Abstract
Levosimendan is an inotropic and vasodilator drug, which is known to improve cardiac function in animal models of right ventricular (RV) failure. The effects of levosimendan on oxygen consumption and myocardial efficiency in the failing RV is unknown. We investigated the effects of levosimendan on RV function, myocardial oxygen consumption, myocardial external efficiency (MEE), and myocardial metabolism in rats with RV hypertrophy and failure. RV hypertrophy and failure were induced by pulmonary trunk banding in rats. Rats were randomized to seven weeks of treatment with vehicle (n = 16) or levosimendan (3 mg/kg/day) (n = 13). Control animals without pulmonary banding received vehicle treatment (n = 11). RV MEE and RV metabolism were evaluated by echocardiography, 11C-acetate positron emission tomography (PET), 18F-FDG PET, and invasive pressure measurements. We found that levosimendan improved RV MEE (26 ± 3 vs. 14 ± 1%, P < 0.01) by increasing RV external work (0.62 ± 0.06 vs. 0.30 ± 0.03 mmHgċmL, P < 0.001) without affecting RV myocardial oxygen consumption (P = 0.64). The improvement in RV MEE was not associated with a change in RV myocardial glucose uptake (1.3 ± 0.1 vs. 1.0 ± 0.1 µmol/g/min, P = 0.44). In conclusion, in the hypertrophic and failing RV of the rat, levosimendan improves RV function without increasing myocardial oxygen consumption leading to improved MEE. The improvement in RV MEE was not associated with a change in myocardial glucose uptake. This study emphasizes the potential therapeutic value of chronic levosimendan treatment RV failure. It extends previous observations on the effect profile of levosimendan and motivates clinical testing of levosimendan in RV failure.
Keywords: calcium sensitizer, right ventricular function, heart failure, oxygen consumption, metabolism
Pulmonary arterial hypertension (PAH) comprises a group of chronic and often progressive pulmonary diseases with a high mortality.1 Although the primary pathophysiological mechanisms in patients with PAH occurs within the pulmonary circulation, right ventricular (RV) function is the most important determinant of prognosis.1,2
Levosimendan is currently used for treatment of acute decompensated left ventricular (LV) failure. In animal experiments, it has recently been demonstrated that acute administration of levosimendan improves the function of the failing RV and that chronic levosimendan treatment prevents the development of RV failure.3,4 These beneficial effects of levosimendan may be explained in part by the fact that levosimendan is an inotropic and vasodilator drug. Levosimendan displays positive inotropic effects by increasing the sensitivity of troponin C to calcium, and vasodilatory effects by opening adenosine triphosphate (ATP)-dependent K+ channels in vascular smooth muscle cells.5 However, the full mechanism behind the beneficial effects in RV failure has never been investigated.
In patients with pulmonary hypertension (PH), the RV is subjected to a chronic pressure overload resulting in increased wall stress and elevated myocardial oxygen consumption (MVO2), triggering the transition to RV contractile dysfunction and failure.6 When administered to patients with LV failure, levosimendan improves RV myocardial external efficiency (MEE) defined as the ratio of stroke work and MVO2.7 However, this improvement in RV MEE was measured in patients without RV failure and it is uncertain whether a similar effect would be observed in patients with a hypertrophic and failing RV secondary to increased RV afterload.
In this study, we investigated the effects of chronic administration of levosimendan on RV function, MVO2, MEE, and myocardial glucose uptake. We performed 11C-acetate PET, 18F-FDG PET, echocardiography, and invasive pressure measurements in rats with pressure-overload induced RV hypertrophy and failure.
Methods
Animals and ethics
The study was performed in male Wistar Galas rats (M&B Taconic, Ry, Denmark) (99 g ± 1.7, n = 40), housed two per cage with unlimited access to food (Altromin #1324, Altromin, Lage, Germany) and water and in 12-h light–dark cycles. The study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1985) and was approved by the Danish Animal Experiments Inspectorate (authorization no. 2012-15-2934-00384).
