Abstract
This brief review assesses the role of Ca2+ signaling in lung endothelium in regulation of endothelial permeability. The disconnect between experimental and clinical outcomes to date may be due, in part, to the use of tools which yield information about aggregate permeability or Ca2+ responses in lung or in endothelial monolayers. The teaching point of this review is to “unpack the box,” i.e. consider the many potential issues which could impact interpretation of outcomes. These include phenotypic heterogeneity and resultant segment-specific permeability responses, methodologic issues related to permeability measures, contributions from Ca2+ channels in cells other than endothelium—such as alveolar macrophages or blood leukocytes), Ca2+ dynamic patterns, rather than averaged Ca2+ responses to channel activation, and the background context, such as changes in endothelial bioenergetics with sepsis. Any or all of these issues might color interpretation of permeability and Ca2+ signaling in lung.
Keywords: ARDS, acute respiratory distress syndromes and acute lung injury, calcium, endothelium
Introduction
While acute lung injury has been extensively investigated, clinical outcomes remain poor. Aside from low volume ventilation, strategies directed at single targets or downstream signaling pathways identified in preclinical studies on acute lung injury have been disappointing when translated to the clinical setting. The disconnect between experimental and clinical outcomes may be due, in part, to the persistent notion when interpreting experimental data that signaling molecules evoke consistent outcomes across the lung. Further clouding our lens for interpretation is the fact that we use tools which yield information about aggregate responses in lung or in endothelial monolayers when assessing the role of Ca2+ signaling in lung endothelium in regulation of endothelial permeability. These tools include measures of total lung wet–dry weight ratio, lung weight gain or the more specific filtration coefficient Kf to assess endothelial permeability in vivo,1 endothelial monolayer resistance to assess permeability in vitro,2–4 or measures of averaged Ca2+ transients to assess mechanisms regulating Ca2+ entry (Fig. 1).5,6
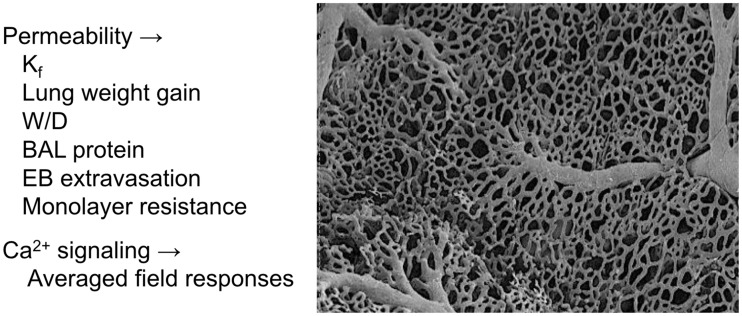
Fig. 1.
Measures designed to assess permeability and Ca2+ signaling in lung endothelium either in vivo or in vitro typically identify averaged outcomes. Permeability measures include the filtration coefficient (Kf) in isolated lung, lung weight gain at constant perfusion pressure, lung wet–dry weight ratio (W/D), protein content in bronchoalveolar lavage fluid (BAL), extravasation of Evan’s blue dye (EB) into lung tissue, and endothelial monolayer resistance.1 In each case, measures are averaged outcomes in the lung or endothelial monolayer as a whole. Similarly, Ca2+ imaging typically only assesses field averaged changes in amplitude over time.5,62 The scanning electron micrograph of a lung vascular corrosion cast (at the right) elucidates compartments in the lung vascular network, including arteries, capillaries, and veins; the network shown here is that at the pleural surface of the lung. We now know that endothelium in these compartments is phenotypically distinct,8,10 which may impact both basal permeability responses and those elicited by Ca2+ channel activation.
Averaged permeability measures may mask phenotypic heterogeneity in endothelium within specific lung vascular compartments.7–10 Similarly, averaged measures of Ca2+ responses will mask variability due to heterogeneity in Ca2+ channel expression or responsiveness even within one vascular compartment or one field of cells in vitro.5,11 While not explored in any detail here, we further need to be cognizant of our assumption that in vitro measures of Ca2+-dependent permeability responses in endothelial cell monolayers replicate or predict responses in the intact lung. Potential for plasticity in Ca2+ channel expression with cell passage,11 localization of Ca2+ channels in endothelium, and related localization of Ca2+-dependent intracellular mechanical forces and substrate stiffness12,13 could color outcomes in a heterogeneous way. To date, these latter issues have not been explored in any detail in lung endothelium.
