Abstract
Purpose
The purpose of this study is to analyze early retinal angiomatous proliferation (RAP) utilizing a novel imaging modality, Projection-Resolved Optical Coherence Tomography Angiography (PR-OCTA).
Observations
Five months prior to the diagnosis of a RAP lesion, cross-sectional PR-OCTA demonstrated flow in the outer retina contiguous with the deep retinal capillary plexus (DCP) and adjacent to a small pigment epithelial detachment. After development of a clinically visible RAP lesion, cross-sectional PR-OCTA demonstrated the RAP lesion connecting DCP and sub-retinal pigment epithelial neovascularization.
Conclusions & importance
This is the first report of PR-OCTA demonstrating abnormal flow in the outer retina prior to the development of a clinically detectable RAP lesion. PR-OCTA may be useful for surveillance and to help further characterize and stage RAP lesions.
Keywords: Optical coherence tomography angiography, Type 3 choroidal neovascularization, Retinal angiomatous proliferation, Neovascular age-related macular degeneration
1. Introduction
Retinal angiomatous proliferation (RAP) or type 3 neovascularization is a well-recognized variant of neovascular age-related macular degeneration (AMD) characterized by intraretinal hemorrhage (IRH) and cystic retinal edema.1, 2, 3, 4, 5, 6 Structural optical coherence tomography (OCT) may additionally demonstrate the presence of accompanying pigment epithelial detachment (PED) or intraretinal pigment migration.3
Optical coherence tomography angiography (OCTA) is a novel functional extension of OCT that enables non-invasive visualization of separate retinal capillary plexuses as well as choroidal neovascularization.7, 8, 9, 10, 11, 12 One limitation of OCTA is that moving red blood cells in the inner retinal vessels project fluctuating shadow artifact onto the deeper layers of the retina creating artificial flow signals. On cross-sectional OCTA, projection artifact appears as “tails” below in situ flow most prominent on hyperreflective structural regions, and en face images contain artificial inner retinal vasculature visible in deeper retinal tissue.
A recent image processing algorithm termed projection-resolved OCTA (PR-OCTA) mitigates projection artifact by resolving the ambiguity between true flow signal and projection artifacts.10 PR-OCTA detects voxels with in situ flow as those where intensity-normalized decorrelation values are higher than all shallower voxels in the same axial scan line, providing artifact resolution for both en face and cross sectional OCTA. In commercial OCT angiography, the flow projection artifact is suppressed with a slab-subtraction (SS) algorithm. A notable limitation of the SS algorithm, in contrast to PR-OCTA, is the failure to remove prominent tail artifacts on cross-sectional OCT angiograms. Adequate resolution of these artifacts is particularly necessary to capture axially directed flow which is characteristic of RAP. We herein present OCTA and PR-OCTA findings of a RAP lesion prior to diagnosis, at clinical diagnosis, and with subsequent therapy.
2. Materials and methods
Following Institutional Review Board approval, multimodal retinal imaging including structural OCT, color fundus photography, and fluorescein angiography was retrospectively reviewed. OCT angiograms were acquired using the commercially available scan protocols with the spectral domain OCT (RTVue-XR Avanti) based on the split-spectrum amplitude decorrelation angiography (SSADA) algorithm.8 These images were subsequently exported to the Casey Eye Reading Center for application of PR-OCTA algorithm and semi-automated segmentation.10 The deep capillary plexus (DCP) of the retina was defined as flow between the outer half of inner nuclear layer and the outer boundary of the outer plexiform layer (OPL). Outer retinal flow was localized between the outer boundary of the OPL and Bruch's membrane (BM). Purple segmentation lines were utilized to depict the inner limiting membrane, yellow lines the interface of OPL and outer nuclear layer, and green lines the interface of retinal pigment epithelium (RPE) and BM. Inner retinal flow was depicted as purple, outer retinal flow as yellow, and choroidal flow as red.
