Abstract
The capacity of existing blood vessels to give rise to new blood vessels via endothelial cell sprouting is called angiogenesis and is a well-studied biologic process. In contrast, little is known about the mechanisms for endothelial cell replacement or regeneration within established blood vessels. Since clear definitions exist for identifying cells with stem and progenitor cell properties in many tissues and organs of the body, several groups have begun to accumulate evidence that endothelial stem and progenitor cells exist within the endothelial intima of existing blood vessels. This paper will review stem and progenitor cell definitions and highlight several recent papers purporting to have identified resident vascular endothelial stem and progenitor cells.
Keywords: Endothelial stem cell, endothelial progenitor cell, vasculogenesis, angiogenesis
Somatic stem and progenitor cells provide a source of tissue-specific cellular elements that permit appropriate tissue and organ functions through replacement of injured, diseased, and senescent cells in many organ systems throughout the lifespan.1 Hematopoietic,2,3 intestinal,4,5 skin,6,7 and skeletal muscle stem cells8,9 have been identified and rigorously studied; however, little is known of the cellular and molecular mechanisms that give rise to the homeostatic repair and replacement of vascular endothelial cells. In 1997, Asahara et al.10 reported on the identification of circulating progenitor cells for the endothelial lineage. These putative endothelial progenitor cells (EPC) were bone marrow-derived cells that displayed an upregulation of “endothelial” cell surface markers and downregulation of “hematopoietic” markers during in vitro culture, suggesting that some hematopoietic stem cells (HSC) displayed the ability to transdifferentiate into EPC to regenerate endothelial cells in vitro.11,12 These putative EPCs also displayed colony-forming activity and were capable of migrating to sites of ischemic injury in vivo in animal models of human disease. Since these putative EPCs were isolated from human blood but participated in neovascularization in the tissues of injured animals, the authors proposed that the cells were engaged in postnatal vasculogenesis as a means to repair the damaged blood vessels. Numerous publications followed where various cell surface markers were identified as enriching for the putative HSCs that could transdifferentiate into EPC subsets to enhance vascular repair13,14 (reviewed in Kovacic et al.15) or could serve as a biomarker for the presence or severity of cardiovascular disease in humans (reviewed in the literature16–19).
Subsequent studies have clarified that those putative EPCs did not possess the capacity to undergo a lineage fate switch (transdifferentiate) from a hematopoietic precursor to a mature endothelial cell. Instead, a series of papers demonstrated that the putative EPCs were in fact events in which labeled donor bone marrow cells either displayed no evidence of plasticity upon rigorous scrutiny,20–22 displayed the host phenotype in tissues following transplantation via spontaneous cell fusion,23,24 were an artifact of host tissue autofluorescence,25 or that were erroneously attributed to becoming integrated in the endothelial intima at sites of injury, when detailed confocal microscopic analysis determined that donor bone marrow cells were localized in peri-endothelial sites and not integrated as endothelial cells.26 Subsequently, the putative bone marrow-derived EPCs were shown to be comprised of numerous stages of hematopoietic stem and progenitor cells (HSPC) that can serve paracrine proangiogenic functions to promote vascular repair and replacement through endogenous endothelial cell mechanisms.20,22,26–35 Indeed, these proangiogenic cells could upregulate some “endothelial cell” markers in vitro and thus give the impression they were becoming endothelial cells; however, functional analysis,36 comparative gene expression profiling,37,38 and analysis of the methylation status of promoters for key known endothelial regulatory genes39 confirmed that the proangiogenic cells remained more closely aligned to HSPC than mature endothelial cells. Thus, a recent position paper has called for the substitution of the term myeloid angiogenic cells (MAC) for the term EPC when considering the reparative role played by bone marrow hematopoietic-derived cells in the neovascularization process.31
Some reports have proposed evidence that vascular endothelial cells may also arise from resident vascular progenitor cells. During murine embryogenesis, mesoangioblasts have been identified that emerge from the dorsal aorta and give rise to endothelial, skeletal muscle, bone, cartilage, and dermal cells.40 The mesoangioblasts display many properties similar to pericytes that are important for controlling the proliferation and differentiation of capillary endothelial cells. Whether these pericytes and mesenchymal stem cells (MSC) within the media and adventitia are resident progenitor cells for all the vascular lineage components has been reviewed on several occasions.41,42 Greiner et al.43 identified stem cell antigen-1 (Sca-1) expressing precursors upon enzymatic digestion of small murine vessels that displayed potential to form endothelial cells in vitro and the endothelial cells displayed vasculogenic functions. However, the location of these cells within the vasculature was not reported. Zengin et al.44 isolated progenitor cells residing between the vascular media and adventitial layers of murine arteries and veins that possessed endothelial cell phenotypic features and capillary tube forming potential in vitro. Similar localization of resident angiogenic mesenchymal progenitor cells were identified in human thoracic aortic samples from humans .45 While interesting, the exact roles played by these resident multi-potent progenitor cells in normal endothelial repair and replacement or following injury has not been pursued or translated into human studies, perhaps due to the requirement for recovery from the host vasculature.
