Abstract
Dysregulated metabolism and rarefaction of the capillary network play a critical role in pulmonary arterial hypertension (PAH) etiology. They are associated with a decrease in perfusion of the lungs, skeletal muscles, and right ventricle (RV). Previous studies suggested that endothelin-1 (ET-1) modulates both metabolism and angiogenesis. We hypothesized that dual ETA/ETB receptors blockade improves PAH by improving cell metabolism and promoting angiogenesis. Five weeks after disease induction, Sugen/hypoxic rats presented severe PAH with pulmonary artery (PA) remodeling, RV hypertrophy and capillary rarefaction in the lungs, RV, and skeletal muscles (microCT angiogram, lectin perfusion, CD31 staining). Two-week treatment with dual ETA/ETB receptors antagonist macitentan (30 mg/kg/d) significantly improved pulmonary hemodynamics, PA vascular remodeling, and RV function and hypertrophy compared to vehicle-treated animals (all P = 0.05). Moreover, macitentan markedly increased lung, RV and quadriceps perfusion, and microvascular density (all P = 0.05). In vitro, these effects were associated with increases in oxidative phosphorylation (oxPhox) and markedly reduced cell proliferation of PAH-PA smooth muscle cells (PASMCs) treated with macitentan without affecting apoptosis. While macitentan did not affect oxPhox, proliferation, and apoptosis of PAH–PA endothelial cells (PAECs), it significantly improved their angiogenic capacity (tube formation assay). Exposure of control PASMC and PAEC to ET-1 fully mimicked the PAH cells phenotype, thus confirming that ET-1 is implicated in both metabolism and angiogenesis abnormalities in PAH. Dual ETA/ETB receptor blockade improved the metabolic changes involved in PAH-PASMCs’ proliferation and the angiogenic capacity of PAH-PAEC leading to an increased capillary density in lungs, RV, and skeletal muscles.
Keywords: pulmonary hypertension, angiogenesis, endothelin, endothelin receptor blockade, macitentan, Sugen/hypoxia, Warburg effect
Introduction
Pulmonary arterial hypertension (PAH) is a life-threatening disease characterized by progressive increases in pulmonary vascular resistance (PVR), right heart failure, and death.1,2 A chronic shift in energy production from mitochondrial oxidative phosphorylation to glycolysis, known as the Warburg effect, has been shown to drive the pro-proliferative phenotype of the pulmonary arteries smooth muscle cells (PASMC) leading to the severe narrowing of distal lung arteries.3,4 In addition, deregulated angiogenic signaling cascades have been reported in PAH, although their precise role in the development and progression of PAH remains elusive. Indeed, enhanced endothelial cell and myofibroblast proliferation have been initially interpreted to reflect an underlying process of dysregulated angiogenesis in PAH,5 contributing to the obliterative arteriolar and multi-channeled “plexiform” lesions. In contrast, administration of Sugen, an anti-angiogenic inhibitor of vascular endothelial growth-factor receptor (VEGF), triggers severe pulmonary vascular remodeling and pulmonary hypertension (PH) in rodents.6 Consistently, impaired angiogenesis and capillary rarefaction have been proposed to contribute to elevated vascular resistances and stimulation of angiogenic response has been shown to correct this elevation.7 As such, initial loss of endothelial cells8 coupled with the reactive proliferation of the remaining vascular cells are believed to contribute to arteriolar obliteration, loss of effective lung microvascular area, and PAH progression.
More recently, similar metabolic/angiogeneic dysregulation was documented within the right ventricle (RV)9 and skeletal muscles10 of human and experimental PAH, fueling the metabolic theory of PAH.3 Capillary rarefaction was also described within the RV11 and skeletal muscles,12 contributing to RV decompensation and exercise intolerance, respectively. This is not surprising as vascular branching signals are coupled with mitochondrial biogenesis.13 However, these observations raised questions on whether the pulmonary and systemic metabolic and angiogenic defects could be mechanistically connected.3,14
Interestingly, in addition to altered levels of circulating metabolic and angiogenic modulatory factors,15,16 PAH patients are characterized by markedly elevated levels of ET-1,17 one of the three major pathways targeted by currently approved PAH therapies.18 ET-1 receptor antagonists have been shown to improve exercise capacity and delay clinical worsening in human PAH.18,19 Although most studies have focused on the effects of ET-1 on vascular smooth muscle cells, ET-1 was recently shown to influence angiogenesis,20–23 while blockade of ETA/ETB receptors improved post-ischemic angiogenesis in vivo.23 We hypothesized that dual ETA/ETB receptors blockade reverses the Warburg effect in PAH-PASMC and promotes angiogenesis in lungs, RV, and skeletal muscles, thus contributing to the clinical improvements observed in human PAH.
Methods
All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and all experiments were performed in accordance with Laval University and Institut Universitaire de Cardiologie et de Pneumologie de Québec biosafety and human (CER-20773) and animal (CPAUL 2014176-1) ethics. Experiments and analyses were performed blinded, as previously recommended.24
Animal model and intervention
Male Sprague-Dawley rats (250–300 g; strain 400; Charles River) were selected for PAH induction using Sugen (SU5416, 20 mg/kg; intraperitoneal [i.p.] injection) at day 0. Rats were placed for three weeks in hypoxia condition (10% oxygen) and then housed under normoxic conditions for two weeks before established PH was confirmed echocardiographically (Philips HD11 XE imaging system/S3-1 probe).25,26 After PH establishment (five weeks after injection), animals were treated with macitentan (30 mg/kg/day, supplied by Actelion Pharmaceuticals Ltd.) or its vehicle (gelatin 7.5%) during two weeks (total study duration of seven weeks), as previously described.27 These rats were compared to rats exposed to Sugen/hypoxia for three weeks and observed for two additional weeks, as well as healthy rats maintained in normoxia for seven weeks.