Study design
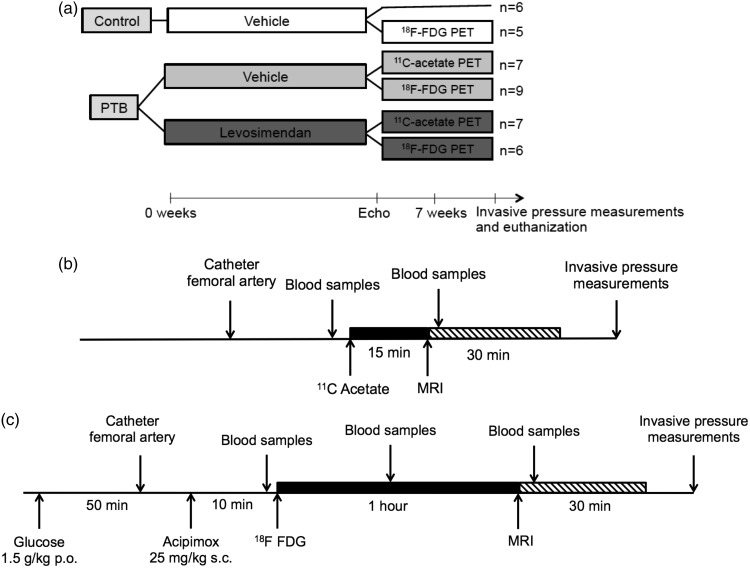
To determine the effect of chronic levosimendan treatment on RV MEE and RV metabolism in rats with RV hypertrophy and failure, 40 rats were randomized to pulmonary trunk (PT) banding (n = 29) or no surgery (n = 11) (Fig. 1a). PT banding was performed with a clip compressed to an inner diameter of 0.6 mm as described previously.4,8 Three days before PT banding surgery, the rats were randomized to treatment with levosimendan 3 mg/kg/day (Orion Pharma, Espoo, Finland) (n = 13 out of 29) or vehicle (n = 16 out of 29) added to the drinking water. Control rats without PT banding received vehicle treatment. Levosimendan and vehicle were prepared and administered as described previously.4 Seven weeks after surgery, the effects on RV function were evaluated by echocardiography, PET, and invasive pressure measurements (Fig. 1).
Fig. 1.
(a) Study design. After seven weeks of treatment, cardiac function and energetics were evaluated by echocardiography, 11C-acetate PET, 18F-FDG PET, and invasive pressure measurements. (b) 11C-acetate PET protocol. See text for explanation. (c) 18F-FDG PET protocol. See text for explanation. PTB, PT banding; FDG, fluorodeoxyglucose; PET, positron emission tomography; MRI, magnetic resonance imaging.
Echocardiography was performed before PET scanning as described elsewhere.4,8 On the day of PET scanning, 20 of the 40 rats were submitted to 11C-acetate PET to determine the effect of levosimendan on RV MEE, and 20 of the 40 rats were submitted to 18F-FDG PET to investigate potential levosimendan-induced changes in RV metabolism (Fig. 1b and 1c). Directly after the scan, invasive pressure measurements were performed as described elsewhere4,8 and the rats were euthanized. Finally, the heart was excised and weighed.4
Positron emission tomography
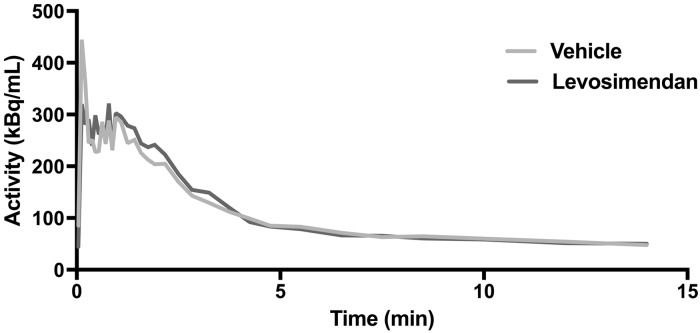
PET scans were performed on a Mediso NanoScan sequential PET/MRI (1 Tesla) (Mediso Ltd., Budapest, Hungary) (Fig. 1b and 1c). Rats were deprived of food, but were allowed free access to water for 24 h before PET scan. The PET scans were performed under general anesthesia (isoflurane and oxygen during induction 4.5% and at maintenance 2%). Before injection of 11C-acetate and 18F-FDG, a catheter was placed in the femoral artery for collection of blood samples, and a second catheter was inserted in the tail vein for intravenous injection of the tracers. Glucose loading was performed by oral gavage (1.5 g/kg) 1 h before injection of 18F-FDG and acipimox (Sigma Aldrich, St Louis, MO, USA) was injected subcutaneously (25 mg/kg) 10 min before 18F-FDG