Abstract
Advances in tissue fixation and imaging techniques have yielded increasing appreciation for the glycosaminoglycan-rich endothelial glycocalyx and its in vivo manifestation, the endothelial surface layer (ESL). Pathological loss of the ESL during critical illness promotes local endothelial dysfunction and, consequently, organ injury. Glycosaminoglycan fragments, such as heparan sulfate, are released into the plasma of animals and humans after ESL degradation and have thus served as a biomarker of endothelial injury. The development of state-of-the-art glycomic techniques, however, has revealed that these circulating heparan sulfate fragments are capable of influencing growth factor and other signaling pathways distant to the site of ESL injury. This review summarizes the current state of knowledge concerning the local (i.e. endothelial injury) and systemic (i.e. para- or endocrine) consequences of ESL degradation and identifies opportunities for future, novel investigations.
Keywords: glycocalyx, glycosaminoglycans, pulmonary endothelium, sepsis/multiple organ failure
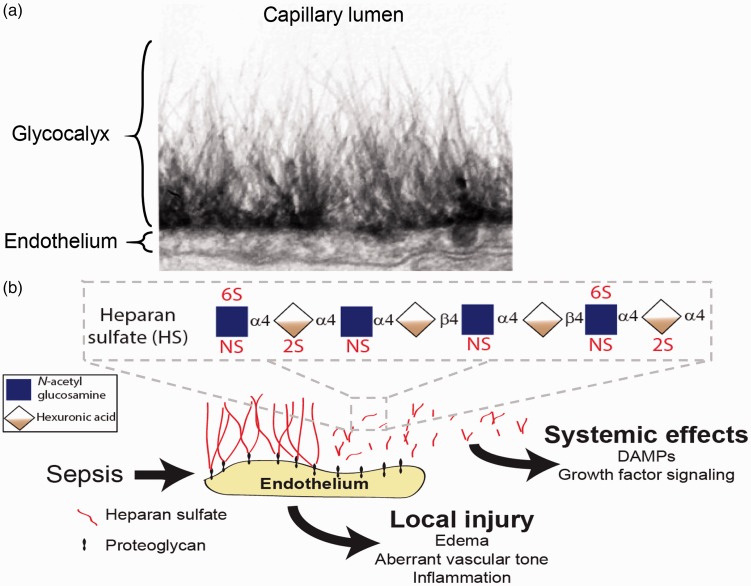
In 1966, John Luft used electron microscopy to observe a 20-nm-thick “endocapillary layer” projecting from the apical surface of mouse diaphragmatic capillary endothelial cells into the vascular lumen.1 This layer was believed to be biologically insignificant, potentially representing a vestigial remnant of the basolateral membrane reflecting onto the apical endothelial surface. In the decades since this observation, however, major advances were made in not only electron microscopy approaches, but also in endothelial-protective tissue fixation techniques.2,3 These advances allowed for the realization that this “insignificant” endocapillary layer was in fact a substantial endothelial glycocalyx (Fig. 1a) that can dwarf the size of the endothelial cell itself.4,5 Furthermore, the emerging use of intravital microscopy revealed that in vivo, the endothelial glycocalyx forms a massive endothelial surface layer (ESL), reaching thicknesses >1 µm and occupying a substantial proportion of the cross-sectional area of the vessel.6
Fig. 1.
Structure of the endothelial glycocalyx/endothelial surface layer. (a) Endothelial glycocalyx thickness is larger than the endothelial cell itself, as demonstrated by electron microscopy of ruthenium-red labeled rat myocardial capillaries. Figure used with permission from van den Berg et al. Circ Res.5 In vivo, the glycocalyx forms an even more substantial ESL, with thickness >1 µm. (b) Pathological degradation of the glycocalyx/ESL during critical illnesses (such as sepsis) causes not only local endothelial injury, but also releases biologically active heparan sulfate fragments into the circulation that may influence signaling processes in an endocrine fashion. For simplicity, chondroitin sulfate and hyaluronic acid are not shown. α4 and β4 refer to glycosidic bonds connecting constituent saccharides. Inset: structure of a heparan sulfate octasaccharide fragment, demonstrating potential sites of sulfation within constituent disaccharide units.
This increasing appreciation of ESL size, coupled with the development of novel techniques capable of interrogating ESL function, has led to an explosion of interest in the impact of ESL integrity on both vascular homeostasis and disease. This review seeks to summarize the current state of knowledge (and identify opportunities for further investigation) concerning ESL structure during health, the impact of ESL degradation on endothelial dysfunction during critical illness, and the emerging appreciation for the paracrine and endocrine signaling capabilities of ESL fragments released during vascular injury. Particular attention will be given to the critical roles played by the glycosaminoglycan heparan sulfate (HS) in the maintenance of ESL integrity and the local and systemic responses to endothelial injury.
The importance of the ESL in endothelial health and injury
As suggested by the name “glycocalyx” (a “sugar layer”), the ESL is enriched in glycosaminoglycans including HS, chondroitin sulfate (CS), and hyaluronic acid (HA).2 HS and CS are anchored to the endothelial surface via covalently binding to cell surface proteins known as proteoglycans.7 HS represents the most common ESL glycosaminoglycan, with HS proteoglycans (HSPGs) accounting for 50–90% of endothelial-associated proteoglycans8 (Fig. 1b). Additionally, HA intercalates itself throughout the ESL via non-covalent interactions with cell surface proteins such as CD44.2 These glycosaminoglycans have the ability to sequester water,9 imparting substantial size and measurable rigidity to the ESL.10 The lungs, known to have particularly high concentrations of HS,11 feature a uniquely thick ESL (1.6–1.7 µm) compared to other vascular beds (e.g. 0.6 µm in the cremaster or mesentery).12,13
This substantial size and gel-like consistency enables the ESL to serve several critical functions relevant to vascular homeostasis:6
Barrier function. By virtue of its enrichment in highly sulfated glycosaminoglycans (such as HS and CS), the ESL forms a negatively-charged fiber “mesh” that overlies cell–cell junctions, limiting protein permeability and (in accordance with Starling forces) opposing fluid flux out of the vascular lumen.14 Accordingly, enzymatic degradation of HS15 and HS-associated proteoglycans16 from isolated perfused vessels can lead to endothelial barrier dysfunction. Comparatively less is known about the role of ESL CS in transvascular fluid flux.
