Abstract
Prior limited research indicates that children with pulmonary hypertension (PH) have higher rates of adverse perioperative outcomes when undergoing non-cardiac procedures and cardiac catheterizations. We examined a single-center retrospective cohort of children with active or pharmacologically controlled PH who underwent cardiac catheterization or non-cardiac surgery during 2006–2014. Preoperative characteristics and perioperative courses were examined to determine relationships between the severity or etiology of PH, type of procedure, and occurrence of major and minor events. We identified 77 patients who underwent 148 procedures at a median age of six months. The most common PH etiologies were bronchopulmonary dysplasia (46.7%), congenital heart disease (29.9%), and congenital diaphragmatic hernia (14.3%). Cardiac catheterizations (39.2%), and abdominal (29.1%) and central venous access (8.9%) were the most common procedures. Major events included failed planned extubation (5.6%), postoperative cardiac arrest (4.7%), induction or intraoperative cardiac arrest (2%), and postoperative death (1.4%). Major events were more frequent in patients with severe baseline PH (P = 0.006) and the incidence was associated with procedure type (P = 0.05). Preoperative inhaled nitric oxide and prostacyclin analog therapies were associated with decreased incidence of minor events (odds ratio [OR] = 0.32, P = 0.046 and OR = 0.24, P = 0.008, respectively), but no change in the incidence of major events. PH etiology was not associated with events (P = 0.24). Children with PH have increased risk of perioperative complications; cardiac arrest and death occur more frequently in patients with severe PH and those undergoing thoracic procedures. Risk may be modified by using preoperative pulmonary vasodilator therapy and lends itself to further prospective studies.
Keywords: pulmonary vascular disease, outcomes, pediatric, bronchopulmonary dysplasia
Pulmonary hypertension (PH) is a heterogeneous and often progressive disorder that can lead to right ventricular failure and death in both adults and children. The distribution of PH etiologies in children is unlike that of the adult population and includes a wide range of developmental pathologies such as persistent PH of the newborn, pulmonary hypoplasia, congenital diaphragmatic hernia, bronchopulmonary dysplasia (BPD), congenital heart disease (CHD), and heritable syndromic or idiopathic pulmonary vascular diseases. Children with PH have increased requirements for medical resources owing to a higher frequency of hospitalizations and escalating costs of care.1 These children frequently receive general anesthesia when undergoing diagnostic and therapeutic procedures required for management of their PH, as well as for procedures related to other co-morbidities.
Perioperative management of patients with PH remains a challenge to anesthesiologists, surgeons, and intensivists.2 The presence of PH adds significant perioperative risk and adult data on perioperative outcomes in this population are much more robust than the pediatric data. Numerous adult studies highlight the fact that patients with PH have higher than normal perioperative mortality and experience more adverse perioperative events.3–7 Because significant differences exist between adult and pediatric PH with regard to pathogenesis and natural history, extrapolating adult data to children may not be appropriate.
A limited body of literature examines the incidence of perioperative complications in children with PH who undergo non-cardiac surgery or cardiac catheterization. It is known that greater impairment in global function caused by PH is associated with adverse outcomes, including mortality and cardiac arrest.8 The few pediatric studies that have evaluated perioperative outcomes found PH to be a major risk factor for adverse perioperative complications, including cardiac arrest and PH crisis. However, in these populations, the etiology of PH in most patients was idiopathic pulmonary arterial hypertension (IPAH).9,10 Today, the etiology of PH is shifting, with increases in non-CHD-related PH and patients undergoing more non-cardiac procedures.1 Therefore, the objective of this study was to examine the incidence of adverse perioperative outcomes after non-cardiac surgical procedures and cardiac catheterizations in our center’s pediatric PH population, which is unique from that of previously published studies because it includes a large number of patients with PH secondary to BPD. Additionally, we sought to assess whether any patient-specific or procedure-specific factors were predictive of adverse event risk. We hypothesized that the severity and etiology of PH would be associated with adverse perioperative outcomes.
Methods
We conducted a single-center retrospective cohort study approved by the Johns Hopkins Institutional Review Board (NA_00051884) and obtained written informed consent from each patient’s parent or guardian. We examined a population of children with PH who underwent non-cardiac surgical procedures, including cardiac catheterizations, in 2006–2014. All patients included in the study were under the care of a pediatric pulmonologist at a BPD specialty clinic and/or were inpatients prescribed sildenafil. All procedures were preplanned and were performed under the care of a pediatric anesthesiologist. Bedside procedures in the intensive care units were excluded.