This review discusses the potential impact of key issues on interpretation of experimental outcomes related to endothelial permeability and Ca2+ signaling: endothelial phenotypic heterogeneity; contributions not related to endothelial cell function; calcium microdomains; and altered context in sepsis. The key teaching point here is to “unpack the box.” In other words, do not necessarily interpret outcomes based on the aggregate response. We should question what’s inside that aggregate measure—in other words, what’s inside the box… and question whether the setting or context may modulate outcomes.
Lung endothelial Ca2+ signaling in heart failure
Historical perspective from our own work on lung endothelial barrier function in heart failure highlights the complexities inherent in interpreting permeability measures and Ca2+ signaling at the whole organ level. We started this work to try to understand adaptations that might allow individuals with heart failure to compensate for the chronic pulmonary venous hypertension, with increased propensity for pulmonary edema. As research will often do, the trail led us to adaptations in endothelial permeability related to Ca2+ signaling, with somewhat surprising outcomes.
In lung endothelium, store-operated channels participate in regulation of endothelial permeability. In cultured rat lung endothelial cells, thapsigargin (TG) or thrombin evoke store depletion, resulting in activation of store-operated channels, endothelial Ca2+ influx, loss of adherence junction integrity, and formation of inter-endothelial cell gaps.14–17 In the intact lung, TG or thrombin increase the filtration coefficient Kf, a measure of water permeability or hydraulic conductivity for the endothelial barrier.7,16,18 This process requires Ca2+ influx into endothelium through store-operated channels. We initially identified a loss of the angiotensin II-dependent permeability responses in lung after pacing-induced heart failure, a model of chronic pulmonary venous hypertension.19 As angiotensin II elicits store-dependent responses in normal endothelium, we used TG to bypass angiotensin II receptors and directly deplete stores in subsequent studies. We found that the increase in Kf associated with TG-induced store depletion is lost after development of chronic heart failure in both the pacing and AV fistula models.20,21 The consensus of more current work suggests that store-operated Ca2+ channels comprise TRPC1 and TRPC4 proteins, members of the canonical subfamily of transient receptor potential (TRP) proteins.22
In parallel, we were investigating the permeability response to high vascular pressure (HiPv) in lung and identified another TRP channel from the vanilloid family—TRPV4—as the target. TRPV4 is gated by mechanical stress, and in many in vitro models, epoxyeicosatrienoic acids or EETs (P450 epoxygenase-derived arachidonic acid metabolites) provide the link between mechanical stress and TRPV4 activation. We subsequently found that the increase in lung Kf with HiPv can be abrogated by pretreatment with a TRPV4 inhibitor or by inhibition of EET synthesis, and is lost in lungs from animals with genetic deletion of TRPV4.23,24 However, in contrast to the abrogation of the store-dependent permeability response in chronic heart failure, we found that the increased Kf response to 14,15-EET is retained.20 Note that Ca2+ influx via TRPV4 is key to the EET-induced increase in permeability.7
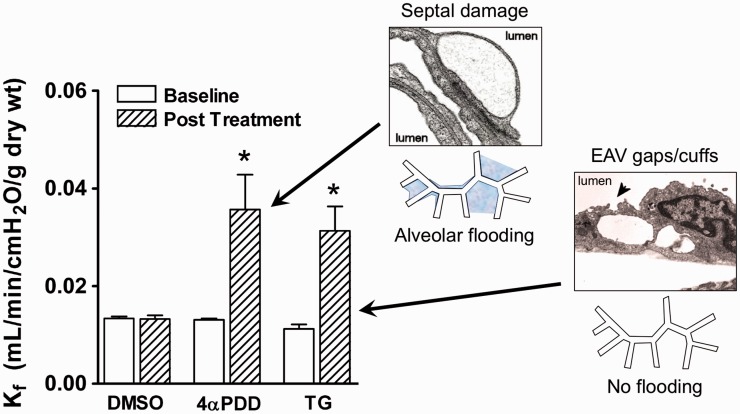
Initially, we assumed that the explanation for these disparate outcomes was simple, i.e. that heart failure led to differential downregulation of store-operated TRP channels but retention of TRPV4 expression. Indeed, we did confirm that the loss of the permeability response to store-depletion in heart failure was associated with downregulation of endothelial TRPC1 and TRPC4.20 In contrast, TRPV4 expression is retained in animals and humans with heart failure.20,24 However, this simple perspective was based in the notion that Kf assesses endothelial barrier function and that all endothelial cells are created equal… Not true. When we “unpacked” that Kf box, we found that the increase in permeability resulting from activation of these two Ca2+ channels was focused in distinct vascular compartments in the lung (Fig. 2). Activation of the store-operated TRPC1/4 channel with TG targeted the extra-alveolar compartment, resulting in the formation of inter-endothelial gaps and perivascular cuffs in extra-alveolar vessels (EAV).7,9 We saw no structural evidence of injury in septal capillaries, nor any evidence of alveolar flooding after activation of store-operated channels. In contrast, activation of TRPV4 with 4α-phorbol-12,13-didecanoate (4αPDD) selectively targeted alveolar septal capillaries. The increase in Kf elicited by TRPV4 activation was associated with injury and derangement of septal capillary endothelium and alveolar flooding.7 This segregation of injury unmasked clear evidence of phenotypic heterogeneity in lung endothelium, a concept that is now well documented.8–10,14,25 The corollary of this story is that increases in Kf or lung water may not be due to homogenous lung injury. Equally plausible explanations include segregated or targeted injury to one vascular compartment or merely increased hydrostatic pressures driving filtration.