Thus, while some data suggest resident vascular progenitor cells may give rise to endothelial cells (among other lineages), what general evidence has been published that would suggest the endothelial intima may give rise directly to reparative or regenerative activity? Obviously, thousands of papers have been published on the topic of angiogenesis over the past three decades and it has become very clear that the systemic circulation can adapt to requirements for more vasculature or less vasculature to meet homeostatic balance or in response to tumor invasion via this mechanism.46–48 More than four decades ago, several reports highlighted the presence of proliferating endothelial cells during rodent development and that in response to vascular injury, clusters of replicating endothelial cells could be identified in areas of “high turnovers”49,50 (reviewed in detail in Yoder51). In the developing mouse embryo, outgrowth of mesoderm-derived precursors displaying endothelial markers displayed a high capacity for prolonged proliferation and ability to incorporate into developing chick embryonic vasculature, but such cells were not prospectively identified within the embryo, studied at a clonal level, or examined for chromosomal abnormalities.52 It has also long been known that among all adult murine tissues, the lung vasculature is one of the most readily amenable to use for isolation and cultivation of endothelial cells that possess proliferative potential.53–55 Several publications have highlighted the capacity for the endogenous endothelial cells within the lung vasculature to give rise to replacement endothelial cell progeny that form new blood vessels upon transplantation56,57 or return vascular function to normal following an acute challenge with lipopolysaccharide (LPS).58 Others have shown that removal of the left lung lobe in a rodent results in compensatory overgrowth of the right lung to rescue lung function, but this lung epithelial expansion is dependent upon endogenous lung vascular endothelial proliferation and vascular expansion.59–61 Likewise, active angiogenesis within the murine skeletal system is required to support osteogenesis.62 Recently, a novel population of proliferative capillaries within the bone metaphysis (1.77% of total endothelium) was identified that function as a precursor population to mediate normal growth of the bone vasculature, to respond to various stressors or metabolic perturbations to rescue damaged skeletal microvasculature, and were shown to be diminished in concentration with aging concomitant with loss of osteoprogenitor cells. These are just a few examples to illustrate the point that accumulating evidence highlights the resident vascular endothelium as a source of regenerative endothelium during development and in response to injury.
The largely unanswered question is whether sufficient evidence exists that well defined endothelial stem and progenitor cells have been identified in murine tissues and organs. What criteria have been used to define such cells? Have the various putative stem and progenitor cells been scrutinized to identify similarities or differences in their location, gene expression, fates, and responses to injury? This paper will now provide a brief overview of common definitions for somatic stem and progenitor cells and then in the light of these terms, review evidence to support the identification of resident vascular stem and progenitor cells in mouse and man.
Assays to identify endothelial stem and progenitor cells
Somatic stem cells can be simply defined as clonally proliferative, self-renewing cells, which give rise to differentiated cell types in a particular tissue or organ.1 A somatic stem cell may be multi-potent (giving rise to multiple types of differentiated cells) or unipotent (differentiating into a single lineage of mature cells). In general, somatic stem cells are located within nurturing niches in the tissue or organ for which they generate replacement progeny; for example, intestinal stem cells are present within the intestinal epithelium and skin stem cells reside within the dermis (hematopoietic stem cells are an exception as they reside in the bone marrow but their progeny are distributed throughout the blood and within tissues).2,63–68 Progenitor cells lack self-renewal potential, however, they may clonally expand prior to differentiation into a single lineage of mature cells. Like somatic stem cells, the majority of somatic progenitor cells reside within the tissues for which they provide replacement mature cells.
Multiple assays have been developed to define stem and progenitor cells that reside in different organs and tissues.1 Stem cells in any tissue must be interrogated for clonal self-renewal. This property is most stringently proven by long-term in vivo persistence with retained capacity for contribution to differentiated progeny. In the murine system, long-term contribution to a particular lineage is best demonstrated through the use of fate mapping strategies (with or without induced perturbations or injuries that require cell replacement). Transplantation assays into primary and secondary recipient hosts are powerful tools to examine clonal self-renewal and long-term contributions to particular cell lineages, but may represent highly stressful and forced expansion of low numbers of transplanted donor stem cells into a system in which endogenous progenitor and mature cells may have been drastically diminished.69–71 Progenitor cells are often measured with colony forming assays in vitro (to prove a precursor product relationship). Stem and progenitor cells may also display distinguishing cell surface markers that can be used to prospectively isolate the cells through use of monoclonal antibodies and flow cytometry or magnetic bead separation. Most recently, stem and progenitor cells from some tissues have become definable by single cell gene expression technologies to permit identification by a unique molecular signature.72–79 Presentation of some selected publications (as examples of detailed approaches to acquire sufficient evidence) identifying putative stem and progenitor cells for the vascular system follows.
