Abstract
Current evidence suggests that exercise training is beneficial in pulmonary arterial hypertension (PAH). Unfortunately, the standard supervised, hospital-based programs limit patient accessibility to this important intervention. Our proof-of-concept study aimed to provide insight into the usefulness of a prescribed walking regimen along with arginine supplementation to improve outcomes for patients with PAH. Twelve PAH patients (all women) in New York Heart Association (NYHA) functional class (FC) II (n = 7) or III (n = 5) and in stable condition for ≥ 3 months were enrolled. Patients performed home- and fitness-center- based walking at 65–75% heart rate (HR) reserve for 45 min, six sessions/week for 12 weeks. Concomitant L-arginine supplementation (6000 mg/day) was provided to maximize beneficial endothelial training adaptations. Cardiopulmonary exercise testing, 6-min walk testing (6MWT), echocardiography, laboratory studies, and quality of life (QoL) survey (SF-36) were performed at baseline and 12 weeks. Eleven patients completed the study (72 session adherence rate = 96 ± 3%). Objective improvement was demonstrated by the 6MWT distance (increased by 40 ± 13 m, P = 0.01), VO2max (increased by 2 ± 0.7 mL/kg/min, P = 0.02), time-to-VO2max (increased by 2.5 ± 0.6 min, P = 0.001), VO2 at anaerobic threshold (increased by 1.3 ± 0.5 mL/kg/min, P = 0.04), HR recovery (reduced by 68 ± 23% in slope, P = 0.01), and SF-36 subscales of Physical Functioning and Energy/Fatigue (increased by 70 ± 34% and 74 ± 34%, respectively, P < 0.05). No adverse events occurred, and right ventricular function and brain natriuretic peptide levels remained stable, suggesting safety of the intervention. This proof-of-concept study indicates that a simple walking regimen with arginine supplementation is a safe and efficacious intervention for clinically stable PAH patients, with gains in objective function and QoL measures. Further investigation in a randomized controlled trial is warranted.
Keywords: aerobic exercise, exercise training, PAH, cardiopulmonary rehabilitation, home exercise program
Until a decade ago, exercise was generally discouraged for patients with pulmonary arterial hypertension (PAH). Since then, this opinion has shifted toward a more liberal recommendation in favor of exercise, including a grade 1 A recommendation at the 5th World Symposium on pulmonary hypertension (PH),1 mainly based on a number of relatively small trials that indicated safety of exercise in PAH and a positive impact on quality of life (QoL) and functional outcomes.2–16 However, the majority of these trials utilized hospital-based training programs. In particular, eight studies included inpatient hospitalization3,5,7,10–12,14,15,17 and the remainder were performed in a medically supervised outpatient setting.4,6,9,13,16 Based on this, current treatment algorithms indicate supervised exercise training;1,18 however, a more home-based exercise program would be expected to reduce barriers to referral and adherence, and be more convenient for many patients, promoting more widespread use and enhancing compliance.19 A recent Cochrane systematic review reporting equal effectiveness of home- vs. center-based cardiac rehabilitation programs recommended continued expansion of evidence-based, home-based programs that refiect the preference of the individual patient.20 A customized rehabilitative exercise program initiated as an independent regimen for patients with PAH has not been investigated. Our goal was to provide a proof-of-concept trial for evaluating the effectiveness of an individually prescribed 12-week home- and fitness-center-based walking program in patients with mild to moderate PAH. Concomitant L-arginine supplementation was provided based on the rationale that many patients with PAH likely experience at least mild systemic or tissue hypoxia during exercise leading to heightened degradation of endothelial nitric oxide synthase (eNOS) substrate L-arginine and potentially blunted vascular endothelial-mediated adaptations.21–24 Therefore, a commercially available L-arginine supplement was included in our prescribed walking regimen, to circumvent this potential limitation and maximize training effects.
Here we provide proof-of-concept effectiveness of a simple, mostly home-based walking program plus supplemental arginine to improve the primary endpoints of physical function and QoL without negative training effects evident on secondary echocardiographic and blood laboratory safety endpoints. Some of the results of these studies have been previously reported in the form of abstracts.25
Methods
This is a prospective pilot interventional study with each patient serving as his or her control. Patients were recruited from the Indiana University Pulmonary Hypertension Clinic (Indianapolis, IN, USA) and additionally at a recruitment booth set up at the 2014 Pulmonary Hypertension Association International Conference held in Indianapolis, Indiana. The Indiana University institutional review board approved the protocol (#1207009162) and all patients gave written informed consent. The following inclusion criteria were applied: (1) PAH (World Health Organization [WHO] Group 1 pulmonary hypertension) established by right heart catheterization (RHC) within two years prior; (2) New York Heart Association (NYHA) functional class (FC) II–III; (3) age 18–75 years; and (4) clinically stable condition (defined as no changes in treatment for the past six months and a stable 6-min walking test (6MWT) (±20 m) during the previous six months. Exclusion criteria consisted of the following: NYHA functional class I or IV; unstable condition; musculoskeletal or other conditions which would limit exercise participation; non-compliance with medical therapies; or enrollment in other clinical trials. The number of patients enrolled was calculated based on a power of 90% using the primary endpoint of 6MWT, expecting at least a 15% change based on 6MWT improvement reported with a 15-week exercise training intervention with a similar population.7 Primary care providers and pulmonologists were responsible for clinical management of patients.
Exercise training
Walking exercise was performed once/day, six days/week, at 65–75% heart rate (HR) reserve, calculated according to Karvonen26 using the patients’ resting and peak HR observed at baseline cardiopulmonary exercise testing (CPET). Exercise session duration initiated at 25 min and increased to 45 min by the end of week 2. Patients were permitted to choose whether to perform over ground vs. treadmill walking. For patients that lived in proximity (<10 miles) to the university (“local individuals,” n = 7), two of the six weekly sessions were performed in the campus fitness center and the remaining four sessions were performed on their own at home. For patients that did not live in proximity to the fitness center (“distance individuals,” n = 5), all six weekly sessions were performed as an independent home program.
Arginine supplementation
To maximize potentially beneficial endothelial training adaptations,27–29 patients received a commercially available L-arginine oral tablet (General Nutrition Centers, Inc.) to self-administer in three daily doses (6000 mg/day)30,31 for 12 weeks, initiated once baseline testing was completed.