Fig. 2.
Segmental permeability responses in lung. Although activation of TRPV4 with 4α-phorbol-12,13-didecanoate (4αPDD) and that of store-operated channels with TG elicits similar increases in the filtration coefficient (Kf) in isolated perfused lungs, these two channels have segment-specific injury patterns. TRPV4 selectively impacts alveolar septal capillaries, leading to derangement of septal endothelium and alveolar flooding. In contrast, TG has no impact in the alveolar septal compartment, but elicits development of inter-endothelial gaps in EAVs, see arrowhead) and perivascular cuffing (not shown). These disparate outcomes provide clear evidence for phenotypic heterogeneity in lung endothelium. Modified from Alvarez et al.7 and Villalta and Townsley.36
Are increases in Kf or lung water (or lack thereof) all about the endothelium?
Mechanistically, we relate Kf to the Starling equation, where this coefficient simply predicts the effectiveness of the net Starling force balance in promoting transvascular fluid filtration. However, we need to consider issues related to the method which might impact outcomes in the lung: vascular compliance; stress relaxation; and the extent of the perfused surface area. We and others have documented significant stress relaxation in the very compliant mammalian lung vasculature following a step increase in vascular pressure (reviewed in Parker and Townsley1). This means that unless the resultant increase in vascular volume is recognized, the measured Kf can be an overestimate of actual endothelial water permeability. Further, measured Kf is a reflection of filtration through the actual perfused surface area in the lung. When the Kf method is applied consistently, we have found that total Kf increases with lung mass across species in normal lungs from mouse to sheep.26 On the other hand, if perfused area decreases, as in the case with ligation of lung lobes, the measured Kf will follow even though the intrinsic permeability of the remaining vascular surface area is normal.27 Thus, measured Kf may underestimate the impact of a challenge on permeability in isolated lung if there is simultaneous derecruitment and loss of perfused surface area.28 Since other averaged measures used as indices of endothelial permeability (e.g. the lung wet–dry weight ratio, lung weight gain, and Evan’s blue dye extravasation) are influenced by transvascular fluid filtration, changes of perfused surface area would impact these measures as well.
Back to Ca2+ channels… We have documented involvement of TRPV4 in two clinically relevant models of acute lung injury—HiPv and ventilator-induced lung injury (VILI). In both scenarios, TRPV4 is activated by mechanical stress.23,24,29 TRPV4 can also be variously activated by heat, acid, EETs and hypotonic-cell swelling.30,31 The widespread expression of TRPV4, along with the broad array of compounds and scenarios in which this channel can be activated, means that any role of TRPV4 can be stimulus- and context-dependent. When we considered the potential for synergistic polymodal gating of TRPV4, we found that the pressure threshold for both HiPv and VILI decreases when tissue temperature is increased.23,29 Since we considered Kf to be a measure of endothelial permeability, we concluded at the time that HiPv and VILI specifically targeted endothelial TRPV4 leading to injury. Perhaps not so simple… The endothelial Ca2+ influx associated with HiPv leads to P-selectin surface expression in lung capillaries,32 raising the possibility of a pro-inflammatory role for endothelial TRPV4. Further, VILI appears to require TRPV4 expression in alveolar macrophages: reconstituting the alveolar macrophage population in TRPV4 null mice with wild-type macrophages completely restores the permeability response to VILI.33 Similarly, while the increase in lung weight (edema) elicited by administration of platelet activating factor (PAF) was abrogated in TRPV4–/– mice, TRPV4 in blood cells was the real culprit behind the PAF-induced edema rather than TRPV4 in lung parenchyma.34 An understanding of signaling complexities in any scenario, and thinking outside of the endothelial Kf box to consider all possible options, are critical to correct interpretation of outcomes in acute lung injury.