To identify stem and progenitor cells for the endothelial lineage, we first need to decide what criteria should be used to define cells belonging to the endothelial lineage. Obviously, there are numerous phenotypic, morphologic, physiologic, genomic, proteomic, and functional parameters that constitute unique and characteristic behaviors of endothelial cells. The point here is that one cannot identify cells of the endothelial lineage by expression of a few cell surface markers or of a few RNA transcripts. For example, when using phenotypic markers to enrich for a stem cell population, functional evidence for enrichment of the particular stem cells must be presented as validation for any of the putative surface markers being highlighted. The best phenotypic markers would be unique and restricted to the particular stem cell being selected; however, this is often not the case and use of a panel of markers can be very useful in discriminating a particular stem cell from progenitor or mature cells within a tissue or organ. Functional evidence for the stem cell should be performed to provide evidence that the putative stem cell directly gives rise to progenitor and mature cells of that tissue or organ (may be conducted by fate mapping studies and/or transplantation as noted above). Transcriptomics and proteomics are increasingly being used at a single cell level to provide novel panels of enriched genes that are expressed by subsets of stem or progenitor cells within the same tissue or organ.72 Thus, in most instances, to fully define a stem or progenitor cell for the endothelial lineage, one must utilize a panel of phenotypic, functional, physiologic, morphologic, and “omics” information concurrently. A few selected exemplary papers that have applied many of these principles to define endothelial stem and progenitor cells will be presented.
Progenitor cells for the endothelial lineage
Patel et al.80 recently proposed several features that could be used to define resident vascular endothelial progenitor cells for the murine vasculature. In experimentally induced cutaneous wounds, most cells expressing CD34 but not CD45 concomitantly expressed CD144, strongly suggesting their endothelial nature. Within the CD34 and CD144 expressing endothelium, the relative level of expression of platelet endothelial cell adhesion molecule (CD31) and vascular endothelial cell growth factor receptor 2 (flk1) permitted discrimination of the endothelial cells into endovascular progenitor cells (EVP), transient amplifying cells (TA), and definitive differentiated cells (D). Of interest, the EVP population did not express c-kit, the mesenchymal marker CD73, or the pericyte marker CD146, but all three populations expressed Tie-2, Sca-1, and CD90 (Table 1). The EVP, TA, and D populations were identified in multiple organs and tissues with varying frequencies. Using the natural kinetics of granulation tissue formation following an experimentally induced cutaneous wound,81,82 the kinetics of appearance in the tissue, change in cell surface marker expression, and loss of certain populations with differentiation and maturation suggested a hierarchy and differentiation sequence from the EVPs which quickly moved into the tissue then changed to the TA and finally the D populations (Fig. 1). Only the EVP population was capable of giving rise to colonies of proliferating endothelium in vitro at a clonal level. While implantation of TA and D populations in Matrigel plugs for seven days was associated with markedly reduced recovery, the EVP implanted plugs formed vessels within the Matrigel in vivo. Lineage tracing experiments using the vascular endothelial cadherin reporter (Cdh5-creERt2/ROSA-YFP) mouse to label the EVP within the center of the wound demonstrated that these precursors gave rise to YFP expressing blood vessels co-expressing FLK1 and CD31. Distinct differences in gene expression were identified in the EVP compared to the D populations. While the EVP population was enriched for matrix metalloproteinases and growth factor signaling, the D population was enriched in endothelial cell surface molecules, Notch signaling target genes, and the SoxF family of genes (Sox7, Sox17, and Sox 18). Of interest, Sox18 expression was upregulated in the EVP population during the initial phases of wound healing but EVP rapidly differentiated into TA and D cells. Loss of Sox18 function studies demonstrated a defect in differentiation of EVP cells to TA and D cells in healing wounds. Thus, evidence for these different populations displaying different functional states was presented using clonogenic in vitro analysis, fate mapping studies in vivo, transplantation analysis, differences in gene expression, differences in phenotypic analysis (flow cytometry and immunofluorescence), and the requirement of certain transcription factors for the differentiation of EVP to D states.80
Table 1.
Cell surface marker expression.
| Marker | Endovascular progenitor | Transit amplifying | Definitive differentiated |
|---|---|---|---|
| VECAD | +++ | +++ | +++ |
| Tie2 | +++ | +++ | +++ |
| Sca1 | +++ | +++ | +++ |
| CD90.2 | +++ | +++ | +++ |
| CD34 | +++ | + | +++ |
| CD31 | –/+ | ++ | +++ |
| VEGFR2 | – | + | +++ |
| CD105 | – | + | +++ |
| Alpha-6 | – | + | +++ |
| CD146 | – | + | +++ |
| CD73 | – | – | ++ |
| CD45 | – | – | – |
+, positive staining; –, negative staining.
Fig. 1.
Evidence for a hierarchy of resident VESCs and progenitor cells. VESCs reside in the endothelial intima of blood vessels. Several markers for the VESCs have been proposed.71,72,74 The EVPs give rise to TA cells that produce definitive differentiated (D) cells.60 VESCs display the greatest proliferative potential while the D cells are non-proliferative.