Self- and telemonitoring
A HR monitor was provided for use during all exercise sessions and patients were taught how to modulate walking effort to stay within their target HR zone. While patients were instructed to use their assigned target HR zone to guide exercise intensity, they were additionally advised that rate of perceived exertion (RPE) should not exceed 8 on a 10-point scale and to adjust accordingly as needed. Patients were also taught to monitor arterial oxygen saturation (SpO2) during exercise with a fingertip pulse oximeter at fitness center visits. Distance individuals were provided a take-home fingertip pulse oximeter (Nonin Medical, Plymouth, MN, USA) for monitoring SpO2 during exercise since they were unable to be checked weekly with the fitness center unit. Patients on sildenafil (n = 9) were provided equipment for daily home blood pressure monitoring. All patients were provided a logbook to record daily: (1) any changes in symptoms or self-perceived wellness; (2) minutes and distance achieved in walking session; (3) RPE during walking session on a 10-point scale; and (4) self-administration of daily L-arginine dosing. For local individuals, logbook entries, HR monitor (Polar Electro, Inc., Woodbury, NY, USA) data, and overall program tolerance were reviewed twice weekly at fitness center visits by a trainer. For distance individuals, these were discussed by telephone and an additional weekly call was performed.
Endpoints
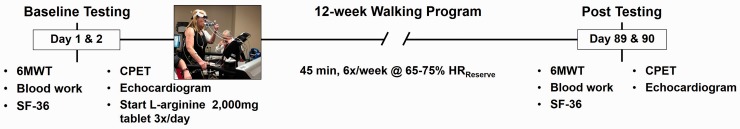
The following measurements were performed within one week, before (“Initial”) and after (“Final”) the 12-week program (Fig. 1): (1) 6MWT to assess distance walked and heart rate recovery (HRrecovery); (2) CPET; 3) physical examination; 4) Doppler echocardiography; 5) fasting blood laboratories; and 6) SF-36 standardized survey to assess health-related QoL. Primary endpoints were change from baseline in 6MWT distance and peak aerobic capacity (VO2max). Secondary endpoints were change in safety indicators of tricuspid annular plane systolic excursion (TAPSE; a surrogate of right ventricular [RV] systolic function) on echocardiography and plasma concentration of brain natriuretic peptide (BNP) as an indicator of cardiac wall stress. Adverse events were defined as hospitalization, death, worsening PH, escalation of therapy, syncope, hypotension, or GI effects.
Fig. 1.
Study protocol indicating pre- and post-intervention measures, separated by the 12-week walking program.
6MWT distance and heart rate recovery
A 6MWT was performed under standardized conditions32 using an indoor corridor or a gymnasium track. Tester and testing conditions were kept identical for initial and final testing. HR via continuous measurement with chest strap (Polar Electro, Inc.) and SpO2 via fingertip oximeter (Nonin Medical, Plymouth, MN, USA) were recorded pre test after 5 min of quiet sitting. HR and SpO2 were recorded every minute during 10 min of sitting immediately after 6MWT completion. HRrecovery was calculated as the slope of the relationship between HR and minutes of recovery (0–10 min, as well as just the first 5 min). HRrecovery is additionally presented as the % of end-exercise (peak) HR at the 10-min recovery time point.
CPET
CPETs were performed using a continuous, speed- and incline-incremented maximal treadmill ramp protocol with 2-min stages until volitional fatigue. Ventilation (VE), oxygen uptake (VO2), and carbon dioxide output (VCO2) were measured continuously by open-circuit spirometry using a Vmax by Carefusion Metabolic Measurement System (BD, Inc., Yorba Linda, CA, USA) and HR was measured via the 12-lead electrocardiogram. The anaerobic threshold was detected with the V-slope method. Resting energy consumption and HR was measured during 5 min of standing on the treadmill before exercise testing. HR and RPE (using a Borg 6–20-point scale) were recorded in the middle and last 30 s of each stage.
Step counts
Pedometer-recorded steps walked in one week (including during exercise sessions) at week 2 and week 11 of the intervention was used to detect any change in overall physical activity habits. Since there is concern that participants of a new exercise regimen might choose more sedentary behaviors in the non-exercise portion of their days which could negate some training benefits, the main purpose of collecting step data was to determine if participants changed their overall daily physical activity as a consequence of the intervention. All individuals received a pedometer (NL-1000 Accelerometer, New Lifestyles, Inc.) calibrated to his/her stride to record steps walked for one week at the beginning of the intervention (week 2) and for one week at the end of the 12-week period (week 11). Participants were instructed to wear the pedometer for all out-of-bed non-bathing/swimming activities, including their exercise walking session, for each seven-day sampling period.
Adherence
Exercise adherence was determined by logbook entries and confirmed by weekly phone call logs and pedometer data and is expressed as the percentage of exercise sessions performed out of the 72 prescribed sessions. L-arginine adherence was also determined by logbook entries and confirmed by weekly phone call logs, as expressed as the percentage of doses taken out of the 216 doses (3 × /day × 72 days) prescribed.
Laboratory values
Venous blood sampling was performed pre and post intervention, collected from patients in the sitting position, on the day of appointment when patient had fasted overnight. Whole blood samples were immediately sent on ice to the pathology laboratory of Indiana University Health for processing and assays which included: C-reactive protein as an indicator of systemic inflammation; BNP as an indicator of RV wall stress; a complete blood count (CBC); a standard metabolic panel and lipid profile; and an amino acid profile including L-arginine and L-citrulline.
Echocardiography
Doppler echocardiography was used to measure PH parameters as recommended by current American Society of Echocardiography guidelines.33,34 M-mode and two-dimensional echocardiography were performed with the patient in the left lateral position using a General Electric Vivid-q system (Fairfield, CT, USA). All studies were scanned in standard views in addition to RV dedicated views when possible. Tricuspid regurgitant (TR) flow was measured in continuous wave mode at the parasternal and apical areas with the highest velocity used for calculation. The peak instantaneous systolic pressure drop from right ventricle to atrium (transvalvular gradient) was calculated from the peak signal velocity of the TR signal by the simplified Bernoulli equation. The final estimation of pulmonary artery systolic pressure (PASP) was obtained by adding the patient’s jugular venous pressure derived from the inferior vena cava size and collapse to the estimate of PASP. RV diameters were measured at the base in the standard apical four-chamber view in their respective times in the cardiac cycle; RA size was measured by planimetry in the apical four-chamber view at end systole. The presence of a notched pattern in the pulse wave through the right ventricular outflow tract (RVOT) was judged by the cardiology team. Impaired RV function was measured with: TAPSE measured using M-mode; and RV fractional area change was calculated based on the difference of end-diastolic area and end-systolic area and divided by end-diastolic area. All studies were processed in digital format and analyzed using a digital offline quantification system.
Statistical analyses
Paired t-testing measured changes between the pre-test and post-test in all outcome variables. Analysis of variance detected differences between local and distance individuals in enrollment characteristics, and in baseline and change values. Data are presented for individuals and as means with standard error (SEM). Statistical analysis utilized SPSS v. 23.0 and differences at α level of 0.05 were considered statistically significant.
