Abstract
Bacterial stem blight caused by Pseudomonas syringae pv. syringae is a common disease of alfalfa (Medicago sativa L). Little is known about host-pathogen interactions and host defense mechanisms. Here, individual resistant and susceptible plants were selected from cultivars Maverick and ZG9830 and used for transcript profiling at 24 and 72 hours after inoculation (hai) with the isolate PssALF3. Bioinformatic analysis revealed a number of differentially expressed genes (DEGs) in resistant and susceptible genotypes. Although resistant plants from each cultivar produced a hypersensitive response, transcriptome analyses indicated that they respond differently at the molecular level. The number of DEGs was higher in resistant plants of ZG9830 at 24 hai than in Maverick, suggesting that ZG9830 plants had a more rapid effector triggered immune response. Unique up-regulated genes in resistant ZG9830 plants included genes encoding putative nematode resistance HSPRO2-like proteins, orthologs for the rice Xa21 and soybean Rpg1-b resistance genes, and TIR-containing R genes lacking both NBS and LRR domains. The suite of R genes up-regulated in resistant Maverick plants had an over-representation of R genes in the CC-NBS-LRR family including two genes for atypical CCR domains and a putative ortholog of the Arabidopsis RPM1 gene. Resistance in both cultivars appears to be mediated primarily by WRKY family transcription factors and expression of genes involved in protein phosphorylation, regulation of transcription, defense response including synthesis of isoflavonoids, and oxidation-reduction processes. These results will further the identification of mechanisms involved in resistance to facilitate selection of parent populations and development of commercial varieties.
Introduction
Alfalfa (Medicago sativa L.) is a key forage crop for dairy producers in the U.S. and in countries around the world. In addition, it is an important component of sustainable agricultural systems because of its high biomass yield, role in soil and water conservation, biological nitrogen fixation that improves soil fertility, interruption of pest and pathogens in crop rotations, and for providing wildlife habitat [1].
Plant pathogens and nematodes that infect alfalfa cause substantial losses in yield and quality of forage and reduce stand life. Largely because of the autotetraploid nature of alfalfa and severe inbreeding depression, alfalfa breeding is traditionally done by phenotypic recurrent selection of plant populations. Genetic mechanisms underlying disease resistance in alfalfa populations obtained by conventional methods remain essentially unknown. Understanding these processes would facilitate selection of resistant parent populations for breeding commercial varieties and increase the percentage of resistant plants in released varieties.
Bacterial stem blight of alfalfa, caused by Pseudomonas syringae pv. syringae, is common in the central and western U.S. and the disease occasionally occurs in eastern states. It has also been reported in Australia, Europe, and recently reported in western Iran [2]. There are two related phases of the disease, localized foliar necrosis (blight) and systemic vascular wilt. Infection of foliage results in water soaked lesions followed by chlorosis and necrosis of leaves. The bacterium penetrates host stems primarily at frost injury sites and forms water-soaked lesions that extend down the stem becoming amber with dried bacterial exudate that blackens with age. Plants with the disease are stunted, with spindly stems that are easily broken. Yield losses from the disease can be as high as 50% of the first harvest [3]. Disease losses in the first harvest are economically damaging because this harvest is typically the highest yielding with the best forage quality. With a changing climate, the possibility exists of expansion in the geographical range and impact of bacterial stem blight in the U.S. and globally.
Pseudomonas syringae is a Gram-negative bacterium that causes disease in practically every cultivated plant species [4]. It has been divided into approximately 50 pathovars (pv.), which are characterized by their host range. P. syringae pv. syringae is a very heterogeneous group whose members can cause disease collectively on over 200 plant species. The bacterium is capable of long distance aerial movement and there is strong evidence for its role in the global water cycle [5]. Long distance movement occurs when bacterial populations on plant surfaces are aerosolized and transported by air currents into the troposphere. They are then re-deposited on plants and in water systems in the form of snow and rainwater. The genetics of the interaction of P. syringae with plant hosts has been studied in both model plants and crop species [6, 7]. The resistance interaction follows the gene-for-gene interaction in which an effector protein from the bacterium is recognized by the resistance gene protein from the host to trigger a cascade of events leading to disease resistance. Initial defense is a hypersensitive response characterized by a rapid collapse/necrosis of infected cells. However, little is known about resistance in perennial plants such as alfalfa.
Previously, bacteria producing a fluorescent pigment were isolated from alfalfa with symptoms of bacterial stem blight from near Cheyenne, WY [8]. The strain ALF3 was identified as P. syringae pv. syringae (PssALF3) and a complete genome sequence was obtained [9]. It was found to be pathogenic on alfalfa, Medicago truncatula (barrel medic), bean seedpods, pear leaves, and beet seedlings but did not cause disease on bean leaves [8].
To date, there are no alfalfa cultivars selected for resistance to this disease and no known chemical control. Little is known about alfalfa-pathogen interactions: the means of bacterial pathogenicity, determinants of virulence, host defense mechanisms, and sources of resistance are absent in the literature. In this study, global transcriptome profiling of resistant and susceptible alfalfa plants was carried out using RNA-seq technology to identify alfalfa genes differentially expressed during infection. Key genes and processes involved in host resistance are proposed.
Results
Evaluation of phenotypic responses to bacterial stem blight in susceptible and resistant genotypes
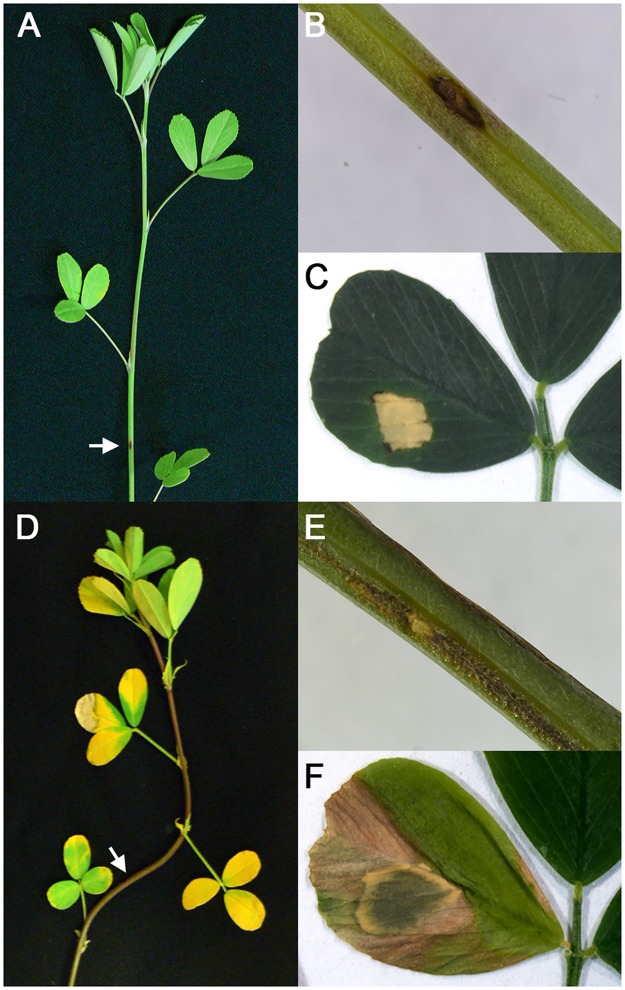
Alfalfa cultivars Maverick and ZG9830 (Materials and methods) used in this work were found to have up to 59% of the plants resistant to bacterial stem blight (Samac, in preparation). Due to the obligate outcrossing genetics of alfalfa, resistant cultivars have a percentage of susceptible plants. To evaluate host resistance and susceptibility in individual alfalfa plants, their leaflets were infiltrated with PssALF3 and scored for disease symptoms. Either susceptible or resistant plants of both cultivars showed a necrotic response at 24 hours. However, for resistant plants the response did not change and in susceptible plants it progressed to water soaking followed by chlorosis and necrosis of cells beyond the site of infiltration (Fig 1). Seven days after inoculation resistant plants of both cultivars displayed browning of cells surrounding the site of inoculation on stems indicating a hypersensitive response (Fig 1). Susceptible plants showed water soaking and collapse at the site of inoculation and systemic chlorosis and necrosis of foliage. Determination of bacterial populations showed that plants scored as susceptible had a mean CFU (colony forming units) = log 8.2 while those scored as resistant had a mean CFU = log 6.4, evidence that susceptible plants with symptoms of disease promoted high bacterial populations. No symptoms and no CFU were obtained from mock-inoculated plants.