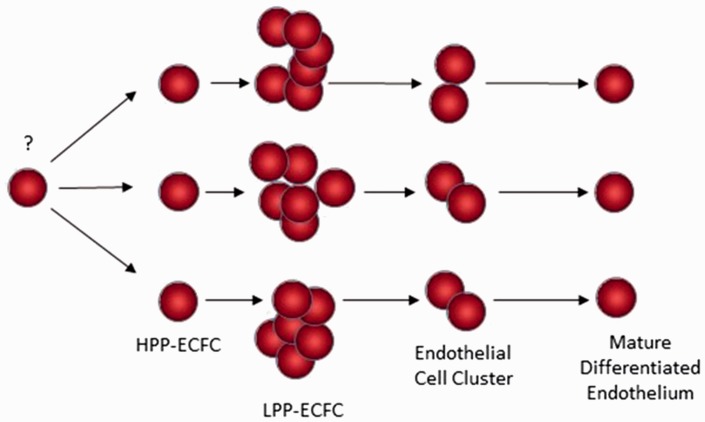
Evidence for the identification of clonogenic human endothelial progenitor cells (circulating or resident) has been published by several groups.83–86 Analysis of the circulating blood of patients following a sex-mismatched bone marrow transplant indicated that most of the circulating blood outgrowth endothelial cells (BOEC) were derived from the host, but the delayed outgrowth cells displayed the most proliferative cells and were donor derived.87 Umbilical cord blood was reported to be enriched for clonogenic endothelial colony forming cells (ECFC) that displayed a hierarchy of proliferative potential and in vivo vessel formation compared to adult peripheral blood (Fig. 2).88 The highly proliferative ECFC from cord blood expressed higher levels of telomerase than the ECFCs cultured from adult peripheral blood cells. Furthermore, resident vascular endothelial cells derived from umbilical veins or human abdominal aorta contained clonogenic ECFCs with vasculogenic potential.89 Very recently, human-induced pluripotent stem cell (hiPSC)-derived ECFCs have been reported with properties that are similar to umbilical cord blood ECFCs but with some distinct differences in gene expression.90 At present, it is unclear where the circulating BOECs and ECFCs are derived from; the most plausible source is the vascular endothelial intima, but convincing evidence is lacking to support this assertion. The lack of distinct cell surface markers to prospectively identify the circulating BOEC and ECFC has been and continues to be a significant impairment to further enrichment and development of these cells as a therapy for treating patients suffering from a host of cardiovascular disorders.
Fig. 2.
Model of a human ECFC hierarchy.68 High proliferative potential-ECFC (HPP-ECFC) display the highest proliferative potential and self-renew with replating potential. Low proliferative potential-ECFC (LPP-ECFC) form colonies greater in size than endothelial cell clusters but less than HPP-ECFC and do not possess self-renewal potential. Mature differentiated endothelial cells do not divide.
Vascular endothelial stem cells
Fang et al.91 reported that stem cells for the endothelial lineage could be isolated from lung blood vasculature. Vascular endothelial stem cells (VESC) expressing the phenotype CD31+CD105+Sca-1+CD117+ (devoid of any mature lineage markers for the hematopoietic system) could be isolated from collagenase digested lung tissue. Only the VESCs displayed clonogenic colony-forming activity in vitro at a frequency of approximately six colony-forming units per 1000 isolated lung endothelial cells. When the green fluorescent protein (GFP)-tagged VESCs were suspended in Matrigel and implanted subcutaneously in mice, the donor GFP-expressing VESCs gave rise to donor derived blood vessels. Indeed, when 15 VESC-expressing GFPs were implanted with B16 tumor tissue, GFP-labeled blood vessels were identified in primary, secondary, tertiary, and quarternary tumor implants. Even a single VESC displayed the capacity to form donor blood vessels in vivo in a Matrigel implant. When mice deficient in CD117 expression were examined for endothelial cell colony-forming activity, a significant tenfold reduction in colony formation was identified. In addition, blood vessel density in CD117-deficient mice inoculated with B16 melanoma tissue was less than half the density observed in wild type-mice with the same tumor implants. Thus, the VESCs identified by Fang et al.91 are marked by CD117 expression (Fig. 1) and loss of CD117 expression causes endothelial cell proliferation and angiogenesis defects. While some flow cytometric estimations of the frequency of the CD117-expressing VESCs in different tissues was reported, no detailed visualization of the precise location of the VESCs in arteries, veins, and capillaries in different tissues was presented. In addition, no fate mapping studies to identify the contributions of the putative VESCs within various vascular beds in homeostatic endothelial turnover was presented.
Stem cells from skin, lung, heart, mammary gland, muscle, and testis have shown evidence for expression of ATP-binding cassette (Abc) transporters that efflux noxious substrates and molecules outside the cells to maintain their viability;92 thus, Hoechst 33342 DNA binding dye rapidly enters into cells but the presence of the Abc transporters results in efflux of the dye in stem cells causing them to display diminished fluorescence (SP) and permitting their identification and isolation by flow cytometric analysis and sorting. Summer et al.93 were one of the first groups to demonstrate that adult murine lung tissue displayed a SP (0.3–0.7% of total cells) that appeared stable up through one year of age. SP cells derived from enzyme-digestion of the lung tissue were mostly blood cells (CD45+) but some non-blood cells also stained. A polyclonal antibody raised against an internal peptide sequence for breast cancer resistance protein 1 (Bcrp1; Abcg2) was used to demonstrate that SP cells were Bcrp1+. While smooth muscle cells and some distal epithelial cells expressed Bcrp1, these cells did not display a SP phenotype, suggesting that in these cells, there was no correlation between Bcrp1 expression and function to confer the SP phenotype. More recently, phenotypic lung SP cells identified as MSCs have been isolated that express Abcg2 and these MSC display properties of pericytes that contribute to fibrotic remodeling following injury.94,95 Lung SP cells isolated from embryos at embryonic day (E) 13.5–18.5 or postnatal day (P) 13–55 murine pups were shown to comprise both blood cell (CD45+) and non-blood cell (CD45–) subsets.96,97 The CD45+ lung cells also co-expressed fetal liver kinase 1 (Flk1) but did not express vascular endothelial cadherin (CD144). Both CD45+ and CD45– subsets gave rise to cells displaying phenotypic and some in vitro functional features of endothelial cells. No clonal analysis of ECFC potential, long-term contribution to lung endothelial retention, or capacity for vascular regeneration or repair by these precursor cells was presented.