injection.9 Tracer was administered as a bolus injection in the tail vein at the beginning of the scan. Frame structures were 12 × 5 s, 6 × 10 s, 4 × 30 s, 4 × 40 s, 6 × 120 s for 18F-FDG and 12 × 5 s, 6 × 50 s, 3 × 20 s, 4 × 30 s, 4 × 60 s, 3 × 120 s for 11C-acetat with mean doses of 30.2 ± 1.6 MBq and 41.1 ± 2.7 MBq, respectively. Images were reconstructed using the TeraTomo 3D algorithm10 (four iterations, six subsets) and 0.4 × 0.4 × 0.4 mm voxels with regularization and all corrections applied. Attenuation maps were obtained using fast gradient echo scout images.10 Post-filtering with a 1-mm Hann filter was performed. Immediately before and after the dynamic PET scan, arterial blood samples were collected for analyses of glucose, lactate, and hemoglobin. Data were analyzed using in-house software. In short, regions of interest (ROIs) corresponding to RV, septum, and LV were drawn in transaxial PET slices. For 18F-FDG, an additional ROI was drawn in the vena cava and used as input function.11 Metabolic rate of glucose uptake was determined using the Gjedde-Patlak method,12 assuming a lumped constant of unity. For 11C-acetate scans, kmono was obtained by linear fitting of the linear portion of a semi-logarithmic plot of 11C-acetate washout curve (typically xs to ys) (Fig. 3). All data were analyzed with the observer blinded to the source of the samples.
Fig. 3.
Representative example of myocardial time activity curve for 11C-acetate PET acquisition. A monoexponential function is fitted to the myocardial clearance starting when most of the blood pool has cleared (around 1 min). This function yields a clearance rate constant kmono which represents the oxidative metabolism and corresponds to myocardial oxygen consumption (MVO2). Note that there is no difference between kmono in the two graphs.
Myocardial external efficiency
MEE of the RV was calculated according to:13
where SV is stroke volume in mL, HR is heart rate in min−1, and RVP mean is the mean RV pressure (RVP) in mmHg. A caloric equivalent of 1 mL × mmHg = 1.33 × 10−4 J was applied to yield J/min.13 Stroke volume (SV) and heart rate measurements used for MEE calculations were obtained during echocardiography. MVO2 (mL/g/min) was calculated using the following equation:14,15
Total RV energy consumption (J/min) was estimated by multiplying RV mass and MVO2 and converted to units of energy using the conversion factor of 20 (1 mL of O2 = 20 J). The healthy control group was excluded from the 11C-acetate PET data because the RV free wall was too thin to obtain valid measurements of MVO2, given the spatial resolution of existing PET scanners.