What really controls Ca2+-dependent outcomes in lung endothelium?
We have shown that direct activation of TRPV4 selectively disrupts the alveolar septal endothelial barrier leading to alveolar flooding.7,23,24,29 Other Ca2+ channels are expressed in lung endothelium, though not all appear to play a role in regulation of endothelial permeability.35,36 As a case in point, activation of TRPV4 with 4αPDD and depolarization-dependent activation of the α1G T-type voltage-gated Ca2+ channel in alveolar septal endothelium lead to apparently equivalent whole-cell Ca2+ transients. While TRPV4-mediated Ca2+ influx increases permeability, that elicited by T-channel activation selectively recruits endothelial surface expression of P-selectin, without increasing permeability.37,38 At present, we do not understand the fundamental basis by which Ca2+ signals target specific functional outcomes in lung endothelium. In lung capillary endothelium, specificity in Ca2+ signaling is particularly challenging due to the attenuated nature of the endothelial barrier.39 Several possibilities should be considered when attempting to investigate this box.
Proximity of Ca2+ channels to their targets? We have little information regarding spatial proximity for T-type Ca2+ channels to sites where P-selectin is sequestered in lung endothelium. Further, P-selectin does not appear to be localized to Weibel-Palade bodies in lung microvascular endothelium,40 so the mechanism underlying P-selectin recruitment to the cell surface with T-channel activation is unclear. We have a little more information regarding TRPV4, which appears to be localized at the base of lung microvascular endothelium.3 Cell–cell tethering at adherence junctions does not appear to be altered on TRPV4 activation, but rather TRPV4-mediated Ca2+ influx elicits activation of MMP2 and MMP9, which contribute to the permeability response.41 TRPV4-mediated endothelial cell detachment from the basement membrane has been observed in vitro and in vivo.36,42 Thus, TRPV4-mediated Ca2+ influx at the cell base would be poised to effect MMP release and untether cell-matrix integrin bonds. However, two factors potentially limit specificity of TRPV4-mediated Ca2+ signals from this perspective: the typical diffusion distance for Ca2+ in cytosol (100–500 nm)43 and the extreme thinness of the septal microvascular endothelial barrier in vivo (100–300 nm).39 Given these factors, one might predict that TRPV4-mediated Ca2+ entry at the endothelial base could elicit P-selectin expression at the apical face of the endothelium, assuming both the channel and the P-selectin source are sited within a finite area across the x-y footprint of the endothelial cell. Since our data do not support that outcome, we conclude that discrete spatial distribution of TRPV4 and the T-type channel is unlikely to solely account for signaling specificity. Other mechanisms must contribute.
Spatial constraints on diffusion due to organellar Ca2+ uptake? Given the predicted diffusion distance for Ca2+ in cytosol mentioned above, localized uptake into intracellular organelles might constrain the local spatial microdomain for a Ca2+ signal elicited by activation of a plasma membrane Ca2+ channel, and thus direct specificity. While not a lot of information exists on this potential mechanism in lung endothelium, there are some hints available in the literature. For example, the effectiveness of plasmalemmal Ca2+ transients in endothelium in gating nearby Ca2+-activated potassium channels is attenuated by mitochondria in close proximity.44 Further, interplay between mitochondria and endoplasmic reticulum shapes cytosolic Ca2+ transients on activation of store-operated channels.31,45,46 Others have argued that the major role of mitochondria in endothelium is to modulate Ca2+-dependent signaling, by provision of ATP for ATPase-dependent Ca2+ sequestration or extrusion from the cytosol or by serving as a Ca2+ sink.47–49 As an example, in fibroblasts from patients with mitochondrial complex I deficiency, resultant mitochondrial depolarization and blunted ATP synthesis delay recovery of agonist-stimulated cytosolic Ca2+ transients.50 A decreased decay rate could lead to increased time for diffusion and thus increased spatial spread of Ca2+ signals. Collectively, these mechanisms should limit Ca2+ dispersion and shape the dynamic patterns of Ca2+ transients51 in lung microvascular endothelium. Our own work has documented that inhibition of mitochondrial complex I to impair overall bioenergetic capacity increased endothelial permeability in lung and in lung microvascular endothelial cells, as assessed by Kf and diffusive permeability, respectively.52,53 Whether this is due to modulation of Ca2+ microdomains remains unclear.