Naito et al.98 have also used the Hoechst staining method and have reported the identification of resident vascular endothelial cells within the side-population (SP). Endothelial SP were identified as dormant cells in the steady state, but possessed clonal colony-forming activity, and produced large numbers of mature endothelial progeny when cultured in vitro. When transplanted into experimentally induced ischemic lesions, the endothelial SP cells restored blood flow and reconstituted de novo blood vessels at the site of injection. Although the surface markers of the SP cells was similar to primary capillary endothelial cells, the gene expression pattern of the SP, and main population (MP) that fail to retain the Hoechst dye, was significantly different. The endothelial SP cells failed to express hematopoietic markers, including c-Kit (CD117), and could not be detected in bone marrow (Fig. 1). Bone marrow transplant studies reconstituted the peripheral blood cell lineages, as would be expected, but did not contribute to vascular endothelial SP cells. Use of the Hoechst method fails to permit identification of the putative VESCs within the organ and tissue vasculature during development or after injury and will rely upon further studies to identify novel cell surface markers. Of interest, several unique cell surface markers were identified in the SP cells that may permit prospective isolation of these putative VESCs.
More recently, Yu et al.99 identified protein C receptor (Procr)-expressing endothelial cells as VESCs in the mammary fat pad, skin, and retina. Procr-expressing cells were identified in the tip and stalk cells of the pubertal stage of the mammary fat pad when robust angiogenesis accompanies rapid epithelial growth. In the adult mammary gland, Procr-expressing cells were predominantly found in the stalk cells in areas of angiogenesis. When single-cell suspensions of the mammary gland tissue were prepared and cells exposed to panels of monoclonal antibodies (all blood cells identified and excluded using CD3e, Ly6G/Ly6C, CD11b, Gr1, CD45R/B220, and Ter119) that included CD105 and CD31, approximately 4% of the vascular endothelium expressed Procr. Of interest, while > 85% of the Procr-expressing cells expressed Sca-1, only 2% of the endothelium expressed c-Kit and expression was in the Procr-negative fraction of cells. When Procr-expressing cells isolated from Actin-GFP reporter mice were suspended in Matrigel and implanted subcutaneously into host animals, significantly more vasculature was formed from cells expressing Procr than from cells not expressing Procr (this fraction contained the c-Kit expressing cells). The Procr-expressing VESCs formed capillary and larger vessels when injected into the empty fat pad of pubertal host animals. Procr-expressing VESC displayed clonal proliferative potential in vitro that was lacking in cells not expressing Procr and the Procr-expressing VESCs produced endothelial progeny through ten passages in vitro (Fig. 3). Lineage tracing studies were conducted in pubertal animals and the Procr-expressing endothelial cells contributed to endothelial cell expansion for up to ten months in vessels within the mammary gland. Surprisingly, the VESCs were determined to be bipotent, with contributions not only to the endothelium but also to pericytes throughout vessels in multiple tissues. The authors suggested that the VESCs identified underwent endothelial to mesenchymal transition to become the pericyte cells in the vascular beds examined.99
Fig. 3.
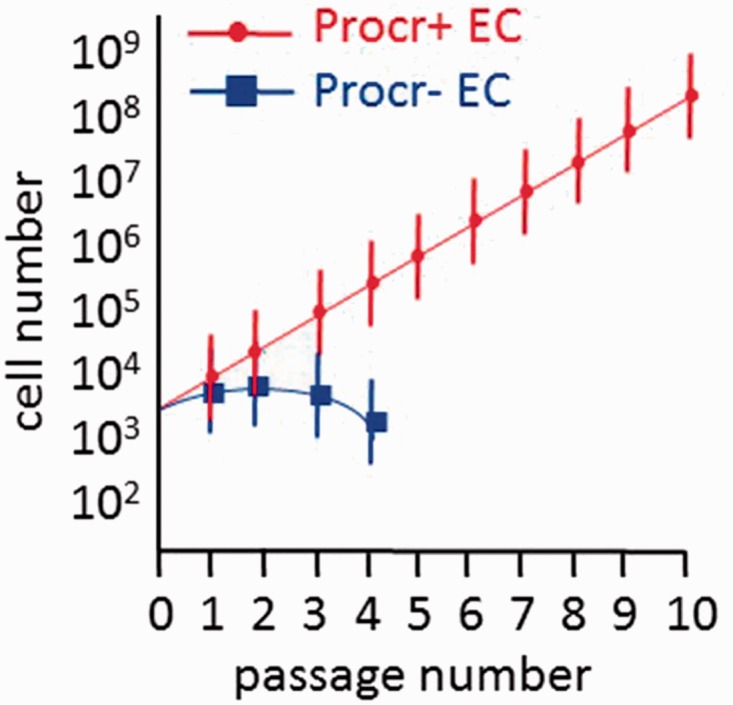
Procr-expressing endothelial cells display the greatest proliferative potential producing progeny through ten passages while the Procr-negative fraction fails to proliferate beyond four passages in vitro74.