Metabolic Rate of Glucose Uptake
Metabolic rate of glucose uptake (µmol/g/min) was calculated from the 18F-FDG PET data using the equation:16
where Ki is the 18F-FDG influx constant derived from the Patlak graphical analysis, Cglu (µmol) represents the average glucose level during 18F-FDG scanning (t = 0 min, t = 30 min), and LC is the lumped constant and assumed to be equal to 1.17,18
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6 (GraphPad software, La Jolla, CA, USA). Unless otherwise stated, normally distributed quantitative data are expressed as mean ± standard error of mean (SEM). Data were tested for normal distribution with a Shapiro–Wilk normality test. One-way ANOVA was applied to data with normal distribution and variance homogeneity and used as gatekeeper-test. Only if the ANOVA was significant, a post hoc Holm-Sidak’s multiple comparisons test was performed (Control vs. Vehicle, Vehicle vs. Levosimendan). This testing procedure controls overall error rate (type 1 error) at level of 5%.19 A non-parametric Kruskal–Wallis test was performed if data were not normally distributed. 11C-acetate PET data were compared using unpaired t-test because the healthy control group had been excluded. In all cases, P < 0.05 was considered statistically significant. Primary data can be obtained online from Pulmonary Circulation’s website.
Results
To assess the effects of PT banding, we compared anatomical and hemodynamic data between the vehicle group and the control group of the 11C-acetate PET rats (Table 1). The PT banding procedure caused a 2.5-fold increase in RV mass in the vehicle group. RV external work was 3.3-fold higher in the vehicle group than in the control group. This was caused by a 4.8-fold increase in RVP mean and a 30% reduction in cardiac index in the vehicle group (Table 1).
Table 1.
Characteristics of the rats.
| Control (n = 6) | Vehicle (n = 7) | Levosimendan (n = 7) | |
|---|---|---|---|
| 11C-ACETATE PET | |||
| Body weight (g) | 317 ± 9 | 320 ± 7 | 324 ± 5 |
| RV mass (g) | 0.17 ± 0.01 | 0.42 ± 0.02* | 0.47 ± 0.03 |
| RV stroke volume (µL) | 258 ± 8 | 232 ± 13 | 371 ± 35‡ |
| Heart rate (bpm) | 391 ± 13 | 302 ± 6* | 314 ± 9 |
| Cardiac index (mL/kg/min) | 317 ± 8.4 | 222 ± 13.8* | 359 ± 28.6‡ |
| Systolic RVP (mmHg) | 22.9 ± 1.5 | 94.3 ± 7.6* | 112.8 ± 3.6 |
| Diastolic RVP (mmHg) | −1.4 ± 1.1 | 1.0 ± 0.9 | 3.59 ± 3.0 |
| Mean RVP (mmHg) | 6.7 ± 0.3 | 32.1 ± 2.4* | 40.0 ± 1.4 |
| kmono (min−1) | 0.32 ± 0.02 | 0.33 ± 0.02 | |
| RV total MVO2 | 2.11 ± 0.1 | 2.45 ± 0.1 | |
| Control (n = 5) | Vehicle (n = 9) | Levosimendan (n = 6) | |
| 18F-FDG PET | |||
| Body weight (g) | 322 ± 11 | 319 ± 4 | 323 ± 4 |
| RV mass (g) | 0.17 ± 0.01 | 0.41 ± 0.02* | 0.43 ± 0.02 |
| Average glucose level (µmol) | 9.39 ± 0.8 | 11.4 ± 0.9 | 9.3 ± 0.8 |
Data are presented as mean ± SEM.
P < 0.05 vs. control.
P < 0.01 vs. vehicle.
RV, right ventricle; RVP, right ventricular pressure; kmono, 11C-acetate clearance rates, MVO2, myocardial oxygen consumption.
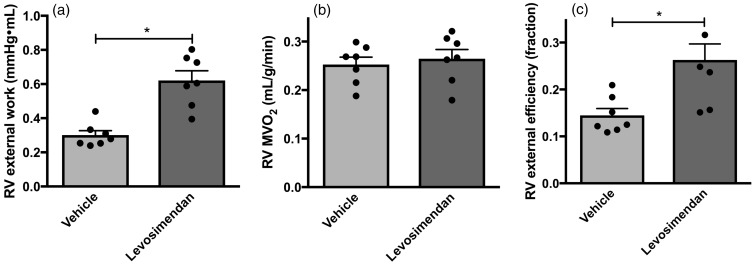
To determine whether chronic treatment with levosimendan improves RV function without increasing RV MVO2, and by that improves RV MEE, we compared the levosimendan group with the vehicle group. In the levosimendan group, RV external work was doubled compared to the vehicle group (Fig. 2a). This was caused by a 25% increase in RVP mean and a 62% increase in cardiac index in the levosimendan group (Table 1). No difference in RV mass was found between the vehicle group and the levosimendan group (P = 0.83). MVO2 did not change (P = 0.64) (Fig. 2b and Fig. 3) and consequently RV MEE in the levosimendan group was significantly higher than in the vehicle group (Fig. 2c).