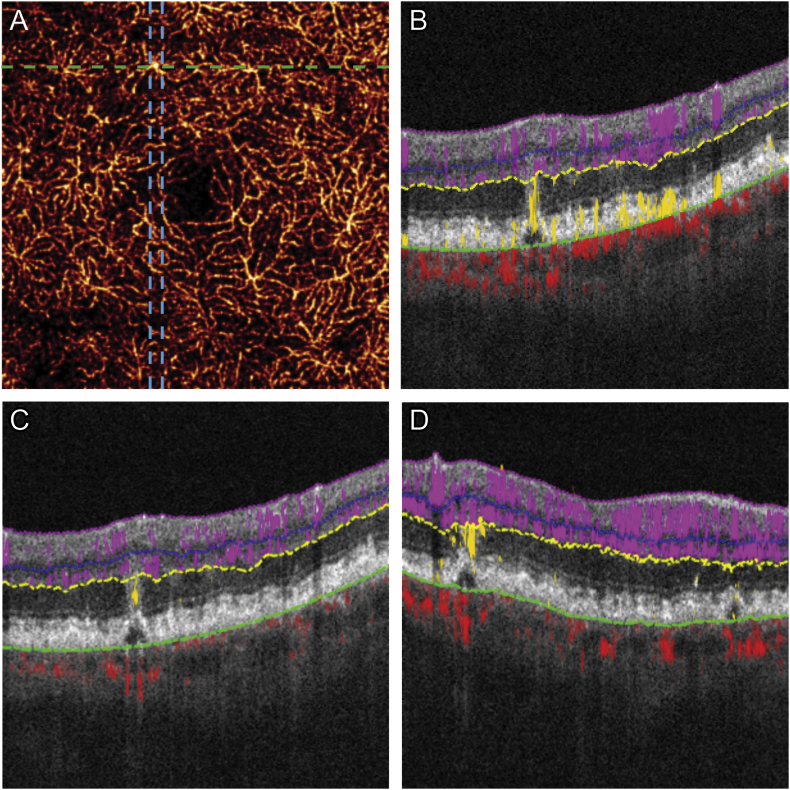
DCP and outer retinal en face angiograms consisted of maximal flow projection along axial (Z) dimension. Thick (100 μm) cross-sectional OCT angiograms consisted of 10 axial frames. Cross-sectional structural OCT images were represented by the reflectance signals in the middle of the thick cross-sectional OCTA (Fig. 2A,D).
Fig. 2.
Outer retinal flow detected five months prior to clinical diagnosis of retinal angiomatous proliferation (RAP).
(A) 3 × 3 mm projection-resolved optical coherence tomography (PR-OCTA) showing a single vessel that was slightly dilated and brighter compared to surrounding deep capillary plexus (B) Conventional OCTA does not clearly depict whether flow is associated with the pigment epithelial detachment (PED) due to extension of tail artifact to the PED (C) Cross-sectional PR-OCTA, in contrast, clearly detected flow in the outer retina associated with the very small PED. (D) Flow was confirmed on thickened perpendicular cross-sectional OCTA composed of 10 axial cross-sectional frames, corresponding to frames between the blue lines in (A). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.1. Case report
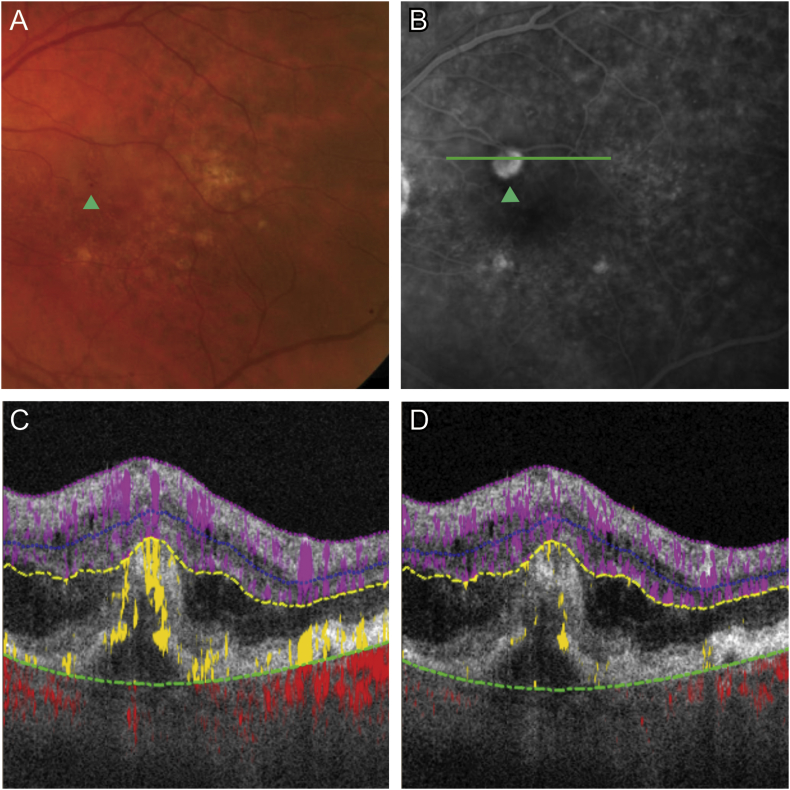
A 79-year-old male with neovascular AMD in the right eye presented with vision loss in the left eye. Visual acuity measured 20/40, IRH was detected superior to the fovea by fundus exam and was associated with leakage on fluorescein angiography (Fig. 1A–B). Cross-sectional OCTA showed intra-retinal fluid (IRF) and abnormal flow in the outer retina, however projection artifact is present on retinal pigment epithelium (RPE) and artifact “tails” of in situ flow limit depth discrimination (Fig. 1C). With cross-sectional PR-OCTA, projection artifact is removed allowing easier discrimination of RAP lesion depth (Fig. 1D). Three-dimensional volume rendering of OCTA illustrates axially directed flow within the RAP lesion (Supplementary Video).