The study population included children aged < 18 years who had echocardiographic evidence of PH verified by the independent reading of an attending pediatric cardiologist, elevated pulmonary vascular resistance (PVR) on cardiac catheterization, or PH that was pharmacologically controlled with targeted pulmonary vasodilators and no evidence of PH on echocardiogram or catheterization. The degree of PH was classified as follows: controlled = active pharmacologic therapy with no evidence of PH on echocardiogram or PVR <2 Wood units (WU) on catheterization; mild = right ventricular pressure (RVP) estimated <2/3 systemic blood pressure on echocardiogram or PVR of 2–4 WU on catheterization; moderate = RVP estimated 2/3 systemic to systemic on echocardiogram or PVR of 5–8 WU on catheterization; and severe = RVP estimated > systemic on echocardiogram or PVR > 8 WU on catheterization. Any category of patient could be prescribed pulmonary vasodilator therapy. We excluded patients if a PH diagnostic procedure (echocardiogram or catheterization) was performed more than 30 days before the operative procedure in order to capture only those who had timely evaluations for preoperative planning.
Outcome definitions
Perioperative events were classified as minor or major and were evaluated up until seven days postoperatively. A minor event was defined as either the need for escalation of chronic vasodilator therapy in the postoperative period or a transient fluctuation in vital signs. If appropriately addressed intraoperatively, these changes should have no long-term effects, but if left uncorrected, they could precipitate or indicate an impending PH crisis. Minor events included hypothermia (core body temperature < 36 ℃), hypoxia (SpO2 < 90% or > 5% less than baseline in cyanotic patients), hypercarbia (end-tidal CO2 > 45 mmHg), and hypotension (systolic blood pressure < 5th percentile for age or > 10% from pre-procedure baseline). A major event was defined as perioperative mortality or a life-threatening deviation requiring immediate treatment, including cardiac arrest, PH crisis, failed planned postoperative extubation, or unanticipated postoperative respiratory failure requiring mechanical ventilation. Clinical data were obtained by reviewing paper and electronic medical records relating to the procedure and/or hospital admission.
Statistical analysis
Statistical analysis was carried out with STATA 11.2 (College Station, TX, USA). For descriptive statistics, continuous variables are described as median and interquartile range (IQR) and categorical variables are expressed as number and percentage. The Mann–Whitney U test was used to determine the relationship between PH diagnosis and PH severity. Major and minor events were compared to severity of PH, etiology of PH, type of procedure, and ASA classification using either chi-square analysis or the Kruskal–Wallis H-test. Univariate and multivariate logistic regression analysis was used to determine the association of individual and combined variables with outcomes. Significance was defined for all tests as a P value of ≤ 0.05. When more than one non-cardiac procedure or cardiac catheterization was performed for an individual patient, each procedure was analyzed independently and clustered by patient to account for the non-independence of procedures. This manuscript adheres to the STROBE guidelines (Strengthening the Reporting of Observational studies in Epidemiology) and checklist.11
Results
Patient characteristics and PH therapy
We identified 77 patients with pharmacologically controlled or active PH (Table 1). The median ASA classification was 3 (53.3%), median age at the time of procedure was six months (IQR = 4–11.3 months), and median weight at the time of procedure was 5.7 kg (IQR = 3.7–8.4 kg). Most patients (55.4%) were classified as having mild PH; however, during the study period, as patient conditions changed, there were 22 instances of downgrading to a less severe PH category and eight instances of upgrading to a more severe category. Etiologies of PH, as classified by the Pulmonary Vascular Research Institute’s consensus approach, included category 1 (19.5%), category 2 (3.9%), category 3 (28.6%), category 4 (46.8%), and category 10 (1.3%).12 Targeted pulmonary vasodilator monotherapy (phosphodiesterase 5 [PDE5] inhibitor ± endothelin receptor antagonist [ETA] ± prostacyclin analog) was prescribed in 97 (66.4%) procedures; two procedures were missing preoperative medication profiles. In 20 (13.7%) procedures, two classes of medications (PDE5 inhibitor and ETA or PDE5 inhibitor and prostacyclin analog) were prescribed and in six (4.1%) procedures, all three classes of medication were prescribed. All patients who received prostacyclin analog therapy were on chronic therapy for at least two weeks before the procedure. Patients who received inhaled nitric oxide (iNO) before the procedure required this therapy acutely or before transition to long-term vasodilator therapy.
Table 1.
Characteristics of patients (n = 77).
| Characteristic | Value* |
|---|---|
| Age at time of procedure, median (IQR) (months) | 6 (4–12) |
| Weight at time of procedure, median (IQR) (kg) | 5.7 (3.7–8.4) |
| Sex | |
| Male | 42 (54.5) |
| Female | 35 (45.5) |
| ASA classification | |
| 2 | 4 (2.9) |
| 3 | 73 (53.3) |
| 4 | 58 (42.3) |
| 5 | 2 (1.5) |
| Severity of PH at time of procedure | |
| Controlled on pharmacologic therapy | 18 (12.2) |
| Mild | 82 (55.4) |
| Moderate | 28 (18.9) |
| Severe | 20 (13.5) |
| PH classification per Pulmonary Vascular Institute Consensus approach12 | |
| Group 1 (prenatal/developmental pulmonary hypertensive vascular disease) | 14 (18.2) |
| Group 2 (perinatal pulmonary vascular maladaptation) | 3 (3.9) |
| Group 3 (pediatric cardiovascular disease) | 22 (28.6) |
| Group 4 (bronchopulmonary dysplasia) | 36 (46.8) |
| Group 5 (isolated pulmonary arterial hypertension) | 1 (1.3) |
| Group 10 (associated with other system disorders) | 1 (1.3) |
All data are presented as n (%) except where noted.