Nitric oxide synthesis. The ESL projects into the vascular lumen and may deform in the presence of shear stresses that accompany increases in vascular flow. This stress is transduced into the endothelial cell, triggering induction of endothelial nitric oxide synthesis (eNOS).17 The subsequent NO-mediated vasorelaxation allows for accommodation of the increased vascular flow responsible for the inciting shear forces. Enzymatic degradation of HS and HA (but not CS) led to loss of flow-mediated dilatation in vivo.18,19
Leukocyte-endothelial adhesion. An intact ESL projects into the vascular lumen beyond the span of cell-surface adhesion molecules.2 Furthermore, its gel-like consistency may impart resistance to penetration by circulating leukocytes. Accordingly, the ESL is anti-adhesive, opposing leukocyte-endothelial adhesion in both systemic20,21 and pulmonary12 vascular beds. Paradoxically, the intact ESL may promote leukocyte rolling (an important step in adhesion in non-pulmonary vascular beds) by serving as a ligand for L-selectin as well as promoting chemokine availability.22 It is unclear how these findings are reconciled with the pro-adhesive effects of enzymatic HS degradation observed in vivo.
Local endothelial impact of ESL degradation during critical illness
Given that an intact ESL contributes to the maintenance of endothelial barrier function, mechanotransduction of shear stress, and prevention of leukocyte-endothelial adhesion, pathological degradation of the ESL would be expected to induce tissue edema, microcirculatory tone dysfunction, and inflammation. These putative consequences of ESL degradation mirror the known pathophysiologic stigmata of several critical illnesses, including sepsis, the acute respiratory distress syndrome, trauma, and ischemia-reperfusion injury. Indeed, animal and human studies have demonstrated a pathogenic role of ESL degradation in the onset of vascular injury during these disease states. Sepsis-associated induction of heparanase triggers degradation of vascular HS, leading to the collapse of the pulmonary12,23 and renal24–26 ESL. The downstream consequences of this degradation appear to vary based upon the affected vascular bed. While pulmonary ESL loss contributes to lung injury via promotion of lung edema and neutrophil adhesion,12,17,23 we observed that heparanase-mediated glomerular HS degradation induces an early loss of glomerular filtration in the absence of kidney edema or inflammation,24 potentially indicating a role for ESL integrity in the control of afferent and efferent glomerular tone (and, consequently, glomerular filtration pressure). These and other local consequences of ESL degradation (as well as the potential roles played by other “sheddases”) have been extensively reviewed elsewhere.13,27,28
Importance of HS structure in cell signaling
While significant effort has been dedicated to understanding the local effects of ESL loss as a mediator of injury to the underlying endothelium, little is known about the distant, systemic consequences of this degradation. ESL loss has been demonstrated to release glycosaminoglycan fragments into the circulation.29–31 While these circulating fragments have been largely regarded as either simple biomarkers of endothelial injury27 or potentially damage-associated molecular patterns,32 emerging data indicate that circulating HS (enriched in highly sulfated hexa- to octasaccharides29) may have a significant impact on both local and systemic signaling pathways.33 These effects may be long-lasting, given that circulating HS fragments may persist for >5 days in patients with respiratory failure.29 Understanding paracrine/endocrine-like effects of circulating HS fragments requires an understanding of HS structure, with particular attention to the localization of sulfation within the disaccharide units that comprise a HS chain.
Heparan sulfate structure
HS is a linear polysaccharide, composed of repeating disaccharide units of N-glucosamine and a hexuronic acid (either glucuronic acid or iduronic acid). This structure of HS is ubiquitous and is conserved across both invertebrates and vertebrates, suggesting evolutionarily important biological functions.34 Although the precise size of HS in vivo is uncertain, chain length is estimated to be in the range of 50–200 saccharides.35,36 As detailed later in this review, constituent disaccharides may be sulfated at distinct sites, enabling a substantial variety of possible HS structures, as determined by variables such as chain length and disaccharide sulfation pattern.37 Indeed, this potential structural heterogeneity is so great that it is feasible that no two glycosaminoglycans in the body are identical.38 Despite this variability, there are organ-specific trends in HS lengths and compositions.36 Despite these organ-specific similarities in HS, there still remains heterogeneity of HS across individual organ substructures, such as the lung airways and alveoli.39 Adding to this complexity, there may be temporal shifts in HS structure, as HS length and sulfation may be dynamically modified in response to cellular and environmental cues.7 The development of new analytical techniques has allowed for an increasing appreciation of the staggering complexity of HS structure and its putative biological relevance.
