Abstract
The aims of this study were to investigate the medication adherence of patients on pulmonary hypertension (PH)-targeted therapies and uncover factors that might influence adherence values. Patients taking at least one specialist medicine (sildenafil, tadalafil, bosentan, ambrisentan, iloprost, epoprostenol, treprostinil) completed a Morisky Medication Adherence Scale-8 (MMAS-8) questionnaire. Participants’ MMAS-8 scores were used to estimate overall medicine adherence. Potential adherence co-factor data were collected from patient databases and hospital discharge summaries. The MMAS-8 questionnaire was completed by 263 patients (mean age = 61.6 ± 14.8 years, 70.6% women). Data from MMAS-8 showed that 47.9% reported high adherence, 40.3% moderate adherence, and 11.8% low adherence. Factors associated with adherence as measured by the MMAS-8 included: older age; taking monotherapy; and having a higher number of co-morbidities or concurrent medicines. Higher administration frequency, greater length of time on targeted therapy, and use of a compliance aid had a negative association with adherence. Overall adherence to PH specialist medicines is relatively high but a proportion of patients report sub-optimal adherence behavior. A number of factors may help to recognize susceptible patients.
Keywords: compliance, medicines, concordance, Morisky, MMAS-8
Pulmonary hypertension (PH) is a rare, debilitating, and potentially fatal disease of the cardiac and respiratory system. It is characterized by increasing resistance in the pulmonary circulation which leads to compensatory hypertrophy and the eventual failure of the right ventricle. Patients develop breathlessness on exertion with a decreased exercise tolerance that steadily deteriorates if not treated appropriately.1 Without specialist treatments, patients have an expected prognosis of 50% survival at three years from diagnosis.2 Thankfully, since targeted therapies for PH have been available, survival rates have much improved.3
There are a number of licensed medical treatments for PH. These medicines vary in the complexity associated with their administration route and dosing frequency (Table 1).
Table 1.
PH specialist medicines: administration, dosing, and complexity.
| Medicine | Medicine class | Administration route | Dosing frequency | Complexity |
|---|---|---|---|---|
| Sildenafil | PDE5i | Oral tablet | Three times daily | |
| Tadalafil | PDE5i | Oral tablet | Once daily | |
| Bosentan | ERA | Oral tablet | Twice daily | Requires monthly blood test for liver function |
| Ambrisentan | ERA | Oral tablet | Once daily | Requires monthly blood test for liver function |
| Macitentan | ERA | Oral tablet | Once daily | Requires monthly blood test for liver function |
| Iloprost | Prostanoid | Nebulizer | Seven times a day (every 3 h while awake) | Nebulizer set-up and frequent cleaning |
| Epoprostenol | Prostanoid | Intravenous (IV) infusion | Continuous infusion changed 12–48 hourly | IV line care and multiple step reconstitution process |
| Treprostinil | Prostanoid | Nebulizer, IV or subcutaneous (SC) | Four times a day nebulizer, continuous IV or SC infusion | Nebulizer set-up and frequent cleaning, IV line care, SC site care |
| Selexipag | IPRA | Oral tablet | Twice daily | |
| Riociguat | SGCS | Oral tablet | Three times daily |
PDE5i, phosphodiesterase type-5 inhibitor; ERA, endothelin antagonist; IPRA, IP receptor agonist; SGCS, soluble guanylate cyclase stimulator.
The first PH sub-diagnostic group to gain a licensed treatment was pulmonary arterial hypertension (PAH). Due to the rarity of PAH (the estimated annual incidence of diagnosed PAH, for example, is in the range of 0.9–7.6 cases per million3), PH specialist medicines are expensive. On drug costs alone, the UK National Health Service (NHS) spends approximately £200–120,000 per patient per year.4 Such expenditure will only improve patient health if medication regimes are well adhered to. Good adherence to medication is generally associated with improved health outcomes;5,6 however, many studies show that poor medication adherence is common.7 The World Health Organization (WHO) has estimated that adherence is only 50% for patients with chronic conditions.8
There are numerous factors that can influence medication adherence such as age, gender, wealth, disease severity, co-morbidities, medication dosing frequency, and side effect profile, as well as health-system related factors such as health-professional to patient communication.8,9 As many of these adherence determinants lack a consistent effect between studies and across populations,9,10 it is important to directly study adherence in any given population of interest.
Adherence to pulmonary hypertension medicines
There are currently few published data on medication adherence to specialist PH therapies. One American study11 has suggested that patients taking once daily tadalafil were more adherent than those taking three times a day sildenafil, but only for patients attending retail pharmacies. In patients receiving specialist pharmacy services, there was no significant difference between these medicines. Another study was an international qualitative ethnographic study12 of PH patients and their daily life which included observation of adherence. The self-selected participants were directly observed to have “high” adherence although the authors did not define what high adherence meant. Additionally, they were only able to observe participants for up to 6 h each, which in many cases may have been less than one dose interval.