ASA, American Society of Anesthesiologists Physical Status; IQR, interquartile range; PH, pulmonary hypertension.
Procedure characteristics
During the study period, 148 procedures were performed and 141 (95.2%) anesthesia data records were available for review. Severity of PH was determined before the procedure via echocardiogram in 81 (54.7%) cases and via cardiac catheterization in 67 (45.3%) cases. Severity categories did not differ with diagnostic modality (P = 0.073).
Each patient underwent one to eight procedures (Table 2). The most common was cardiac catheterization (39.2%). Of those, 84.4% were vasoreactivity studies and the remainder were interventional. Abdominal procedures (29.1%) were the second most common and included Nissen fundoplication, gastrostomy tube, inguinal hernia repair, cholecystectomy, and lysis of adhesions. Central venous access procedures (8.8%) were next in frequency. Thoracic procedures accounted for 6.8% and included repair of congenital diaphragmatic hernia, video-assisted thoracoscopic surgery, and diaphragm plication. Neurologic procedures also accounted for 6.8% and included ventricular shunts and craniosynostosis repair. Airway procedures (4.1%) included tracheostomy, tonsillectomy (and/or adenoidectomy), and bronchoscopy. Other procedures (5.4%) included scar excision and grafting, osteotomies, myringotomy tubes, hearing tests, and cystoscopies. Nearly all patients (93.6%) received general anesthesia with a combination of inhalation and intravenous anesthetics. Median anesthesia time, from the time the anesthesia team assumed care to exit from the operating room, was 176 min (IQR = 107.8–258.5 min).
Table 2.
Characteristics of 148 procedures.
| Characteristic | Value* |
|---|---|
| Type of procedure | |
| Cardiac catheterization | 58 (39.2) |
| Abdominal | 43 (29.1) |
| Central venous access | 13 (8.8) |
| Thoracic | 10 (6.8) |
| Neurologic | 10 (6.8) |
| Airway | 6 (4.1) |
| Other | 8 (5.4) |
| Airway management | |
| Endotracheal tube | 132 (93.6) |
| Mature tracheostomy | 4 (2.8) |
| Natural airway | 4 (2.8) |
| Laryngeal mask airway | 1 (0.7) |
| Anesthesia duration, median (IQR), minutes | 176 (107.8–258.5) |
| Post-procedure disposition | |
| Neonatal ICU | 45 (30.8) |
| Pediatric ICU | 82 (56.2) |
| General pediatrics floor | 11 (7.5) |
| Home | 8 (5.5) |
All data are presented as n (%) except where noted.
ICU, intensive care unit.
Intra- and post-procedure events
The incidence of major and minor perioperative events is outlined in Table 3. A total of 201 minor events occurred, in the range of 0–4 per procedure, and 21 major events occurred, in the range of 0–3 per procedure. Intraoperative hypercarbia on continuous end-tidal CO2 monitoring and hypotension were the two most frequent minor events, occurring in 68 (45.9%) and 58 (39.2%) procedures, respectively. No patient died intraoperatively. The characteristics of patients who experienced perioperative cardiac arrest and/or postoperative death in the first seven days are summarized in Table 4. Though most events occurred postoperatively, we cannot directly attribute them to a PH crisis, as no real-time measurements of pulmonary artery pressure or PVR were available.
Table 3.
Perioperative events per procedure.
| Type of event | n (% of procedures) |
|---|---|
| Intraoperative events – minor | |
| Hypercarbia: end-tidal CO2 > 45 mmHg | 68 (45.9) |
| Hypotension: SBP < 5th percentile for age or > 10% decrease from pre-procedure baseline | 58 (39.2) |
| Hypothermia: core temp < 36℃ | 34 (23) |
| Hypoxia: SpO2 < 90% or decrease > 5% from baseline in cyanotic patients | 27 (18.2) |
| Intraoperative events – major | |
| Failed planned extubation (out of 54 planned) | 3 (5.6) |
| Cardiac arrest | 4 (2.7) |
| PH crisis | 3 (2) |
| Postoperative events – minor | |
| Escalation of PH therapy | 14 (9.5) |
| Postoperative events – major | |
| Cardiac arrest | 7 (4.7) |
| Respiratory failure | 2 (1.4) |
| Death | 2 (1.4) |
PH, pulmonary hypertension; SBP, systolic blood pressure.
Table 4.