HS synthesis largely occurs within the Golgi apparatus.40 HS biosynthesis begins with the xylosyltransferase-mediated addition of an anchoring xylose (from a UDP-xylose donor) onto a serine residue of a putative proteoglycan. The efficiency of xylosylation is controlled by the availability of UDP-xylose, xylosyltransferase activity, and other competing reactions, yielding variable numbers of glycosaminoglycan chains attached to a proteoglycan backbone.41 After xylosylation, a core tetrasaccharide is formed by the sequential addition of two galactose residues (by β1-4 galactosyl and β1-3 galactosyltransferases) followed by a glucuronic acid (by β1-3 glucuronosyltransferase).41 This tetrasaccharide then undergoes modifications such as phosphorylation and sulfation by an undiscovered process. At this point, biosynthetic processes diverge, allowing for the development of either CS or HS proteoglycans. The addition of α1-4 N-acetyl-glucosamine by exostosin-like glycosyltransferase 3 (EXTL3) commits the nascent glycosaminoglycan strand to becoming a HS proteoglycan.41 Subsequent polymerization of HS with alternating N-glucosamine and hexuronic acid are catalyzed by a complex of exostosin 1 (EXT1) and 2 (EXT2).42
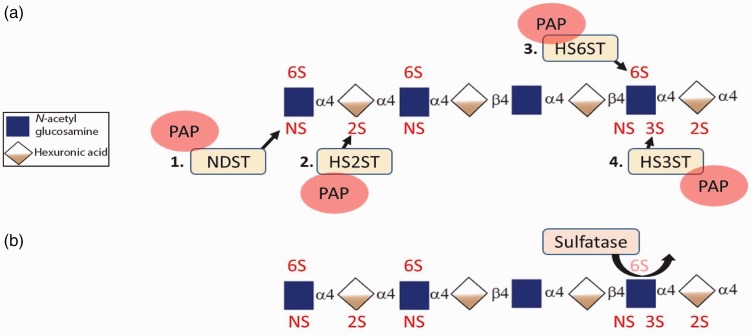
As HS polymerization progresses, there is immediate (and coordinated) modification of constituent disaccharides including sulfation and epimerization (Fig. 2). N-glucosamine may be sulfated at the N-position by N-deacetylase/N-sulfotransferases (NDSTs).42 These sites of N-sulfation are most likely to become highly sulfated regions of the mature HS polysaccharide. N-sulfation may be followed by epimerization of glucuronic acid to iduronic acid by C5-epimerase, at sites typically adjacent to N-sulfated glucosamines.42 Hexuronic acids (iduronic acid and, rarely, glucuronic acid) are then sulfated at 2-O position by 2-O sulfotransferases (HS2ST), which stops further epimerization of glucuronic acid.41 Sulfation of N-glucosamine residues at the 6-O position follows, catalyzed by heparan sulfate-6-sulfotransferases (HS6ST). The final step of HS modification is the rare (but important) 3-O sulfation of glucosamine residues by 3-O sulfotransferases (HS3ST). Despite the rarity of 3-O sulfation, HS3STs represent the largest family of sulfotransferase enzymes.43 These modifications of HS occur in clusters along the chain, separated by regions devoid of sulfation.41 Accordingly, HS chains may be geographically divided into NS domains (N-sulfated), NA domains (N-acetylated), and NS/NA domains (mixed).41
Fig. 2.
HS sulfation patterns and catalyzing enzymes. (a) Sulfate groups from the high energy donor, 3’-phosphoadenyl-5’-phosphosulfate (PAP) are transferred to specific positions of HS. Sulfation generally progresses in order as depicted. (b) HS is further modified post-synthetically at cell membrane and extracellular space by sulfatase which specifically cleaves 6-O sulfates from N-glucosamine residues. NDST, N-deacetylase/N-sulfotransferase; HS2ST, heparan sulfate-2-sulfotransferase; HS6ST, heparan sulfate-6-sulfotransferase; HS3ST, heparan sulfate-3-sulfotransferase; α4 and β4: glycosidic bonds.
After completion of polymerization, sulfation, and epimerization, HS proteoglycans are transported from the Golgi to the cell membrane, where they contribute to the endothelial glycocalyx or other extracellular matrix structures. At this point, HS can be further modified by either heparanase-mediated cleavage or selective removal of 6-O sulfates by sulfatase-1 and -2.44,45 Notably, other sites of sulfation (N-, 3-O, 2-O) do not have known extracellular sulfatases, suggesting an evolutionarily conserved function of dynamic regulation of HS 6-O sulfation.
Functional significance of HS sulfation
The importance of HS synthesis and modification is demonstrated not only by the redundancy of enzymes in the aforementioned biosynthetic processes, but also by the fact that mutations that interfere with these processes have dramatic phenotypes. Indeed, Ext1 null mice (which lack the ability to polymerize HS) are embryonically lethal,46 and human mutations in EXT1 and 2 cause hereditary multiple exostoses, a disorder characterized by the formation of multiple cartilaginous benign tumors in bones.47
Similarly, the importance of HS sulfation is demonstrated by the critical roles played by sulfotransferases in development. While D. melanogaster and C. elegans only have one ortholog of NDST, vertebrates have four isoforms of NDST.48 Vertebrate isoforms of NDSTs are differentially expressed in a tissue-specific and developmental stage-specific manner, and most cells express two isoforms.49 NDST-1 is widely expressed and its deficiency in mice is lethal.50 Most NDST-1 deficient mice survive to birth, but become cyanotic and die within 10 h from neonatal respiratory distress.50,51 Other NDST-1 knockout mice die prenatally with various severe defects in skull and eye development.48,52 In NDST-1 deficient mouse embryos, abnormal vascular development is evident. This results from the inability of PDGF-BB to bind to HS, which causes impaired pericyte recruitment.53 Deletion of NDST2 in mice yields an abnormal phenotype in mast cells, which is the site of heparin production.48,54,55 Double knockout of NDST-1 and 2 is embryonically lethal.48,56 However, mutation of NDST3 or NDST4 does not appear to affect development, yielding nearly normal and fertile mice, suggesting that loss of these isoforms is well-compensated by other isoforms.57,58
