Abstract
Purpose
To report a case of uveitic glaucoma with a congested optic disc where optical coherence tomography angiography (OCT-A) provided diagnostic utility in assessing glaucomatous damage but optical coherence tomography (OCT) alone had limited utility.
Observations
We report a case of a 33-year-old Caucasian female referred to the USC Roski Eye Institute for uncontrolled intraocular pressure (IOP) in the left eye. She was managed by an outside provider for 6 months, where her IOP ranged from 28 to 42 mm Hg in the left eye on maximally tolerated medical therapy. Her clinical exam was consistent with Herpes family trabeculitis, optic nerve congestion, and possible glaucomatous damage. Initial evaluation of the optic nerve by standard modalities (fundus exam and OCT) was limited by optic nerve congestion; however, OCT-A showed peripapillary hypoperfusion, as commonly observed in glaucomatous eyes. She underwent aqueous shunt implantation for elevated IOPs poorly controlled by medications.
Conclusions and importance
OCT-A can be a useful tool in the evaluation of glaucoma in instances where disc congestion masks both nerve excavation and retinal nerve fiber thinning normally seen on exam and on standard OCT of the optic nerve.
Keywords: Glaucoma, Optical coherence tomography angiography, Glaucoma drainage implant, Optic nerve perfusion
1. Introduction
The diagnosis of glaucoma is multifaceted and involves the consideration of several factors including intraocular pressure (IOP), central corneal thickness (CCT), cup-disc ratio (CDR), visual field defects, nerve fiber layer thickness, and ganglion cell-inner plexiform layer (GC-IPL) thickness using a number of modalities. Optical coherence tomography angiography (OCT-A) of the optic nerve head is a novel means of evaluating optic nerve and peripapillary microvasculature. Reduced disc and peripapillary perfusion are associated with glaucomatous optic neuropathy.1 We report the case of a patient where evaluation of glaucoma by conventional imaging modalities was limited by optic disc congestion, and OCT-A demonstrated greater ability to detect glaucomatous changes.
2. Case report
A 33-year-old Caucasian female was referred for uncontrolled IOP in the left eye. She reported gradual blurring of vision and pressure-like pain in her left eye over the course of 1–2 years with more noticeable progression in the six months prior to presentation. The patient had been managed by an outside optometrist for the last six months where her maximum IOP was 42 mm Hg, decreased to 26 mm Hg briefly on maximally tolerated medical therapy, but then increased to 39 mm Hg prior to referral. Previous treatments included various combinations of travoprost, bimatoprost, timolol, brinzolamide/brimonidine, and brimonidine/timolol. At the time of our examination, the patient was actively using travoprost. She reported having had a recent MRI brain, which was negative for any mass lesions or other abnormalities. Her medical history was notable only for aphthous ulcers. Social history was unremarkable, and there was no family history of glaucoma.
The patient's best-corrected visual acuity was 20/20 in each eye. IOP was 12 and 35 mm Hg and CCT was 537 and 542 μm in the right and left eye, respectively. There was a mild relative afferent pupillary defect in the left eye. Color plates, red saturation, brightness saturation, and extraocular movements were normal bilaterally. Slit lamp examination was normal in the right eye and remarkable for endothelial pigment suggestive of old stellate keratic precipitates, 1 + flare, and rare cell in the left eye. Gonioscopy demonstrated open angles in both eyes (D40r1+ in all quadrants in the right eye and D40r1-2+ in the left eye by Spaeth grading).