Fig 1. Symptoms of bacterial stem blight at 7 days after inoculation.
(A) Response of resistant plant. (B) Stem of resistant plant at inoculation site. (C) Leaf of resistant plant at site of infiltration. (D) Response of susceptible plant. (E) Stem of susceptible plant at inoculation site. (F) Leaf of susceptible plant at site of infiltration. Arrows indicate point of inoculation.
To uncover molecular mechanisms behind observed disease resistance or susceptibility and identify genes expressed by host plants in response to infection, RNA extracted from alfalfa plants was subjected to Illumina RNA sequencing. Individual resistant and susceptible plants were selected using disease symptoms that developed later in the course of infection, after RNA samples were collected at 24 and 72 hai.
Transcriptome profiling of alfalfa plants in response to inoculation with PssALF3
The following hypotheses were tested: (1) host defense responses will be up-regulated in response to pathogen inoculation in resistant plants; and (2) host defense responses in susceptible plants will be suppressed and/or different from resistant plants upon inoculation.
A total of 6,176,469,338 pair-end reads were generated from 36 strand-specific cDNA libraries, averaging 171,568,593 reads per library (S1 Table). A total of 99.98% of all reads mapped to the reference CADL genome. Because cDNA libraries were generated using a poly(A) selection protocol, a minuscule number of bacterial transcripts mapped to the genome of PssALF3 (0.0017% of the total read counts) and were considered negligible. Therefore, the data obtained by RNA-seq were considered to be clean and sufficient for gene expression profiling.
Differentially expressed genes in ‘Maverick’ plants
Total counts of differentially expressed genes (DEGs) in Maverick plants at each time point (24 and 72 hai) are shown in S2 Table and quantitative estimates of DEGs in response to inoculation with PssALF3 are shown in Table 1. Four general observations can be made: (i) the number of DEGs at 72 hai is substantially larger than at 24 hai; (ii) the susceptible response generated more DEGs; (iii) the number of up-regulated DEGs in resistant plants is considerably higher at 72 hai than at 24 hai, and (iv) the proportion of DEGs up-regulated at 72 hai vs 24 hai in resistant plants is ~two-fold higher than in susceptible plants.
Table 1. Counts of differentially expressed genes in cv. Maverick.
| Time point | Susceptible/Mock | Resistant/Mock | ||||
|---|---|---|---|---|---|---|
| up | down | total | up | down | Total | |
| 24 hrs | 1,851 | 554 | 2,405 | 962 | 114 | 1,076 |
| 72 hrs | 2,098 | 985 | 3,083 | 2,079 | 583 | 2,662 |
| Unique | ||||||
| 24 | 760 | 427 | 1,187 | 321 | 108 | 429 |
| 72 | 1,007 | 858 | 1,865 | 1,438 | 577 | 2,015 |
| common | 1,091 | 127 | 1,218 | 641 | 6 | 647 |
To further investigate this quantitative variability in expression we identified unique and common DEGs at both time points in susceptible and resistant interactions and between susceptible and resistant plants. Between 24 and 72 hai the number of unique DEGs increased in both interactions, especially in resistant plants (more than a four-fold increase). If the defense response in resistant Maverick plants is at its maximum at 72 hai, these unique DEGs, particularly those up-regulated, are likely to include genes implicated in resistance pathways, whereas common DEGs potentially include genes implicated in a general response to infection (S3 Table).
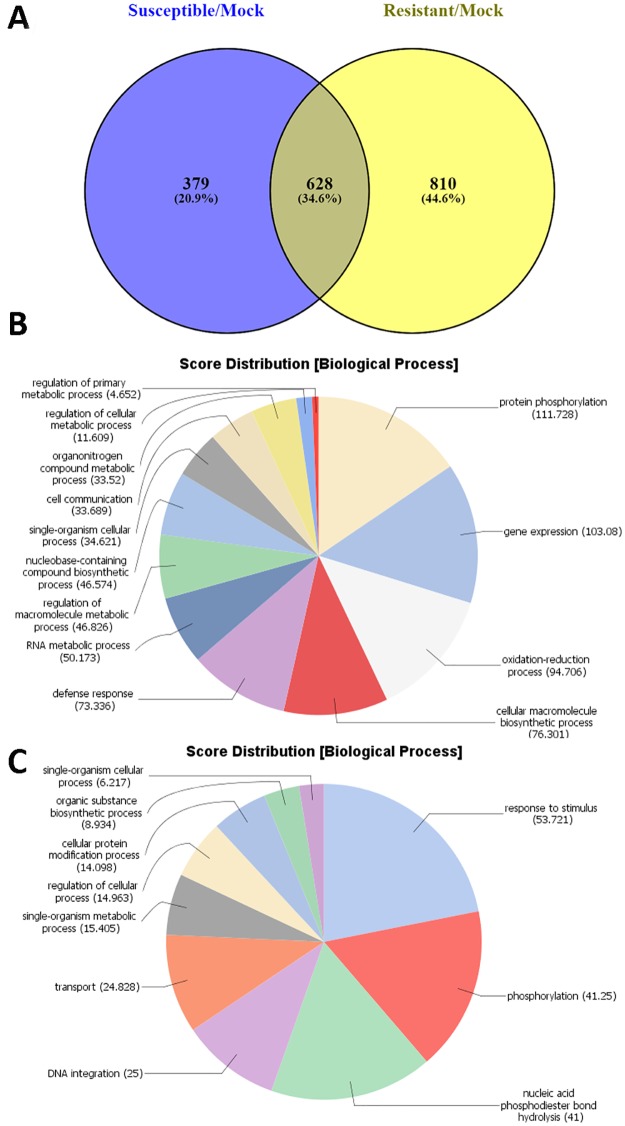
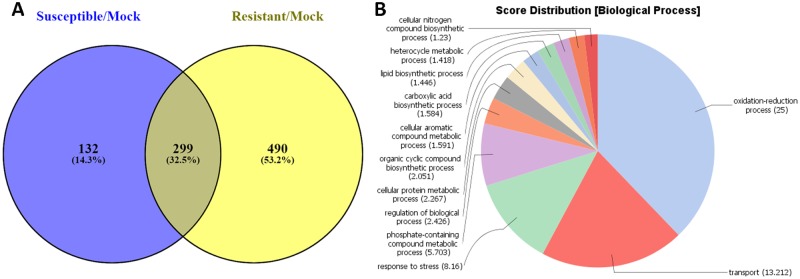
A comparison between the unique up-regulated genes at 72 hai in resistant/mock vs susceptible/mock interactions (1,438 DEGs vs 1,007 DEGs, respectively) (Table 1), identified 810 genes up-regulated only in resistant plants (Fig 2A and S4 Table). Based on the BLASTX hits and gene annotations, at least 56 (6.9%) out of these unique up-regulated DEGs in resistant plants represent genes putatively encoding disease resistance proteins (blastx E-value cut-off ≤ 1e-10). Most of them (~44 DEGs) encode major classes of plant resistance (R) genes. Approximately forty-seven DEGs (5.8%) are putative transcription factors (TFs); among them highly-induced TFs of WRKY (20), MYB (8) and NAC (7) families that are implicated in stress responses and developmental programs. A large group of DEGs (~82 genes, 10.1%) putatively encodes receptor-like kinases (RLK), a superfamily of proteins involved in elicitor perception and R gene mediated responses [10]. One of these (Medtr0602s0020.1), a likely ortholog of FLS2 (E-value = 0.0, identity 100%) encoding the receptor of flg22 (GeneBank accession XP_013441712), a component of bacterial flagellin and a potent elicitor of defense responses [11], was uniquely up-regulated at 72 hai in resistant plants. In addition, a substantial number of genes involved in isoflavonoid biosynthesis were found among 810 unique DEGs up-regulated in Maverick (S4 Table).
Fig 2. Differentially expressed genes (DEGs) in alfalfa plants from cultivar Maverick in response to inoculation with P. syringae pv. syringae ALF3.
(A) Venn diagram depicting the unique and common up-regulated DEGs between susceptible and resistant plants at 72 hours after inoculation. (B) Functional profiling of the unique genes up-regulated genes in resistant plants (810 DEGs) using the Blast2Go tool. (C) Functional profiling of the unique genes up-regulated genes in susceptible plants (379 DEGs).