Conclusions
There is a growing body of work to support the concept that endothelial stem and progenitor cells exist within the endothelial intima of resident tissue vasculature. At present, limited comparisons among the different approaches used by the authors has been accomplished, but some limitations of the present work can be identified. While the work of Patel et al.80 has shown that endothelial progenitors can be identified by applying stringent criteria, the specific sites of EVP, TA, and D cell localization in organs and tissues at homeostasis (artery, vein, or capillary bed), the contributions of EVP to TA and D cells during homeostasis, differences in the EVP among different organs across the lifespan of the mouse, and determination of whether the EVP represents an endothelial stem cell remain to be addressed. Human endothelial progenitor cells (ECFCs) have been identified;87,88,90 however, no unique identifying markers have permitted prospective isolation of ECFCs from circulating blood or blood vascular endothelium to permit identification of the site of origin of ECFC in humans and determination of whether these cells display stem cell activity for the endothelial lineage. Several papers have published evidence for the presence of resident VESCs in mice; however, the relationship between the unipotent VESC identified by Fang et al.91 and Naito et al.,98 and the bipotent VESC identified by Yu et al.99 remains unclear. It is clear that the expression of c-Kit as a marker for VESC differs in these three papers as it is a critical marker in the work of Fang et al.,91 but is not expressed on the SP VESC of Naito et al.98 or the Procr-expressing VESC of Yu et al.99 Identification of unique and perhaps more distinguishing characteristics of the VESCs that discriminate these stem cells from progenitor and mature endothelial elements awaits additional study. Finally, no cell surface antigen has yet been reported that can be used to prospectively identify VESCs in mice and man. This is an exciting and emerging theme that will impact our understanding of how the vascular endothelium is organized and replenished throughout the lifespan and may offer new insights into mechanisms of acquired endothelial dysfunction and development of cardiovascular disease.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
2017 Grover Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the educational grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institutes of Health.
References
- 1.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001; 17: 387–403. [DOI] [PubMed] [Google Scholar]
- 2.Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 2015; 125: 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivanovs A, Rybtsov S, Ng ES, et al. Human haematopoietic stem cell development: from the embryo to the dish. Development 2017; 144: 2323–2337. [DOI] [PubMed] [Google Scholar]
- 4.Beumer J, Clevers H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 2016; 143: 3639–3649. [DOI] [PubMed] [Google Scholar]
- 5.Grun D, Muraro MJ, Boisset JC, et al. De novo prediction of stem cell identity using single-cell transcriptome data. Cell Stem Cell 2016; 19: 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs E. Epithelial skin biology: three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Curr Top Dev Biol 2016; 116: 357–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge Y, Gomez NC, Adam RC, et al. Stem cell lineage infidelity drives wound repair and cancer. Cell 2017; 169: 636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chal J, Pourquie O. Making muscle: skeletal myogenesis in vivo and in vitro. Development 2017; 144: 2104–2122. [DOI] [PubMed] [Google Scholar]
- 9.Joanisse S, Parise G. Cytokine mediated control of muscle stem cell function. Adv Exp Med Biol 2016; 900: 27–44. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–967. [DOI] [PubMed] [Google Scholar]
- 11.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85: 221–228. [DOI] [PubMed] [Google Scholar]
- 12.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 1999; 18: 3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001; 107: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majka SM, Jackson KA, Kienstra KA, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest 2003; 111: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacic JC, Moore J, Herbert A, et al. Endothelial progenitor cells, angioblasts, and angiogenesis–old terms reconsidered from a current perspective. Trends Cardiovasc Med 2008; 18: 45–51. [DOI] [PubMed] [Google Scholar]
- 16.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 2012; 110: 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 2003; 23: 1185–1189. [DOI] [PubMed] [Google Scholar]
- 18.Ribatti D. The discovery of endothelial progenitor cells. An historical review. Leuk Res 2007; 31: 439–444. [DOI] [PubMed] [Google Scholar]
- 19.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2012; 2: a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinkevich Y, Lindau P, Ueno H, et al. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 2011; 476: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Udani VM, Santarelli JG, Yung YC, et al. Hematopoietic stem cells give rise to perivascular endothelial-like cells during brain tumor angiogenesis. Stem Cells Dev 2005; 14: 478–486. [DOI] [PubMed] [Google Scholar]
- 22.Wagers AJ, Sherwood RI, Christensen JL, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002; 297: 2256–2259. [DOI] [PubMed] [Google Scholar]
- 23.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002; 416: 542–545. [DOI] [PubMed] [Google Scholar]
- 24.Ying QL, Nichols J, Evans EP, et al. Changing potency by spontaneous fusion. Nature 2002; 416: 545–548. [DOI] [PubMed] [Google Scholar]
- 25.Jackson KA, Snyder DS, Goodell MA. Skeletal muscle fiber-specific green autofluorescence: potential for stem cell engraftment artifacts. Stem Cells 2004; 22: 180–187. [DOI] [PubMed] [Google Scholar]