Other sites of sulfation similarly have importance in development. Germline deletion of HS2ST (responsible for 2-O sulfation) in mice is also lethal.59 These mice survive until birth, but die perinatally due to kidney agenesis.59,60 Deletion of HS2ST is also shown to affect FGF2 signaling in brain development.61 There are three isoforms of HS6STs, which are responsible for 6-O sulfation.62,63 Most HS6ST-1 deficient mice die during embryonic and perinatal stages, and the mice that survive show developmental abnormalities such as reduction of vasculature in the placenta, mediated by reduction of Wnt signaling, and enlarged alveoli in lungs.64 Vascular branching pattern defects have been reported in HS6ST-2 knockout zebrafish.65
The importance of 3-O sulfation is reflected by the presence of seven (seemingly redundant) HS3ST isoforms in vertebrates. HS3ST-1 and HS3ST-5 are necessary to create the pentasaccharide sequence essential for antithrombin binding, as described later in this review.66,67 Knocking out HS3ST-1 causes postnatal lethality that is genetic-background-dependent.68 No obvious phenotype was reported in HS3ST-2 knock out mice.69
Finally, knocking out sulfatase-1 and 2 (that selectively remove 6-O sulfation from mature HS proteoglycans post-synthetically) results in viable mice, but they show postnatal growth defects and an enlarged, dysfunctional esophagus, and double knockout results in reduced fertility.70
Impact of HS sulfation on cellular signaling
The importance of HS sulfation to development has been attributed to the influence of HS on growth factor signaling. One of the well-described pathways that illustrates the importance of HS sulfation on cellular signaling is the fibroblast growth factor (FGF) pathway.42 HS facilitates FGF signaling (thereby promoting cell migration, proliferation, and differentiation) by binding to FGFs and their cognate FGF receptors (FGFRs), bringing these ligands and receptors into close proximity.71 Alternatively, HS binding may sequester FGFs away from their receptors, creating a repository that can be released by HS degradation during physiological or pathological stimuli.71 The interaction between HS and FGF2 required HS sequences of five to six saccharides enriched in N-sulfation of glucosamine and 2-O sulfation of iduronic acid.72,73 In fibroblasts with impaired heparan sulfate synthesis, Guimond et al. reported that FGF-induced mitogenic activity required HS > 10 saccharides in length with 2-O and 6-O sulfation.74 Heparin (a highly sulfated variant of HS) depleted of 6-O sulfation competitively inhibited FGF-2 mediated mitogenic activity. Furthermore, FGF-1 or FGF-4 activity was sensitive to saccharides of various sulfation patterns.74,75 In vivo, FGF2-mediated angiogenesis within chick embryos was inhibited by excess 6-O desulfated heparin, attributed to binding and sequestering of FGF2.76 Binding of FGFR to HS is also required for effective signaling and is dependent upon 6-O sulfation of glucosamine.77
Antithrombin is another well-described example of a protein whose function is shaped by electrostatic interactions with HS. Antithrombin inhibits thrombin, Factor Xa, and other procoagulant factors in a slow, progressive manner.78 Antithrombin activity is accentuated when bound to specific forms of HS (such as heparin), but a specific pentasaccharide sequence with 3-O sulfation of glucosamine residues is a requisite for this interaction to occur. This sequence variant is observed in only 1–10% of glycosaminoglycans.42,78–80 The inhibitory effect of antithrombin on Factor Xa only requires interaction with this pentasaccharide HS sequence,81 whereas an additional, separate hexasaccharide sequence is required for accelerating inactivation of thrombin.42
Systemic consequences of circulating HS fragments released during ESL degradation
Endothelial glycocalyx degradation, as happens during sepsis, ischemia-reperfusion injury, and other hyper-inflammatory states, induces not only local endothelial dysfunction (as described previously) but also releases fragments of HS and HSPG extracellular domains (ectodomains) into the circulation.29,31,82 These now-soluble factors are thought to be biologically active, having the ability to affect endothelial signaling processes in an autocrine, paracrine, and/or endocrine manner.83 As described above, HS (both soluble and cell-surface bound) is able to bind to over 100 different signaling mediators in a oligosaccharide length and sulfation-dependent manner, in part through electrostatic interactions between negatively charged sulfate groups on HS and positively charged amino acids within signaling mediators.84 These interactions between HS and proteins (i.e. signaling ligands and receptors) allow soluble HS to both positively and negatively regulate various endothelial signaling pathways. While there are few studies that describe the effect of circulating HS and HSPGs on endothelial signaling in vivo, there are several in vitro studies that demonstrate the ability of soluble HS and HSPGs to affect endothelial cell signaling.
As previously noted, endothelial growth factor signaling is heavily influenced by the presence or absence of HS. The effect of HS on endothelial FGF and vascular endothelial growth factor (VEGF) signaling have been the most extensively investigated. Given sufficient sulfation and chain length, HS binds both growth factor ligands and receptors and acts as a scaffolding molecule to facilitate growth factor ligand-receptor binding.84 Indeed, the formation of a HS-growth factor ligand-growth factor receptor ternary complex is required for receptor activation and downstream signaling of several pathways including FGF2-FGFR1 signaling.85 However, despite HS being a requirement for several signaling pathways, soluble HS and HSPGs can both activate and inhibit FGF and VEGF signaling under different circumstances.