While the sets of unique DEGs up-regulated in resistant and susceptible plants of Maverick at 72 hai (810 and 379 DEGs, respectively) include a number of tentative R genes, most members of the TIR-NBS-LRR class, only resistant plants had differentially expressed R genes of the CC-NBS-LRR (CNL) class (nine R genes, E-value = 0 for all). Both TIR-NBS-LRR and CNL proteins are involved in pathogen recognition, although their sequence and signaling pathways are different (McHale et al., 2006). The N-terminal domain of R proteins (TIR or CC) is important for interaction with different proteins, including TFs [12] and can define specificity of pathogen recognition [13].
We attempted to identify orthologs of several well-known resistance genes, including R genes against P. syringae in the 810 unique genes up-regulated in Maverick. The genes included RPM1 and RPS2 from Arabidopsis, Rpg1-b from soybean, and an ortholog for a rice disease resistance gene Xa21, conferring resistance to Xanthomonas oryzae pv. oryzae [10, 14,15,16]. The respective sequences were used as queries against the entire CADL genome using standalone BLAST. Because of the conserved motifs in R genes, the search yielded numerous hits with different gene IDs in our DEG sets. When only sequences with the lowest E-value, higher bit scores and percent identities were considered, three candidates were predicted as potential orthologs of RPM1, RPS2, and Xa21: Medtr5g027900.1 (log2 fold change = 5.1, E-value = 1E-139, identity 31.1%, bit score = 446), Medtr4g073840.1 (log2 fold change = 2.1, E-value = 9E-38, percent identity = 26.1%, bit score = 155), and Medtr8g470400.1 (log2 fold change = 2.64, E-value = 4E-156, bit score = 491, identity = 37%), respectively. Low E-values, significant percent identities, and high expression levels indicate that RPM1 and Xa21 orthologs may be involved in the resistance response to PssALF3 in Maverick plants. No putative orthologs were identified for Rpg1-b.
Functional categorization using the Blast2Go tool [17] supported roles of DEGs in disease resistance. The 810 DEGs from resistant plants were predominately in five categories of the Gene Ontology (GO) domain Biological Process: ‘defense responses,’ ‘gene expression,’ ‘protein phosphorylation,’ ‘oxidation-reduction process,’ and ‘cellular macromolecule biosynthetic process’ (Fig 2B). The functional category ‘defense response’ was absent from the 379 unique DEGs up-regulated in susceptible plants (Fig 2C).
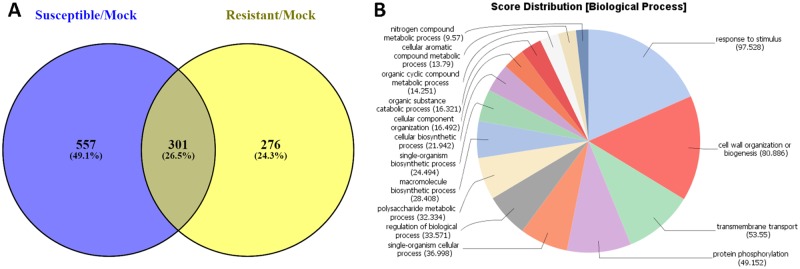
When unique down-regulated DEGs were compared between susceptible and resistant plants at 72 hai, 557 genes were found in the susceptible plants (Fig 3A). It is possible that down-regulation of these DEGs played a role in susceptibility to the infection, while common repressed genes between susceptible and resistant plants were not involved in the process. A list of the unique DEGs down-regulated in susceptible plants is shown in S5 Table. The unique down-regulated genes in susceptible plants were predominantly in the functional categories ‘response to stimulus,’ ‘cell wall organization and biogenesis,’ ‘transmembrane transport,’ and ‘protein phosphorylation’ (Fig 3B). GO term ‘response to stimulus’ include subcategories ‘response to biotic stimulus’ and ‘stress response.’ Down-regulation of the respective processes in susceptible plants may contribute to predisposition to infection.
Fig 3. Down-regulated differentially expressed genes (DEGs) in alfalfa plants from cultivar Maverick.
(A) Venn diagram depicting the unique and common down-regulated DEGs between susceptible and resistant plants at 72 hours after inoculation. (B) Functional profiling of the unique genes down-regulated in susceptible Maverick plants.
Differentially expressed genes in ‘ZG9830’ plants
Total counts of DEGs found in ZG9830 plants in response to inoculation with PssALF3 are shown in S6 Table. While the susceptible response was similar to that observed in susceptible Maverick plants, the reaction of resistant plants to the infection was characterized by increased numbers of DEGs at 24 hai and decreased numbers of DEGs at 72 hai (Table 2). Most of the DEGs up-regulated in the resistant response at 24 hai were unique (61%). Resistant responses to infection appear to occur more rapidly in the selected ZG9830 plants than in the Maverick plants and expressed genes may be important in development of host resistance (S7 Table).
Table 2. Counts of differentially expressed genes in cv. ZG9830.
| Time point | Susceptible/Mock | Resistant/Mock | ||||
|---|---|---|---|---|---|---|
| up | down | total | up | down | total | |
| 24 hrs | 2,106 | 522 | 2,628 | 2,731 | 830 | 3,561 |
| 72 hrs | 2,609 | 999 | 3,608 | 1,307 | 193 | 1,500 |
| Unique | ||||||
| 24 | 659 | 431 | 1,090 | 1,721 | 789 | 2,510 |
| 72 | 1,162 | 908 | 2,070 | 297 | 152 | 449 |
| common | 1,447 | 91 | 1,538 | 1,010 | 41 | 1,100 |
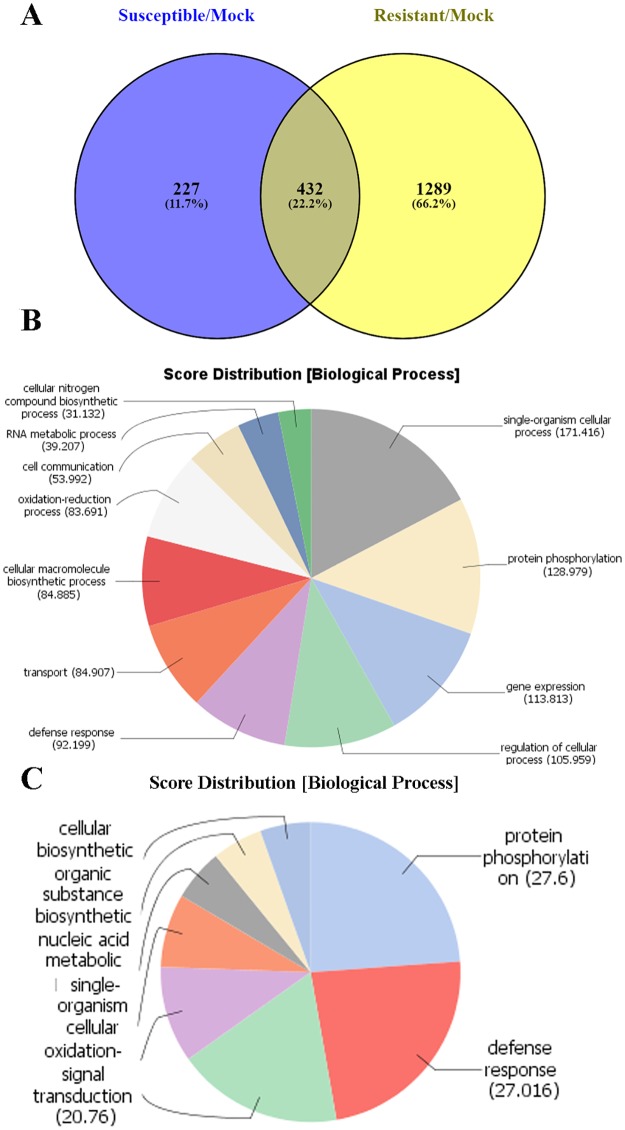
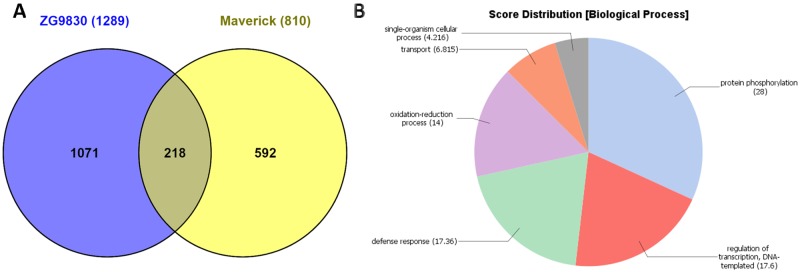
When we compared unique DEGs up-regulated at 24 hai in resistant and susceptible plants (1,721 and 659, respectively, in Table 2), 1,289 genes were induced only in resistant plants and 227 DEGs were induced only in susceptible plants (Fig 4A and S8 Table). At least 134 of the unique DEGs (10.3%) up-regulated in resistant plants at 24 hai represented genes putatively encoding R proteins. Of these, 83% (110 DEGs) belonged to the major classes of R genes, largely of the TIR-NBS-LRR class. Among up-regulated DEGs encoding putative R proteins in resistant plants, there were three nematode resistance HSPRO2-like proteins (E-values = 0, 0, and 1E-143), which were notably absent in the DEGs from susceptible plants. HSPRO2 has an imperfect LRR domain and lacks a nucleotide-binding site that is a characteristic feature of R proteins [18]. HSPRO2 was reported to interact with WRKY TFs positively regulating basal resistance in Arabidopsis against P. syringae pv. tomato [18]. It has also been previously suggested that Arabidopsis HSPRO2-like proteins could be implicated in a more general resistance process, not only nematode attack [19]. Also, TIR-containing genes lacking both NBS and LRR domains were up-regulated only in resistant ZG9830 plants. These TIR-unknown, or TX proteins, have been proposed to participate in plant basal defense responses [20]. Unlike the response in Maverick plants, R genes of the CNL type were up-regulated in both susceptible and resistant plants. Forty-nine DEGs (3.8%) encoded TFs, including members from the WRKY (13), MYB (8), AP2/ERF (6), and NAC (4) families. A substantial portion of the DEGs encoded receptor kinases (~140 genes, 10.8%). As in cv. Maverick, resistant plants of ZG9830 contained a group of up-regulated genes involved in the phenylpropanoid pathway leading to production of antimicrobial isoflavonoid phytoalexins (S8 Table) [21, 22].