- 26.Purhonen S, Palm J, Rossi D, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A 2008; 105: 6620–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005; 8: 211–226. [DOI] [PubMed] [Google Scholar]
- 28.Dudley AC, Udagawa T, Melero-Martin JM, et al. Bone marrow is a reservoir for proangiogenic myelomonocytic cells but not endothelial cells in spontaneous tumors. Blood 2010; 116: 3367–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gothert JR, Gustin SE, Hall MA, et al. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 2005; 105: 2724–2732. [DOI] [PubMed] [Google Scholar]
- 30.Hagensen MK, Shim J, Thim T, et al. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation 2010; 121: 898–905. [DOI] [PubMed] [Google Scholar]
- 31.Medina RJ, Barber CL, Sabatier F, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 2017; 6: 1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuno Y, Nakamura-Ishizu A, Kishi K, et al. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 2011; 117: 5264–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol 2007; 27: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 34.Wickersheim A, Kerber M, de Miguel LS, et al. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer 2009; 125: 1771–1777. [DOI] [PubMed] [Google Scholar]
- 35.Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res 2004; 94: 230–238. [DOI] [PubMed] [Google Scholar]
- 36.Sieveking DP, Buckle A, Celermajer DS, et al. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol 2008; 51: 660–668. [DOI] [PubMed] [Google Scholar]
- 37.Desai A, Glaser A, Liu D, et al. Microarray-based characterization of a colony assay used to investigate endothelial progenitor cells and relevance to endothelial function in humans. Arterioscler Thromb Vasc Biol 2009; 29: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medina RJ, O’Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 2010; 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtani K, Vlachojannis GJ, Koyanagi M, et al. Epigenetic regulation of endothelial lineage committed genes in pro-angiogenic hematopoietic and endothelial progenitor cells. Circ Res 2011; 109: 1219–1229. [DOI] [PubMed] [Google Scholar]
- 40.Minasi MG, Riminucci M, De Angelis L, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 2002; 129: 2773–2783. [DOI] [PubMed] [Google Scholar]
- 41.Kovacic JC, Boehm M. Resident vascular progenitor cells: an emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res 2009; 2: 2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeager ME, Frid MG, Stenmark KR. Progenitor cells in pulmonary vascular remodeling. Pulm Circ 2011; 1: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grenier G, Scime A, Le Grand F, et al. Resident endothelial precursors in muscle, adipose, and dermis contribute to postnatal vasculogenesis. Stem Cells 2007; 25: 3101–3110. [DOI] [PubMed] [Google Scholar]
- 44.Zengin E, Chalajour F, Gehling UM, et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 2006; 133: 1543–1551. [DOI] [PubMed] [Google Scholar]
- 45.Pasquinelli G, Tazzari PL, Vaselli C, et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells 2007; 25: 1627–1234. [DOI] [PubMed] [Google Scholar]
- 46.Cantelmo AR, Brajic A, Carmeliet P. Endothelial metabolism driving angiogenesis: emerging concepts and principles. Cancer J 2015; 21: 244–249. [DOI] [PubMed] [Google Scholar]
- 47.Vandekeere S, Dewerchin M, Carmeliet P. Angiogenesis revisited: an overlooked role of endothelial cell metabolism in vessel sprouting. Microcirculation 2015; 22: 509–517. [DOI] [PubMed] [Google Scholar]
- 48.Wong BW, Marsch E, Treps L, et al. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J 2017; 36: 2187–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz SM, Benditt EP. Cell replication in the aortic endothelium: a new method for study of the problem. Lab Invest 1973; 28: 699–707. [PubMed] [Google Scholar]
- 50.Schwartz SM, Benditt EP. Clustering of replicating cells in aortic endothelium. Proc Natl Acad Sci U S A 1976; 73: 651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol 2010; 30: 1094–1103. [DOI] [PubMed] [Google Scholar]
- 52.Hatzopoulos AK, Folkman J, Vasile E, et al. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 1998; 125: 1457–1468. [DOI] [PubMed] [Google Scholar]
- 53.Dong QG, Bernasconi S, Lostaglio S, et al. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol 1997; 17: 1599–1604. [DOI] [PubMed] [Google Scholar]
- 54.Fehrenbach ML, Cao G, Williams JT, et al. Isolation of murine lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 2009; 296: L1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin Y, Liu Y, Antonyak M, et al. Isolation and characterization of vascular endothelial cells from murine heart and lung. Methods Mol Biol 2012; 843: 147–154. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez DF, Huang L, King JA, et al. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 2008; 294: L419–430. [DOI] [PubMed] [Google Scholar]
- 57.Schniedermann J, Rennecke M, Buttler K, et al. Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol 2010; 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawasaki T, Nishiwaki T, Sekine A, et al. Vascular repair by tissue-resident endothelial progenitor cells in endotoxin-induced lung injury. Am J Respir Cell Mol Biol 2015; 53: 500–512. [DOI] [PubMed] [Google Scholar]
- 59.Cao Z, Lis R, Ginsberg M, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med 2016; 22: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding BS, Nolan DJ, Guo P, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 2011; 147: 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voswinckel R, Ziegelhoeffer T, Heil M, et al. Circulating vascular progenitor cells do not contribute to compensatory lung growth. Circ Res 2003; 93: 372–379. [DOI] [PubMed] [Google Scholar]