Current data on adherence to PH medicines are limited and the different study methodologies used do not permit robust conclusions. The following study aimed to quantify adherence to specialist PH medicines. It also sought to address a gap in our current state of knowledge regarding the usefulness of adherence measurement tools in a PH population and uncover medication and patient factors that can predict adherence.
Methods
This study aimed to measure the level of adherence to specialist PH medicines present within the PH population at a single specialist UK treatment center. The study used the WHO’s definition of adherence:8 “the extent to which a person’s behavior – taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a healthcare provider.”
The European taxonomy stage of adherence13 under investigation was implementation. The study used an observational methodology as there was no overall published description of adherence in PH at the time.
Participants for the study were all patients of Papworth Hospital, a 240-bed specialist heart and lung hospital situated in Cambridgeshire, UK, which cares for over 800 patients with PH, approximately half of whom are on specialist PH medication.3 The participants were selected by screening for inclusion characteristics within a Papworth Hospital PH patient database.
Participants were adult PH patients receiving at least one targeted PH therapy (Table 1) on 1 July 2014. Macitentan tablets were not originally included in the inclusion criteria as they were not a funded PH medicine in the UK until several months after July 2014. However, some patients transitioned on to macitentan during the data collection phase and these data were included. Selexipag and riociguat were not available therapies in the UK at the time of the study and are not included. Nebulized treprostinil was only available for a small number of patients as continuation therapy after participation in previous clinical trials. All therapies were prescribed by the Pulmonary Vascular Disease Unit team at Papworth Hospital and were delivered by a homecare company (Healthcare at Home or Bupa Home Healthcare). Homecare companies are commercial pharmacy companies that are involved in the dispensing and delivering of high-cost, specialist, or restricted supply chain medicines to patients in England. The medicines are funded by the government health system and are provided free of charge to patients. Patients without capacity to consent or on current PH trial medication were excluded unless they were merely continuing on unlicensed medicines post trial completion.
This quantitative cross-sectional study utilized an adherence questionnaire. There are many questionnaires in the literature that measure medication adherence behaviors for various health conditions, although none are validated for use in PH. The Morisky Medication Adherence Scale-8 (MMAS-8) questionnaire was chosen for this study over other possible candidates (such as the Adherence to Refills and Medication Scale14 or Beliefs about Medicines Questionnaire15) because it is well validated against blood pressure readings16 and pharmacy refill data17 for measurement of medication adherence among systemic hypertension patients. It has a reported internal reliability (Cronbach’s alpha = 0.83) that is within acceptable limits5 and includes questions that help distinguish between intentional and non-intentional adherence.
Data were collected between July 2014 and February 2015. Three data collection tools (The MMAS-8 questionnaire, pill count data obtained from homecare companies, and postal surveys [separate surveys from the MMAS-8] which include self-reported pill counts) were initially used; however, the homecare data and postal surveys proved unfeasible and are not presented here. More than one data collection tool was chosen to satisfy study design recommendations from the WHO and others who advocate multi-method approaches to investigating adherence.8,9
Participants completed the MMAS-8 questionnaire either in hospital during their next hospital appointment or at home via post. Participants received one reminder letter if they did not return the questionnaire within three weeks.
An adherence score was calculated by the participant’s answers to questions 1–8. As per Morisky,16 a score of <6 indicated low adherence, a score 6– < 8 indicated moderate adherence, and a score of 8 indicated high adherence. For statistical tests which required dichotomized results, two different definitions of adherence were used for comparison. Within these definitions, either scores of ≥6 or scores of 8 were deemed to indicate adherence.
Two additional questions (questions 9 and 10) were used to collect data on the independent variables “use of carer to help with medicines” and “use of a compliance aid” (including dosette systems or pill organizers) to confirm whether these factors may predict adherence.
Data on patient demographics, PH sub-diagnosis, and start date of specialist therapy were taken from a clinical database. Further data on each participant’s number of concurrent medicines and current co-morbidities were obtained from electronic copies of the participants discharge summary that were filled in by medical staff as part of routine care. The discharge summary that was closest in time to the date that the MMAS-8 was filled in was used, whether this was before or after the MMAS-8 date. Co-morbidities were collated from the participants’ “diagnoses” listed within the discharge summary. For the purposes of this study, co-morbidity was defined as “a health problem currently affecting the participant.” As such, diagnoses for past medical problems that had since resolved were not included. Answers to individual MMAS-8 questions were analyzed to evaluate if forgetfulness (questions 1 and 8) or side effects (question 3) attributed to non-adherence.