Fig 4. Differentially expressed genes (DEGs) in alfalfa plants from cultivar ZG9830 in response to inoculation with P. syringae pv. syringae ALF3.
(A) Venn diagram depicting a number of unique and common up-regulated DEGs between susceptible and resistant plants at 24 hours after inoculation. (B) Functional categorization of the unique genes up-regulated in resistant plants. (C) Functional categorization of the unique DEGs induced in susceptible plants at 24 hai.
The same orthologous R genes (RPM1, Medtr5g027900.1 and RPS2, Medtr4g073840.1) that were predicted in Maverick plants were found in the set of unique 1,289 DEGs up-regulated in ZG9830 plants (S8 Table) as well as several tentative Xa21 orthologs. The best match to Xa21 was Medtr5g025890.1, (log2 fold change = 4.14) with an E-value = 0 and 46% percent identity. We were also able to identify a putative ortholog of the soybean resistance gene Rpg1-b [14, 23]: Medtr8g038570.1 (E-value = 0, identity 49.5%, bit score 1027 and log2 fold change = 2.16). Thus, several potential orthologs of previously identified genes involved in resistance to bacterial pathogens were identified in resistant alfalfa plants and may participate in defense reaction against PssALF3.
Functional categorization of the unique genes up-regulated in resistant ZG9830 plants (1,289 DEGs) revealed at least eight prominent categories in the GO term Biological Process: ‘single-organism cellular process,’ ‘protein phosphorylation,’ ‘gene expression,’ ‘regulation of cellular process,’ ‘defense responses,’ ‘transport,’ ‘cellular macromolecule biosynthetic process,’ and ‘oxidation-reduction process’ (Fig 4B). Three of these categories were underrepresented in resistant Maverick plants: ‘single-organism cellular process,’ ‘transport,’ and ‘regulation of cellular process.’ This might indicate a more quantitative type of disease resistance in ZG9380 plants as compared to Maverick plants, with a greater number of genes contributing to resistance in the selected ZG9380 plants [24, 25]. Functional characterization of the unique DEGs up-regulated in susceptible ZG9380 plants at 24 hai (227 DEGs) revealed four prevalent GO terms: ‘protein phosphorylation,’ ‘defense response,’ ‘signal transduction,’ and ‘oxidation’ (Fig 4C). Most likely, up-regulated DEGs from these categories are part of the basal resistance pathways that are effectively overcome by the pathogen [26].
Comparison of the unique down-regulated DEGs between resistant and susceptible ZG9830 plants at 24 hai (789 vs 431 DEGs, respectively) identified 132 genes in the susceptible response (Fig 5A and S9 Table). As in the case with Maverick plants, these down-regulated genes could play a role in susceptibility to the infection, whereas the 299 down-regulated genes in common between susceptible and resistant plants may not be involved in the infection process. Functional profiling of the unique genes down-regulated genes in susceptible plants (132 DEGs) identified three predominant categories: ‘oxidation reduction,’ ‘transport,’ and ‘response to stress’ (Fig 5B). Genes in these categories play key roles during plant acclimation to stress [27, 28].
Fig 5. Down-regulated differentially expressed genes (DEGs) in alfalfa plants from cultivar ZG9830.
(A) Venn diagram depicting a number of unique and common down-regulated DEGs between susceptible and resistant plants 24 hours after inoculation. (B) Functional categorization of the unique genes down-regulated genes in susceptible plants.
Comparison of the unique up-regulated DEGs from Maverick and ZG9830
To determine if the same genes participate in resistance responses in both cultivars, we compared unique DEGs up-regulated in Maverick and ZG9830 at 72 hai (810 DEGs) and 24 hai (1,289 DEGs). A total of 218 DEGs were in common (Fig 6A and S10 Table). Functional annotation of biological processes associated with these DEGs identified four prevalent GO terms, accentuating their roles in both genotypes: ‘protein phosphorylation,’ ‘regulation of transcription, DNA-templated,’ ‘defense response,’ and ‘oxidation-reduction process’ (Fig 6B). Among these common up-regulated DEGs were 20 R genes, including four of the CNL class. A putative homolog of the gene encoding the nematode resistance HSPRO2 protein was up-regulated in resistant plants of both cultivars. Common TFs included eight WRKY, five MYB, and five members of other TF families. Therefore, it appears that WRKY and MYB TFs have a dominant role in regulating disease resistance networks against P. syringae in both cultivars [29].
Fig 6. Comparison of differentially expressed genes (DEGs) in resistant plants from Maverick and ZG9830.
(A) Venn diagram depicting the number of common DEGs between resistant plants. (B) Functional categorization of biological processes associated with resistance responses to P. syringae in plants from cv. Maverick and cv. ZG9830.
Confirmation of the transcriptomic data by quantitative real-time PCR (qPCR)
qPCR was performed with 23 arbitrarily selected genes identified as differentially expressed based on analysis of the transcriptome (Table 3 and S11 Table). DEGs that were unique or common between the two cultivars were evaluated. qPCR data and the corresponding RNA-seq values were similar for all genes tested (Table 3). Importantly, up-regulation of genes encoding disease-resistance proteins was confirmed by qPCR.
Table 3. Confirmation of the transcriptomic data by quantitative real-time PCR.
