In a similar study, children vaccinated with ASO3-adjuvanted monovalent A(H1N1)pdm09 vaccine demonstrated higher frequencies of IFN-γ–producing cells according to ELISPOT, which persisted into the 2010–2011 season; those vaccinated with unadjuvanted whole-virion vaccine demonstrated a much lower level of IFN-γ–producing cells [33]. Similar to our results, vaccination with 2010–2011 seasonal vaccine further expanded T-cell responses to levels higher than those in subjects who were not previously vaccinated (Figure 5). While the studies performed in adults vaccinated with 2007–2008 seasonal vaccine were performed prior to A(H1N1)pdm09 circulation [32], our results cannot definitively attribute preexisting CD4+ and CD8+ cellular responses solely to prior 2009–2010 vaccination; our results suggest prior pandemic infection and therefore the possibility of a prime-boost effect from seasonal vaccine followed by pandemic infection (Table 2, Figure 1). However, only subjects receiving prior seasonal vaccination exhibited these enhanced T-cell responses to pandemic virus, demonstrating the major role of prior seasonal vaccination in this effect.
The effect of prior vaccination was analyzed by grouping all previously vaccinated subjects regardless of receiving TIV or LAIV. Vaccine type exerts differential effects on immune responses. LAIV stimulates higher T-cell responses in young children, compared with TIV [34, 35]. The 2 types of vaccines also influence responses in different lymphoid tissues and stimulate different antibody repertoires, as well as promote differential T- and B-cell phenotypes [36, 37]. Although the type of vaccine received the previous year potentially influenced responses in the subsequent influenza season, sample size precluded further subdividing this group to examine such interactions.
Our study has several limitations. The young age of subjects limited blood sample volumes, restricting the number of analyses that could be performed, especially T- and B-cell analyses. PBMCs were collected on different days after vaccination, to accommodate critical time points for plasmablasts, memory B cells, and T cells. While subdividing our cohort into different sample days allowed a more comprehensive evaluation, it reduced statistical power. Our cohort also had limited racial diversity and was slightly skewed toward males (Table 1).
Children in this study exhibited serological and cell-mediated immunity broader than components present in that year’s vaccine. Vaccination boosted serological responses to strains experienced not only in the past, likely through cross-reactivity and/or the back-boost effect, but also against strains that would circulate in the subsequent season. Although vaccination of subjects who had not received the previous year’s seasonal vaccine induced HI titers comparable to subjects who received prior vaccination, subjects who were previously vaccinated were likely afforded some level of preexisting protection prior to receipt of the vaccine in the following year. In contrast to serological responses, children receiving prior seasonal vaccination appeared to have enhanced T-cell responses upon vaccination in the subsequent year. The increased breadth of immunity to future strains is likely important for children who do not consistently receive annual influenza vaccination and in years when vaccine delivery is delayed or the influenza season gets off to an early start. Prior seasonal vaccination also stimulated IFN-γ– and TNF-α–producing T cells responsive to serologically distinct A(H1N1)pdm09. Whether these cross-reactive cells are the result of vaccination with prior seasonal vaccine alone or due to a prime-boost effect from vaccination followed by infection is uncertain. Nevertheless, the ability of seasonal vaccines to induce cross-reactive HI titers and T cells against future strains stresses the importance of yearly vaccination and warrants further investigation.
Supplementary Material
Acknowledgments
We sincerely thank Dr Michael Decker (Sanofi Pasteur, Swiftwater, PA) for providing monovalent influenza vaccines to assess cell-mediated immune responses.
Financial support
This work was supported by the CDC.
Footnotes
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC) or the Agency for Toxic Substances and Disease Registry.