Results
Enrollment
A total of 378 patients were screened; 93 had group I PAH and 12 met the criteria and were enrolled (Fig. 2, Table 1). Of these, 11 completed the study; the dropout of one patient was due to time constraints.
Fig. 2.
Flowchart indicating the number of patients screened for enrollment, the number of patients and reasons for exclusion, and the number of patients completed.
Table 1.
Participant characteristics at enrollment.
| Age (years) | 44 ± 4.0 |
| Gender (n) | |
| Women | 12 |
| Men | 0 |
| Height (cm) | 160 ± 1.0 |
| Weight (kg) | 79 ± 6.8 |
| BMI | 30 ± 2.8 |
| 6MWD (m) | 482 ± 17 |
| PAH etiology (n) | |
| Idiopathic | 8 |
| Connective tissue disease | 1 |
| Congenital heart disease | 3 |
| NYHA class (n) | |
| II | 6 |
| III | 6 |
| Co-morbidities (n) | |
| Hypertension | 3 |
| Diabetes mellitus | 1 |
| Hyperlipidemia | 3 |
| Obstructive sleep apnea | 3 |
| Oxygen use (n) | 2 |
| PAH therapy (n) | |
| Prostacyclin | 4 |
| Ca + channel blocker | 2 |
| ERA | 2 |
| PDE5 inhibitor | 9 |
| Hemodynamics | |
| mPAP (mmHg) | 44 ± 5 |
| PAWP (mmHg) | 12 ± 2 |
| PVR (WU) | 6.6 ± 1.5 |
| Cardiac index (mL/min/m2) | 3.1 ± 0.3 |
Chart values obtained at time of enrollment for the 12 enrolled patients are presented as n, or as means ± SEM where indicated.
BMI, body mass index; 6MWD, 6-min walk distance; PAH, pulmonary arterial hypertension; NYHA, New York Heart Association; ERA, endothelin receptor antagonist; PDE, phosphodiesterase; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance.
Adherence
Adherence to the prescribed exercise regimen was > 95% in nine participants, and 64% and 94% adherence in the remaining two participants. Adherence to the prescribed L-arginine was > 90% in eight individuals and at least 80% adherence in the remaining individuals, except one participant who had an adherence of 58%. Weekly average step count (Table 2) moved in the anticipated direction over the intervention period with a slight steps-per-week increase between weeks 2 and 11, expected as pedometer collection included steps during walking sessions.
Table 2.
Change in hemodynamics, weekly step count, 6MWT, and CPET.
| Variable | Initial | Final | Absolute change | P value | Change (%) |
|---|---|---|---|---|---|
| Weight (kg) | 79 ± 7 | 79 ± 7 | –0.2 ± 0.7 | 0.81 | 0.0 ± 0.9 |
| Resting HR (bpm) | 83 ± 3 | 79 ± 3 | –4.0 ± 3.7 | 0.32 | –3.7 ± 4.6 |
| Systolic BP (mmHg) | 113 ± 5 | 113 ± 5 | –0.1 ± 4.6 | 0.98 | 0.7 ± 3.9 |
| Diastolic BP (mmHg) | 81 ± 5 | 75 ± 3 | –6.8 ± 5.9 | 0.27 | –5.6 ± 5.3 |
| Pedometer 1-week average (steps/day) | 7717 ± 809 | 8276 ± 917 | 559 ± 522 | 0.31 | 3.8 ± 6.9 |
| 6MWT | |||||
| Distance (m) | 481 ± 18 | 521 ± 23 | 40 ± 13.4* | 0.01 | 8.6 ± 2.6 |
| Peak HR (bpm) | 112 ± 4 | 126 ± 8 | 14 ± 4.0* | 0.01 | 7.7 ± 4.8 |
| HRrecovery (slope 0–5 min) | –4 ± 0.5 | –7 ± 3 | –2.7 ± 0.8** | 0.008 | 65.0 ± 22 |
| HRrecovery (slope 0–10 min) | –2 ± 0.3 | –3 ± 0.4 | –1.2 ± 0.4* | 0.01 | 68.0 ± 19 |
| HRrecovery (%peak HR at 10 min) | 80 ± 2.4 | 68 ± 2.7 | –12 ± 2.5** | 0.001 | –14.2 ± 3 |
| CPET | |||||
| Time to VO2max (min) | 9.5 ± 0.6 | 12.0 ± 1.1 | 2.5 ± 0.6** | 0.001 | 24.4 ± 4.9 |
| VO2max (mL/kg/min) | 15.7 ± 1.2 | 17.5 ± 1.1 | 1.8 ± 0.7* | 0.02 | 13.2 ± 4.7 |
| VO2max (L/min) | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.13 ± 0.1* | 0.02 | 12.7 ± 4.3 |
| VO2max %predicted | 51.4 ± 3.7 | 58.1 ± 3.8 | 6.6 ± 2.5* | 0.03 | 13.7 ± 5.0 |
| Ventilation max (L/min) | 45.8 ± 4.7 | 49.7 ± 5.6 | 6.0 ± 3.2 | 0.11 | 13.0 ± 6.0 |
| Peak METs | 5.3 ± 0.4 | 5.4 ± 0.3 | 0.2 ± 0.2 | 0.38 | 5.0 ± 3.6 |
| HR at rest (bpm) | 93 ± 6 | 89 ± 4 | –4.9 ± 4.8 | 0.33 | –3.3 ± 4.8 |
| HRmax (bpm) | 155 ± 6 | 152 ± 4 | –2.5 ± 3.0 | 0.49 | –1.1 ± 2.3 |
| Borg scale at max | 16.5 ± 0.5 | 15.2 ± 1.3 | –1.1 ± 1.1 | 0.36 | –7.2 ± 7.5 |
| VO2 at AT (mL/kg/min) | 13.4 ± 1.5 | 14.4 ± 1.2 | 1.3 ± 0.5* | 0.047 | 9.9 ± 4.3 |
| ΔSBP (mmHg) / ΔVO2 (mL/kg/min) | 3.7 ± 0.6 | 3.4 ± 0.6 | –0.2 ± 0.5 | 0.76 | 13.0 ± 6.0 |
| VE/VCO2 mid | 38.2 ± 0.4 | 36.4 ± 0.3 | –1.3 ± 1.2 | 0.31 | 5.0 ± 3.6 |
| 65–75% HRreserve | 133-9 ± 7 | 130-6 ± 7 | –3.2 ± 3 | 0.40 | –1.8 ± 2.6 |
Values are means ± SEM for the 11 of 12 patients that completed the study. Paired t-testing was used to analyze changes between the pre-test (Initial) and post-test (Final) at the end of 12 weeks in all outcome variables, with bold type and asterisks indicating a significant change (*P < 0.05 and **P < 0.01) in comparison to baseline.