| Cultivar | Gene ID/Medtr ID | Description [Medicago truncatula] | Primers | qPCR | RNA-seq |
|---|---|---|---|---|---|
| Log2-Fold Change | |||||
| Maverick | ID = g27133/Medtr0014s0220.1 | PIF1-like helicase | LN460-461 | -6.26 | -6.6 |
| Maverick | ID = g50748/Medtr2g079990.1 | NAC transcription factor-like protein | LN464-465 | 4.61 | 4.43 |
| Maverick | ID = g77527/Medtr5g065843.1 | Transmembrane protein, putative | LN468-469 | -8.63 | -6.61 |
| Maverick | ID = g80095/Medtr2g035150.1 | Disease-resistance response protein | LN472-473 | 9.91 | 8.5 |
| Maverick | ID = g85508/Medtr5g010640.1 | Pathogenesis-related thaumatin family protein | LN474-475 | 7.64 | 6.65 |
| Maverick | ID = g98659/Medtr1g067650.1 | C2H2 type zinc finger transcription factor family protein | LN476-477 | 8.18 | 7.17 |
| Maverick | ID = g124797/Medtr2g035150.1 | Disease-resistance response protein | LN486-487 | 1.05 | 8.9 |
| ZG9830 | ID = g5392/Medtr2g026710.1 | Nuclear transcription factor protein Y protein | LN452-453 | -2.56 | -4.08 |
| ZG9830 | ID = g20713/Medtr4g123990.1 | ABC transporter B family protein | LN458-459 | 7.38 | 8.41 |
| ZG9830 | ID = g134174/Medtr3g467270.1 | Salicylic acid carboxyl methyltransferase | LN492-493 | -1.22 | -4.03 |
| ZG9830 | ID = g8661/Medtr6g472230.1 | Disease resistance protein (TIR-NBS-LRR class), putative | LN500-501 | 6.52 | 6.01 |
| ZG9830 | ID = g79942/Medtr6g472230.1 | Disease resistance protein (TIR-NBS-LRR class), putative | LN502-503 | 5.11 | 5.82 |
| ZG9830 | ID = g91572/Medtr3g011390.1 | NBS-LRR disease resistance protein | LN504-505 | 3.93 | 3.08 |
| ZG9830 | ID = g102799/Medtr5g037700.1 | Disease resistance protein (TIR-NBS-LRR class) | LN506-507 | 3.49 | 2.28 |
| ZG9830 | ID = g107375/Medtr4g073840.1 | NBS-LRR type disease resistance protein | LN508-509 | 1.96 | 2.22 |
| ZG9830 | ID = g60077/Medtr1g090680.1 | LRR and NB-ARC domain disease resistance protein | LN466-467 | -2.31 | -3.37 |
| ZG9830 | ID = g79257/Medtr5g013440.1 | Expansin-B1-like protein | LN470-471 | -6 | -6.6 |
| ZG9830 | ID = g130558/Medtr5g095840.1 | Transmembrane protein, putative | LN490-491 | -4.67 | -5.4 |
| ZG9830 | ID = g139043/Medtr6g465530.1 | Dehydration-responsive element-binding protein | LN494-495 | -4.5 | -5.28 |
| ZG9830 | ID = g110113/Medtr4g080777.1 | Disease resistance protein (TIR-NBS-LRR class), putative | LN510-511 | 5.13 | 3.17 |
| ZG9830 | ID = g128367/Medtr8g020300.1 | Disease resistance protein (TIR-NBS-LRR class) | LN512-513 | 3.63 | 2.28 |
| ZG9830 | ID = g136515/Medtr4g018940.1 | Disease resistance family protein/LRR protein | LN514-515 | 3.87 | 2.71 |
| ZG9830 | ID = g150897/Medtr6g472230.1 | Disease resistance protein (TIR-NBS-LRR class), putative | LN516-517 | 7.06 | 5.63 |
Detection of simple sequence repeats (SSRs)
Simple sequence repeats can provide valuable information on genetic polymorphism between resistance and susceptible plants. We restricted the analysis of SSRs to a few unique up-regulated genes, which presumably play key roles in resistance to PssALF3 in each cultivar. We focused on nine up-regulated R genes of the CNL class and the Xa21 ortholog from Maverick plants and seven genes encoding TX and HSPRO-2-like proteins, and orthologs of the Xa21 and Rpg1-b genes from ZG9830. Mining of SSRs, including hexamers with a minimum number of repeats two in the selected genes resulted in 1,207 and 758 SSRs in Maverick and ZG9830, respectively. Data are presented in S12 Table.
Discussion
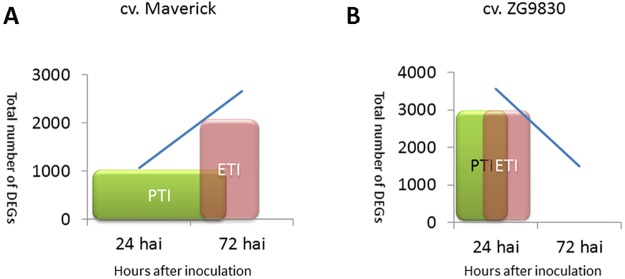
The interaction of P. syringae with plant hosts has been studied extensively and has informed much of the current understanding of the plant immune system [30, 31, 32, 33]. However, only a few studies on molecular interactions between P. syringae and different legume species have been described [15, 34, 35]. This is the first investigation of molecular responses of alfalfa to P. syringae. Here, we report that a highly pathogenic strain of P. syringae pv. syringae isolated from alfalfa caused transcriptional reprogramming of thousands of genes in susceptible and resistant genotypes of both cultivars used in this study. Although resistant plants from the two cultivars responded similarly to inoculation with a hypersensitive response at the site of inoculation, gene transcript profiling revealed that they respond differently at the molecular level (Tables 1 and 2) suggesting that resistance mechanisms are different between the two cultivars.
Two distinct but overlapping pathways of plant immune system have been previously defined: pattern-triggered immunity (PTI) and effector triggered immunity (ETI) [31, 32]. In PTI, conserved pathogen components, termed pathogen-associated molecular patterns (PAMPs) are recognized by host receptors, triggering a large transcriptional response. In susceptible plants, effectors produced by specialized pathogens can suppress PTI-based defenses and promote disease. In ETI, recognition of pathogen effectors by plant R proteins triggers the resistance response [31]. Up-regulated genes early in PTI and ETI are similar in Arabidopsis-P. syringae interactions but differ in magnitude with ETI having an earlier, stronger, and more extended response than PTI [36]. Similar sets of genes and patterns of gene expression occur in legumes responding to P. syringae with some exceptions. In soybean and M. truncatula, resistant plants have a strong and sustained up-regulation of genes in the phenylpropanoid pathway leading to production of isoflavonoid phytoalexins and there is a down-regulation of chloroplast related genes, which may favor production of reactive oxygen species involved in defense [35, 37]. Legumes also strongly up-regulate a group of pathogenesis-related proteins, named PR-10 proteins, with undefined functions in defense responses. While some of these host-pathogen responses were observed in our analysis of the alfalfa-PssALF3 interaction, many unique aspects were identified.
The observation of a hypersensitive response at the site of inoculation in resistant plants suggests that both cultivars utilize ETI, although temporally different. The transcriptomic responses of resistant ZG9830 plants indicate they may have a stronger and more rapid ETI than resistant Maverick plants, which had a later response to the infection with more differentially expressed genes at 72 hai compared to 24 hai (Table 1). In contrast, resistant plants from ZG9830 had more differentially expressed genes at 24 hai compared to 72 hai (Table 2). Whether resistance in ZG9830 is due to a quick and robust ETI response [38], a highly effective PTI-mediated signaling initiated early in host-pathogen interaction [31, 39], or a combination of ETI and PTI, is currently unclear. Although examples of strong PTI are rare, it can sometimes result in a hypersensitive response [38]. Data of expression of key resistance response genes from additional earlier and intervening time points are needed to confirm these observations.
Our analysis of DEGs in resistant ZG9830 plants identified a group of up-regulated genes with homology to known R genes that may be instrumental in resistance to PssALF3: four TIR-containing R genes lacking both NBS and LRR domains (TIR-unknown, or TX proteins [20], three up-regulated genes encoding nematode resistance HSPRO2-like proteins containing an imperfect LRR domain but no NBS [18], Medtr5g025890.1, encoding a putative ortholog for the rice Xa21 gene, and Medtr8g038570.1, a proposed ortholog of the soybean resistance gene Rpg1-b (Table 4). In a scenario in which ZG9830 primarily utilizes ETI, the observed up-regulation of DEGs orthologous to the Xa21 and Rpg1-b resistance genes could imply mechanisms similar to Xa21 signaling in rice, in which the R protein acts as a pathogen recognition receptor for the avrXa21 effector from Xanthomonas oryzae pv. oryza [10, 40] or to Rpg1-b signaling in soybean, where it mediates detection of the P. syringae pv. glycinea effector protein AvrB [15]. The up-regulation of genes encoding TX and HSPRO2-like proteins, that were proposed to participate in or positively regulate plant basal defense responses [18, 20] may signify the importance of PTI in resistant ZG9830 plants. The large number of functional categories of induced DEGs in resistant ZG9830 plants is indicative of a diverse type of resistance response that might be driven not by a single gene but rather by a contribution of multiple individual genes, each playing a role in the outcome [24, 25].