Fig. 2.
Effect of levosimendan on RV energetics. (a) RV external work. Levosimendan increases RV external work mainly by an increase in SV. (b) RV myocardial oxygen consumption (MVO2) estimated from kmono achieved from 11C-acetate PET scans. Levosimendan did not affect RV MVO2. (c) RV myocardial external efficiency (RV MEE), calculated as the ratio of RV external work and total RV MVO2. Levosimendan improved RV MEE. Data are presented as mean ± SEM. Vehicle: n = 7 and Levosimendan: n = 7. *P < 0.01, unpaired t-test.
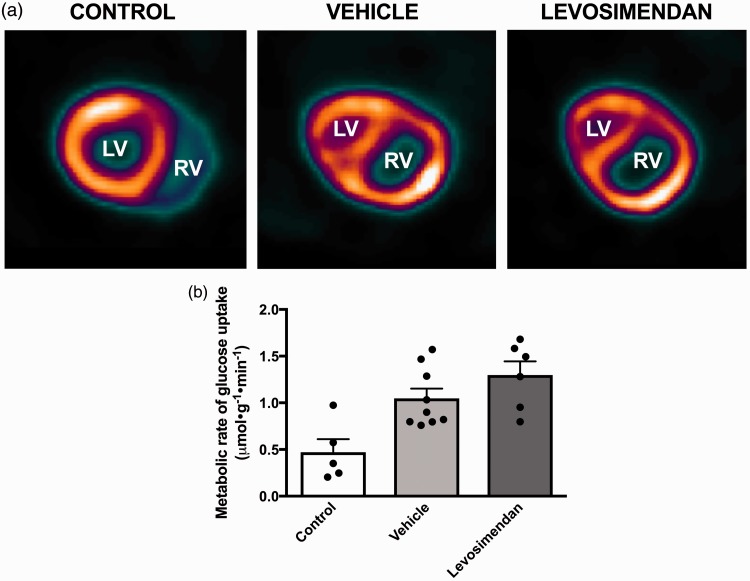
Next, we examined whether chronic administration of levosimendan changes RV metabolism in rats with RV hypertrophy and failure. In the PT banded vehicle group, metabolic rate of glucose uptake was more than doubled compared to the control group (Fig. 3a and b), though the difference was not statistically significant due to an outlier in the small control group. No difference was found in metabolic rate of glucose uptake between the vehicle group and the levosimendan group (P = 0.30) (Fig. 4a and b). Metabolic rate of glucose uptake in the LV, septum, and total myocardium was comparable between the control, vehicle, and levosimendan group.
Fig. 4.
Effect of levosimendan on RV metabolism. (a) Representative data from an 18F-FDG PET scan in a control rat, a vehicle treated rat, and a levosimendan-treated rat. (b) The metabolic rate of glucose uptake was achieved from 18F-FDG PET scans. Note that levosimendan does not affect RV metabolic rate of glucose uptake. Data are presented as mean ± SEM. Controls: n = 5, Vehicle: n = 9, and Levosimendan: n = 6.
Discussion
In this study, we provide evidence that chronic treatment with levosimendan increases RV stroke work without affecting RV MVO2, leading to an improvement in RV MEE in rats with RV hypertrophy and dysfunction.