HR, heart rate; bpm, beats per minute; BP, blood pressure; 6MWT, 6-min walk test; VO2max, maximum oxygen consumption; MET, metabolic equivalent; Peak HR, highest HR achieved; HRrecovery, HR relative to minutes of recovery; AT, anaerobic threshold; ΔSBP, change in systolic blood pressure from rest to peak exercise; ΔVO2, change in VO2 from rest to peak exercise; VE/VCO2, ventilatory equivalent for carbon dioxide; 65–75%HRreserve, calculated according to formula of Karvonen.26
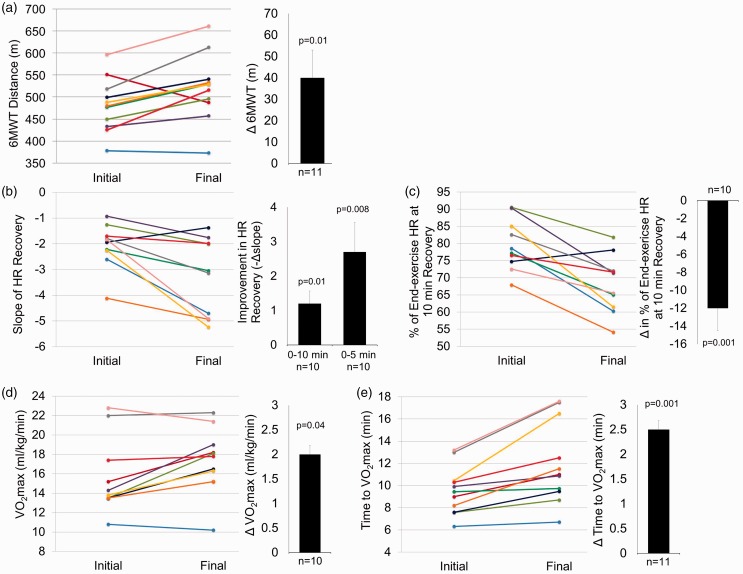
Training effects on functional status
6MWT (Fig. 3a, Table 2) distance increased by a mean of 40 m. Eight patients (73%) surpassed the minimum clinically important difference (MCID) for this population of 33 m.35 The slope of HRrecovery (Fig. 3b, Table 2) was improved (more negative) in all but one participant, with the majority of improvement occurring within the first 5 min of recovery. Faster HRrecovery after training was also evidenced by patients being at a lower percentage of end-exercise HR at the 10-min recovery time point (Fig. 3c, Table 2). Favorable changes were also observed in CPET parameters, including VO2max, time-to-VO2max (Fig. 3d, e, Table 2), and anaerobic threshold (AT) (Table 2).
Fig. 3.
Training improvements in aerobic exercise tolerance and cardiorespiratory fitness. Individual responses are presented as different colored lines from initial to final values for 11 patients who completed the study. Paired t-testing was used to analyze changes between the pre-test (Initial) and post-test at the end of 12 weeks (Final) in all outcome variables. Mean ± SEM and P values for pre- to post-intervention change are presented in bar graphs adjacent to individual responses. (a) 6MWT distance for n = 11; (b) HRrecovery as slope of relationship between HR and 0–10 min of recovery following 6MWT for n = 10 (missed collection in one participant), including an additional bar graph indicating mean ± SEM for slope of relationship between HR and 0–5 min of recovery; (c) HRrecovery % of end-6MWT HR at the 10-min recovery time point for n = 10 (missed collection in one individual); (d) maximal rate of oxygen consumption relative to kg of body mass (VO2max) in CPET for n = 10 (unable to collect expired gases in one participant that required supplemental oxygen during exercise); (e) number of minutes completed of CPET incremental treadmill protocol for n = 11.
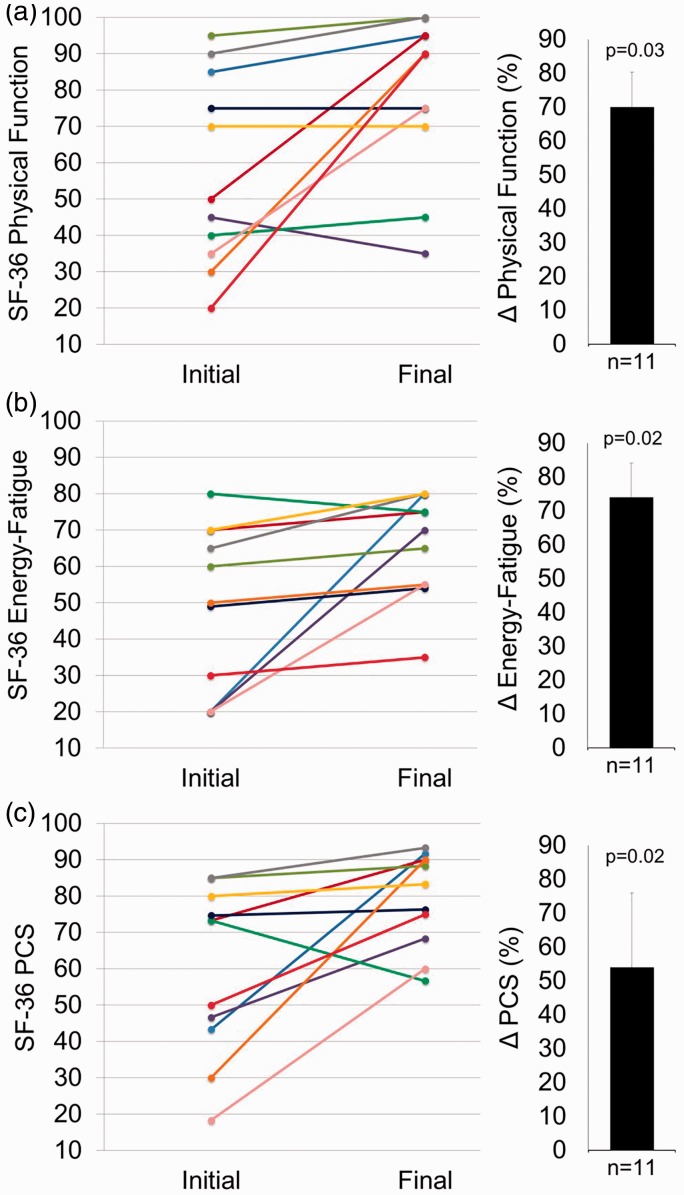
Training effects on quality of life
QoL scores increased by > 70% for SF-36 subscales Physical Functioning and Energy/Fatigue (Fig. 4a, b, Table 3) with a tendency for increase in Emotional Wellbeing (P = 0.12) and Role Limitation Physical Health (P = 0.19). The Physical Component Summation (PCS) score, an aggregation of subscales specific to physical health (Fig. 4c), was also increased.