Fig. 1.
Retinal angiomatous proliferation (RAP) at the time of diagnosis.
(A) Color photo and (B) fluorescein angiography of RAP lesion (green arrow). (C) Cross-sectional (corresponds to green line in B) optical coherence tomographic angiography (OCTA) revealed abnormal flow in the outer retina (yellow), however projection artifact is present. With cross-sectional PR-OCTA, projection artifact is removed allowing easier discrimination of RAP lesion depth (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajoc.2017.10.001.
The following is the supplementary data related to this article:
Three-dimensional volume rendering of optical coherence tomography angiography (OCTA) in a retinal angiomatous proliferation (RAP) lesion (.mpg). Volume rendering of OCTA data preserves anatomic three-dimensional relationships and enables enhanced visualization of retinal vascular abnormalities. Three-dimensional volume rendering of OCTA in our case of RAP well-illustrates axially directed flow which is typical for this lesion.
Five months prior, the patient had received OCTA imaging of the left eye as part of routine evaluation. Scans were reviewed focusing on the region superior to the fovea: en face PR-OCTA of DCP showed a single vessel that was slightly dilated and brighter compared to surrounding DCP (Fig. 2A). Flow could not be confirmed by conventional OCTA due to tail artifact (Fig. 2B). Cross-sectional PR-OCTA detected flow in the outer retina associated with a very small PED and no IRF was present (Fig. 2D). Thick cross-sectional OCTA revealed outer retinal flow was contiguous with DCP without extension into the sub-retinal pigment epithelial (RPE) space (Fig. 3). The axially oriented RAP lesion appeared small with en face OCTA (Fig. 3).
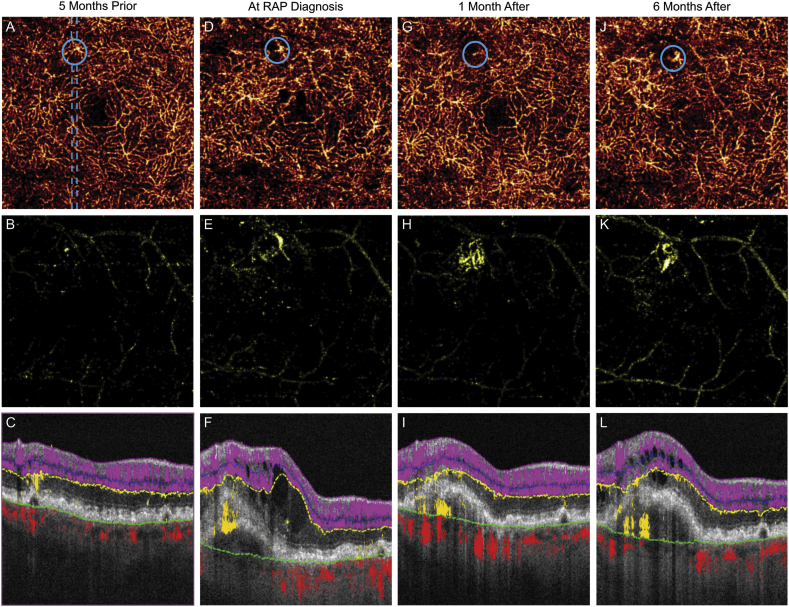
Fig. 3.