Characteristics of children who experienced cardiac arrest or death in the first seven days post procedure.
| Age (months)* | PH etiology | PH severity at time of procedure, diagnostic procedure | Operative procedure | Minor events during procedure | Major events during or post procedure | Post-procedure outcome |
|---|---|---|---|---|---|---|
| 1 | CDH | Severe, echocardiogram | Thoracic | Hypotension, hypoxia | Two cardiac arrests during procedure, one cardiac arrest post procedure, post-procedure death | Death on day 0 |
| 1 | CDH | Severe, echocardiogram | Thoracic | None (on ECMO) | Post-procedure death | Death on day 6 |
| 3 | BPD | Mild, echocardiogram | Abdominal | Hypothermia | Post-procedure cardiac arrest day 0 | Survived to discharge on day 8 |
| 5 | BPD | Mild, echocardiogram | Abdominal | None | Post-procedure cardiac arrest day 0 | Survived to discharge on day 15 |
| 6 | BPD | Severe, echocardiogram | Central venous access | Hypotension | Post-procedure cardiac arrest day 2 | Survived to discharge on day 62 |
| 6 | BPD | Severe, echocardiogram | Central venous access | ADR unavailable for review | Post-procedure cardiac arrest day 1 | Survived to discharge on day 66 |
| 9 | CHD | Moderate, catheterization | Abdominal | None | Induction cardiac arrest | Survived to discharge on day 5 |
| 15 | CHD | Moderate, echocardiogram | Airway | Hypothermia | Post-procedure cardiac arrest day 0 | Survived to discharge on day 4 |
| 22 | CHD | Mild, catheterization | Cardiac catheterization | Hypercarbia | Post-procedure cardiac arrest day 0 | Survived to discharge on day 65 |
| 58 | CHD | Moderate, catheterization | Central venous access | Hypotension, hypoxia, hypercarbia | Induction cardiac arrest | Survived to discharge on day 5 |
Each represents one patient.
ADR, anesthesia data record; BPD, bronchopulmonary dysplasia; CDH, congenital diaphragmatic hernia; CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation; PH, pulmonary hypertension.
Minor events
We found no correlation between the cumulative occurrence of minor events per procedure and the following patient variables: age, weight, sex, PH etiology, PH severity, procedure type, or pre-procedure medications. Preoperative medications included diuretics, targeted pulmonary vasodilators, digoxin, systemic steroids, and supplemental oxygen. Neither ASA classification nor total anesthesia duration was associated with minor events. However, in patients undergoing procedures other than cardiac catheterization, pre-procedure PDE5 inhibitors were predictive of minor events (OR = 5.88; P = 0.03). In patients with BPD-associated PH, preoperative PDE5 inhibitor therapy was also predictive of minor events (OR = 5.88; P = 0.014); however, this association was seen in patients with BPD-associated PH undergoing both cardiac catheterization and non-cardiac surgery.
Major events
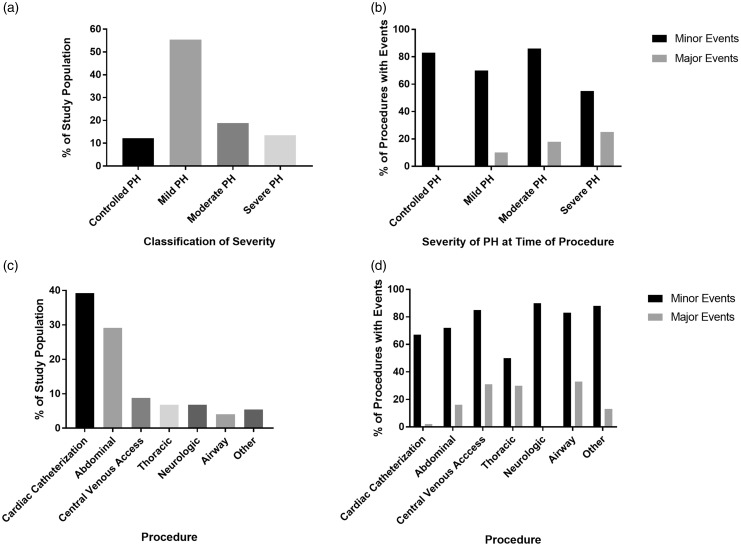
Major events were associated with severity of PH (OR = 2.04; P = 0.006) but not with etiology of PH. Patients with severe PH accounted for 13.5% of the population but experienced 42.1% of the major events. One-quarter of patients with severe PH experienced at least one major event per procedure (Fig. 1). Regression analysis indicated that the incidence of major events was also associated with the type of procedure (P = 0.05). Most notably, only 6.8% of the patients underwent thoracic procedures, but this group accounted for 28.6% of all major events. Moreover, at least one major event occurred in three of the ten (30%) thoracic procedures performed. This number is striking when compared with the fact that 39.2% of patients underwent cardiac catheterization but accounted for only 4.7% of major complications. Furthermore, in all patients undergoing procedures other than cardiac catheterization, the perioperative use of iNO and PH severity were each predictive of major events (OR = 15.23; P = 0.003 and P < 0.001, respectively). No individual PH etiology was associated with occurrence of major events. However, when patients with BPD (as the etiology of PH) were excluded, severity of PH (P = 0.041), type of surgery (P = 0.05), and ASA class (P = 0.0018) were all associated with the occurrence of major events.