At low concentrations, soluble HS is thought to activate FGF2 and VEGF signaling by facilitating ligand-receptor binding as previously described.86–88 However, at high concentrations, soluble HS compete to bind both growth factor ligand and receptor, reducing the formation of ternary HS-growth factor ligand-growth factor receptor complexes and inhibiting downstream signaling.89 Furthermore, if HS is present on the endothelial cell surface, as occurs in healthy endothelial cells and endothelial cells with retained and recovered glycocalyces, soluble HS can also compete with endothelial cell-surface HS for growth factor ligand, sequestering the ligand away from its cognate receptor and inhibiting signaling.90
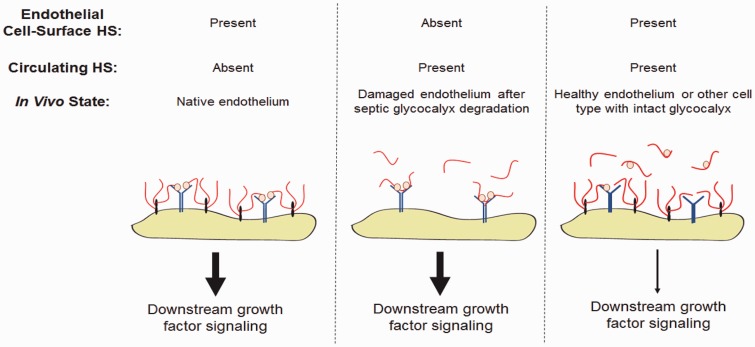
As such, the effect of circulating HS may not only depend on the presence of biologically active fragments (with activity determined by specific sulfation pattern and length), but also the absence of competing cell-surface HS (Fig. 3). We recently reported that circulating HS has the ability to activate endothelial FGF2 signaling in mice after septic endothelial glycocalyx degradation, facilitating endothelial glycocalyx recovery.83 The effect of circulating HS on FGF2 and VEGF signaling on endothelial cells with a replete ESL is uncertain. Directly testing this hypothesis is limited by the absence of an intact ESL in cultured cells;91,92 thus, most in vitro models would be expected to approximate an injured, ESL-degraded endothelial surface as opposed to a healthy endothelium.
Fig. 3.
Growth factor signaling may be shaped by both cell-surface and soluble HS. During homeostasis, cell-surface HSPGs provide cis-activation of growth factor signaling by stabilizing ligand-receptor interactions. In the absence of cell-surface HSPGs, this activation may be salvaged by the presence of soluble HS fragments of sufficient size and sulfation to engage growth factor ligands and receptors. In the presence of both cell-surface HSPGs and circulating HS, we hypothesize that the excess of HS sequesters ligands, attenuating downstream growth factor signaling.
While there are many studies that highlight the varied effects of HS on endothelial growth factor signaling, there are far fewer publications studying the effect of soluble shed HSPG ectodomains on endothelial FGF and VEGF signaling and angiogenesis. However, from these limited studies it appears that soluble HSPGs also have the ability to both activate and inhibit endothelial growth factor signaling. In a study by Kato et al., the soluble extracellular domain of syndecan-1 (a HSPG) was shown to inhibit FGF2 signaling and cell proliferation; however, heparitinase treatment of the ectodomain transformed syndecan-1 into a facilitator of FGF2 signaling.93 The authors suggest that the degradation of undersulfated HS regions and release of heavily sulfated HS domains from the syndecan-1 ectodomain allow the freed heavily sulfated HS to bind and facilitate FGF2-FGFR1 signaling.93 Similarly, another group has recently shown that shed syndecan-2 inhibits angiogenesis, although the inhibitory effect of syndecan-2 studied may be independent of the HS chains on syndecan-2 and growth factor signaling.94 In contrast to these studies, Purushothaman et al. have reported that shed syndecan-1 can enhance endothelial cell invasion and angiogenesis. In this study, the authors show that while both the syndecan-1 core protein and the associated HS may each be partially responsible for this effect, that the HS attached to the shed syndecan-1 enhances VEGF signaling in endothelial cells, as this effect can be abolished by HS degradation from syndecan-1 by heparinase-III.95 Although the studies performed by Purushothaman et al. and Kato et al. examine the effect of syndecan-1 associated HS on signaling initiated by two different growth factor ligands, VEGF and FGF2, these findings starkly contradict each other. As such, more research is needed to understand the complex role of shed HSPG ectodomains on endothelial growth factor signaling and elucidate the distinct roles of the HSPG core protein, the associated HS, and HS after cleavage from the core protein.
A second family of signaling mediators that HS is known to regulate is inflammatory signaling mediators, thereby affecting endothelial cell activation and inflammation. As is seen with growth factor signaling, HS may both enhance and reduce inflammatory signaling depending on its mode of action. HS is thought to enhance inflammation by binding to interferon-γ (IFN-γ) and protecting it from degradation, thus increasing its half-life and activity in circulation.96 Additionally, HS fragments generated by heparanase digestion are thought to act as damage-associated molecular pattern (DAMP) ligands themselves, binding to Toll-like receptor 4 (TLR-4) and increasing pro-inflammatory cytokine release.32 Furthermore, HS in serum from septic shock patients has been shown to induce mitochondrial dysfunction in cardiomyocytes in a TLR-4 dependent manner.97
Alternatively, soluble HS is also able to reduce inflammation by binding and inhibiting downstream signaling of circulating inflammatory mediators, including histones and high mobility group protein B1 (HMGB1), in a mechanism similar to that of high concentration soluble HS-inhibited growth factor signaling. During tissue injury, dying cells and neutrophils (via neutrophil extracellular traps) release nuclear proteins, including histones and HMGB1, which, when extracellular, can be detected as DAMPs. Endothelial cell-surface HS is known to facilitate the binding of both histones and HMGB1 to the cell surface where they can signal through TLR-2, TLR-4, and/or the receptor for advanced glycation end products (RAGE) to enhance inflammatory signaling and cytokine release.98–101 Preliminary data suggest that soluble HS, like HS released during endothelial glycocalyx degradation, can intercept HMGB1 and histones and prevent their binding to the endothelial cell-surface.100,102,103 In a model of histone-induced lung injury, we recently reported that HS attenuates lung injury initiated by intravascular histone injection; however, this affect was observed with HS oligosaccharides incapable of directly binding histones, suggesting that there may be alternative protective roles of HS against histone-induced injury.103
Given their important and promiscuous roles, circulating HSPGs and HS in vivo likely impart complex, context-dependent roles in health and disease. These roles remain to be further defined.