- 62.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014; 507: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atlasi Y, Looijenga L, Fodde R. Cancer stem cells, pluripotency, and cellular heterogeneity: a WNTer perspective. Curr Top Dev Biol 2014; 107: 373–404. [DOI] [PubMed] [Google Scholar]
- 64.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 2013; 154: 274–284. [DOI] [PubMed] [Google Scholar]
- 65.Ge Z, Lal S, Le TYL, et al. Cardiac stem cells: translation to human studies. Biophys Rev 2015; 7: 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lloyd-Lewis B, Harris OB, Watson CJ, et al. Mammary stem cells: premise, properties, and perspectives. Trends Cell Biol 2017; 27: 556–567. [DOI] [PubMed] [Google Scholar]
- 67.Rycaj K, Tang DG. Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res 2015; 75: 4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valent P, Bonnet D, De Maria R, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer 2012; 12: 767–775. [DOI] [PubMed] [Google Scholar]
- 69.Busch K, Klapproth K, Barile M, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 2015; 518: 542–546. [DOI] [PubMed] [Google Scholar]
- 70.Pei W, Feyerabend TB, Rossler J, et al. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 2017; 548: 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep 2011; 12: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bian Q, Cahan P. Computational tools for stem cell biology. Trends Biotechnol 2016; 34: 993–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giustacchini A, Thongjuea S, Barkas N, et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat Med 2017; 23: 692–702. [DOI] [PubMed] [Google Scholar]
- 74.Kirschner K, Chandra T, Kiselev V, et al. Proliferation drives aging-related functional decline in a subpopulation of the hematopoietic stem cell compartment. Cell Rep 2017; 19: 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Natarajan KN, Teichmann SA, Kolodziejczyk AA. Single cell transcriptomics of pluripotent stem cells: reprogramming and differentiation. Curr Opin Genet Dev 2017; 46: 66–76. [DOI] [PubMed] [Google Scholar]
- 76.Rizvi AH, Camara PG, Kandror EK, et al. Single-cell topological RNA-seq analysis reveals insights into cellular differentiation and development. Nat Biotechnol 2017; 35: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi Z, Geng Y, Liu J, et al. Single-cell transcriptomics reveals gene signatures and alterations associated with aging in distinct neural stem/progenitor cell subpopulations. Protein Cell 2017. DOI: 10.1007/s13238-017-0450-2. [DOI] [PMC free article] [PubMed]
- 78.Shlush LI, Mitchell A, Heisler L, et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 2017; 547: 104–108. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, Yosef N. A new way to build cell lineages. eLife 2017; 6: e25654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel J, Seppanen EJ, Rodero MP, et al. Functional definition of progenitors versus mature endothelial cells reveals key SoxF-dependent differentiation process. Circulation 2017; 135: 786–805. [DOI] [PubMed] [Google Scholar]
- 81.Streit M, Velasco P, Riccardi L, et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J 2000; 19: 3272–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang N, Fang Z, Contag PR, et al. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood 2004; 103: 617–626. [DOI] [PubMed] [Google Scholar]
- 83.Bompais H, Chagraoui J, Canron X, et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood 2004; 103: 2577–2584. [DOI] [PubMed] [Google Scholar]
- 84.Guillevic O, Ferratge S, Pascaud J, et al. A novel molecular and functional stemness signature assessing human cord blood-derived endothelial progenitor cell immaturity. PloS One 2016; 11: e0152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solovey A, Lin Y, Browne P, et al. Circulating activated endothelial cells in sickle cell anemia. New Engl J Med 1997; 337: 1584–1590. [DOI] [PubMed] [Google Scholar]
- 86.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007; 109: 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 2000; 105: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004; 104: 2752–2760. [DOI] [PubMed] [Google Scholar]
- 89.Ingram DA, Mead LE, Moore DB, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005; 105: 2783–2786. [DOI] [PubMed] [Google Scholar]
- 90.Prasain N, Lee MR, Vemula S, et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol 2014; 32: 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang S, Wei J, Pentinmikko N, et al. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol 2012; 10: e1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells 2006; 24: 3–12. [DOI] [PubMed] [Google Scholar]
- 93.Summer R, Kotton DN, Sun X, et al. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol 2003; 285: L97–104. [DOI] [PubMed] [Google Scholar]
- 94.Jun D, Garat C, West J, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells 2011; 29: 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marriott S, Baskir RS, Gaskill C, et al. ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am J Physiol Cell Physiol 2014; 307: C684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Irwin D, Helm K, Campbell N, et al. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol 2007; 293: L941–951. [DOI] [PubMed] [Google Scholar]
- 97.Summer R, Kotton DN, Liang S, et al. Embryonic lung side population cells are hematopoietic and vascular precursors. Am J Respir Cell Mol Biol 2005; 33: 32–40. [DOI] [PubMed] [Google Scholar]
- 98.Naito H, Kidoya H, Sakimoto S, et al. Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J 2012; 31: 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu QC, Song W, Wang D, et al. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res 2016; 26: 1079–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]