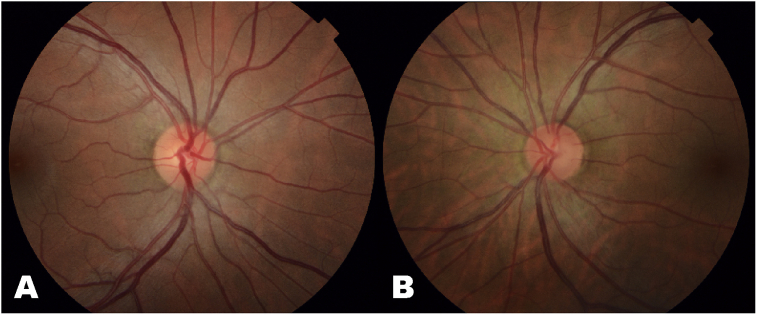
The optic nerve exam of the right eye was normal with a 0.4 CDR. The left eye revealed a congested disc with prominent venous pulsations. Despite the nerve's congested appearance, subtle excavation of the inferior neuroretinal rim was appreciable on careful stereoscopic exam, and the CDR was approximated to be 0.55. On undilated, non-stereoscopic exam, this excavation could not be appreciated. Mild swelling of the surrounding retinal nerve fiber layer (RNFL) and gliotic changes without active disc edema were also appreciated (Fig. 1). The macula, vessels, and periphery were within normal limits in both eyes.
Fig. 1.
Fundus photographs of the right (A) and left (B) eyes of the patient with Herpes trabeculitis. The right eye (A) is normal compared to the left eye (B) which demonstrates gliosis of the neuroretinal rim and surrounding retinal nerve fiber layer. On stereoscopic fundus exam, there was subtle excavation of the inferior neuroretinal rim appreciated in the left eye, but this is difficult to appreciate on fundus photography.
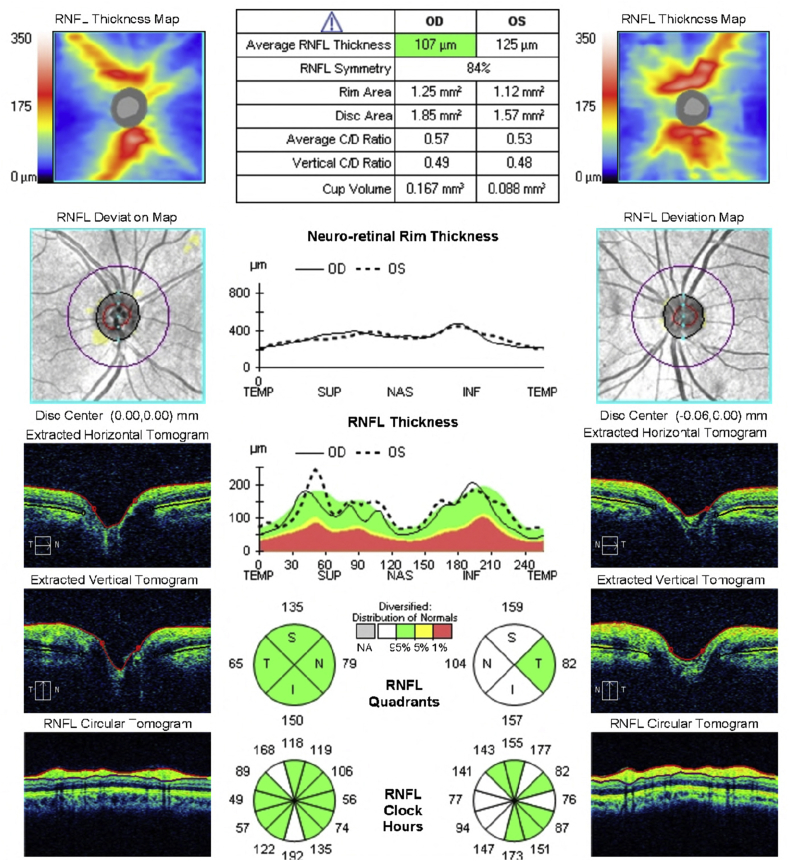
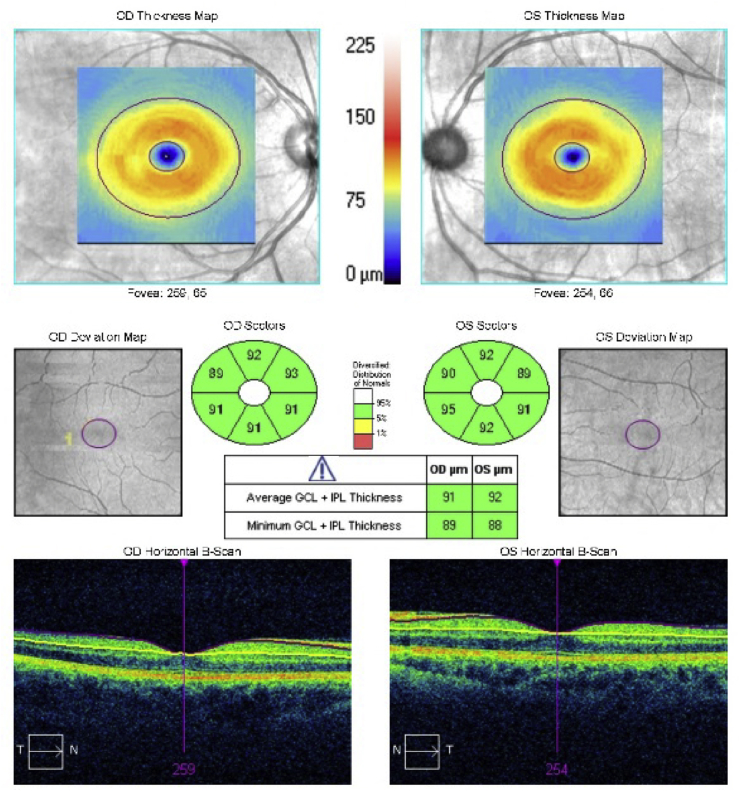
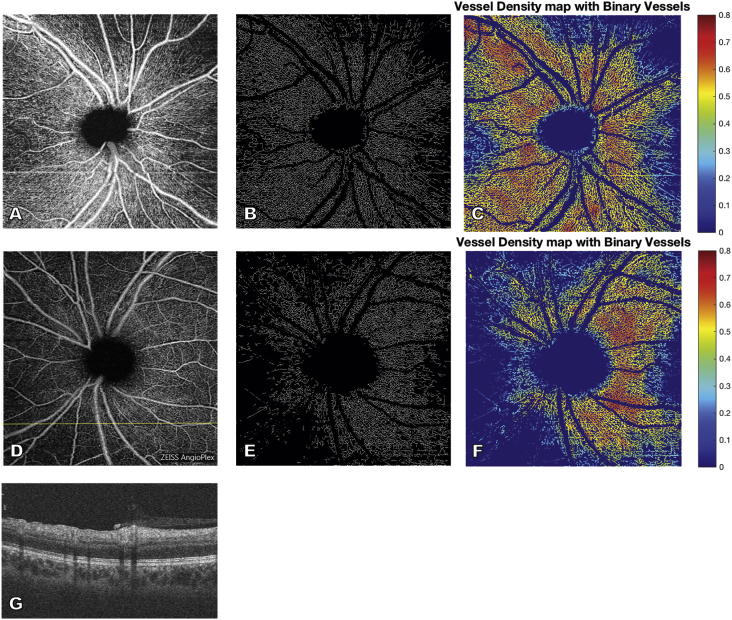
Humphrey visual field 24–2 examination demonstrated a normal visual field in the right eye and an early superior arcuate defect and nasal defects in the left eye (Fig. 2). Optical coherence tomography (OCT) suggested thickening of the RNFL in the left eye (Fig. 3) with preservation of the ganglion cell complex (GCC) in both eyes (Fig. 4). The RNFL thickening was suspected to be due to the gliosis seen on exam, so OCT-A using a 6 mm × 6 mm scanning protocol was obtained to assess for glaucomatous damage. Using prototype software, semiautomatic segmentation of the RNFL layer was performed to generate an en face image of vasculature in the RNFL layer in the peripapillary area (also termed the “radial peripapillary capillaries”).2 The peripapillary vasculature was quantified using prototype software by calculating vessel skeleton density (i.e. capillary density), vessel area density, and flux (i.e. flow signal intensity of the peripapillary area) in an annulus with an outer diameter of 5.6mm and an inner diameter determined by the optic nerve head border. Visual examination of these en face images qualitatively demonstrated a decrease in global peripapillary vessel density and flux in the left eye compared to the right eye (Fig. 5).