To uncover the predictors of adherence, two different binary logistic regression analyses were conducted with different definitions of adherence as the dependent variable. The two ways of defining adherence included: (1) MMAS-8 score of ≥6; and (2) MMAS-8 score equal to 8 only.
An MMAS-8 cut-off point of 6 allows investigation into predictors of low adherence vs. moderate to high adherence, while a cut-off point of 8 separates high adherence from low to moderate adherence.
The independent variables entered into each analysis included: medication regime (phosphodiesterase type 5 inhibitor [PDE5i] monotherapy, endothelin receptor antagonist [ERA] monotherapy, ERA + PDE5i, nebulized iloprost + PDE5i, intravenous/subcutaneous prostanoid + PDE5i, “trial drug” + ERA ± PDE5i); monotherapy vs. dual (or triple) therapy; currently taking unlicensed “trial” medicines; administration frequency of the specialist PH medicine (for participants on monotherapy); administration frequency of the most frequent PH specialist medicine in the participant’s regime (hereafter called maximum frequency) (all participants); age; gender (sex); WHO class; diagnosis; sub-diagnosis; number of co-morbidities; psychological illness as a co-morbidity; psychological illness as a co-morbidity or taking psychiatric medicine; number of concurrent medicines (including PH therapies); length of time on PH specialist medicine (longest time was chosen if on more than one therapy); use of a compliance aid; and help from other people (e.g. family) with organizing medicines.
Statistical tests
A computer statistical software package (IBM SPSS Statistics version 22) was used for performing the statistical analysis. Descriptive statistics used to explore the adherence level of the sample included means, standard deviations, medians, and interquartile ranges (IQR). Descriptive statistics were also used to explore other sample characteristics such as types of medication taken, duration of treatment, and number of co-morbidities. The two different binary measures of adherence were used as dependent variables in logistic regressions with demographic, medication, and patient-related factors used as predictor variables. Participants with missing data were excluded from statistical tests involving the missing variable. Regression models were obtained by utilizing a stepwise backward regression to find factors that reached a significance of P < 0.1. Factors were then manually entered to regression models to find a parsimonious model where all factors in the model achieved a significance of P < 0.05.
Ethics and patient consent
Ethical approval for the study was given by the NRES Committee North West – Greater Manchester East Research Ethics Committee (reference 14/NW/0271, protocol number P1928). The study also obtained approval from Papworth Hospital’s Research & Development Department. Written informed consent was provided by all participants.
Results
A total of 420 potential participants were selected from a hospital clinical database. A number of these patients were then removed from the study for either meeting exclusion criteria or due to clinical events such as death/transplantation or due to practical/social aspects (Fig. 1).
Fig. 1.
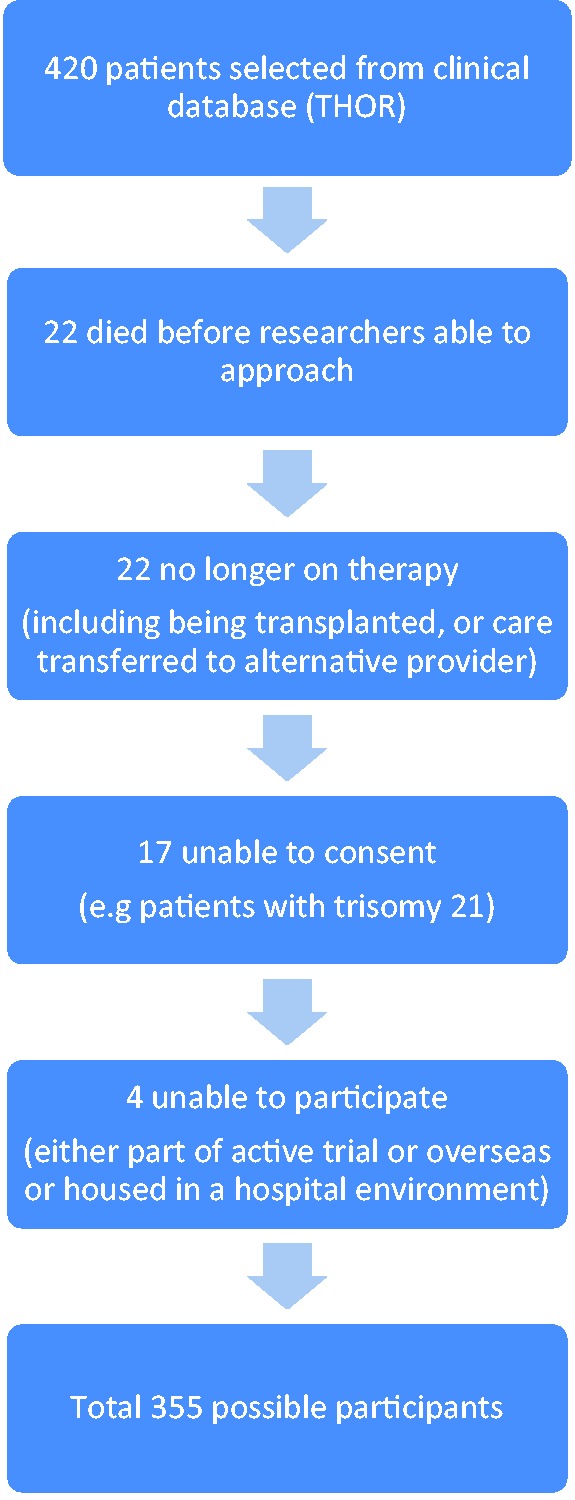
Sample selection.