Levosimendan increased RV cardiac index in the hypertrophic and failing RV as observed previously.4 By using a model with a fixed afterload, the increase in cardiac index is explained by a direct cardiac effect including increased contractility, as the model excludes any influence from pulmonary vasodilatation or changes in RV afterload.20–23
Furthermore, levosimendan improved RV MEE by increasing RV stroke work without affecting RV MVO2. This finding is in accordance with a human study by Ukkonen et al.,7 who showed that levosimendan had a neutral effect on RV MVO2 and improved RV MEE in patients with LV failure. In this human study, it was not possible to determine whether the improved RV MEE was due to unloading of the RV by pulmonary vasodilation or a direct myocardial effect of levosimendan. Our results do, however, emphasize that levosimendan can directly improve the function of the failing RV without increasing RV MVO2.
It could be argued that prolonged increase in RV external work could be harmful to the RV despite the fact that no increase in RV metabolism was observed. Our data do not support whether levosimendan treatment is beneficial or harmful in the long term, only that it increases the external work of the RV. To determine this, it would be relevant to perform survival studies. It is, however, relevant to consider that an increase in external work will most likely not be an issue in human pulmonary disease, since it will be balanced by a pulmonary vasodilation leading to unloading of the RV. We have experimental data confirming this in the Sugen 5416/hypoxia rat model of PAH,24 but clinical confirmation of the experimental data is still lacking.
Despite an increase in RV stroke work, glucose uptake rate was not changed (Fig. 4a and b). Thus, it could be speculated that the improvement in RV function is not caused by altered metabolism, although we have no data that support this hypothesis directly.
One limitation in our study is the assumption we made for the measurement of the metabolic rate of glucose uptake, where we assumed that a partial volume effect was not present. This assumption is reasonable as we solely compared the metabolic rate of glucose uptake between the vehicle group and the levosimendan group. Since RV mass is similar in the two groups (Table 1), a partial volume effect will not affect the comparison between the two groups. We were not able to investigate the effect of levosimendan on myocardial blood flow from the 11C-acetate PET scans because of the partial volume effect. However, recent evidence suggests that levosimendan is a potent vasodilator of human coronary arteries,25 which may also influence RV MEE.
Another limitation of the study is that RV failure in the PT banding model only reproduces proximal pulmonary artery disease. However, RV failure is more commonly seen in pulmonary vascular diseases, which are not evaluated in this model. Thus, it is likely that the PT banding model is limited in its application to pulmonary vascular disease. The benefit of the PT banding model is that it allows for investigation of cardiac-specific effects of levosimendan treatment without influence from the pulmonary vasodilation, which has been the main interest in this study.
In conclusion, our results demonstrate that levosimendan improves RV stroke work and myocardial external efficiency in the failing RV without increasing myocardial oxygen consumption. Furthermore, these changes were not accompanied by a change in RV glucose uptake rate.
Appendix
Detailed methods
Levosimendan treatment
Levosimendan (Orion Pharma, Espoo, Finland) was administered 3 mg/kg body weight/day in drinking water and administration was initiated three days before the PT banding operation. This dose was demonstrated effective in previous rat studies.1,2 To dissolve levosimendan, a solution of dehydrated ethanol 99.9% (VWR – Bie & Berntsen A/S, Herlev, Denmark), polyvinylpyrrolidone (10000 KD), and citric acid powder (both Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) was used. Vehicle treatment consisted of this solution without levosimendan added. To maintain the target dose of levosimendan and vehicle treatment, the drinking water concentration was adjusted twice weekly according to water consumption and body weight.
Pulmonary trunk banding
A PT banding operation was performed to induce an accurate and fixed increase in RV afterload resulting in RV hypertrophy and failure seven weeks after the operation. Rats were anesthetized with sevoflurane (Abbott Scandinavia, AB, Solona, Sweden) (induction: 7.0% in 2:1 O2/N2O mix; maintenance: 3.5% in 2:1 O2/N2O mix) and placed on a heated pad, keeping body temperature at 37℃. To maintain sufficient ventilation, the rats were intubated artificially and ventilated using a rodent ventilator (UGO Basile S.R.L., Comerio VA, Italy) at 75 breaths/min and a tidal volume of 10 mL/kg (7%). A lateral thoracotomy was performed on the left side of the sternum and the pericardium was opened. Using a dual-view surgical microscope, the PT was separated carefully from the aorta. The banding was done with a horizon applier pre-modified to compress a titanium clip to a preset inner diameter of 0.6 mm around the PT. The thorax was closed in three separate layers and excess air evacuated. The rats received a subcutaneous injection of buprenorphine (0.12 mg/kg) (Reckitt Benckiser, Berkshire, UK) preoperatively and was treated with buprenorphine in the drinking water (7.4 µg/mL) for three days postoperatively to relieve pain. Control rats did not undergo operation.