Fig. 2.
Humphrey visual field examination from initial evaluation. Right eye showed excellent reliability while left eye showed good reliability. The right eye had a normal visual field result while the left eye had an early superior arcuate and inferior nasal defect.
Fig. 3.
Optical coherence tomography of the retinal nerve fiber layer (RNFL) showing normal average RNFL thickness in the right eye and thickening of the RNFL in the inferior, superior, and nasal quadrants in the left eye.
Fig. 4.
Optical coherence tomography of the ganglion cell complex (GCC) demonstrating normal thickness of the ganglion cell layer in both eyes.
Fig. 5.
En face optical coherence tomography angiography image of the radial peripapillary capillaries of the retinal nerve fiber layer (RNFL) of the right (A) and left (D) eye demonstrate a reduction in peripapillary perfusion in the left eye compared to the right. Vessel skeleton maps and vessel area density maps of the peripapillary vasculature of the right (B, C) and left (E, F) eye demonstrate decreased vessel density in the left eye compared to the right, consistent with glaucomatous damage in the left eye. B-scan of the left eye (G) at the location demarcated by the yellow horizontal line in D demonstrates thickening in the region of the RNFL (due to gliosis) in regions of poor perfusion. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Briefly, to calculate vessel skeleton density, large vessels were blacked out and all remaining vessels were skeletonized to a one pixel width; vessel skeleton density was calculated as the proportion of pixels taken up by this skeletonized OCT-A signal and represents density of the radial peripapillary capillaries in the RNFL layer. Vessel area density was the proportion of pixels taken up by any OCT-A signal from the binarized image with large vessels excluded. Flux was determined as the average of normalized flow intensity values over the peripapillary area.1, 3 Vessel skeleton density, vessel area density, and flux were reduced in the left eye compared to the right eye (Table 1). Quadrantal analysis of these same indices in the inferior, superior, nasal, and temporal quadrants demonstrated a pronounced decrease in vessel skeleton density and vessel area density in the nasal quadrant and modest decrease in the inferior quadrant of the left eye when compared to the right eye (data not shown).
Table 1.
Quantification of vascular parameters obtained by optical coherence tomography angiography demonstrating decreased vessel area density, vessel skeleton density, and flux in the left eye compared to the right eye.
| OD | OS | |
|---|---|---|
| Vessel Area Density | 0.423 | 0.309 |
| Vessel Skeleton Density | 0.188 | 0.130 |
| Flux | 0.281 | 0.222 |
The patient was started on prednisolone acetate QID, valacyclovir 1000mg TID, and acetazolamide extended-release 500mg BID for presumptive herpetic trabeculitis and IOP control. Travoprost was discontinued due to the risk of increasing inflammation. The patient was seen by the neuro-ophthalmology service to rule out non-glaucomatous optic neuropathy, and they agreed that this patient had secondary glaucoma in the left eye with concurrent disc “hyperemia” due to her underlying uveitic etiology. In her subsequent visits, IOP fluctuated significantly—from 12 mm Hg at 3 weeks following initial presentation to 33 mm Hg on dorzolamide-timolol, brimonidine, and acetazolamide at 8 weeks after presentation, despite the fact that the anterior chamber inflammation had resolved and prednisolone was tapered. A uveitic work-up was completed and serologies resulted positive only for CMV and VZV IgG. Negative labs included: CMV IgM, VZV IgM, HSV1/2 IgM and IgG, HIV, RPR, FTA-ABS, and Quantiferon. The patient underwent placement of an Ahmed glaucoma valve and valacyclovir was subsequently tapered to 500mg BID. Postoperative IOP improved following glaucoma valve implantation.