Summary and future directions
In the past 50 years, our understanding of the ESL has evolved from being an insignificant remnant of the basolateral membrane to a critical (and massive) contributor to endothelial function. With the advent of new analytical techniques, we are poised to make additional discoveries about the significance of ESL degradation in disease: not only does ESL loss cause local endothelial dysfunction, but the release of ESL components into the circulation shapes systemic responses to critical illness. Further mechanistic investigations are needed not only to understand these systemic responses, but also to identify therapeutic targets that can improve meaningful, multi-system outcomes in critical illness.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
NIH R01 HL125371 (to EPS) and R01 GM125095 (to EPS and PSH).
2017 Grover Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the educational grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institutes of Health.
References
- 1.Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 1966; 25(6): 1773–1783. [PubMed] [Google Scholar]
- 2.Schmidt EP, Kuebler WM, Lee WL, et al. Adhesion molecules: master controllers of the circulatory system. Compr Physiol 2016; 6(2): 945–973. [DOI] [PubMed] [Google Scholar]
- 3.Ebong EE, Macaluso FP, Spray DC, et al. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioslcer Thromb Vasc Biol 2011; 31(8): 1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Teeffelen JW, Brands J, Stroes ES, et al. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med 2007; 17(3): 101–105. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res 2003; 92(6): 592–594. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Schmidt EP. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers 2013; 1(1): 23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454(3): 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ihrcke NS, Wrenshall LE, Lindman BJ, et al. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today 1993; 14(10): 500–505. [DOI] [PubMed] [Google Scholar]
- 9.Negrini D, Passi A, Moriondo A. The role of proteoglycans in pulmonary edema development. Intensive Care Med 2008; 34(4): 610–618. [DOI] [PubMed] [Google Scholar]
- 10.Wiesinger A, Peters W, Chappell D, et al. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One 2013; 8(11): e80905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledin J, Staatz W, Li JP, et al. Heparan sulfate structure in mice with genetically modified heparan sulfate production. J Biol Chem 2004; 279(41): 42732–42741. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012; 18(8): 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeger SM, Yang Y, Schmidt EP. Heparan sulfate in the developing, healthy, and injured lung. Am J Respir Cell Mol Biol 2016; 55(1): 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng 2012; 40(4): 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dull RO, Cluff M, Kingston J, et al. Lung heparan sulfates modulate K(fc) during increased vascular pressure: evidence for glycocalyx-mediated mechanotransduction. Am J Physiol Lung Cell Mol Physiol 2012; 302(9): L816–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol 1990; 428: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florian JA, Kosky JR, Ainslie K, et al. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 2003; 93(10): e136–e142. [DOI] [PubMed] [Google Scholar]
- 18.Pahakis MY, Kosky JR, Dull RO, et al. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 2007; 355(1): 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med 2006; 259(4): 339–350. [DOI] [PubMed] [Google Scholar]
- 20.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 2003; 23(9): 1541–1547. [DOI] [PubMed] [Google Scholar]
- 21.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol 2002; 283(4): H1282–1291. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Fuster M, Sriramarao P, et al. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol 2005; 6(9): 902–910. [DOI] [PubMed] [Google Scholar]
- 23.Han S, Lee SJ, Kim KE, et al. Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med 2016; 8(335): 335ra55. [DOI] [PubMed] [Google Scholar]
- 24.Lygizos MI, Yang Y, Altmann CJ, et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep 2013; 1(6): e00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt EP, Overdier KH, Sun X, et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med 2016; 194(4): 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garsen M, Benner M, Dijkman HB, et al. Heparanase Is Essential for the Development of Acute Experimental Glomerulonephritis. Am J Pathol 2016; 186(4): 805–815. [DOI] [PubMed] [Google Scholar]
- 27.Colbert JF, Schmidt EP. Endothelial and microcirculatory function and dysfunction in sepsis. Clin Chest Med 2016; 37(2): 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker BF, Jacob M, Leipert S, et al. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol 2015; 80: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt EP, Li G, Li L, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem 2014; 289(12): 8194–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson A, Berkestedt I, Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol Scand 2014; 58(1): 36–43. [DOI] [PubMed] [Google Scholar]
- 31.Nelson A, Berkestedt I, Schmidtchen A, et al. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock 2008; 30(6): 623–627. [DOI] [PubMed] [Google Scholar]
- 32.Goodall KJ, Poon IK, Phipps S, et al. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One 2014; 9(10): e109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Haeger SM, Suflita MA, et al. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol 2017; 56(6): 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros GF, Mendes A, Castro RA, et al. Distribution of sulfated glycosaminoglycans in the animal kingdom: widespread occurrence of heparin-like compounds in invertebrates. Biochim Biophys Acta 2000; 1475(3): 287–294. [DOI] [PubMed] [Google Scholar]