Overall, 282 participants consented out of the possible 355 selected (79.4% response rate). A total of 263 participants fully completed the MMAS-8 questionnaire (93%). Study participants had a mean age of 61.6 years (±standard deviation [SD] 14.8) and 199 of them were women (70.6%). The majority of participants were in WHO functional class III (209, 74.1%) and most had sub-diagnoses of idiopathic PAH (IPAH) (63, 22.4%), PAH associated with congenital heart disease (48, 17.0%), PAH associated with connective tissue disease (37, 13.1%), or PH due to chronic thromboembolic disease (proximal 61, 21.6%; distal 35, 12.4%). The mean number of co-morbidities recorded in the patient notes was 4.5 (±SD 2.1, n = 281). Psychological illness (anxiety, depression, bipolar, schizophrenia, or dementia) was recorded in the medical notes of 17 participants (6%). Participants took a mean of 8.52 (±SD 3.68) concurrent medicines (range = 1–23).
The medication regimes used by participants are shown in Table 2. Eleven participants were discovered to be taking various unlicensed (in the UK) medicines for PH as continuation therapy after being participants in previous clinical trials. These participants are labeled as taking a “trial” medicine in Table 2. In all these cases, the associated trial had finished and participants were not part of an active ongoing trial process (with the associated increased exposure to the medical team and other impacts on adherence) but were receiving stock as a courtesy from the manufacturers.
Table 2.
Participants’ medication regime.
| PH medicine(s) | Frequency | Percent |
|---|---|---|
| Ambrisentan monotherapy | 23 | 8.2 |
| Bosentan monotherapy | 33 | 11.7 |
| Sildenafil monotherapy | 104 | 36.9 |
| Tadalafil monotherapy | 2 | 0.7 |
| Iloprost monotherapy | 1 | 0.4 |
| Epoprostenol monotherapy | 1 | 0.4 |
| ERA + PDE5i | 86 | 30.5 |
| Iloprost (nebulized) + PDE5i | 7 | 2.5 |
| IV/SC Prostanoid + ERA | 1 | 0.4 |
| IV/SC Prostanoid + PDE5i | 13 | 4.6 |
| Trial drug + ERA + /− PDE5i | 11 | 4.0% |
| Total | 282 |
Most patients had been on specialist PH medication for several years (mean time since first starting treatment was 4.8 [±SD 3.2] years, range = 2.4 months–14.4 years). For the 166 participants taking more than one therapy, dual therapy had started on average (mean) 2.9 (±SD 2.2) years previously (range = 0–9.1 years). Compliance aids such as pill organizers were used by 51.2% (144/281) of participants.
Overall adherence levels
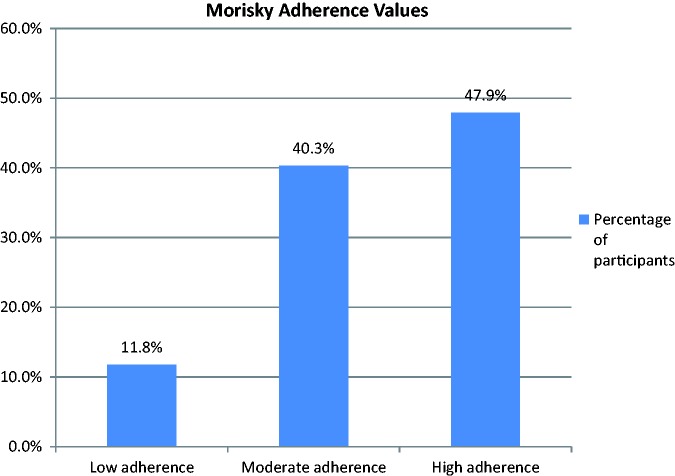
Participants’ scores from the MMAS-8 questionnaire were in the range of 2–8 (out of 8) with a median score of 7.75 (IQR = 1.00). With values grouped as recommended by Morisky,16 the number of participants scoring low, moderate, and high for adherence were 31, 106, and 126, respectively. Fig. 2 shows these in percentage terms.