Fig. 1.
PH severity and thoracic procedures were associated with major events. (a) Severity of PH at the time of procedure; most had mild PH. (b) Incidence of major and minor events by PH severity classification. Patients with severe PH had a higher incidence of major events (P = 0.006). The incidence of minor events did not differ significantly between groups. (c) Numbers of each procedure type performed. (d) The incidence of major and minor events by procedure. Approximately one-third of central venous access, thoracic, and airway procedures were marked by a major event. The incidence of major events was disproportionately high for thoracic procedures (P = 0.05). No differences in the incidence of minor events were noted between groups.
Factors associated with decreased incidence of events on univariate analysis
Based on evaluation of all patient data, two preoperative interventions were associated with fewer minor events on univariate analysis: prostacyclin analog therapy (OR = 0.24; P = 0.008) and perioperative iNO therapy (OR = 0.32; P = 0.046; Table 5). In subgroup analyses based on procedure type and PH etiology, univariate analysis showed that patients undergoing cardiac catheterization had fewer minor events when they were receiving prostacyclin analog therapy (OR = 0.13, P < 0.001) or had received pre-procedure digoxin therapy (OR = 0.2; P = 0.05). In patients undergoing any procedure other than cardiac catheterization, preoperative therapy with supplemental oxygen was associated with a lower incidence of major events (OR = 0.23; P = 0.038). Among patients with BPD-associated PH, no patient characteristic or intervention was associated with a decreased number of major events. In patients who did not have BPD, preoperative prostacyclin analog therapy (OR = 0.27; P = 0.021) and perioperative iNO (OR = 0.28; P = 0.025) were associated with fewer minor events on univariate analysis.
Table 5.
Univariate analysis of preoperative patient and procedure characteristics and minor and major events.
| Variable | Minor events |
Major events |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Demographics | ||||
| Age < 1 month | 1.23 (0.23–7.25) | 0.75 | 0.68 (0.072–6.41) | 0.74 |
| Age < 12 months | 1.26 (0.56–2.86) | 0.58 | 0.84 (0.27–2.57) | 0.76 |
| Weight < 5 kg | 1.03 (0.47–2.21) | 0.95 | 0.67 (0.25–1.75) | 0.41 |
| Weight < 10 kg | 0.95 (0.37–2.42) | 0.92 | 0.84 (0.22–3.20) | 0.8 |
| Sex | 1.12 (0.53–2.39) | 0.77 | 2.2 (0.74–6.55) | 0.15 |
| Preoperative medication therapies | ||||
| Diuretic therapy | 0.74 (0.28–1.99) | 0.56 | 0.77 (0.24–2.44) | 0.65 |
| Systemic steroid | 1.83 (0.78–4.27) | 0.16 | 1.44 (0.48–4.32) | 0.51 |
| Supplemental oxygen | 1.52 (0.64–3.61) | 0.34 | 0.47 (0.13–1.64) | 0.24 |
| Phosphodiesterase 5 inhibitors | 1.45 (0.65–3.44) | 0.35 | 1.41 (0.45–4.37) | 0.55 |
| Inhaled nitric oxide | 0.32 (0.11–0.98) | 0.046 | 3.92 (0.87–17.60) | 0.074 |
| Endothelin receptor antagonists | 0.63 (0.26–1.55) | 0.32 | 0.34 (0.045–2.55) | 0.29 |
| Prostacyclin analog | 0.24 (0.087–0.69) | 0.008 | 1.19 (0.11–13.36) | 0.89 |
| Digoxin | 0.47 (0.19–2.61) | 0.61 | 1.41 (0.13–15.4) | 0.78 |
| Anesthesia | ||||
| ASA class | n/a | 0.5 | n/a | 0.21 |
| Repeated airway attempts | 1.26 (0.36–4.41) | 0.72 | 0.64 (0.12–3.50) | 0.61 |
| Prolonged anesthesia (> 75th percentile) | 0.55 (0.2–1.55) | 0.26 | 0.93 (0.27–3.27) | 0.91 |
| Minor events | n/a | n/a | 1.39 (0.42–4.57) | 0.59 |
ASA, American Society of Anesthesiologists Physical Status; CI, confidence interval; n/a, not applicable.
Factors associated with decreased incidence of events on multivariate analysis
On multivariate analysis, after controlling for age, sex, preoperative prostacyclin analog use, and preoperative iNO therapy in all patients, preoperative prostacyclin analog therapy remained associated with fewer minor events (OR = 0.31; P = 0.024), but no pre-procedure characteristic was associated with a reduced incidence of major events. Also, in patients who underwent cardiac catheterization only, preoperative prostacyclin analog therapy was associated with fewer minor events (OR = 0.15; P = 0.018). Finally, in patients with PH of non-BPD etiology, perioperative iNO was associated with fewer minor events (OR = 0.24; P = 0.033).