Table 4. Candidate genes involved in the resistance response against PssALF3 in two alfalfa cultivars.
| cv. Maverick | cv. ZG9830 | ||||
|---|---|---|---|---|---|
| Medtr ID | Log2 Fold Change | Description | Medtr ID | Log2 Fold Change | Description |
| Medtr5g027860.1 | 5.79 | CC-NBS-LRR | Medtr2g079950.1 | 7.90 | TIR-unknown (TX) |
| Medtr5g027900.1* | 5.15 | CC-NBS-LRR | Medtr2g079950.1 | 6.87 | TIR-unknown (TX) |
| Medtr5g027900.1* | 4.70 | CC-NBS-LRR | Medtr3g070230.1 | 3.59 | HSPRO2-like |
| Medtr8g079520.1 | 3.07 | CCR-NBS-LRR | Medtr0277s0020.3 | 2.76 | TIR-unknown (TX) |
| Medtr2g038510.1 | 2.74 | CC-NBS-LRR | Medtr5g092220.1 | 2.66 | TIR-unknown (TX) |
| Medtr5g018120.1 | 2.61 | CC-NBS-LRR | Medtr5g082150.1 | 2.42 | HSPRO2-like |
| Medtr2g038510.1 | 2.52 | CC-NBS-LRR | Medtr5g082150.1 | 2.08 | HSPRO2-like |
| Medtr5g027910.1 | 2.48 | CC-NBS-LRR | Medtr5g025890.1 | 4.14 | LRR-RLK |
| Medtr5g018210.1 | 2.29 | CCR-NBS-LRR | Medtr8g038570.1 | 2.16 | NBS-LRR |
| Medtr8g470400.1 | 2.64 | LRR-RLK | |||
*unique alfalfa genes that map to the same gene in the M. truncatula genome
In Maverick plants, the transcriptome analysis suggests that an early and relatively weak PTI does not contain the pathogen in either susceptible or resistant plants and is overpowered by secreted pathogen effectors [32]. In this scenario, one of the nine genes encoding a group of CNL proteins up-regulated at 72 hai in resistant plants could potentially be responsible for recognition of bacterial effectors and triggering an ETI response that contains the pathogen. Importantly, two of the nine CNL class genes encode atypical CCR domains [41], which alone are sufficient for induction of defense responses in Arabidopsis (Collier et al., 2011) [42], are strongly up-regulated in resistant Maverick plants at 72 hai (Table 4). The CCR domain in Medtr8g079520.1 and Medtr5g018210.1 resembles The CCR domain in the Arabidopsis RPW8 protein (locus RESISTANCE TO POWDERY MILDEW8) that mediates broad-spectrum mildew resistance [43]. Putative orthologs for the Arabidopsis RPM1 gene conferring resistance to P. syringae and for rice Xa21 gene, conferring resistance to Xanthomonas oryzae pv. oryzae [16], could also play a role in the resistance response in Maverick plants, although errors in identification of homologous genes remain a possibility due to highly conserved features in predicted candidate genes. Functional categorization of the unique DEGs up-regulated at 72 hai showed that susceptible Maverick plants lacked a GO category ‘defense response,’ whereas this term was one of the most prevalent in resistant plants.
A significant number of induced R genes found in both cultivars suggests possible roles in the PssALF3-alfalfa interaction. Regulation of the expression of R genes has been found to occur through transcriptional and post-transcriptional mechanisms to maintain the proper level to sufficiently activate resistance without having negative effects on fitness or plant growth [44, 45]. Transcripts of many R genes have been shown to accumulate in response to pathogen attack as well as abiotic stresses [46]. Defense-associated up-regulation of R genes contributes to PTI and ETI, may enhance potency of pathogen recognition, and has been observed in other plant species [44, 47].
Functional characterization of DEGs found in common in resistant plants of the two cultivars showed that four major biological processes predominated: ‘protein phosphorylation,’ ‘regulation of transcription,’ ‘defense response,’ and ‘oxidation-reduction.’ A substantial portion of the DEGs was associated with each of these categories (S9 Table). These four processes appear critical to plant defense signaling and activity against PssALF3 in alfalfa. Among differentially expressed TFs in the category ‘regulation of transcription,’ WRKY TFs appear to play a critical role in regulation of defense mechanisms against P. syringae in both cultivars. WRKY family TFs are involved in regulation of key constituents of the plant immune system including PTI, ETI, and systemic acquired resistance [48]. Redox signaling and production of reactive oxygen species also appear to be at the core of defense response in both cultivars, particularly in resistant Maverick plants (Fig 6A and 6B).
Conclusions
In summary, evaluation of resistant and susceptible responses to P. syringae pv. syringae showed that timing of the resistance response and candidate genes involved in resistance differed between the two cultivars. The development of a hypersensitive response and suppression of bacterial populations in plants categorized as resistant suggests the plants rely on ETI, particularly in the plants selected from cultivar ZG9830. The response of resistant plants from cultivar ZG9830 to the pathogen occurred earlier and may be coordinated by NBS-LRR, TX, and HSPRO2-like R proteins. Resistant plants from the cultivar Maverick responded later and R genes in the CNL class may be important in triggering resistance. Based on our interpretation of the data we propose a schematic representation of defense signaling in response to PssALF3 in plants of both alfalfa cultivars (Fig 7). Other interpretations of the data using different comparisons are possible and may reveal additional outcomes and conclusions. Additionally, our focus on selected components of the plant immune system may not reflect the complexity of this adaptive host-pathogen interaction. Notwithstanding, this study provides the first comprehensive study of the transcriptional response of alfalfa plants to P. syringae pv. syringae, an agriculturally important bacterial pathogen of alfalfa. Our results suggest that utilization of ZG9830 as a source of resistance for developing bacterial blight resistant cultivars may be more productive than use of Maverick as a source germplasm.
Fig 7. Hypothetical mechanisms of resistance to PssALF3.
(A) Resistant plants of cv. Maverick. (B) Resistant plants of cv. ZG9830. PTI, pathogen-associated molecular patterns (PAMP)-triggered immunity. ETI, effector-triggered immunity [31].
Methods
Plant material and bacterial inoculation
Resistant and susceptible alfalfa plants for transcript profiling were selected from cultivars Maverick [49] and ZG9830 [50]. To select plants, scarified seed were planted in a peat-based potting mix (Sungro LC8, SunGrow Horticultural Distribution, Agawam, MA) and grown for 5 weeks in the greenhouse at approximately 25°C with a 16 h photoperiod until they had formed four true leaves. The bacterial inoculum was prepared by culturing PssALF3 on King’s B agar for 2 days at 25°C. Cells were harvested in sterile distilled water and adjusted to an optical density OD600 = 0.1, approximately 1.5 x 108 colony forming units (CFU)/ml. The third internode above the cotyledons was wounded at a single site using a 22-gauge needle and the wound swabbed using a sponge moistened with the bacterial inoculum. After symptoms appeared, diseased material was removed and plants were allowed to re-grow. Vegetative clones of selected plants were made and re-tested for disease symptoms by stem inoculation, leaflet infiltration and determination of bacterial populations. Leaflets were infiltrated with the bacterial inoculum on the abaxial side using a needless syringe. To determine bacterial populations, inoculated internodes were scored for disease symptoms at 7 d after inoculation then excised using a sterile razor blade. These stem sections were placed in sterile water for 1 h and the resulting bacterial suspension used to make serial dilutions. Bacteria were cultured King’s B agar and CFU determined after 2 d incubation at 25°C. For transcript profiling, plants were inoculated at three sites per internode by wounding with a 22-gauge needle and swabbing with a suspension of PssALF3 at OD600 = 0.1. Mock-inoculated plants were wounded with a 22-gauge needle and swabbed with sterile water. Internodes were excised at 24 and 72 h after inoculation (hai), frozen immediately in liquid nitrogen, then stored at -80°C until used for RNA extraction.
RNA extraction and RNA-seq
Three resistant, three susceptible, and three mock-inoculated plants from cvs. Maverick and ZG9830 were chosen for transcript profiling at each time point. RNA was extracted using the RNAeasy Plant Mini Kit (Qiagen) and treated with DNase to remove any remaining DNA from total RNA samples. Purity and quantity of the samples was checked with a NanoDrop spectrophotometer (Thermo Scientific, USA) and Agilent 2100 BioAnalyzer. After total RNA was extracted from plants at 24 and 72 hai, they were maintained in the greenhouse to observe symptoms and classify plants as susceptible or resistant. RNA sequencing was performed by the Center for Computational Genomics, Next Generation Sequencing Center, Johns Hopkins University. cDNA libraries were generated using a poly (A) selection method and TruSeq RNA Library Preparation kit (Illumina, Inc.) Paired-end reads (2 x 150 bp) were generated using the Illumina HiSeq 2500 sequencing system. Four samples were pooled into each individual lane.