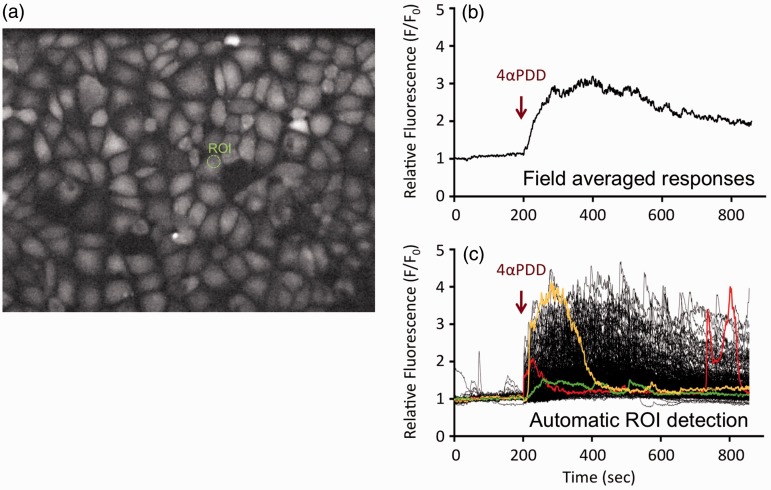
Dynamic patterning of Ca2+ transients? We have observed dynamic patterning in Ca2+ signals elicited by TRPV4 activation in monolayers of lung microvascular endothelium which is masked when analyzing field-averaged Ca2+ responses (Fig. 3). The dynamics recorded using automated region of interest (ROI) analysis show substantial diversity in the temporal responses elicited by activation of TRPV4 with 4αPDD. While signal amplitude after channel activation in individual ROIs does vary, there is much more variability in the temporal patterning, i.e. when a transient appears, its duration, and its rate of decay. In coronary arteries, substance P-mediated vasodilation was found to correlate precisely with dynamic Ca2+ patterns in the endothelium, even though there was poor correlation of vasodilation with Ca2+ responses averaged across the field of cells.6 While critical to unpack, understanding this box will require development of tools to assess the integrity of the endothelial barrier and Ca2+ signaling with a high degree of spatial and temporal specificity. We will need to explore whether specific cellular targets which regulate endothelial permeability in EAVs and in septal capillaries are regulated solely by changes in Ca2+ signal amplitude or whether regulation is dependent upon critical frequency coding in Ca2+ transients.
Fig. 3.
Ca2+ dynamics in lung endothelium. Lung microvascular endothelium were loaded with Fluo4AM for assessment of Ca2+ responses to activation of TRPV4 with 4αPDD (a). Dynamic patterning in Ca2+ signals elicited by TRPV4 activation is masked when analyzing field-averaged Ca2+ responses (b). The dynamics (c) recorded using automated ROI analysis show substantial diversity in the temporal responses elicited by activation of TRPV4 with 4αPDD. In this analysis, each line shows the Ca2+ signal over time in an individual ROI. Several ROI responses are color-coded to highlight the diversity in local Ca2+ responses after TRPV4 activation. Such attention to individual response coding shows that while signal amplitude after channel activation in individual ROIs does vary, there is much more variability in the temporal pattering, i.e. when a transient appears, its duration and its rate of decay.