Fig. 4.
Training improvements in QoL as assessed by the SF-36. Individual responses are presented as different colored lines from initial to final values for 11 patients who completed the study. Paired t-testing was used to analyze changes between the pre-test (Initial) and post-test at the end of 12 weeks (Final) in all outcome variables. Mean ± SEM and P values for pre- to post-intervention change is presented in bar graphs adjacent to individual responses. (a) SF-36 subscale for Physical Function; (b) SF-36 subscale for Energy/Fatigue; and (c) SF-36 Physical Component Summation (PCS) score.
Table 3.
Quality of life.
| SF-36 Scale | Initial | Final | Absolute change | P value | Change (%) |
|---|---|---|---|---|---|
| Physical functioning | 58 ± 8 | 79 ± 7 | 21 ± 8* | 0.03 | 70 ± 34 |
| Energy/Fatigue | 48 ± 7 | 66 ± 4 | 17 ± 6* | 0.02 | 74 ± 34 |
| Role limitations due to physical health | 75 ± 12 | 91 ± 6 | 16 ± 11 | 0.19 | 53 ± 37 |
| Bodily pain | 81 ± 5 | 83 ± 5 | 2 ± 2 | 0.29 | 3 ± 2 |
| General health perception | 53 ± 4 | 55 ± 7 | 2 ± 5 | 0.68 | 4 ± 11 |
| Social functioning | 76 ± 7 | 78 ± 6 | 2 ± 8 | 0.79 | 14 ± 16 |
| Role limitations due to emotional | 97 ± 3 | 91 ± 6 | 0 ± 0 | 0.34 | 0 ± 0 |
| Emotional wellbeing | 72 ± 5 | 83 ± 3 | 11 ± 6 | 0.12 | 28 ± 19 |
Values are means ± SEM for the 11 of 12 patients that completed the study. Paired t-testing was used to analyze changes between the pre-test (Initial) and post-test (Final) at the end of 12 weeks in all SF-36 quality of life scales, with bold type and asterisks indicating a significant change (*P < 0.05) in comparison to baseline.
No adverse effects of training
No adverse events occurred. Echocardiographic and blood laboratory data demonstrated that the intervention was not associated with negative effects (Table 4). In addition to unchanged BNP, echocardiographic parameters of RV function such as TAPSE and RV fractional area change also remained stable over the 12 weeks, suggesting safety of the intervention. Plasma L-arginine increased markedly as expected, but no other blood values changed from baseline.
Table 4.
Change blood laboratory and echocardiography parameters (n = 11).
| Initial | Final | Absolute change | P value | Change (%) | |
|---|---|---|---|---|---|
| Blood laboratory | |||||
| WBC | 7.71 ± 0.7 | 7.42 ± 0.6 | –0.3 ± 0.5 | 0.57 | –2.1 ± 5.2 |
| Hemoglobin (g/dL) | 13.60 ± 0.4 | 13.45 ± 0.4 | –0.2 ± 0.2 | 0.57 | 1 ± 1.9 |
| Platelets | 250.36 ± 25.6 | 266.18 ± 27.3 | 15.8 ± 11.5 | 0.20 | 6.8 ± 4.7 |
| Sodium (mmol/L) | 138.18 ± 0.7 | 137.45 ± 0.7 | –0.7 ± 0.6 | 0.27 | –0.5 ± 0.5 |
| Bicarbonate (mmol/L) | 23.20 ± 1.2 | 24.27 ± 0.9 | 0.9 ± 0.5 | 0.12 | 4.7 ± 2.5 |
| Creatinine (mg/dL) | 0.84 ± 0.1 | 0.82 ± 0.1 | –0.01 ± 0.01 | 0.34 | –1.3 ± 1.6 |
| BNP (pg/mL) | 109.82 ± 58.1 | 96.00 ± 44.2 | –13.8 ± 17.9 | 0.46 | 16.4 ± 25 |
| L-arginine (nmol/mL) | 48.82 ± 4.4 | 81.82 ± 10.7 | 33.0 ± 9.6* | 0.006 | 72.1 ± 18 |
| L-citrulline (nmol/mL) | 31.91 ± 3.7 | 37.36 ± 4.7 | 5.5 ± 3.8 | 0.19 | 19.9 ± 11.3 |
| Insulin sensitivity (TG/HDL) | 2.82 ± 0.4 | 2.52 ± 0.2 | –0.3 ± 0.2 | 0.29 | –1.7 ± 10.3 |
| C-reactive protein (mg/L) | 5.63 ± 1.9 | 5.32 ± 1.4 | –0.3 ± 0.9 | 0.74 | 43.7 ± 26.2 |
| Echocardiography | |||||
| RA area | 17.62 ± 2.1 | 18.92 ± 1.8 | 1.3 ± 1.3 | 0.35 | 11.2 ± 8.6 |
| Estimated RA pressure | 7.10 ± 0.8 | 6.60 ± 0.9 | –0.5 ± 0.9 | 0.59 | 4.2 ± 19.8 |
| TR jet gradient | 38.04 ± 4.6 | 41.06 ± 6.9 | 3.0 ± 3.6 | 0.43 | 5.7 ± 9.1 |
| TAPSE | 1.57 ± 0.2 | 1.56 ± 0.1 | –0.01 ± 0.1 | 0.93 | 4 ± 8.2 |
| RV EDD | 4.14 ± 0.3 | 4.19 ± 0.2 | 0.05 ± 0.1 | 0.76 | 2.6 ± 4 |
| RV ESD | 3.38 ± 0.3 | 3.40 ± 0.2 | 0.020 ± 0.1 | 0.90 | 2.5 ± 4.1 |
| RV FS | 0.19 ± 0.0 | 0.19 ± 0.0 | 0.001 ± 0.03 | 1.0 | 23.8 ± 27.4 |
| RV EDA | 27.35 ± 2.4 | 28.39 ± 2.5 | 1.04 ± 1.4 | 0.47 | 4.7 ± 5.2 |
| RV ESA | 19.20 ± 2.9 | 19.21 ± 2.9 | 0.007 ± 1.06 | 0.99 | 2.1 ± 5.7 |
| RV FA change | 0.32 ± 0.0 | 0.34 ± 0.0 | 0.02 ± 0.03 | 0.43 | 11.6 ± 10.8 |
| L atrial AP diameter | 3.16 ± 0.1 | 3.15 ± 0.1 | –0.01 ± 0.1 | 0.92 | 0.5 ± 3 |
| LV EDD | 4.31 ± 0.2 | 4.05 ± 0.3 | –0.3 ± 0.2 | 0.25 | –6.2 ± 5.5 |
| LV ESD | 2.82 ± 0.2 | 2.72 ± 0.2 | –0.1 ± 0.1 | 0.60 | –1.3 ± 7.3 |
| LV FS | 34.95 ± 3.1 | 31.86 ± 3.2 | –3.1 ± 3.7 | 0.43 | –4.3 ± 9.9 |
| LV EDV | 93.03 ± 8.6 | 92.39 ± 6.3 | –3.4 ± 9.1 | 0.72 | 0.7 ± 9.8 |
| LV ESV | 34.23 ± 4.9 | 42.01 ± 4.4 | 2.9 ± 5.0 | 0.58 | 18.5 ± 16.5 |
| LV EF | 64.22 ± 2.8 | 58.62 ± 1.8 | –4.8 ± 2.1 | 0.07 | –7.1 ± 3.3 |
| LV SV | 61.38 ± 4.8 | 52.75 ± 3.0 | –9.4 ± 5.1 | 0.11 | –11.6 ± 7.4 |
| AVA | 2.70 ± 0.1 | 2.84 ± 0.3 | 0.30 ± 0.4 | 0.68 | 10.2 ± 7.4 |
| E/A | 1.28 ± 0.1 | 1.15 ± 0.1 | –0.13 ± 0.09 | 0.20 | –7.2 ± 7 |
| E/E’ | 8.01 ± 0.7 | 7.80 ± 0.6 | –0.27 ± 1.6 | 0.84 | 3.8 ± 12.4 |
Values are means ± SEM for the 11 of 12 patients that completed the study. Paired t-testing was used to analyze changes between the pre-test (Initial) and post-test (Final) at the end of 12 weeks in all outcome variables, with bold type and asterisks indicating a significant change (*P < 0.05) in comparison to baseline.