Potential conflicts of interest
J. M. K. has received research funding from GlaxoSmithKline and Juvaris (now Colby Pharmaceuticals). L. A. C. is an employee of Abbott Laboratories. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Taubenberger JK, Morens DM. Influenza: the once and future pandemic. Public Health Rep. 2010;125(suppl 3):16–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–62. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Flannery B, Clippard J, Zimmerman RK, et al. Early estimates of seasonal influenza vaccine effectiveness—United States, January 2015. MMWR Morb Mortal Wkly Rep. 2015;64:10–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DJ, Lapedes AS, de Jong JC, et al. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305:371–6. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 5.Fonville JM, Wilks SH, James SL, et al. Antibody landscapes after influenza virus infection or vaccination. Science. 2014;346:996–1000. doi: 10.1126/science.1256427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuler CM, Iwamoto M, Bridges CB, et al. Vaccine effectiveness against medically attended, laboratory-confirmed influenza among children aged 6 to 59 months, 2003–2004. Pediatrics. 2007;119:e587–95. doi: 10.1542/peds.2006-1878. [DOI] [PubMed] [Google Scholar]
- 7.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 9.McIntyre AF, Gonzalez-Feliciano AG, Bryan LN, et al. Seasonal influenza vaccination coverage - United States, 2009–10 and 2010–11. MMWR Surveill Summ. 2013;62(suppl 3):65–8. [PubMed] [Google Scholar]
- 10.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 11.Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis. 2015 doi: 10.1093/ofid/ofv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 13.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng S, Fang VJ, Ip DK, et al. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J Infect Dis. 2013;208:1320–4. doi: 10.1093/infdis/jit372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black S, Nicolay U, Vesikari T, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30:1081–5. doi: 10.1097/INF.0b013e3182367662. [DOI] [PubMed] [Google Scholar]
- 16.Richards KA, Topham D, Chaves FA, Sant AJ. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J Immunol. 2010;185:4998–5002. doi: 10.4049/jimmunol.1001395. [DOI] [PubMed] [Google Scholar]
- 17.Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis. 2013;207:297–305. doi: 10.1093/infdis/jis684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–52. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest BD, Pride MW, Dunning AJ, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol. 2008;15:1042–53. doi: 10.1128/CVI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson M, Risi G, Davis M, et al. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis. 2012;205:733–44. doi: 10.1093/infdis/jir641. [DOI] [PubMed] [Google Scholar]
- 22.Song JY, Cheong HJ, Seo YB, et al. Comparison of the long-term immunogenicity of two pandemic influenza A/H1N1 2009 vaccines, the MF59-adjuvanted and unadjuvanted vaccines, in adults. Clin Vaccine Immunol. 2012;19:638–41. doi: 10.1128/CVI.00026-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yates JL, Racine R, McBride KM, Winslow GM. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J Immunol. 2013;191:1240–9. doi: 10.4049/jimmunol.1300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–9. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 26.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–7. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert M, Przekopowitz M, Taudien S, et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A. 2015;112:E546–55. doi: 10.1073/pnas.1416276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luytjes W, Enouf V, Schipper M, et al. HI responses induced by seasonal influenza vaccination are associated with clinical protection and with seroprotection against non-homologous strains. Vaccine. 2012;30:5262–9. doi: 10.1016/j.vaccine.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 29.Vesikari T, Pellegrini M, Karvonen A, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J. 2009;28:563–71. doi: 10.1097/INF.0b013e31819d6394. [DOI] [PubMed] [Google Scholar]
- 30.Vesikari T, Knuf M, Wutzler P, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–16. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 31.Greenbaum JA, Kotturi MF, Kim Y, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009;106:20365–70. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbramanian RA, Basha S, Shata MT, Brady RC, Bernstein DI. Pandemic and seasonal H1N1 influenza hemagglutinin-specific T cell responses elicited by seasonal influenza vaccination. Vaccine. 2010;28:8258–67. doi: 10.1016/j.vaccine.2010.10.077. [DOI] [PubMed] [Google Scholar]
- 33.Lambe T, Spencer AJ, Mullarkey CE, et al. T-cell responses in children to internal influenza antigens, 1 year after immunization with pandemic H1N1 influenza vaccine, and response to revaccination with seasonal trivalent-inactivated influenza vaccine. Pediatr Infect Dis J. 2012;31:e86–91. doi: 10.1097/INF.0b013e318255e443. [DOI] [PubMed] [Google Scholar]
- 34.He XS, Holmes TH, Zhang C, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–66. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoft DF, Babusis E, Worku S, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis. 2011;204:845–53. doi: 10.1093/infdis/jir436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He XS, Holmes TH, Mahmood K, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197:803–11. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 37.Sealy R, Webby RJ, Crumpton JC, Hurwitz JL. Differential localization and function of antibody-forming cells responsive to inactivated or live-attenuated influenza virus vaccines. Int Immunol. 2013;25:183–95. doi: 10.1093/intimm/dxs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