3. Discussion
The clinical evaluation of glaucoma involves assessment of the patient's history, IOP, corneal thickness, visual field changes, and optic nerve properties on fundus examination and by OCT.4 The culmination of these indices can be used to make the diagnosis, monitor for progression of glaucoma, and guide treatment.5, 6 RNFL thinning is predictive of visual field loss in glaucoma patients.7 Ganglion cell-inner plexiform layer (GC-IPL) and ganglion cell complex (comprised of the RNFL and GC-IPL) thinning has been shown to sometimes precede changes in the RNFL and is a predictor of glaucomatous visual field loss in glaucoma suspects and early glaucoma.6, 8 The most sensitive RNFL parameter across multiple spectral domain OCT (SD-OCT) devices is the inferior sector with sensitivities between 0.66 and 0.80 and specificities between 0.88 and 0.94. GCC parameters are slightly less sensitive than RNFL parameters with highest sensitivities between 0.64 and 0.78 and specificities between 0.90 to 0.94.9 Nonetheless, evaluation by OCT is dependent on the ability to segment the retinal layers and assumes that no other conditions are affecting the thickness of the RNFL and GC-IPL or GCC. Pathological changes that distort the retinal architecture or that add volume to each layer, such as edema or gliosis, will affect the diagnostic ability of RNFL or GC-IPL thicknesses in diagnosing glaucoma.
OCT-A provides an additional noninvasive tool to evaluate a number of ocular diseases, including glaucoma. The loss of both the optic nerve and peripapillary microvascular network has been shown to accompany glaucomatous damage.10, 11, 12 Preperimetric glaucoma patients also demonstrated a significant reduction in optic nerve head perfusion compared to normal patients.13 With increasing disease severity, there is a correlated decrease in vessel density and perfusion.1, 14 Measurements of peripapillary flow and vessel density by OCT-A demonstrate high reproducibility and high correlation with visual field loss and glaucoma staging; in contrast, RNFL thickness does not correlate with visual field loss or glaucoma staging.15 In the presented patient, the history of chronic IOP elevation, an afferent pupillary defect in the absence of color desaturation, and characteristic visual field changes, taken together, were suggestive of glaucomatous damage. However, the optic nerve head in the left eye, because of the inflammatory gliotic changes, showed only subtle glaucomatous excavation that could have been easily missed on fundus exam. OCT of the RNFL was thickened in this eye and so was unhelpful and even misleading and could have caused some to conclude that there was an absence of glaucomatous damage. However, with OCT-A, it was possible to demonstrate hypoperfusion and loss of peripapillary vasculature, a finding characteristic and supportive of glaucomatous damage in light of this patient's clinical history. OCT-A was able to provide diagnostic utility in the diagnosis of glaucomatous damage in this patient, where optic nerve changes were masked by conventional imaging modalities.
4. Conclusions
To our knowledge, this is the first report of OCT-A imaging acquired during an acute, uveitic, glaucomatous episode. In uveitic patients, assessment of the optic nerve head for glaucomatous damage is difficult when optic disc congestion or gliosis of the RNFL is present. As in our case, disc congestion has been previously shown to alter measurements of the neuroretinal rim and peripapillary RNFL, giving elevated values of RNFL thickness by SD-OCT and increased rim area and volume by Heidelberg Retinal Tomograph (HRT).16 Such findings may give the false impression that there is an absence of glaucomatous damage.
This report demonstrates the limitations of conventional OCT imaging and clinical utility of OCT-A imaging in patients with a congested disc being assessed for glaucomatous damage. We still do not know if the reduced microvasculature of the disc and peripapillary area seen in glaucoma is simply the result of ganglion cell loss in glaucomatous optic neuropathy or whether reduced microvasculature and reduced ocular blood flow plays a causal role in the pathophysiology of glaucoma, as suggested by several studies.17 Regardless of what future longitudinal studies demonstrate regarding ocular blood flow and glaucoma, OCT-A is proving to serve an important role in glaucoma diagnosis, and its role may increase in the future as we determine additional unique roles that OCT-A can serve in glaucoma diagnosis.