Echocardiography
Transthoracic echocardiography was performed to evaluate cardiac function. Echocardiography was performed on a Vevo2100 echocardiographic system (VisualSonics Inc., Toronto, ON, Canada) using a MS250 line array transducer scanning at a frequency of 14–21 MHz. A parasternal long-axis view was performed for visualization of the right ventricle outflow tract and the PT. Pulsed wave Doppler was used to obtain the velocity time integral from the PT (VTIPT) and SV was calculated as the average of three heart beats according to [SV = PT radius2 × 3.14 × VTIPT]. All images were analyzed off-line (Vevo® 2100, Fujifilm VisualSonics Inc., Amsterdam, The Netherlands) with the observer blinded to the source of the sample.
Invasive right ventricular pressure measurements
RVP measurements were performed for the calculation of right ventricular myocardial external efficiency. RVP was recorded using Miliar MikroTip conductance catheters (SPR-869NR, Millar Instruments Inc., Houston, TX, USA) and performed in general anesthesia (Isoflurane and oxygen: Induction: 4.5%; maintenance 2.5%) with the rats intubated and ventilated while placed on a heating pad. The catheter was pre-soaked for approximately 20 min in sterile saline, connected to a signal-conditioning box (PCU-2000; Millar Instruments Inc.), and calibrated before insertion. The catheter was inserted into the apex of the RV in the anesthetized rat using an open chest approach allowing for RVP to be measured in the beating heart. Diastolic and systolic RVP was measured after approximately 10 min of stabilization. Analysis was performed using software from Notocord (Notocord Systems SAS, Midland, NC, USA).
Supplemental references
Revermann M, Schloss M, Mieth A, et al. Levosimendan attenuates pulmonary vascular remodeling. Intensive Care Med 2011; 37: 1368–1377.
Hillgaard TK, Andersen A, Andersen S, et al. Levosimendan prevents pressure-overload-induced right ventricular failure. J Cardiovasc Pharmacol 2016; 67: 275–282.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was supported by The Danish Heart Association (grant no. 15-R99-A5898-22953), Aarhus University Hospital and Novo Nordisk Foundation (grant no. NNF17OC0024868). The funding sources had no involvement in study design, data analyses, writing of the report, or the submission decision.