Fig. 2.
Morisky adherence values (percentage).
The pattern of answers to the individual questions of the MMAS-8 can give insight into potential reasons for non-adherence. Table 3 shows the number and percentage of participants answering yes or no to each MMAS-8 question.
Table 3.
Percentage answers to each MMAS-8* question.
| Yes | No | Missing | ||
|---|---|---|---|---|
| Q1 Do you sometimes forget to take your PH medicines? | 158 (20.6%) | 223 (79.1%) | 1 (0.4%) | |
| Q2 Thinking over the past two weeks, were there any days when you did not take your PH medicine? | 23 (8.2%) | 258 (91.5%) | 1 (0.4%) | |
| Q3 Have you ever cut back or stopped taking your PH medication without telling your doctor because you felt worse when you took it? | 9 (3.2%) | 273 (96.8%) | 0 | |
| Q4 When you travel or leave home, do you sometimes forget to bring along your PH medication? | 25 (8.9%) | 256 (90.8%) | 1 (0.4%) | |
| Q5 Did you take your PH medicine yesterday? | 208 (73.8%) | 67 (23.8%) | 7 (2.5%) | |
| Q6 When you feel like your PH is under control, do you sometimes stop taking your medicine? | 3 (1.1%) | 273 (96.8%) | 6 (2.1%) | |
| Q7 Do you ever feel hassled about sticking to your PH treatment plan? | 30 (10.6%) | 249 (88.3%) | 3 (1.1%) | |
| Q8 How often do you have difficulty remembering to take all your medications? | Never/Rarely 224 (79.4%) | Once in a while 45 (16.0%) | Sometimes 10 (3.5%) | All the time 3 (1.1%) |
Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772, USA.
Predictors of adherence
Binary logistic regression analyses were conducted to uncover predictors of adherence. Low numbers in some levels of independent variables required those variables to be collapsed as recommended by Kirkwood and Sterne.18 When the collapsed variables still rendered small classes, these were excluded and models fitted to a reduced dataset.
Binary logistic regression for adherence as MMAS-8 score ≥ 6
The parsimonious model from a logistic regression to assess predictors for a MMAS-8 score > 6 contained five factors: use of a dosette box or other compliance aid; age; number of co-morbidities; maximum frequency of specialist PH medicine; and length of time on PH specialist medicine. The other factors tested did not reach significance. The full model was statistically significant (P < 0.001) with a Nagelkerke R2 of 34.4%.
The factors “age” and “number of co-morbidities” had a positive effect on adherence. For every year of increased age or each additional co-morbidity, the odds of scoring ≥6 on the MMAS-8 questionnaire (and being labeled as adherent) increased. In contrast, “use of a dosette box or other compliance aid,” “maximum frequency of PH specialist medication,” and “length of time on PH specialist medicine” had negative predictive effects on adherence (odds ratios [ORs] < 1). For every increase in administration time for the most frequent PH specialist medicine (e.g. from twice a day to three times a day), the odds of being labeled as adherent reduced by 2.54 (95% confidence interval [CI] = 1.54–4.18). Similarly, for every year spent taking a PH specialist medicine there was a corresponding decrease in the odds of being adherent by 1.16 (95% CI = 1.02–1.33).
Sub-diagnosis as a group also reached statistical significance. Participants with a diagnosis of distal chronic thromboembolic disease or PH due to connective tissue disease were 19.60 (95% CI = 3.26–111.10) and 11.76 (95% CI = 2.15–62.50) times, respectively, less likely to be adherent than participants with idiopathic disease. Participants with PH due to proximal thromboembolic disease, PAH associated with congenital heart disease, and participants with “other” sub-diagnoses were not shown to have statistically different adherence to participants with idiopathic disease.
Binary logistic regression for adherence as MMAS-8 score of 8
Logistic regression was utilized to analyze predictors to adherence as defined as MMAS-8 scores of 8 (and scores of < 8 defined as non-adherent). The variables age, maximum frequency of specialist PH medicine, and number of concurrent medicines reached statistical significance. The model was statistically significant (P < 0.001) and had a Nagelkerke R2 of 12.4%.
The factor “number of concurrent medicines” had a positive predictive effect on scoring 8 on the MMAS-8 questionnaire while negative predictive ability was associated with the maximum frequency of the specialist PH therapy and being on multiple targeted therapies. Compared to taking only one targeted therapy, the odds of being highly adherent on dual or triple therapy decreased by 1.98 (95% CI = 1.15–3.39).
Table 4 provides a summary of the various predictors of adherence against the two adherence definitions with their associated ORs.
Table 4.