Patient consent
Personal identifying information has been removed from this report because consent to publish such information was not obtained.
Acknowledgements and disclosures
Funding
Mentoring for the Advancement of Physician Scientists, American Glaucoma Society (GMR); Carl Zeiss Meditec (provided research imaging device).
Conflict of interest
The authors have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Bojikian K.D., Chen C.-L., Wen J.C. Optic disc perfusion in primary open angle and normal tension glaucoma eyes using optical coherence tomography-based microangiography. Vavvas D.G., editor. PLoS One. 2016;11(5):e0154691. doi: 10.1371/journal.pone.0154691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin X., Chao J.R., Wang R.K. User-guided segmentation for volumetric retinal optical coherence tomography images. J Biomed Opt. 2014;19(8):86020. doi: 10.1117/1.JBO.19.8.086020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C.-L., Bojikian K.D., Gupta D. Optic nerve head perfusion in normal eyes and eyes with glaucoma using optical coherence tomography-based microangiography. Quant Imaging Med Surg. 2016;6(2):125–133. doi: 10.21037/qims.2016.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinreb R.N., Aung T., Medeiros F.A. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon M.O., Beiser J.A., Brandt J.D. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol (Chic Ill 1960) 2002;120(6) doi: 10.1001/archopht.120.6.714. 714–720-30. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X., Loewen N., Tan O. Predicting development of glaucomatous visual field conversion using baseline fourier-domain optical coherence tomography. Am J Ophthalmol. 2015:29–37. doi: 10.1016/j.ajo.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu M., Lin C., Weinreb R.N. Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: a 5-year prospective study. Ophthalmology. 2016;123(6):1–10. doi: 10.1016/j.ophtha.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Shin H.Y., Park H.Y.L., Jung K.I., Choi J.A., Park C.K. Glaucoma diagnostic ability of ganglion cell-inner plexiform layer thickness differs according to the location of visual field loss. Ophthalmology. 2014;121(1):93–99. doi: 10.1016/j.ophtha.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Oddone F., Lucenteforte E., Michelessi M. Macular versus retinal nerve fiber layer parameters for diagnosing manifest glaucoma: a systematic review of diagnostic accuracy studies. Ophthalmology. 2016;123(5):939–949. doi: 10.1016/j.ophtha.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Lévêque P., Zéboulon P., Brasnu E., Baudouin C., Labbé A. Optic disc vascularization in glaucoma: value of spectral-domain optical coherence tomography angiography. J Ophthalmol. 2016;2016:6956717. doi: 10.1155/2016/6956717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia Y., Wei E., Wang X. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322–1332. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelson G., Langhans M.J., Groh M.J. Perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open angle glaucoma. J Glaucoma. 1996;5(2):91–98. [PubMed] [Google Scholar]
- 13.Jia Y., Morrison J.C., Tokayer J. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3(12):3127–3137. doi: 10.1364/BOE.3.003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Jiang C., Ko T. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefe’s Arch Clin Exp Ophthalmol. 2015;253(9):1557–1564. doi: 10.1007/s00417-015-3095-y. [DOI] [PubMed] [Google Scholar]
- 15.Liu L., Jia Y., Takusagawa H.L. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015:4197. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinz C., Kogelboom K., Heiligenhaus A. Influence of optic disc leakage on objective optic nerve head assessment in patients with uveitis. Graefe’s Arch Clin Exp Ophthalmol. 2016;254(2):361–364. doi: 10.1007/s00417-015-3218-5. [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa T. Ocular blood flow and influencing factors for glaucoma. Asia-Pacific J Ophthalmol Phila Pa) 2016;5(1):38–44. doi: 10.1097/APO.0000000000000183. [DOI] [PubMed] [Google Scholar]