Read mapping, quantification, and functional analysis
The whole genome sequence of cultivated alfalfa at the diploid level (CADL, 2n = 2x = 16; CADL_HM342.v0.95P) was obtained from the Medicago HapMap project (http://www.medicagohapmap.org/home/view) and putative gene predictions were made using the AUGUSTUS (2.7) (http://augustus.gobics.de/) gene prediction tool. Gene IDs used in this manuscript were extracted from the AUGUSTUS output. The strand-specific paired-end reads were mapped onto the CADL gene predictions using Bowtie aligner version 2.2.9 (http://bowtie-bio.sourceforge.net/index.shtml). The paired-end reads were mapped using the –nofw and –norc parameters to best capture strand specific sequencing. Gene annotation was based on BLASTX hits with the M. truncatula genome database downloaded from the NCBI Genbank (https://www.ncbi.nlm.nih.gov/). The M. truncatula IDs (Medtr) were assigned using the BLASTX program with Mt4.0, which can be downloaded from the Medicago truncatula Genome Database (http://www.medicagogenome.org/home). The DESeq 2 package from Bioconductor [51] was used to estimate sample quality and expression level of the genes. The raw counts obtained from the Bowtie alignments were normalized using the DESeq 2 program that calculated the size factors of each library based on the raw counts. The size factors were calculated by dividing each column of raw counts by the geometric mean of the rows between comparisons. The median of these ratios was used as the size factor for the column. Genes with fold change more than 2, false discovery rate (FDR) less than 0.05, and number of mapped reads more than 50 were counted as a differentially expressed. The Blast2GO tool was used for functional categorization of differentially expressed genes [17].
The distribution scores in the Gene Ontology (GO) charts represent the sum of sequences directly or indirectly associated to a given GO category weighted by the distance of the category to the term of "direct annotation". It was computed by the software according to the formula
where seq is the number of different sequences annotated in a child GO term and dist is the distance to the node of the child term (https://biobam.atlassian.net/wiki/display/BFCD/Gene+Ontology+Graph+Visualization).
Verification of transcriptome data
Quantitative real-time PCR (qPCR) was performed with arbitrarily selected genes to confirm transcriptomic data. Primers were designed using the online Realtime PCR tool (Integrated DNA Technologies Inc., San Diego, USA; https://www.idtdna.com/scitools/Applications/RealTimePCR/) and alfalfa sequences generated in this work. cDNA for qPCR analyses was made using the SuperScript™ III First-Strand Synthesis System with oligo d(T) (ThermoFisher Scientific) and the same RNA samples that were used for RNA sequencing. Amplification was conducted with a Rotor Gene Q real time PCR cycler (Qiagen) using the Rotor-Gene SYBR® Green PCR kit (Qiagen) with three biological replicates using the following parameters: 95°C for 10 min (one cycle), 95°C for 10 s and 60°C for 45 s (40 cycles). The Delta Delta C(T) method (2−ΔΔCT) was used for analysis of relative expression [52]. To obtain a final ratio for any given gene, an average and a standard deviation for all biological replicates was calculated. The reference gene in all qPCR experiments was NP_001237047, a gene of unknown function with little variation in expression levels [53].
Detection of simple sequence repeats (SSR) markers
Identification of di-, tri-, tetra-, penta-, and hexanucleotide SSR loci, with a minimum repeat numbers of two was performed using the Simple Sequence Repeat Identification Tool (SSRIT, http://archive.gramene.org/db/markers/ssrtool).
Supporting information
(XLSX)
[Maverick-resistant 24 hrs: 1076 DEGs; Maverick-resistant 72 hrs: 2,662 DEGs; Maverick-susceptible, 24 hrs: 2,405; Maverick-susceptible, 72 hrs: 3,083].
(XLSX)
[Maverick-resistant 24 hrs: 429 DEGs; Maverick-resistant 72 hrs: 2,015 DEGs; Maverick-susceptible, 24 hrs: 1,187 DEGs; Maverick-susceptible, 72 hrs: 1.865 DEGs].
(XLSX)
(XLSX)
(XLSX)
[ZG9830-resistant 24 hrs: 3.561 DEGs; ZG9830-resistant 72 hrs: 1,500 DEGs; ZG9830-susceptible, 24 hrs. 2,628; ZG9830-susceptible, 72 hrs: 3,608].
(XLSX)
[ZG9830-resistant 24 hrs: 2,461 DEGs; ZG9830-resistant 72 hrs: 400 DEGs; ZG9830-susceptible, 24 hrs: 1,090 DEGs; ZG9830-susceptible, 72 hrs: 2,070 DEGs].
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We acknowledge the excellent technical assistance of Melinda Dornbusch and Susan Miller in plant maintenance and RNA isolation. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Data Availability
The data reported in this study were submitted to the NCBI’s sequence read archive (SRA), BioProject number PRJNA407947.
Funding Statement
This work was supported by funds from USDA-ARS-CRIS projects 8042-21000-268-00D and 5062-12210-002D.
References
- 1.Putnam DH, Russelle M, Orloff S, Kuhn J, Fitzhugh L, Godfrey L, et al. Alfalfa, wildlife and the environment—the importance and benefits of alfalfa in the 21st century. Novato, CA: California Alfalfa and Forage Association; 2001. [Google Scholar]
- 2.Harighi B. Occurrence of alfalfa bacterial stem blight diseases in Kurdistan Province, Iran. J Phytopathol. 2007;155:593–595. [Google Scholar]
- 3.Gray FA, Hollingsworth CR. Bacterial stem blight In: Samac DA, Rhodes LH, and Lamp WO, editors. Compendium of Alfalfa Diseases and Pests, Third Edition St. Paul, MN: APS Press, 2015. p. 61–62. [Google Scholar]
- 4.Young JM. Taxonomy of Pseudomonas syringae. J Plant Pathol. 2010;92:S1.5–S1.14. [Google Scholar]
- 5.Morris CE, Monteil CL, Berge O. The life history of Pseudomonas syringae: Linking agriculture to earth system processes. Annu Rev Phytopathol. 2013;51:85–104. doi: 10.1146/annurev-phyto-082712-102402 [DOI] [PubMed] [Google Scholar]
- 6.Alfano JR, Collmer A. Bacterial pathogens in plants: Life up against the wall. Plant Cell. 1996;8:1683–1698. doi: 10.1105/tpc.8.10.1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quirino BF, Bent AF. Deciphering host resistance and pathogen virulence: the Arabidopsis/Pseudomonas interaction as a model. Mol Plant Pathol. 2003; 4:517–530. doi: 10.1046/j.1364-3703.2003.00198.x [DOI] [PubMed] [Google Scholar]
- 8.Samac DA, Studholme DJ, Ao S. Characterization of the bacterial stem blight pathogen of alfalfa, Pseudomonas syringae pv. syringae ALF3. Phytopathology. 2014;104:102. [Google Scholar]
- 9.Harrison J, Dornbusch MR, Samac DA, Studholme DJ. Draft genome sequence of Pseudomonas syringae pv. syringae ALF3 isolated from alfalfa. Genome Announc. 2016;4:e01722–15. doi: 10.1128/genomeA.01722-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff KE, Ramonell KM. The role and regulation of receptor-like kinases in plant defense. Gene Regul Syst Bio. 2007;1:167–175. [PMC free article] [PubMed] [Google Scholar]
- 11.Bent AF, Mackey D. Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol. 2007;45:399–436. doi: 10.1146/annurev.phyto.45.062806.094427 [DOI] [PubMed] [Google Scholar]
- 12.Loutre C, Wicker T, Travella S, Galli P, Scofield S, Fahima T, et al. Two different CC-NBS-LRR genes are required for Lr10-mediated leaf rust resistance in tetraploid and hexaploid wheat. Plant J. 2009;60:1043–1054. doi: 10.1111/j.1365-313X.2009.04024.x [DOI] [PubMed] [Google Scholar]
- 13.McHale L, Tan X, Koehl P, Michelmore RW. Plant NBS-LRR proteins: adaptable guards. Genome Biol. 2006;7:212 doi: 10.1186/gb-2006-7-4-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashfield T, Keen NT, Buzzell RI, Innes RW. Soybean resistance genes specific for different Pseudomonas syringae avirulence genes are allelic, or closely linked, at the RPG1 locus. Genetics. 1995;141:1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashfield T, Redditt T, Russell A, Kessens R, Rodibaugh N, Galloway L, et al. Evolutionary relationship of disease resistance genes in soybean and Arabidopsis specific for the Pseudomonas syringae effectors AvrB and AvrRpm1. Plant Physiol. 2014;166: 235–251. doi: 10.1104/pp.114.244715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. [DOI] [PubMed] [Google Scholar]