Summary of predictors and associated ORs for different definitions of adherence.
| Definition of adherence → Predictor ↓ | Morisky score ≥ 6 | Morisky score = 8 |
|---|---|---|
| Dual or triple therapy vs. monotherapy | ns | 0.51 (P = 0.013) |
| (95% CI = 0.30–0.87) | ||
| Maximum frequency of therapies | 0.39 (P < 0.001) | 0.70 (P = 0.027) |
| (95% CI = 0.24–0.65) | (95% CI = 0.52–0.96) | |
| Age | 1.07 (P = 0.001) | ns |
| (95% CI = 1.03–1.11) | ||
| Sub-diagnosis (collapsed) | Various (P = 0.016) | ns |
| Co-morbidities (n) | 1.38 (P = 0.012) | ns |
| (95% CI = 1.07–1.78) | ||
| Concurrent medicines (n) | ns | 1.11 (P = 0.007) |
| (95% CI = 1.03–1.19) | ||
| Length of time on therapy | 0.86 (P = 0.023) | ns |
| (95% CI = 0.75–0.98) | ||
| Use of compliance aid | 0.36 (P = 0.036) | ns |
| (95% CI = 0.14–0.94) |
ns, not a significant predictor; CI, confidence interval.
Reliability of MMAS-8 Scale in this study
According to Morisky et al.,16 the MMAS-8 has acceptable internal consistency, with a Cronbach’s alpha coefficient reported of 0.83. In the current study, the Cronbach’s alpha coefficient was 0.53. Values >0.7 are considered to be acceptable.19
Discussion
This study set out to investigate the medication adherence of patients on PH-targeted therapies and explore the medication and patient-related factors that might influence adherence values.
The primary finding was the overall level of adherence to targeted PH therapies. A median MMAS-8 score of 7.75 (mean = 7.19, n = 263) out of 8.00 indicates a relatively high average level of adherence for the study population. Compared to other authors, the percentage of patients achieving a high adherence score in this study is broadly in agreement with results in the literature for systemic hypertension (range = 15.9–58%),16,17,20 and is slightly higher than results for patients with diabetes (range = 19–43.1%).21,22 Nevertheless, stratified into low adherence (11.8% of participants), moderate adherence (40.3%), and high adherence (47.9%), it can be concluded that a significant proportion of study participants have suboptimal adherence. The literature is not clear whether moderate adherence to these medicines is sufficient to achieve acceptable health outcomes, so it is possible that over half of these patients would benefit from improved medication adherence.
Medicine-related factors that might influence adherence
Previous authors10 have reported that increased dose frequency has a negative effect on adherence. The data from this study suggests that a related predictor—the maximum frequency of the participant’s regime—also has a measurable negative effect. Similarly, taking a combination of PH medicines compared to taking monotherapy seems to have a detrimental association with adherence. These are important findings as combination therapy has been confirmed as an important treatment strategy in PH23 and the current results suggest that to avoid the detrimental effects of combination therapy on adherence, the PH therapies chosen should have as low a frequency of administration as possible.
Patient-related and other factors that might influence adherence
The negative effect on adherence of the length of time on treatment has been observed by other adherence authors;10 however, the mild positive effect of age seen in this study is far from universal. The WHO8 and others10 have highlighted that there is no overwhelming evidence that age is generally predictive of adherence. Nevertheless, in this population, it would seem that younger patients may be the most at risk of poor adherence.
A number of adherence predictors produced unexpected results in this study. The number of a participant’s co-morbidities being associated with a positive effect on adherence seems counterintuitive. Increasing co-morbidities is usually accompanied by increased medication regime complexity which in turn can negatively affect adherence.8,10 The outcome in this study seems to refute the findings of other authors24,25 who have reported a negative relationship between adherence and co-morbidities in other conditions and make the positive relationship seen in the current study difficult to explain.
The effect of sub-diagnosis on adherence is also challenging. The results suggest that participants with a sub-diagnosis of distal chronic thromboembolic PH or PAH associated with connective tissue disease are less likely to be adherent than participants with IPAH. It is difficult to see a clear reason for this result. Some authors18 strongly caution against extrapolating too much meaning from the results of a logistic regression where categorical variables are not binary and only one or two categories are statistically significant. As such, this finding is intriguing but requires further validation.
The use of a compliance aid reducing the likelihood of being labeled as adherent was a surprising result given that many participants actively use compliance aids in order to increase their adherence. However, other authors using a four-question version of the Morisky adherence tool had similar findings with participants who used pill organizers.26 This may be due to the way the MMAS-8 measures adherence. Pill organizers are helpful for simplifying complicated medication regimes and for providing visual feedback to patients about whether a dose has been taken but most do not inherently change the medication taking behaviors (e.g. forgetfulness) that the MMAS-8 attempts to measure.