WBC, white blood cell count; BNP, brain natriuretic peptide; TG, triclycerides; HDL, high-density lipoprotein; RA, right atrial; TR, tricuspid regurgitant; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; LV, left ventricle; EDD, end-diastolic diameter, ESD, end-systolic diameter; FS, fractional shortening; EDA, end-diastolic area; ESA, end-systolic area; FA change, fractional area change; AP, anteroposterior; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; SV, stroke volume; AVA, aortic valve area; E/A, average ratio of transmitral Doppler E wave velocity to A wave velocity; E/E’, ratio of Doppler-derived peak E velocity and early diastolic E' velocity at mitral annulus.
Outcomes for completely home-based vs. partially fitness-center-based program
A little less than half of the individuals were distance individuals and performed all walking sessions independently at home. While not intended a priori, this provided an additional opportunity to compare adherence and outcomes between a partially fitness-center-based vs. completely home-based program. There was no difference in enrollment characteristics (Table 1) or initial values (Table 2) between these two groups except that the distance vs. the local individuals tended to be older (52 ± 5 vs. 37 ± 4 years, P = 0.05) with lower initial systolic blood pressure (101 ± 6 vs. 124 ± 6 mmHg, P = 0.03) and higher % age-predicted VO2max (60 ± 5 vs. 44 ± 2% predicted, P = 0.02). There was no difference in adherence (93.3 ± 6 vs. 98.3 ± 1%, P = 0.47) or pre-post change values between local and distance individuals for any functional, QoL, echocardiographic, or blood laboratory outcomes.
Discussion
Our uncontrolled, prospective interventional pilot study demonstrates that a simple walking program with arginine supplementation resulted in positive impact on functional and QoL outcomes for PAH. The improvement is equivalent or better to previously reported more highly structured training interventions conducted in a medically supervised setting. In addition, no adverse events occurred and echocardiographic and blood indicators of RV function and wall stress remained stable over the 12-week period, suggesting safety of this mostly home-based intervention in a PAH patient cohort.
Improvements in functional status
Compared to that reported for trials of supervised cycling or treadmill exercise in PH, similar or greater gains were attained in 6MWT distance2–6,9,10,12,13,16,36 and VO2max or time to VO2max3–7,12–14,16,36 following our mostly home-based walking program. Improvement in 6MWT and/or VO2max was also as good or better than those reported in exercise trials that included add-ons such as respiratory2,5,7,11,12,14,36 or resistance2–7,10–12,14,36 training. Of the patients, 73% surpassed the 6MWT distance MCID for PAH (33 m),35 as well as for chronic obstructive pulmonary disease (COPD) and heart failure (54–55 m),37 with a mean improvement of almost twice the MCID. Improved aerobic fitness was also evidenced by a higher VO2 at AT and faster HRrecovery, a parameter that has not been customarily reported in exercise trials for PAH but has been proposed to be an important marker predictive of clinical worsening and time to clinical worsening in patients with idiopathic PAH.38 A possible association of autonomic dysfunction with survival in PAH39–41 makes the improvement observed in HRrecovery after our simple walking program even more relevant and important.
Improvements in quality of life
Patient gain in QoL was as good as or better than that reported for exercise trials in PH that have included patient counseling or mental training/coaching sessions.5,7,10,12–14,36 However, we cannot rule out that even further gains in QoL or functional indicators would have occurred if such additional components had been included in the 12-week program.
Adherence and feasibility
While adherence to prescribed exercise for patients with PAH is only reported for a limited number of trials to date, the 96 ± 3% adherence rate for our 72-session walking program was as good as4 or better3,12,13,16 compared to that in trials utilizing more highly structured interventions that report adherence rates. The close verbal communication with patients and the tools provided (HR monitors, training logs, pedometers) may have contributed to our exceptionally high adherence. Weekly average step count remained consistent between week 2 and week 11 indicating that outcomes were not confounded by large swings in the sedentary or physical activity habits of patients over this time. Since pedometer recordings included exercise sessions, the slight steps-per-week increase observed may be accounted for by walking speed increases with patient conditioning as expected, but the methodology precludes this assessment.
Adherence was equally good for local vs. distance individuals, suggesting that this is not dependent on in-person interaction between patient and trainer. The high exercise adherence of our trial not only reduced risk of patients not being dosed as intended with the intervention,42,43 but also suggests that this approach may have favorable patient compliance when implemented in practice. Indeed, the financial and logistical burden on patients, clinicians, caregivers, and third-party payers, was quite low for our intervention especially compared to training approaches previously investigated for PAH that encompassed inpatient hospitalization and issue of a cycle ergometer for each patient upon discharge.7 For example, the cost per patient for our 12-week exercise intervention was under US$800 and no work hours were missed for employed patients to participate. Though exercise trials for PH conducted in an outpatient setting4,6,9,13,16 have a clear feasibility advantage by not requiring an inpatient component, these too can present significant challenges to implementation and compliance44,45 such as transportation and location46,47 and long wait periods for availability.46 These are eliminated with home-based exercise programs. Further, due to newer and more available technologies (e.g. wearable accelerometers, pulse oximeters, personalized web-based exercise tracking and coaching applications) home-based programs now have much more feedback and interaction with patients; this may enhance preference, compliance, safety, and effectiveness.48,49 Feasibility of home-based exercise interventions has been demonstrated for patients with COPD,50 myocardial infarction,51,52 and heart failure,53 and our results suggest feasibility for patients with PAH as well.