Optical coherence tomography angiography (OCTA) in an evolving retinal angiomatous proliferation (RAP) lesion.
(A,D,G,J) Serial projection-resolved OCTA (PR-OCTA) of the deep capillary plexus reveals evolution of a dilated vessel (blue circles). En face outer retinal slab (B,E,H,K) and cross-sectional OCT/PR-OCTA (C,F,I,L) corresponding to dashed blue line demonstrate axial neovascular flow continuous with the dilated DCP vessel. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At the time of RAP diagnosis, monthly treatment with aflibercept was initiated. After three injections, treatment interval was extended to every six weeks. Serial PR-OCTA demonstrates RAP lesion evolution and response to treatment. En face DCP PR-OCTA shows enlargement of the DCP vessel compared to five months prior to clinical appearance of RAP lesion (Fig. 3A). One month after treatment, the vessel appears less dilated (Fig. 3D). Six months following initiation of treatment and during longer treatment interval, the DCP vessel becomes more dilated and prominent (Fig. 3J). En face outer retinal PR-OCTA reveals growth of neovascularization and lesion persistence while under treatment (Fig. 3 B,E,H,K). Serial thick cross-sectional PR-OCTA reveals extension of outer retinal flow into the sub-RPE space leading up to RAP diagnosis (Fig. 3C,F). One month following treatment, despite resolution of IRF, flow persists above and below the PED (Fig. 3I). Subsequent to extension of treatment interval, the PED enlarges and IRF reoccurs. Thick cross-sectional PR-OCTA demonstrates persistent sub-RPE flow consistent with neovascular tissue.
3. Discussion
This is the first case report using PR-OCTA demonstrating the development of a RAP lesion prior to symptoms and classically described clinical features. Vessels in early RAP lesions are small and primarily axially oriented, rendering them difficult to detect with en face OCTA (Fig. 1B); cross-sectional OCTA is particularly useful in this setting. Further, RAP lesions often have associated hyper-reflective material and pigment migration in the outer retina.1, 2, 3, 4, 5 These features are highly susceptible to projection artifact and potentially may lead to false positive flow detection in the outer retina. Previous studies utilized slab subtraction projection artifact removal in which en face superficial retinal vessel slab is subtracted from outer retinal slab.13 This technique removes large vessel projection artifact for en face images only, whereas PR-OCTA mitigates artifact in both en face and cross-sectional OCTA. PR-OCTA in this case improved interpreter confidence to recognize true flow signal from projection artifact with cross-sectional OCTA, an invaluable strength of the PR algorithm (Fig. 1C-D). A single cross-sectional PR-OCTA frame reveals a fine cut through the RAP lesion, whereas thick cross-sectional OCTA aided in identifying the extent of the lesion (Fig. 2C-D).
Previous reports have described RAP with conventional en face OCTA.7, 11, 12 At the time of clinical diagnosis and while under treatment, our case had a curvilinear morphology as previously described.12 Two previous studies demonstrated reduction of flow after treatment of RAP lesions11, 12 In our case, the sub-RPE component of the RAP lesion appeared less responsive to treatment at one month (Fig. 3F,I) compared to the DCP component of the RAP lesion (Fig. 3D,G). The DCP component of the RAP lesion enlarged while the treatment interval was extended. Further longitudinal study with PR-OCTA is needed to determine if RAP lesion flow changes under anti-vascular endothelial growth factor may be useful for clinicians attempting to optimize treatment intervals.