The positive effect on adherence of the number of concurrent medicines taken by the patient suggests that taking a high number of medicines is advantageous. It is possible that if patients are already in a medication-taking routine with other medicines then taking a PH specialist medicine is little extra burden. Conversely, however, this result also implies that participants taking a low number of concurrent medicines are at risk of non-adherence despite their simpler regimes. This is in contrast with other authors who have predominantly either found no effect or a negative effect with the number of prescribed medicines.10 There is also disparity with this study’s finding that participants on PH monotherapy were more likely to be adherent than those on a combination of PH treatments. Most participants on dual or triple therapy had three times daily sildenafil as a co-medicine and it is possible that the maximum frequency effect discussed previously was influencing the negative effect of combination treatment. It is also possible that an unknown confounder is playing a role.
A number of factors did not predict adherence, including gender, disease severity (WHO functional class), diagnosis, and help from others. These are in keeping with the mixed outcomes of other research.8,10
Limitations of the study
The study relies on self-reported data which cannot be independently verified. It must be assumed that participants filled in the questionnaire as the researchers intended. Other authors have highlighted that participants can overestimate their adherence with self-reported tools and can suffer from poor recall of behavior.5,6,8,9 In addition, the three-month period measured in this study may not be reflective of the participants’ adherence over a longer time period.
Participation bias cannot be ruled out in this patient population as the sampling technique was a convenience sample of all possible participants at the study center. Certain characteristics, such as English language ability may have been under-/overestimated and patients who were non-adherent may have been less likely to participate. Participants’ number of co-morbidities and concurrent medicines were extracted from discharge summaries by a single operator with their own interpretation biases. Discharge summaries were written by the participant’s medical team as part of routine care with no record of the sources of data.
In contrast with the reliability published by Morisky,16 the internal reliability for the MMAS-8 in this study was calculated to be poor with a Cronbach’s alpha lower than that considered acceptable. This study looked at several adherence predictors and compared them to different definitions of adherence. It is therefore possible that some of the significant findings of this study may be Type-1 errors. There are large numbers of possible predictors of adherence published in the literature, many of which it has not been possible to measure as part of this research. Some of these confounders and/or other as yet unknown predictors may have potentially played a role in the results seen.
Conclusions
The results of this research study suggest that overall adherence to PH specialist medicines is relatively high and that the MMAS-8 questionnaire, while having limitations, may be helpful in recognizing under-adherence in a clinical setting. There is, however, potential for improvement in medication adherence in this patient population and the results have suggested several factors that may be helpful in targeting patients at particular risk of under adherence. Younger age, taking high-frequency PH medicines such as sildenafil or nebulized iloprost, and having taken a targeted therapy for a long time may be particular factors increasing the likelihood of non-adherence. Other risk factors may include having a low number of concurrent medicines or co-morbidities and taking more than one PH therapy. Having a sub-diagnosis of distal chronic thromboembolic PH or PH associated with connective tissue disease may possibly increase the risk of under-adherence but further confirmation of these risk factors is required.
The results imply that it may be advantageous to regularly screen PH patients for potential under-adherence so that it can be recognized and interventions provided to improve adherence for these patients. Research into appropriate interventions is needed.
Acknowledgements
The authors would like to express their gratitude to the following people for their help with this research: The Pulmonary Hypertension Association UK, Dr Robert Mackenzie-Ross, Natalie Doughty, Nicky Speed, Fiona Page, Chris McCorquodale, Jackie Yates, Papworth Hospital Library staff and all of the patients who provided their time.
Appendix 1 – Adjustments of predictor categories for logistic regression
Below are the details of how predictor categories were collapsed:
Diagnosis - Collapsed:
“Pulmonary hypertension with unclear mechanisms” and “pulmonary veno-occlusive disease or capillary haemangiomatosis” were set to “missing”
Sub-diagnosis collapsed:
IPAH, PAH secondary to connective tissue disease, chronic thromboembolic disease (distal and proximal), and PAH secondary to congenital heart disease were kept the same. All other subcategories were reclassified as “other” (Table 5).
Table 5.
Reclassification of subcategories for logistic regressions.
| Sub-diagnosis | Frequency | Reclassified as |
|---|---|---|
| Idiopathic | 63 | Kept the same |
| Heritable – BMPR2 | 3 | Other |
| Heritable – ALK1 endoglin | 2 | Other |
| Heritable – unknown | 3 | Other |
| Drugs and toxins induced | 1 | Other |
| Connective tissue disease | 37 | Kept the same |
| HIV infection | 3 | Other |
| Portal hypertension | 6 | Other |
| Congenital heart disease | 48 | Kept the same |
| Chronic hemolytic anemia | 1 | Other |
| Pulmonary veno-occlusive disease | 5 | Other |
| Chronic thromboembolic – proximal | 61 | Kept the same |
| Chronic thromboembolic – distal | 35 | Kept the same |
| Sarcoidosis | 8 | Other |
| Pulmonary Langerhans cell histiocytosis | 1 | Other |
| Vasculitis | 1 | Other |
| Fibrosing mediastinitis | 1 | Other |
| Chronic renal failure on dialysis | 2 | Other |
| Other | 1 | Other |
Medication regime collapsed:
prostanoid monotherapy and “Prostanoid + ERA” set as “missing” – removing two participants’ data.