- 35.Fuster MM, Wang L. Endothelial heparan sulfate in angiogenesis. Prog Mol Biol Transl Sci 2010; 93: 179–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi X, Zaia J. Organ-specific heparan sulfate structural phenotypes. J Biol Chem 2009; 284(18): 11806–11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marth JD. A unified vision of the building blocks of life. Nat Cell Biol 2008; 10(9): 1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J 2006; 20(1): 9–22. [DOI] [PubMed] [Google Scholar]
- 39.Smits NC, Robbesom AA, Versteeg EM, et al. Heterogeneity of heparan sulfates in human lung. Am J Respir Cell Mol Biol 2004; 30(2): 166–173. [DOI] [PubMed] [Google Scholar]
- 40.Pinhal MA, Smith B, Olson S, et al. Enzyme interactions in heparan sulfate biosynthesis: uronosyl 5-epimerase and 2-O-sulfotransferase interact in vivo. Proc Natl Acad Sci U S A 2001; 98(23): 12984–12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esko JD, Kimata K, Lindahl U. Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, et al.(eds). Essentials of Glycobiology, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2009, pp. 229–247. [PubMed] [Google Scholar]
- 42.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 2002; 71: 435–471. [DOI] [PubMed] [Google Scholar]
- 43.Thacker BE, Xu D, Lawrence R, et al. Heparan sulfate 3-O-sulfation: a rare modification in search of a function. Matrix Biol 2014; 35: 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhoot GK, Gustafsson MK, Ai X, et al. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 2001; 293(5535): 1663–1666. [DOI] [PubMed] [Google Scholar]
- 45.Morimoto-Tomita M, Uchimura K, Werb Z, et al. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem 2002; 277(51): 49175–49185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin X, Wei G, Shi Z, et al. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol 2000; 224(2): 299–311. [DOI] [PubMed] [Google Scholar]
- 47.Nadanaka S, Kitagawa H. Heparan sulphate biosynthesis and disease. J Biochem 2008; 144(1): 7–14. [DOI] [PubMed] [Google Scholar]
- 48.Grobe K, Ledin J, Ringvall M, et al. Heparan sulfate and development: differential roles of the N-acetylglucosamine N-deacetylase/N-sulfotransferase isozymes. Biochim Biophys Acta 2002; 1573(3): 209–215. [DOI] [PubMed] [Google Scholar]
- 49.Aikawa J, Grobe K, Tsujimoto M, et al. Multiple isozymes of heparan sulfate/heparin GlcNAc N-deacetylase/GlcN N-sulfotransferase. Structure and activity of the fourth member, NDST4. J Biol Chem 2001; 276(8): 5876–5882. [DOI] [PubMed] [Google Scholar]
- 50.Fan G, Xiao L, Cheng L, et al. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett 2000; 467(1): 7–11. [DOI] [PubMed] [Google Scholar]
- 51.Ringvall M, Ledin J, Holmborn K, et al. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem 2000; 275(34): 25926–25930. [DOI] [PubMed] [Google Scholar]
- 52.Pallerla SR, Pan Y, Zhang X, et al. Heparan sulfate Ndst1 gene function variably regulates multiple signaling pathways during mouse development. Dev Dyn 2007; 236(2): 556–563. [DOI] [PubMed] [Google Scholar]
- 53.Abramsson A, Kurup S, Busse M, et al. Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes Dev 2007; 21(3): 316–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humphries DE, Wong GW, Friend DS, et al. Heparin is essential for the storage of specific granule proteases in mast cells. Nature 1999; 400(6746): 769–772. [DOI] [PubMed] [Google Scholar]
- 55.Forsberg E, Pejler G, Ringvall M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 1999; 400(6746): 773–776. [DOI] [PubMed] [Google Scholar]
- 56.Forsberg E, Kjellen L. Heparan sulfate: lessons from knockout mice. J Clin Invest 2001; 108(2): 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pallerla SR, Lawrence R, Lewejohann L, et al. Altered heparan sulfate structure in mice with deleted NDST3 gene function. J Biol Chem 2008; 283(24): 16885–16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jao TM, Li YL, Lin SW, et al. Alteration of colonic epithelial cell differentiation in mice deficient for glucosaminyl N-deacetylase/N-sulfotransferase 4. Oncotarget 2016; 7(51): 84938–84950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson VA, Gallagher JT, Merry CL. Heparan sulfate 2-O-sulfotransferase (Hs2st) and mouse development. Glycoconj J 2002; 19(4–5): 347–354. [DOI] [PubMed] [Google Scholar]
- 60.Bullock SL, Fletcher JM, Beddington RS, et al. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev 1998; 12(12): 1894–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan WK, Howe K, Clegg JM, et al. 2-O heparan sulfate sulfation by Hs2st is required for Erk/Mapk signalling activation at the mid-gestational mouse telencephalic midline. PLoS One 2015; 10(6): e0130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Habuchi H, Tanaka M, Habuchi O, et al. The occurrence of three isoforms of heparan sulfate 6-O-sulfotransferase having different specificities for hexuronic acid adjacent to the targeted N-sulfoglucosamine. J Biol Chem 2000; 275(4): 2859–2868. [DOI] [PubMed] [Google Scholar]
- 63.Habuchi H, Miyake G, Nogami K, et al. Biosynthesis of heparan sulphate with diverse structures and functions: two alternatively spliced forms of human heparan sulphate 6-O-sulphotransferase-2 having different expression patterns and properties. Biochem J 2003; 371(Pt 1): 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Habuchi H, Kimata K. Mice deficient in heparan sulfate 6-O-sulfotransferase-1. Prog Mol Biol Transl Sci 2010; 93: 79–111. [DOI] [PubMed] [Google Scholar]
- 65.Chen E, Stringer SE, Rusch MA, et al. A unique role for 6-O sulfation modification in zebrafish vascular development. Dev Biol 2005; 284(2): 364–376. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Pedersen LC. Anticoagulant heparan sulfate: structural specificity and biosynthesis. Appl Microbiol Biotechnol 2007; 74(2): 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia G, Chen J, Tiwari V, et al. Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus, type 1. J Biol Chem 2002; 277(40): 37912–37919. [DOI] [PubMed] [Google Scholar]