Complexities in sepsis and acute lung injury
Attributing outcomes in sepsis and acute lung injury to activation of one Ca2+ channel can be problematic, even when the direct impact of channel activation seems clear cut. For example, while we have a good understanding of TRPV4’s role in simple models with direct channel activation, studies in more complex models of acute lung injury, such as that elicited by chemical inhalation exposure or in models of sepsis have not yielded a consistent picture of TRPV4’s involvement.54–57 A further complication in the setting of sepsis may be the resultant alteration in endothelial “context” for interpreting Ca2+ channel activation. For example, sepsis and trauma are commonly associated with mitochondrial bioenergetic dysfunction. Serum from patients with trauma and/or sepsis has deleterious impact on bioenergetics of vascular or pulmonary endothelial cells in culture.58–61 If mitochondria are indeed critical to shaping of Ca2+ microdomains in normal lung endothelium, then endothelial bioenergetic impairment in sepsis could increase the spread of Ca2+ signals and impair Ca2+ signaling specificity. Yet another box to unpack…
Conclusion
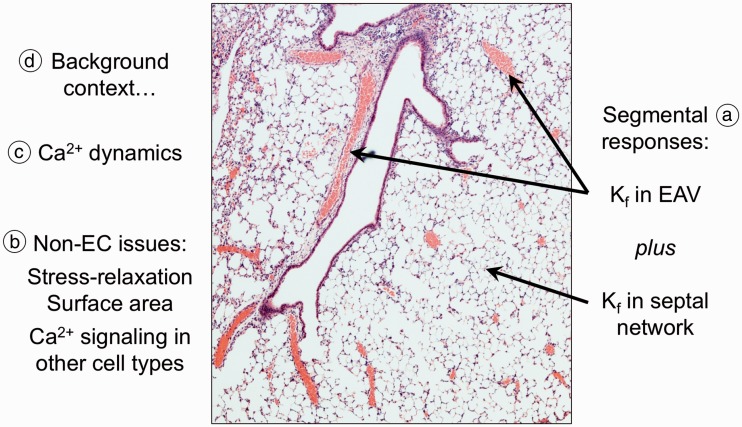
In summary, there are many issues to consider when “unpacking” the Ca2+-dependent permeability box (Fig. 4), including potential contributions from differential segmental responses in the lung vasculature, methodologic issues, contributions from other cells in lung beyond endothelial cells, dynamic patterning in Ca2+ transients, and the background state of the lung and lung endothelium in disease.
Fig. 4.
Summary of issues in unpacking the Ca2+ dependent permeability box. These issues are many, as noted on this image of a hematoxylin and eosin-stained lung section. (a) The filtration coefficient Kf in an isolated lung is in reality the sum of those in all perfused vascular compartments. So, coefficients in EAV and in septal capillaries are separately dictated by barrier integrity in those compartments. This issue similarly impacts interpretation of other averaged measures of lung edema and permeability. (b) Issues not related to the endothelium can color interpretation. These include stress relaxation and changes in surface area which can impact the measure of permeability per se. In addition, Ca2+ channels in cells other than endothelium (e.g. alveolar macrophages, blood leukocytes, and/or airway epithelium) might, in reality, contribute to the apparent permeability response in lung. (c) Ca2+ dynamic patterns, rather than averaged Ca2+ responses to channel activation, might yield more specific information regarding the contribution of any Ca2+ channel to regulation of lung permeability. Nonetheless, not all Ca2+ signals target pathways which regulate permeability. (d) The background context, such as changes in endothelial bioenergetics with sepsis, might color interpretation and integration of Ca2+ signals in lung. Responses to challenge with Ca2+ channel agonists might be lost or amplified, or perhaps the specificity of those signals might be altered. A small portion of this image appeared in black and white in Townsley and Stevens.63
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
2017 Grover Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the educational grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institutes of Health.