- 17.Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray SL, Ingle RA, Petersen LN, Denby KJ. Basal resistance against Pseudomonas syringae in Arabidopsis involves WRKY53 and a protein with homology to a nematode resistance protein. Mol Plant-Microbe Interact. 2007;20:1431–1438. doi: 10.1094/MPMI-20-11-1431 [DOI] [PubMed] [Google Scholar]
- 19.Gissot L, Polge C. Jossier M. Girin T, Bouly JP, Kreis M, et al. AKINβγ contributes to SnRK1 heterotrimeric complexes and interacts with two proteins implicated in plant pathogen resistance through its KIS/GBD sequence. Plant Physiol. 2006;142:931–944. doi: 10.1104/pp.106.087718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandety RS, Caplan JL, Cavanaugh K, Perroud B, Wroblewski T, Michelmore RW, et al. The role of TIR-NBS and TIR-X proteins in plant basal defense responses. Plant Physiol. 2013;162:1459–1472. doi: 10.1104/pp.113.219162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreyra MLF, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012;3:222 doi: 10.3389/fpls.2012.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106 [DOI] [PubMed] [Google Scholar]
- 23.Ashfield T, Bocian A, Held D, Henk AD, Marek LF, Danesh D, et al. Genetic and physical localization of the soybean Rpg1-b disease resistance gene reveals a complex locus containing several tightly linked families of NBS-LRR genes Mol. Plant-Microbe Interact. 2003;16:817–826. [DOI] [PubMed] [Google Scholar]
- 24.Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ. Shades of gray: the world of quantitative disease resistance. Trends Plant Sci. 2009; 14:21–29. doi: 10.1016/j.tplants.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 25.Toueni M, Ben C, Le Ru A, Gentzbittel L, Rickauer M. Quantitative resistance to Verticillium wilt in Medicago truncatula involves eradication of the fungus from roots and is associated with transcriptional responses related to innate immunity. Front Plant Sci. 2016; 7:1431 doi: 10.3389/fpls.2016.01431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niks RE, Marcel TC. Nonhost and basal resistance: how to explain specificity? New Phytol. 2009;182:817–828. doi: 10.1111/j.1469-8137.2009.02849.x [DOI] [PubMed] [Google Scholar]
- 27.Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299 [DOI] [PubMed] [Google Scholar]
- 28.Conde A, Chaves MM, Gerós H. Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol. 2011;52:1583–1602. doi: 10.1093/pcp/pcr107 [DOI] [PubMed] [Google Scholar]
- 29.Pandey SP, Somssich IE. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009; 150:1648–1655. doi: 10.1104/pp.109.138990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006; 124:803–814. doi: 10.1016/j.cell.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 31.Jones JD, Dangl JL. The plant immune system. Nature 2006;444:323–329. doi: 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- 32.Katagiri F, Tsuda K. Understanding the plant immune system. Mol Plant-Microbe Interact. 2010; 23:1531–1536. doi: 10.1094/MPMI-04-10-0099 [DOI] [PubMed] [Google Scholar]
- 33.Xin XF, He SY. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol. 2013;251:473–498. [DOI] [PubMed] [Google Scholar]
- 34.Arnold DL, Lovell HC, Jackson RW, Mansfield JW. Pseudomonas syringae pv. phaseolicola: from ‘has bean’ to supermodel. Mol Plant Pathol. 2011;12:617–627. doi: 10.1111/j.1364-3703.2010.00697.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bozsó Z, Maunoury N, Szatmari A, Mergaert P, Ott PG, Zsíros LR, et al. Transcriptome analysis of a bacterially induced basal and hypersensitive response of Medicago truncatula. Plant Mol Biol. 2009; 70:627–646. doi: 10.1007/s11103-009-9496-8 [DOI] [PubMed] [Google Scholar]
- 36.Tsuda K, Katagiri F. Comparing signaling mechanisms engaged in pattern triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010; 13, 459–465. doi: 10.1016/j.pbi.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 37.Zou J, Rodriguez-Zas S, Aldea M, Li M, Zhu J, Gonzalez DO, et al. Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol Plant-Microbe Interact. 2005;18:1161–1174. doi: 10.1094/MPMI-18-1161 [DOI] [PubMed] [Google Scholar]
- 38.Thomma BP, Nürnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 2011;23:4–15. doi: 10.1105/tpc.110.082602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grennan AK. Plant response to bacterial pathogens. Overlap between innate and gene-for-gene defense response. Plant Physiol. 2006;142:809–811. doi: 10.1104/pp.106.900207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SW, Han SW, Bartley LE, Ronald PC. Unique characteristics of Xanthomonas oryzae pv. oryzae AvrXa21 and implications for plant innate immunity. Proc Natl Acad Sci USA. 2006;103:18395–18400. doi: 10.1073/pnas.0605508103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones JD, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science 2016;354: aaf6395 doi: 10.1126/science.aaf6395 [DOI] [PubMed] [Google Scholar]
- 42.Collier SM, Hamel LP, Moffett P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol Plant-Microbe Interact. 2011;24:918–931. doi: 10.1094/MPMI-03-11-0050 [DOI] [PubMed] [Google Scholar]
- 43.Xiao S, Ellwood S, Calis O, Patrick E, Li T, Coleman M, Turner JG. Broad-spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Science. 2001;291:118–120. doi: 10.1126/science.291.5501.118 [DOI] [PubMed] [Google Scholar]
- 44.Lai Y, Eulgem T. Transcript-level expression control of plant NLR genes. Mol Plant Pathol. 2017. doi: 10.1111/mpp.12607 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Kapos P, Zhang Y. NLRs in plants. Curr Opin Immunol. 2015;32:114–121. doi: 10.1016/j.coi.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 46.MacQueen A, Bergelson J. Modulation of R-gene expression across environments. J Exp Bot. 2016;67:2093–2105. doi: 10.1093/jxb/erv530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohr TJ, Mammarella ND, Hoff T, Woffenden BJ, Jelesko JG, McDowell JM. The Arabidopsis downy mildew resistance gene RPP8 is induced by pathogens and salicylic acid and is regulated by W box cis elements. Mol Plant-Microbe Interact. 2010;23:1303–1315. doi: 10.1094/MPMI-01-10-0022 [DOI] [PubMed] [Google Scholar]
- 48.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 49.Moutray JB. Maverick Alfalfa. Crop Sci. 1983;23:801. [Google Scholar]
- 50.McCaslin M, Woodward T, Undersander D. Winter survival. In: Standard tests to characterize alfalfa cultivars. North American Alfalfa Improvement Conference. 2004. https://www.naaic.org/resource/stdtests.php. Accessed 21 Sept 2017.
- 51.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106 doi: 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 53.Postnikova OA, Shao J, Nemchinov LG. Analysis of the alfalfa root transcriptome in response to salinity stress. Plant Cell Physiol. 2013;54:1041–1055. doi: 10.1093/pcp/pct056 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
[Maverick-resistant 24 hrs: 1076 DEGs; Maverick-resistant 72 hrs: 2,662 DEGs; Maverick-susceptible, 24 hrs: 2,405; Maverick-susceptible, 72 hrs: 3,083].
(XLSX)
[Maverick-resistant 24 hrs: 429 DEGs; Maverick-resistant 72 hrs: 2,015 DEGs; Maverick-susceptible, 24 hrs: 1,187 DEGs; Maverick-susceptible, 72 hrs: 1.865 DEGs].
(XLSX)
(XLSX)
(XLSX)
[ZG9830-resistant 24 hrs: 3.561 DEGs; ZG9830-resistant 72 hrs: 1,500 DEGs; ZG9830-susceptible, 24 hrs. 2,628; ZG9830-susceptible, 72 hrs: 3,608].
(XLSX)
[ZG9830-resistant 24 hrs: 2,461 DEGs; ZG9830-resistant 72 hrs: 400 DEGs; ZG9830-susceptible, 24 hrs: 1,090 DEGs; ZG9830-susceptible, 72 hrs: 2,070 DEGs].
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The data reported in this study were submitted to the NCBI’s sequence read archive (SRA), BioProject number PRJNA407947.