Concomitant arginine supplementation
This study provides initial insight into usefulness of concomitant L-arginine for patients participating in an exercise regimen, on the basis that positive training effects may be augmented by provision of increased substrate for eNOS, thus increasing nitric oxide bioavailability in the pulmonary vasculature.27,29,54–56 Plasma L-arginine was effectively increased by the supplementation provided and our findings agree with a previous report of VO2max improvement with L-arginine supplementation in pre-capillary PH.28 However, our small proof-of-concept study design precludes evaluation of L-arginine’s specific impact on endpoints. Nonetheless, it is possible that some of the functional gains observed were augmented by L-arginine attenuating pulmonary pressure rise relative to workload, which is a known exercise limiter for this population.57,58 While this pilot study was not powered or designed to test adherence and tolerance to L-arginine, both were observed to be good across all patients and no patients experienced gastrointestinal distress, hypotension, or other adverse symptoms. Further placebo-controlled studies examining the impact of this readily available and inexpensive supplement, or other potential enhancers of endothelial-mediated positive exercise responses in PAH, are warranted, as has been demonstrated with L-arginine plus training for cardiac and skeletal muscle of healthy trained rats29,54 and pulmonary arteries of pulmonary hypertensive rats.27
Limitations
This proof-of-concept study was intended to merely pilot feasibility for use of a mostly home-based training approach plus arginine supplementation. As such, there was no control comparative group for the walking intervention and each participant served as their own control. Further, as discussed above, this small feasibility study included no group receiving L-arginine only or exercise only, with the intention that these variables be examined independently in a subsequent larger controlled trial.
The enrollment number was small and the presence of a selection bias toward more motivated and higher functioning patients is likely, supported by slightly higher 6MWT distances at baseline than is typical for a PAH population.59 It is possible that observations reported here following training may have been affected differently in a cohort with more impairment at baseline. For instance, improvement in 6MWT may have been even greater if we had enrolled a more functionally limited patient cohort.60 On the other hand, more impaired individuals may have resulted in lower adherence due to greater difficulty completing the protocol.
Since exclusion criteria included non-compliance with medical therapies, it is possible that examining a low-structure intervention approach that specifically aims to remove barriers to compliance as we have done here may not have contributed as much to the outcomes as it would for less compliant patients. Also, regarding compliance, there is inherent weakness in relying primarily on self-report for exercise adherence which was the case for 10 out of the 12 weeks of the intervention, since pedometer counts were only collected during weeks 2 and 11. A subsequent trial would be strengthened with the inclusion of devices such as wearable accelerometer activity monitors to measure daily adherence to the exercise intervention.
Lastly, screening of 110 patients was required to enroll 12 patients, and five of these patients lived too far away to conveniently utilize the fitness center, therefore performing all their walking sessions on their own at home. Comparable outcomes for these patients suggests that a completely home-based program is as efficacious as a partially fitness-center-based program; however, the study was not adequately powered a priori to make meaningful comparisons in outcomes between these two groups.
In conclusion, our mostly home-based prescribed walking regimen plus arginine supplementation for clinically stable PAH patients elicited good adherence and gains in function and QoL on par with those reported for more highly structured exercise interventions. The proof-of-concept feasibility demonstrated here supports the need for a subsequent randomized clinical trial to further investigate this potentially paradigm-changing approach to exercise prescription for PAH.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was entirely funded by the American Thoracic Society-Pulmonary Hypertension Association Proof-of-Concept grant to MBB. The funders provided no input or contributions in the development of the research and manuscript.
References
- 1.Galie N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62: D60–72. [DOI] [PubMed] [Google Scholar]
- 2.Uchi M, Saji T, Harada T. [Feasibility of cardiopulmonary rehabilitation in patients with idiopathic pulmonary arterial hypertension treated with intravenous prostacyclin infusion therapy]. J Cardiol 2005; 46: 183–193. [PubMed] [Google Scholar]
- 3.de Man FS, Handoko ML, Groepenhoff H, et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2009; 34: 669–675. [DOI] [PubMed] [Google Scholar]
- 4.Fox BD, Kassirer M, Weiss I, et al. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. J Card Fail 2011; 17: 196–200. [DOI] [PubMed] [Google Scholar]
- 5.Grunig E, Maier F, Ehlken N, et al. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Res Ther 2012; 14: R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mainguy V, Maltais F, Didier S, et al. Effects of a rehabilitation program on skeletal muscle function in idiopathic pulmonary hypertension. J Cardiopulm Rehabil Prev 2010; 30: 319–323. [DOI] [PubMed] [Google Scholar]
- 7.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006; 114: 1482–1489. [DOI] [PubMed] [Google Scholar]
- 8.Zafrir B. Exercise training and rehabilitation in pulmonary arterial hypertension: rationale and current data evaluation. J Cardiopulm Rehabil Prev 2013; 33: 263–273. [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker M, Wilt J, Dasgupta R, et al. Exercise training in patients with pulmonary arterial hypertension: a case report. Cardiopulm Phys Ther J 2009; 20: 12–18. [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Quintana E, Miranda-Calderin G, Ugarte-Lopetegui A, et al. Rehabilitation program in adult congenital heart disease patients with pulmonary hypertension. Congenit Heart Dis 2010; 5: 44–50. [DOI] [PubMed] [Google Scholar]
- 11.Grunig E, Ehlken N, Ghofrani A, et al. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration 2011; 81: 394–401. [DOI] [PubMed] [Google Scholar]
- 12.Nagel C, Prange F, Guth S, et al. Exercise training improves exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. PLoS One 2012; 7: e41603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan L, Chin LM, Kennedy M, et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013; 143: 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker-Grunig T, Klose H, Ehlken N, et al. Efficacy of exercise training in pulmonary arterial hypertension associated with congenital heart disease. Int J Cardiol 2013; 168: 375–381. [DOI] [PubMed] [Google Scholar]
- 15.Ley S, Fink C, Risse F, et al. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur Radiol 2013; 23: 324–331. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein AA, Chin LM, Keyser RE, et al. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir Med 2013; 107: 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlken N, Lichtblau M, Klose H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J 2016; 37: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 19.Piotrowicz E, Baranowski R, Bilinska M, et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail 2010; 12: 164–171. [DOI] [PubMed] [Google Scholar]