References
- 1.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 2004; 286: L231–246. [DOI] [PubMed] [Google Scholar]
- 2.Creighton J, Jian M, Sayner S, et al. Adenosine monophosphate-activated kinase α1 promotes endothelial barrier repair. FASEB J 2011; 25: 3356–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker JC, Hashizumi M, Kelly SV, et al. TRPV4 calcium entry and surface expression attenuated by inhibition of myosin light chain kinase in rat pulmonary microvascular endothelial cells. Physiol Rep 2013; 1: e00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldeck-Weiermair M, Alam MR, Khan MJ, et al. Spatiotemporal correlations between cytosolic and mitochondrial Ca2+ signals using a novel red-shifted mitochondrial targeted cameleon. PloS One 2012; 7: e45917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis M, Qian X, Charbel C, et al. Automated region of interest analysis of dynamic Ca2+ signals in image sequences. Am J Physiol Cell Physiol 2012; 303: C236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis M, Waldrup JR, Qian X, et al. Functional tuning of intrinsic endothelial Ca2+ dynamics in swine coronary arteries. Circ Res 2016; 118: 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez DF, King JA, Weber D, et al. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier. Circ Res 2006; 99: 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King J, Hamil T, Creighton J, et al. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvas Res 2004; 67: 139–151. [DOI] [PubMed] [Google Scholar]
- 9.Lowe K, Alvarez DF, King JA, et al. Perivascular fluid cuffs decrease lung compliance by increasing tissue resistance. Crit Care Med 2010; 38: 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens T, Rosenberg R, Aird W, et al. NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol Cell Physiol 2001; 281: C1422–1433. [DOI] [PubMed] [Google Scholar]
- 11.Kohler R, Brakemeier S, Kuhn M, et al. Expression of ryanodine receptor type 3 and TRP channels in endothelial cells: comparison of in situ and cultured human endothelial cells. Cardiovasc Res 2001; 51: 160–168. [DOI] [PubMed] [Google Scholar]
- 12.Hardin C, Fredberg JJ, Krishnan R. Real estate of monolayer permeability: location location location. Lab Invest 2013; 93: 148–150. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan R, Klumpers DD, Park CY, et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 2011; 300: C146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chetham PM, Babal P, Bridges JP, et al. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol 1999; 276: L41–50. [DOI] [PubMed] [Google Scholar]
- 15.Cioffi DL, Moore TM, Schaack J, et al. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 2002; 157: 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore TM, Norwood NR, Creighton JR, et al. Receptor-dependent activation of store-operated calcium entry increases endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 2000; 279: L691–698. [DOI] [PubMed] [Google Scholar]
- 17.Tiruppathi C, Ahmmed GU, Vogel SM, et al. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 2006; 13: 693–708. [DOI] [PubMed] [Google Scholar]
- 18.Tiruppathi C, Freichel M, Vogel SM, et al. Impairment of store-operated Ca2+ entry in TRPC4-/- mice interferes with increase in lung microvascular permeability. Circ Res 2002; 91: 70–76. [DOI] [PubMed] [Google Scholar]
- 19.Roy BJ, Pitts VH, Townsley MI. Pulmonary vascular response to angiotensin II in canine pacing-induced heart failure. Am J Physiol 1996; 271: H222–227. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Resp Crit Care Med 2005; 172: 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivey CL, Roy BJ, Townsley MI. Ablation of lung endothelial injury after pacing-induced heart failure is related to alterations in Ca2+ signaling. Am J Physiol 1998; 275: H844–851. [DOI] [PubMed] [Google Scholar]
- 22.Cioffi DL, Wu S, Chen H, et al. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ Res 2012; 110: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jian MY, King JA, Al-Mehdi AB, et al. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 2008; 38: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorneloe KS, Cheung M, Bao W, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 2012; 4: 159ra148. [DOI] [PubMed] [Google Scholar]
- 25.Parker JC, Stevens T, Randall J, et al. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 2006; 291: L30–37. [DOI] [PubMed] [Google Scholar]
- 26.Parker JC, Townsley MI. Physiological determinants of the pulmonary filtration coefficient. Am J Physiol Lung Cell Mol Physiol 2008; 295: L235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsley MI, Parker JC, Korthuis RJ, et al. Alterations in hemodynamics and Kf,c during lung mass resection. J Appl Physiol 1987; 63: 2460–2466. [DOI] [PubMed] [Google Scholar]
- 28.Shibamoto T, Parker JC, Taylor AE, et al. Derecruitment of filtration surface area in paraquat-injured isolated dog lungs. J Appl Physiol 1990; 68: 1581–1589. [DOI] [PubMed] [Google Scholar]
- 29.Hamanaka K, Jian MY, Weber DS, et al. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 2007; 293: L923–932. [DOI] [PubMed] [Google Scholar]
- 30.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 2010; 103: 2–17. [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Vriens J, Prenen J, et al. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol 2004; 286: C195–205. [DOI] [PubMed] [Google Scholar]