Discussion
Our data support prior evidence that pediatric patients with PH have an elevated risk of perioperative morbidity and mortality and that this risk increases with PH severity.7,8,13 We found that PH-targeted pharmacologic therapy was associated with a decreased incidence of minor intraoperative events. However, neither BPD nor any other etiology of PH was associated with major or minor events.
Several single-center studies have reported the risk of cardiac arrest during cardiac catheterization or non-cardiac surgeries to be more than tenfold higher in children with PH than in patients without PH undergoing the same procedures.7,13 Based on perioperative data from 77 PH patients undergoing 148 procedures, we report intra- and postoperative cardiac arrest rates of 2% and 4.7%, respectively, a 1.4% perioperative mortality rate, and a reintubation rate of 5.6%. Our findings are consonant with those reported by others. Large prospective multicenter registries of over 1.8 million anesthetic occurrences, including the Pediatric Perioperative Cardiac Arrest and the Wake Up Safely registries, report the incidence of perioperative cardiac arrest in the general pediatric population to be in the range of 0.014–0.033%, with a mortality of 0.0036–0.011%.14,15 In contrast, previously documented risk of perioperative cardiac arrest or need for cardiac massage was between 1.4% and 5.7% in children with PH, with subsequent rates of mortality or need for mechanical cardiac support of 0–3.5%.8,14,16–18 Data from over 50,000 children in the American College of Surgeons National Surgical Quality Improvement Program showed a reintubation rate of 1.7–2.3% in children without PH, but an increase to 5–5.4% in children with PH. It is hoped that insights from the current study might inform the prospective planning of perioperative care by designated subspecialty teams and improve upon these outcomes in the future.
Avoidance of PH crisis and resultant right heart failure represents one of the overriding goals in the peri-procedural care of patients with PH.19 Pre-emptive initiation of pulmonary vasodilators before non-cardiac surgery is not unheard of, yet recent studies have not demonstrated a positive impact of this practice.20,21 A common progression of vasodilator therapy at our institution for patients with BPD is initiation of PDE5 inhibitors, followed by ETA, and then prostacyclin analogs for patients with right ventricular dysfunction or refractory elevation of PVR. Preoperative prostacyclin analog therapy was associated with fewer minor perioperative events in this study, suggesting that multimodal preoperative pharmacologic therapy may be beneficial. A multicenter prospective study that examines the use of perioperative prostacyclin analog therapy would help to determine generalizability of these interventions for reducing perioperative events.
Inhaled NO and prostacyclin analog therapies were associated with a decreased frequency of minor adverse events in our study. However, in the subset of patients undergoing non-catheterization procedures, we noted an increase in minor events in children receiving PDE5 inhibitors and/or nitric oxide. Most of these minor events occurred when optimization of therapy was limited because it was initiated too close to the procedural date, doses or numbers of therapies were inadequate, or the agents were used for acute rescue. Supplemental oxygen therapy decreased the risk of major events for all procedures except cardiac catheterization. Others have reported that chronic pulmonary vasodilator therapy decreased risk of perioperative complications in the general PH population.19,22 Inclusion of these interventions in preoperative protocols for patients with PH may be a valuable consideration, but optimizing the timing of initiation for this pre-procedural intervention remains a future challenge.
In our study, 93.6% of patients received a balanced anesthetic composed of volatile anesthetics and intravenous agents. This was associated with low rates of intraoperative arrest (2%) and PH crisis (2%). However, there is currently no reported best practice anesthetic for pediatric PH. Inhaled gases, particularly isoflurane, attenuate hypoxic pulmonary vasoconstriction and may even provide some degree of vasodilation.2,21,23–26 Opioids have a good safety profile and blunt the pulmonary vascular response to noxious stimuli; meanwhile, ketamine may be associated with fewer changes in pulmonary hemodynamics.23,27–30 While no specific anesthetic technique is known to be superior, our study suggests that a balanced technique may be safest but this remains an area for further study.3,7,8,21
The postoperative period in our study was fraught with risk for major cardiopulmonary events. Despite an 87% direct ICU admission rate for postoperative care, 4.7% of patients experienced a cardiac arrest within the first seven postoperative days. One arrest occurred on the general care ward on postoperative day 0, triggering patient transfer to the PICU. Failed extubation occurred in 5.6% percent of patients and 1.4% experienced postoperative respiratory failure. Adult patients with PH also experience more postoperative complications than case-matched controls, including heart failure, delayed extubation, and death.3 These data emphasize the importance of comprehensive cardiopulmonary monitoring and teamwork for planning and execution of the postoperative stage of care, including consideration of disposition to an ICU.