- 68.HajMohammadi S, Enjyoji K, Princivalle M, et al. Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. J Clin Invest 2003; 111(7): 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasegawa H, Wang F. Visualizing mechanosensory endings of TrkC-expressing neurons in HS3ST-2-hPLAP mice. J Comp Neurol 2008; 511(4): 543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ai X, Kitazawa T, Do AT, et al. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development 2007; 134(18): 3327–3338. [DOI] [PubMed] [Google Scholar]
- 71.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 2001; 22(4): 201–207. [DOI] [PubMed] [Google Scholar]
- 72.Turnbull JE, Fernig DG, Ke Y, et al. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem 1992; 267(15): 10337–10341. [PubMed] [Google Scholar]
- 73.Maccarana M, Casu B, Lindahl U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem 1993; 268(32): 23898–23905. [PubMed] [Google Scholar]
- 74.Guimond S, Maccarana M, Olwin BB, et al. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem 1993; 268(32): 23906–23914. [PubMed] [Google Scholar]
- 75.Kreuger J, Prydz K, Pettersson RF, et al. Characterization of fibroblast growth factor 1 binding heparan sulfate domain. Glycobiology 1999; 9(7): 723–729. [DOI] [PubMed] [Google Scholar]
- 76.Lundin L, Larsson H, Kreuger J, et al. Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogenicity and angiogenesis. J Biol Chem 2000; 275(32): 24653–24660. [DOI] [PubMed] [Google Scholar]
- 77.Loo BM, Kreuger J, Jalkanen M, et al. Binding of heparin/heparan sulfate to fibroblast growth factor receptor 4. J Biol Chem 2001; 276(20): 16868–16876. [DOI] [PubMed] [Google Scholar]
- 78.Weitz JI. Heparan sulfate: antithrombotic or not? J Clin Invest 2003; 111(7): 952–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atha DH, Lormeau JC, Petitou M, et al. Contribution of 3-O- and 6-O-sulfated glucosamine residues in the heparin-induced conformational change in antithrombin III. Biochemistry 1987; 26(20): 6454–6461. [DOI] [PubMed] [Google Scholar]
- 80.Marcum JA, Atha DH, Fritze LM, et al. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem 1986; 261(16): 7507–7517. [PubMed] [Google Scholar]
- 81.Petitou M, Imberty A, Duchaussoy P, et al. Experimental proof for the structure of a thrombin-inhibiting heparin molecule. Chemistry 2001; 7(4): 858–873. [DOI] [PubMed] [Google Scholar]
- 82.Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007; 116(17): 1896–1906. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y, Haeger SM, Suflita MA, et al. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol 2017; 56: 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu D, Esko JD. Demystifying heparan sulfate-protein interactions. Annu Rev Biochem 2014; 83: 129–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiao D, Meyer K, Mundhenke C, et al. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem 2003; 278(18): 16045–16053. [DOI] [PubMed] [Google Scholar]
- 86.Quarto N, Amalric F. Heparan sulfate proteoglycans as transducers of FGF-2 signalling. J Cell Sci 1994; 107(Pt 11): 3201–3212. [DOI] [PubMed] [Google Scholar]
- 87.Gitay-Goren H, Soker S, Vlodavsky I, et al. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem 1992; 267(9): 6093–6098. [PubMed] [Google Scholar]
- 88.Fannon M, Forsten KE, Nugent MA. Potentiation and inhibition of bFGF binding by heparin: a model for regulation of cellular response. Biochemistry 2000; 39(6): 1434–1445. [DOI] [PubMed] [Google Scholar]
- 89.Krufka A, Guimond S, Rapraeger AC. Two hierarchies of FGF-2 signaling in heparin: mitogenic stimulation and high-affinity binding/receptor transphosphorylation. Biochemistry 1996; 35(34): 11131–11141. [DOI] [PubMed] [Google Scholar]
- 90.Ostrovsky O, Berman B, Gallagher J, et al. Differential effects of heparin saccharides on the formation of specific fibroblast growth factor (FGF) and FGF receptor complexes. J Biol Chem 2002; 277(4): 2444–2453. [DOI] [PubMed] [Google Scholar]
- 91.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res 2008; 102(7): 770–776. [DOI] [PubMed] [Google Scholar]
- 92.Chappell D, Jacob M, Paul O, et al. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res 2009; 104(11): 1313–1317. [DOI] [PubMed] [Google Scholar]
- 93.Kato M, Wang H, Kainulainen V, et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med 1998; 4(6): 691–697. [DOI] [PubMed] [Google Scholar]
- 94.De Rossi G, Evans AR, Kay E, et al. Shed syndecan-2 inhibits angiogenesis. J Cell Sci 2014; 127(Pt 21): 4788–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Purushothaman A, Uyama T, Kobayashi F, et al. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood 2010; 115(12): 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lortat-Jacob H, Baltzer F, Grimaud JA. Heparin decreases the blood clearance of interferon-gamma and increases its activity by limiting the processing of its carboxyl-terminal sequence. J Biol Chem 1996; 271(27): 16139–16143. [DOI] [PubMed] [Google Scholar]
- 97.Martin L, Peters C, Schmitz S, et al. Soluble heparan sulfate in serum of septic shock patients induces mitochondrial dysfunction in murine cardiomyocytes. Shock 2015; 44(6): 569–577. [DOI] [PubMed] [Google Scholar]
- 98.Xu J, Zhang X, Monestier M, et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 2011; 187(5): 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park JS, Svetkauskaite D, He Q, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 2004; 279(9): 7370–7377. [DOI] [PubMed] [Google Scholar]
- 100.Freeman CG, Parish CR, Knox KJ, et al. The accumulation of circulating histones on heparan sulphate in the capillary glycocalyx of the lungs. Biomaterials 2013; 34(22): 5670–5676. [DOI] [PubMed] [Google Scholar]
- 101.Xu D, Young J, Song D, et al. Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE). J Biol Chem 2011; 286(48): 41736–41744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu D, Fuster MM, Lawrence R, et al. Heparan sulfate regulates VEGF165- and VEGF121-mediated vascular hyperpermeability. J Biol Chem 2011; 286(1): 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y, Haeger SM, Yang Y, et al. Circulating heparan sulfate fragments attenuate histone-induced lung injury independently of histone binding. Shock 2017; 48: 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]