- 32.Kuebler WM, Ying X, Singh B, et al. Pressure is proinflammatory in lung venular capillaries. J Clin Invest 1999; 104: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamanaka K, Jian MY, Townsley MI, et al. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2010; 299: L353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin J, Michalick L, Tang C, et al. Role of transient receptor potential vanilloid 4 in neutrophil activation and acute lung injury. Am J Respir Cell Mol Biol 2016; 54: 370–383. [DOI] [PubMed] [Google Scholar]
- 35.Townsley MI, King JA, Alvarez DF. Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation 2006; 13: 725–739. [DOI] [PubMed] [Google Scholar]
- 36.Villalta PC, Townsley MI. Transient receptor potential channels and regulation of lung endothelial permeability. Pulm Circ 2013; 3: 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Jian MY, Xu YC, et al. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 2009; 297: L650–L657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou C, Chen H, King JA, et al. α1G T-type calcium channel selectively regulates P-selectin surface expression in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 2010; 299: L86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Townsley MI. Structure and composition of pulmonary arteries, capillaries, and veins. Compr Physiol 2012; 2: 675–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S, Zhou C, King JA, et al. A unique pulmonary microvascular endothelial cell niche revealed by Weibel-Palade bodies and Griffonia simplicifolia. Pulm Circ 2014; 4: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villalta PC, Rocic P, Townsley MI. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2014; 307: L652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willette RN, Bao W, Nerurkar S, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 2008; 326: 443–452. [DOI] [PubMed] [Google Scholar]
- 43.Clapham DE. Calcium signaling. Cell 1995; 80: 259–268. [DOI] [PubMed] [Google Scholar]
- 44.Malli R, Frieden M, Osibow K, et al. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J Biol Chem 2003; 278: 10807–10815. [DOI] [PubMed] [Google Scholar]
- 45.Graier WF, Frieden M, Malli R. Mitochondria and Ca2+ signaling: old guests, new functions. Pflugers Archiv 2007; 455: 375–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malli R, Frieden M, Osibow K, et al. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem 2003; 278: 44769–44779. [DOI] [PubMed] [Google Scholar]
- 47.Demaurex N, Poburko D, Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochim Biophys Acta 2009; 1787: 1383–1394. [DOI] [PubMed] [Google Scholar]
- 48.Groschner LN, Waldeck-Weiermair M, Malli R, et al. Endothelial mitochondria–less respiration, more integration. Pflugers Archiv 2012; 464: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheitlin CG, Julian JA, Shanmughapriya S, et al. Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress. Am J Physiol Cell Physiol 2016; 310: C479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Distelmaier F, Visch HJ, Smeitink JA, et al. The antioxidant Trolox restores mitochondrial membrane potential and Ca2+-stimulated ATP production in human complex I deficiency. J Mol Med 2009; 87: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filadi R, Pozzan T. Generation and functions of second messengers microdomains. Cell Calcium 2015; 58: 405–414. [DOI] [PubMed] [Google Scholar]
- 52.Bongard RD, Townsley MI, Merker MP. The effects of mitochondrial complex I blockade on ATP and permeability in rat pulmonary microvascular endothelial cells in culture (PMVEC) are overcome by coenzyme Q1 (CoQ1). Free Rad Biol Med 2015; 79: 69–77. [DOI] [PubMed] [Google Scholar]
- 53.Bongard RD, Yan K, Hoffmann RG, et al. Depleted energy charge and increased pulmonary endothelial permeability induced by mitochondrial complex I inhibition are mitigated by coenzyme Q1 in the isolated perfused rat lung. Free Rad Biol Med 2013; 65: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balakrishna S, Song W, Achanta S, et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2014; 307: L158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalsgaard T, Sonkusare SK, Teuscher C, et al. Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice. Sci Rep 2016; 6: 33841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sand CA, Starr A, Nandi M, et al. Blockade or deletion of transient receptor potential vanilloid 4 (TRPV4) is not protective in a murine model of sepsis. F1000 Res 2015; 4: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Townsley MI, Alvarez DF. Pseudomonas aeruginosa-induced pulmonary edema - early impact on periarterial cuffs independent of TRPV4. Am J Resp Crit Care Med 2012; 185: A5509. [Google Scholar]
- 58.Boulos M, Astiz ME, Barua RS, et al. Impaired mitochondrial function induced by serum from septic shock patients is attenuated by inhibition of nitric oxide synthase and poly(ADP-ribose) synthase. Crit Care Med 2003; 31: 353–358. [DOI] [PubMed] [Google Scholar]
- 59.Hsieh YC, Athar M, Chaudry IH. When apoptosis meets autophagy: deciding cell fate after trauma and sepsis. Trends Mol Med 2009; 15: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kozlov AV, Bahrami S, Calzia E, et al. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intens Care 2011; 1: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010; 464: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor MS, Francis M. Decoding dynamic Ca2+ signaling in the vascular endothelium. Front Physiol 2014; 5: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Townsley MI, Stevens T. Lung endothelium, San Francisco, CA: Morgan and Claypool Publishers, 2015. [Google Scholar]