Limitations of our study include the small number of patients from a single center and the use of large registry studies as controls. Definitions of PH and its severity were based on qualitative echocardiographic evidence in just over half the procedures because these data were more commonly collected and quantitative echocardiographic metrics such as tricuspid annular plane systolic excursion and ventriculoarterial coupling were not available in most of the patient records. Because cardiac catheterization remains the gold standard, patients without cardiac catheterization data may have been misclassified. Patients with active or pharmacologically controlled PH may have been excluded if their most recent PH diagnostics were obtained more than 30 days before the surgical procedure. Additionally, use of the Panama Functional Classification of Pulmonary Hypertension was limited here, given patient age, heterogeneity of PH etiology,23 and the fact that most patients in this study had no recent outpatient performance data for scoring.31 Furthermore, the small sample might have restricted associations between patient risk factors and pre-, intra-, and postoperative care as they relate to adverse events. Associations may have been more thoroughly delineated by a larger sample size. Baseline rates of major events differ at our institution from reported national trends. The absence of an association between vasodilator therapy and major events may be due to a low frequency of such events.
Heterogeneity of PH etiology was another challenge in this study, and might limit applicability of our results to some subgroups. A consistent feature that differentiates studies of pediatric PH from studies in adults is that children with IPAH are a minority. Most patients in our study had PH as a sequela of prematurity or other non-cardiac causes. The emphasis on infants with BPD-associated PH is a strength of this study and defining perioperative risk in children with PH unrelated to CHD is a pressing priority. However, one consequence of this large BPD cohort is that end-tidal CO2 monitoring might have underestimated intraoperative hypercarbia, given the increased dead space ventilation in these patients, and arterial blood gas analyses and serum bicarbonate levels were not available for all patients or procedures. The missing values may limit the applicability of our data on event incidence to other care settings. Further, our study of a convenience-based patient sample was underpowered to definitively discern differences in perioperative outcomes and management priorities between children with differing PH etiologies. The use of our inpatient prescribing data for sildenafil to identify non-BPD subjects did not capture patients that were not on pharmacologic therapy or those undergoing only outpatient procedures. The impact of subspecialty training in pediatric cardiac anesthesiology on PH patient outcomes was not studied here and remains an important open question. Finally, it remains unknown if the degree or type of prescribed pulmonary vasodilator therapy creates an implicit bias in providers that would cause them to be more cautious or prudent in their care.
In summary, our data indicate that children with PH are at increased risk for major perioperative complications, including cardiac arrest and death. Moreover, this risk is increased by increasing PH severity. Thoracic procedures, including congenital diaphragmatic hernia repair, diaphragm plication, and video-assisted thoracoscopic surgery were associated with increased numbers of major adverse events. Scrutiny at the time of perioperative screening, dedicated multidisciplinary care that incorporates input from PH specialists, and pharmacologic optimization may enhance perioperative outcomes. Strong consideration for postoperative cardiopulmonary ICU monitoring care is warranted because most of the cardiac arrests and deaths occurred in the postoperative period. Additional studies will be necessary to elucidate ideal anesthetic techniques for this population, the role of subspecialty cardiac anesthesiologists and intensivists in their perioperative care, other modifiable risk factors, and the definition of optimal clinical pathways for perioperative care.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
Meghan Bernier received support from Ruth L. Kirschstein NRSA Institutional Research Training Grant (NRSA 5T32HD044355-12). Joseph M. Collaco received support from Johns Hopkins All Children’s Hospital via an intramural Johns Hopkins Department of Pediatrics grant award.
References
- 1.Maxwell BG, Nies MK, Ajuba-Iwuji CC, et al. Trends in hospitalization for pediatric pulmonary hypertension. Pediatrics 2015; 136: 241–250. [DOI] [PubMed] [Google Scholar]
- 2.Blaise G, Langleben D, Hubert B. Pulmonary arterial hypertension: pathophysiology and anesthetic approach. Anesthesiology 2003; 99: 1415–1432. [DOI] [PubMed] [Google Scholar]
- 3.Lai HC, Lai HC, Wang KY, et al. Severe pulmonary hypertension complicates postoperative outcome of non-cardiac surgery. Br J Anaesth 2007; 99: 184–190. [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 2005; 45: 1691–1699. [DOI] [PubMed] [Google Scholar]