References
- 1.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 2.Voelkel NF, Gomez-Arroyo J, Abbate A, et al. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J 2012; 40: 1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vildbrad MD, Andersen A, Holmboe S, et al. Acute effects of levosimendan in experimental models of right ventricular hypertrophy and failure. Pulm Circ 2014; 4: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillgaard TK, Andersen A, Andersen S, et al. Levosimendan prevents pressure-overload-induced right ventricular failure. J Cardiovasc Pharmacol 2016; 67: 275–282. [DOI] [PubMed] [Google Scholar]
- 5.Papp Z, Edes I, Fruhwald S, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012; 159: 82–87. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E. Control of myocardial oxygen consumption: physiologic and clinical considerations. Am J Cardiol 1971; 27: 416–432. [DOI] [PubMed] [Google Scholar]
- 7.Ukkonen H, Saraste M, Akkila J, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther 2000; 68: 522–531. [DOI] [PubMed] [Google Scholar]
- 8.Axelgaard S, Holmboe S, Ringgaard S, et al. Effects of chronic treprostinil treatment on experimental right heart hypertrophy and failure. Cardiol Young 2017; 27: 90–100. [DOI] [PubMed] [Google Scholar]
- 9.Poussier S, Maskali F, Tran N, et al. ECG-triggered 18F-fluorodeoxyglucose positron emission tomography imaging of the rat heart is dramatically enhanced by acipimox. Eur J Nucl Med Mol Imaging 2010; 37: 1745–1750. [DOI] [PubMed] [Google Scholar]
- 10.Nagy K, Toth M, Major P, et al. Performance evaluation of the small-animal nanoScan PET/MRI system. J Nucl Med 2013; 54: 1825–1832. [DOI] [PubMed] [Google Scholar]
- 11.Lanz B, Poitry-Yamate C, Gruetter R. Image-derived input function from the vena cava for 18F-FDG PET studies in rats and mice. J Nucl Med 2014; 55: 1380–1388. [DOI] [PubMed] [Google Scholar]
- 12.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 1985; 5: 584–590. [DOI] [PubMed] [Google Scholar]
- 13.Knaapen P, Germans T, Knuuti J, et al. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation 2007; 115: 918–927. [DOI] [PubMed] [Google Scholar]
- 14.Ng CK, Huang SC, Schelbert HR, et al. Validation of a model for [1-11C]acetate as a tracer of cardiac oxidative metabolism. Am J Physiol 1994; 266: H1304–1315. [DOI] [PubMed] [Google Scholar]
- 15.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J 1980; 186: 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen R, Jorsal A, Iversen P, et al. Heart failure patients with prediabetes and newly diagnosed diabetes display abnormalities in myocardial metabolism. J Nucl Cardiol 2016. Doi: 10.1007/s12350-016-0622-0. [DOI] [PubMed]
- 17.Choi Y, Brunken RC, Hawkins RA, et al. Factors affecting myocardial 2-[F-18]fluoro-2-deoxy-D-glucose uptake in positron emission tomography studies of normal humans. Eur J Nucl Med 1993; 20: 308–318. [DOI] [PubMed] [Google Scholar]
- 18.Bull U, Foroutan Y, Hellwig D, et al. Comparison of relative 18FDG uptake with metabolic rate (MRGlucose) in the myocardium in CAD, classified by 99m-Tc-MIBI. Nuklearmedizin 1995; 34: 223–228. [PubMed] [Google Scholar]
- 19.Hancock GR, Klockars AJ. The quest for α: developments in multiple comparison procedures in the quarter century since games (1971). Rev Educ Res 1996; 66: 269–306. [Google Scholar]
- 20.Haikala H, Kaivola J, Nissinen E, et al. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J Mol Cell Cardiol 1995; 27: 1859–1866. [DOI] [PubMed] [Google Scholar]
- 21.Haikala H, Linden IB. Mechanisms of action of calcium-sensitizing drugs. J Cardiovasc Pharmacol 1995; 26 Suppl. 1: S10–19. [PubMed] [Google Scholar]
- 22.Pollesello P, Ovaska M, Kaivola J, et al. Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C. A molecular modelling, fluorescence probe, and proton nuclear magnetic resonance study. J Biol Chem 1994; 269: 28584–28590. [PubMed] [Google Scholar]
- 23.Sorsa T, Pollesello P, Solaro RJ. The contractile apparatus as a target for drugs against heart failure: interaction of levosimendan, a calcium sensitiser, with cardiac troponin c. Mol Cell Biochem 2004; 266: 87–107. [DOI] [PubMed] [Google Scholar]
- 24.Hansen MS, Andersen A, Holmboe S, et al. Levosimendan prevents and reverts right ventricular failure in experimental pulmonary arterial hypertension. J Cardiovasc Pharmacol 2017; 70: 232–238. [DOI] [PubMed] [Google Scholar]
- 25.Michaels AD, McKeown B, Kostal M, et al. Effects of intravenous levosimendan on human coronary vasomotor regulation, left ventricular wall stress, and myocardial oxygen uptake. Circulation 2005; 111: 1504–1509. [DOI] [PubMed] [Google Scholar]