- 20.Taylor RS, Dalal H, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2015; 8: CD007130. [DOI] [PubMed] [Google Scholar]
- 21.Goret L, Reboul C, Tanguy S, et al. Training does not affect the alteration in pulmonary artery vasoreactivity in pulmonary hypertensive rats. Eur J Pharmacol 2005; 527: 121–128. [DOI] [PubMed] [Google Scholar]
- 22.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2009; 121: 2045–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krotova K, Patel JM, Block ER, et al. Hypoxic upregulation of arginase II in human lung endothelial cells. Am J Physiol Cell Physiol 2010; 299: C1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, Kaneko FT, Zheng S, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 2004; 18: 1746–1748. [DOI] [PubMed] [Google Scholar]
- 25.Brown M, Kempf A, Collins C, et al. A simple daily walking program plus l-arginine supplementation improves aerobic capacity and quality of life in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 193: A2316. [Google Scholar]
- 26.Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med 1988; 5: 303–511. [DOI] [PubMed] [Google Scholar]
- 27.Goret L, Tanguy S, Guiraud I, et al. Acute administration of l-arginine restores nitric oxide-mediated relaxation in isolated pulmonary arteries from pulmonary hypertensive exercise trained rats. Eur J Pharmacol 2008; 581: 148–156. [DOI] [PubMed] [Google Scholar]
- 28.Nagaya N, Uematsu M, Oya H, et al. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med 2001; 163: 887–891. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki J. Microvascular angioadaptation after endurance training with l-arginine supplementation in rat heart and hindleg muscles. Exp Physiol 2005; 90: 763–771. [DOI] [PubMed] [Google Scholar]
- 30.Jabecka A, Ast J, Bogdaski P, et al. Oral L-arginine supplementation in patients with mild arterial hypertension and its effect on plasma level of asymmetric dimethylarginine, L-citruline, L-arginine and antioxidant status. Eur Rev Med Pharmacol Sci 2012; 16: 1665–1674. [PubMed] [Google Scholar]
- 31.Jablecka A, Bogdanski P, Balcer N, et al. The effect of oral L-arginine supplementation on fasting glucose, HbA1c, nitric oxide and total antioxidant status in diabetic patients with atherosclerotic peripheral arterial disease of lower extremities. Eur Rev Med Pharmacol Sci 2012; 16: 342–350. [PubMed] [Google Scholar]
- 32.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166: 111–117. [DOI] [PubMed]
- 33.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39. [DOI] [PubMed] [Google Scholar]
- 34.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713; quiz 86–88. [DOI] [PubMed] [Google Scholar]
- 35.Mathai SC, Puhan MA, Lam D, et al. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunig E, Lichtblau M, Ehlken N, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012; 40: 84–92. [DOI] [PubMed] [Google Scholar]
- 37.Rasekaba T, Lee AL, Naughton MT, et al. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J 2009; 39: 495–501. [DOI] [PubMed] [Google Scholar]
- 38.Minai OA, Gudavalli R, Mummadi S, et al. Heart rate recovery predicts clinical worsening in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 185: 400–408. [DOI] [PubMed] [Google Scholar]
- 39.Velez-Roa S, Ciarka A, Najem B, et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004; 110: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 40.Naeije R, van de Borne P. Clinical relevance of autonomic nervous system disturbances in pulmonary arterial hypertension. Eur Respir J 2009; 34: 792–794. [DOI] [PubMed] [Google Scholar]
- 41.Wensel R, Jilek C, Dorr M, et al. Impaired cardiac autonomic control relates to disease severity in pulmonary hypertension. Eur Respir J 2009; 34: 895–901. [DOI] [PubMed] [Google Scholar]
- 42.Conraads VM, Deaton C, Piotrowicz E, et al. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2012; 14: 451–458. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston K, Grimmer-Somers K. Pulmonary rehabilitation: overwhelming evidence but lost in translation? Physiother Can 2010; 62: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis 2011; 8: 89–99. [DOI] [PubMed] [Google Scholar]
- 46.Keating A, Lee AL, Holland AE. Lack of perceived benefit and inadequate transport influence uptake and completion of pulmonary rehabilitation in people with chronic obstructive pulmonary disease: a qualitative study. J Physiother 2011; 57: 183–190. [DOI] [PubMed] [Google Scholar]
- 47.Pirkis J, Ftanou M, Williamson M, et al. Australia’s better access initiative: an evaluation. Aust N Z J Psychiatry 2011; 45: 726–739. [DOI] [PubMed] [Google Scholar]
- 48.Brouwers RW, Kraal JJ, Traa SC, et al. Effects of cardiac telerehabilitation in patients with coronary artery disease using a personalised patient-centred web application: protocol for the SmartCare-CAD randomised controlled trial. BMC Cardiovasc Disord 2017; 17: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattila J, Ding H, Mattila E, et al. Mobile tools for home-based cardiac rehabilitation based on heart rate and movement activity analysis. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 6448–6452. [DOI] [PubMed] [Google Scholar]
- 50.Coquart JB, Grosbois JM, Olivier C, et al. Home-based neuromuscular electrical stimulation improves exercise tolerance and health-related quality of life in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noites A, Freitas CP, Pinto J, et al. Effects of a phase IV home-based cardiac rehabilitation program on cardiorespiratory fitness and physical activity. Heart Lung Circ 2017; 26: 455–462. [DOI] [PubMed] [Google Scholar]
- 52.Matos-Garcia BC, Rocco IS, Maiorano LD, et al. A home-based walking program improves respiratory endurance in patients with acute myocardial infarction: a randomized controlled trial. Can J Cardiol 2017; 33: 785–791. [DOI] [PubMed] [Google Scholar]
- 53.McNamara RJ, McKeough ZJ, Mo LR, et al. Community-based exercise training for people with chronic respiratory and chronic cardiac disease: a mixed-methods evaluation. Int J Chron Obstruct Pulmon Dis 2016; 11: 2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki J. L-arginine supplementation causes additional effects on exercise-induced angiogenesis and VEGF expression in the heart and hind-leg muscles of middle-aged rats. J Physiol Sci 2006; 56: 39–44. [DOI] [PubMed] [Google Scholar]
- 55.Huang C-C, Tsai S-C, Lin W-T. Potential ergogenic effects of l-arginine against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp Gerontol 2008; 43: 571–577. [DOI] [PubMed] [Google Scholar]
- 56.Maxwell AJ, Ho H-KV, Le CQ, et al. l-Arginine enhances aerobic exercise capacity in association with augmented nitric oxide production. J Appl Physiol (1985) 2001; 90: 933–938. [DOI] [PubMed] [Google Scholar]
- 57.Janicki JS, Weber KT, Likoff MJ, et al. The pressure-flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation 1985; 72: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 58.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 59.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 60.Frost AE, Langleben D, Oudiz R, et al. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol 2005; 43: 36–39. [DOI] [PubMed] [Google Scholar]