- 5.Gerson MC, Hurst JM, Hertzberg VS, et al. Prediction of cardiac and pulmonary complications related to elective abdominal and noncardiac thoracic surgery in geriatric patients. Am J Med 1990; 88: 101–107. [DOI] [PubMed] [Google Scholar]
- 6.Reich DL, Bodian CA, Krol M, et al. Intraoperative hemodynamic predictors of mortality, stroke, and myocardial infarction after coronary artery bypass surgery. Anesth Analg 1999; 89: 814–822. [DOI] [PubMed] [Google Scholar]
- 7.Warner MA, Lunn RJ, O’Leary PW, et al. Outcomes of noncardiac surgical procedures in children and adults with congenital heart disease. Mayo Perioperative Outcomes Group. Mayo Clin Proc 1998; 73: 728–734. [DOI] [PubMed] [Google Scholar]
- 8.Balkin EM, Olson ED, Robertson L, et al. Change in pediatric functional classification during treatment and morbidity and mortality in children with pulmonary hypertension. Pediatr Cardiol 2016; 37: 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmosino MJ, Friesen RH, Doran A, et al. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg 2007; 104: 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faraoni D, Zurakowski D, Vo D, et al. Post-operative outcomes in children with and without congenital heart disease undergoing noncardiac surgery. J Am Coll Cardiol 2016; 67: 793–801. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 12.Cerro MJ, Abman S, Diaz G, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ 2011; 1: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor CJ, Derrick G, McEwan A, et al. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth 2007; 98: 657–661. [DOI] [PubMed] [Google Scholar]
- 14.Morray JP, Geiduschek JM, Ramamoorthy C, et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesthesiology 2000; 93: 6–14. [DOI] [PubMed] [Google Scholar]
- 15.Kurth CD, Tyler D, Heitmiller E, et al. National pediatric anesthesia safety quality improvement program in the United States. Anesth Analg 2014; 119: 112–121. [DOI] [PubMed] [Google Scholar]
- 16.Ramamoorthy C, Haberkern CM, Bhananker SM, et al. Anesthesia-related cardiac arrest in children with heart disease: data from the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesth Analg 2010; 110: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 17.Bennett D, Marcus R, Stokes M. Incidents and complications during pediatric cardiac catheterization. Paediatr Anaesth 2005; 15: 1083–1088. [DOI] [PubMed] [Google Scholar]
- 18.O’Byrne ML, Glatz AC, Hanna BD, et al. Predictors of catastrophic adverse outcomes in children with pulmonary hypertension undergoing cardiac catheterization: a multi-institutional analysis from the pediatric health information systems database. J Am Coll Cardiol 2015; 66: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kameny RJ, Fineman J, Adatia I. Perioperative management of pediatric pulmonary hypertension. Advances in Pulmonary Hypertension 2016; 15: 87–91. [Google Scholar]
- 20.Taylor K, Moulton D, Zhao XY, et al. The impact of targeted therapies for pulmonary hypertension on pediatric intraoperative morbidity or mortality. Anesth Analg 2015; 120: 420–426. [DOI] [PubMed] [Google Scholar]
- 21.Friesen RH, Twite MD, Nichols CS, et al. Hemodynamic response to ketamine in children with pulmonary hypertension. Paediatr Anaesth 2016; 26: 102–108. [DOI] [PubMed] [Google Scholar]
- 22.Fischer LG, Van Aken H, Burkle H. Management of pulmonary hypertension: physiological and pharmacological considerations for anesthesiologists. Anesth Analg 2003; 96: 1603–1616. [DOI] [PubMed] [Google Scholar]
- 23.Friesen RH, Williams GD. Anesthetic management of children with pulmonary arterial hypertension. Paediatr Anaesth 2008; 18: 208–216. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson AJ, Bindslev L, Hedenstierna G. Hypoxia-induced pulmonary vasoconstriction in the human lung. The effect of isoflurane anesthesia. Anesthesiology 1987; 66: 312–316. [DOI] [PubMed] [Google Scholar]
- 25.Cheng DC, Edelist G. Isoflurane and primary pulmonary hypertension. Anaesthesia 1988; 43: 22–24. [DOI] [PubMed] [Google Scholar]
- 26.Hickey PR, Hansen DD, Strafford M, et al. Pulmonary and systemic hemodynamic effects of nitrous oxide in infants with normal and elevated pulmonary vascular resistance. Anesthesiology 1986; 65: 374–378. [DOI] [PubMed] [Google Scholar]
- 27.Williams GD, Maan H, Ramamoorthy C, et al. Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Paediatr Anaesth 2010; 20: 28–37. [DOI] [PubMed] [Google Scholar]
- 28.Williams GD, Friesen RH. Administration of ketamine to children with pulmonary hypertension is safe: pro-con debate: Pro Argument. Paediatr Anaesth 2012; 22: 1042–1052. [DOI] [PubMed] [Google Scholar]
- 29.Oklu E, Bulutcu FS, Yalcin Y, et al. Which anesthetic agent alters the hemodynamic status during pediatric catheterization? Comparison of propofol versus ketamine. J Cardiothorac Vasc Anesth 2003; 17: 686–690. [DOI] [PubMed] [Google Scholar]
- 30.Hickey PR, Hansen DD, Cramolini GM, et al. Pulmonary and systemic hemodynamic responses to ketamine in infants with normal and elevated pulmonary vascular resistance. Anesthesiology 1985; 62: 287–293. [DOI] [PubMed] [Google Scholar]
- 31.Lammers AE, Adatia I, Cerro MJ, et al. Functional classification of pulmonary hypertension in children: Report from the PVRI pediatric taskforce, Panama 2011. Pulm Circ 2011; 1: 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]