WHO collapsed:
Classes III and IV were collapsed into a combined Class III/IV.
Conflict of interests
Duncan Grady has received educational grants from Actelion Pharmaceuticals and Merck Sharp & Dohme Limited and advisory board honoraria from Actelion Pharmaceuticals.
Dr Pepke-Zaba and her institution have received research and education grants from Bayer plc, Merck Sharp and Dohme Limited and Actelion Pharmaceuticals. Dr Pepke-Zaba has received advisory board honoraria from Bayer plc, Merck Sharp & Dohme Limited and Actelion Pharmaceuticals.
Funding
This work was financially supported by the Pulmonary Hypertension Association UK.
References
- 1.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004; 351: 1425–36. [DOI] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 3.Health and Social Care Information Centre. Pulmonary Hypertension Association UK, National Pulmonary Hypertension Centres of the UK and Ireland Physicians Committee, NHS England, National Services Division Scotland, Welsh Health Specialised Services Committee. National Audit of Pulmonary Hypertension 2014, Leeds: HSCIC, 2015. [Google Scholar]
- 4.NHS England. Clinical Commissioning Policy: National policy for targeted therapies for the treatment of pulmonary hypertension in adults, London: NHS England, 2014. [Google Scholar]
- 5.Garfield S, Clifford S, Eliasson L, et al. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol 2011; 11: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008; 2: CD000011. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Moser DK, Lennie TA, et al. Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin North Am 2008; 43: 133–154. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action, Geneva: WHO, 2003. [Google Scholar]
- 9.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 10.Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol 2013; 4: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waxman A, Chen SY, Boulanger L, et al. Factors associated with adherence to phosphodiesterase type 5 inhibitors for the treatment of pulmonary arterial hypertension. J Med Econ 2013; 16: 298–306. [DOI] [PubMed] [Google Scholar]
- 12.Kingman M, Hinzmann B, Sweet O, et al. Living with pulmonary hypertension: unique insights from an international ethnographic study. BMJ Open 2014; 4: e004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kripalani S, Risser J, Gatti ME, et al. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health 2009; 12: 118–123. [DOI] [PubMed] [Google Scholar]
- 15.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999; 14: 1–24. [Google Scholar]
- 16.Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008; 10: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Krousel-Wood M, Islam T, Webber LS, et al. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care 2009; 15: 59–66. [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkwood BR and Sterne JAC. Essential Medical Statistics, Malden, MA: Blackwell Science, 2003. [Google Scholar]
- 19.Pallant J. SPSS Survival manual - A step by step guide to data analyis using IBM SPSS, Maidenhead: Open University Press, 2013. [Google Scholar]
- 20.Pandey A, Raza F, Velasco A, et al. Comparison of Morisky Medication Adherence Scale with therapeutic drug monitoring in apparent treatment-resistant hypertension. J Am Soc Hypertens 2015; 9: 420–426. [DOI] [PubMed] [Google Scholar]
- 21.Tandon S, Chew M, Eklu-Gadegbeku CK, et al. Validation and psychometric properties of the 8-item Morisky Medication Adherence Scale (MMAS-8) in Type 2 diabetes patients in sub-Saharan Africa. Diabetes Res Clin Pract 2015; 110: 129–136. [DOI] [PubMed] [Google Scholar]
- 22.Zongo A, Guenette L, Moisan J, et al. Predictive validity of self-reported measures of adherence to noninsulin antidiabetes medication against control of glycated hemoglobin levels. Can J Diabetes 2016; 40: 58–65. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz G, Besinque GM, Lickert CA, et al. Combination therapy in pulmonary arterial hypertension: is this the new standard of care? Am J Manag Care 2015; 21: s151–161. [PubMed] [Google Scholar]
- 24.Wong MC, Liu J, Zhou S, et al. The association between multimorbidity and poor adherence with cardiovascular medications. Int J Cardiol 2014; 177: 477–482. [DOI] [PubMed] [Google Scholar]
- 25.Rolnick SJ, Pawloski PA, Hedblom BD, et al. Patient characteristics associated with medication adherence. Clin Med Res 2013; 11: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitano F, Napolitano P, Angelillo IF. Medication adherence among patients with chronic conditions in Italy. Eur J Public Health 2016; 26: 48–52. [DOI] [PubMed] [Google Scholar]