The origin and evolution of RAP lesions is controversial. It has been suggested with some histopathological evidence that RAP arises from the DCP with subsequent extension into the sub-RPE space, while others have postulated a choroidal origin.1, 2, 5, 6 In this longitudinal OCTA series, abnormal flow was clearly detected initially in the outer retina and contiguous with a small dilated vessel in the DCP. A small PED was present, but no sub-RPE flow was identified, suggesting potential origin from DCP. Further, progression over time depicted extension of flow into the sub-RPE space. RAP lesions are commonly bilateral, and frequent scanning of asymptomatic fellow eyes with PR-OCTA may help determine the origin and further define RAP pathogenesis.
4. Conclusions
Projection-Resolved OCTA improves depth discrimination by removing artifact. In this case, a RAP lesion arising from the deep capillary plexus was confirmed by PR-OCTA when adequate artifact resolution could not be obtained with conventional OCTA (SS algorithm). This case clearly demonstrates extension of flow initiating from the outer retina into the sub-RPE space over time.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
This work was supported by grant R01 EY024544, DP3 DK104397, R01 EY023285, P30 EY010572 from the National Institutes of Health (Bethesda, MD), and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY).
Kavita V. Bhavsar receives financial support from the Portland VA Healthcare System.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Conflict of interest
The following authors have no financial disclosures: KVB, YJ, JW, RCP, AKL, DH, STB.
Acknowledgements
None.
References
- 1.Yannuzzi L.A., Negrão S., Iida T. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–434. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Yannuzzi L.A., Freund K.B., Takahashi B.S. Review of retinal angiomatous proliferation or type 3 neovascularization. Retina. 2008;28(3):375–384. doi: 10.1097/IAE.0b013e3181619c55. [DOI] [PubMed] [Google Scholar]
- 3.Nagiel A., Sarraf D., Sadda S.R. Type 3 neovascularization: evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina. 2015;35(4):638–647. doi: 10.1097/IAE.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 4.Viola F., Massacesi A., Orzalesi N., Ratiglia R., Staurenghi G. Retinal angiomatous proliferation: natural history and progression of visual loss. Retina. 2009;29(6):732–739. doi: 10.1097/IAE.0b013e3181a395cb. [DOI] [PubMed] [Google Scholar]
- 5.Shimada H., Kawamura A., Mori R., Yuzawa M. Clinicopathological findings of retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2007;245(2):295–300. doi: 10.1007/s00417-006-0367-6. [DOI] [PubMed] [Google Scholar]
- 6.Monson D.M., Smith J.R., Klein M.L., Wilson D.J. Clinicopathologic correlation of retinal angiomatous proliferation. Arch Ophthalmol. 2008;126(12):1664–1668. doi: 10.1001/archopht.126.12.1664. [DOI] [PubMed] [Google Scholar]
- 7.Dansingani K.K., Naysan J., Freund K.B. En face OCT angiography demonstrates flow in early type 3 neovascularization (retinal angiomatous proliferation) Eye. 2015;29(5):703–706. doi: 10.1038/eye.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia Y., Tan O., Tokayer J. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokayer J., Jia Y., Dhalla A.H., Huang D. Blood flow velocity quantification using split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Biomed Opt Express. 2013;4(10):1909–1924. doi: 10.1364/BOE.4.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M., Hwang T.S., Campbell J.P. Projection-resolved optical coherence tomographic angiography. Biomed Opt Express. 2016;7(3):816–828. doi: 10.1364/BOE.7.000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Carlo T.E., Romano A., Waheed N.K., Duker J.S. A review of optical coherence tomography angiography (OCTA) Int J Retina Vitreous. 2015;1:5. doi: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuehlewein L., Dansingani K.K., de Carlo T.E. Optical coherence tomography angiography of type 3 neovascularization secondary to age-related macular degeneration. Retina. 2015;35(11):2229–2235. doi: 10.1097/IAE.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 13.Jia Y., Bailey S.T., Wilson D.J. Quantiative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-dimensional volume rendering of optical coherence tomography angiography (OCTA) in a retinal angiomatous proliferation (RAP) lesion (.mpg). Volume rendering of OCTA data preserves anatomic three-dimensional relationships and enables enhanced visualization of retinal vascular abnormalities. Three-dimensional volume rendering of OCTA in our case of RAP well-illustrates axially directed flow which is typical for this lesion.