Right heart catheterization: Before sacrifice, invasive closed-chest right heart catheterization (RHC) (Scisense ADV500 Pressure-Volume Measurement System; analyzed with labscibe2 software; Work System, Inc.) was performed under 3% Isoflurane (Abbvie 173803, Saint-Laurent, QC, Canada) anesthesia. Mean pulmonary artery (PA) pressure, RV systolic pressure, RV cardiac output, stroke volume, and total pulmonary resistance (or TPR) were assessed, as previously described.11,12,25,28
Lectin perfusion assay: Fluorescein-conjugated lectin (Vector laboratories FL-1081, Burlingame, CA, USA) that binds uniformly to the luminal surface of endothelial cells was injected in half of all animals after RHC and before sacrifice, as previously described.12 Briefly, an i.p. injection with heparin (3 mL, 80 U) was done and, 5 min later, lectin was injected through the jugular vein (5 mg/mL – 0.25 mg per rat). The animals were sacrificed after 20 min, allowing lectin to circulate through the whole body. The RV, lungs, and quadriceps were retrieved and processed for optimal cutting temperature freezing. Cryosections (5–10 µm) were done and mounted on slides with a mounted media nuclei counterstain (Dapi) (EMS 17984-24). Images were acquired using a Carl Zeiss Micro Imaging microscopy workstation. For the RV, ImageJ automatically quantified signal intensity. For quadriceps, the number of lectin-positive cells under the total number of cells was manually calculated in ten different fields per specimen at 20X magnification. For the lung, the number of lectin positive vessels manually calculated in ten different fields per specimen at 20X magnification.
Angiogram: In the other half of the animals, the vasculature was filled through the jugular vein with the radiopaque silicone rubber Microfil (Flow Tech, MV-112, Carver, MA, USA) to form a vascular cast for further CT-scan analyses using micro-CT (eXplore CT-120 scanner; Gamma Medica Inc., Northridge, CA, USA). Three-dimensional reconstruction of the organs was performed using the OsiriX software. Vascular perfusion and the total volume of the organ of interest were measured using the Microview software. Percentage of perfusion was calculated by dividing the vasculature signal volume (Microfil) on the total organ volume.
Histological analyses, confocal microscopy and immunofluorescence: Following sacrifice, vascular remodeling was assessed in lung sections stained with hematoxylin and eosin (H&E) according to standard histological procedures.11,12,25,28,29 PA wall thickness was calculated by the percentage of the PA wall area on total artery area. A minimum of five arteries/animal were measured. Pulmonary arterial muscularization was assessed on H&E-stained sections. Briefly, 30–50 vessels of <100 µm diameter were counted in each lung section. Arteries in which the lumen was partially (50%) or fully obstructed were defined as a muscularized artery. The proportion of muscularized arteries was expressed as a percentage of total arteries counted. CD31 immunofluorescence was performed on 4 -µm paraffin-embedded tissues. Briefly, the vascular network was revealed with a rabbit polyclonal antibody against CD31/PECAM (1:100; Abcam, AB28364, Cambridge, MA, USA) in the lungs, RV, and quadriceps.11,12 Antigen retrieval was performed by microwaving samples in 0.01 M citrate buffer at pH 6.0. Secondary antibody was conjugated to Alexafluor 594 nm (Goat anti-rabbit IgG, Life Technologies, A110037, Burlington, ON, Canada) and nuclei were visualized by DAPI staining. Negative controls were also performed with each immunostaining experiment. Images were acquired using a Carl Zeiss MicroImaging microscopy workstation. For the RV, ImageJ software automatically quantified signal intensity as the percentage of positive pixels in five fields per specimen at 40× magnification. For quadriceps, the number of CD31 positive cells under the total number of cells was manually calculated in ten different fields per specimen at 40× magnification. For the lungs, the number of CD31 positive vessels was manually calculated in ten different fields per specimen at 40× magnification.
Human cell experiments
Cell isolation and culture: Human PAH–PASMCs and PAH–PAECs were isolated from PAH patients by enzymatic digestion from distal PA (<1000 µm) at the time of lung transplant. PAH–PASMCs were isolated from one idiopathic and two heritable PAH patients (M52, F32, and M39, respectively) while PAH–PAECs were isolated from three heritable PAH patients (F26, F32, and F35). All patients had RHC that confirmed PAH and gave informed consent. PAECs were isolated using DYNABEADS CD31 positive selection (Invitrogen 11155D, Carlsbad, CA, USA) following the manufacturer’s recommendations. CD31+ cells were subsequently grown in EBM-2 media (Lonza, CC-3156, Switzerland) according to the recommendations of the supplier (Endothelial Cells Growth Media Kits, CC-3162) and incubated at 37℃, 5% CO2 atmosphere. PASMCs were grown in human smooth muscle cells media (#310-470, Cell applications San Diego, CA, USA) supplemented according to the manufacturer (Smooth Muscle Cells Growth Media Kits, #311-GS). Control PASMC and PAEC lines were purchased from Cell Applications (San Diego, CA, USA). PASMC and PAEC phenotypes were confirmed using α-smooth muscle actin staining and CD31 labeling, respectively, and were used from third to sixth passage.11,12,25,30 PAH–PAECs and PAH–PASMCs were then treated with vehicle (DMSO or water), dual ETA/ETB (macitentan 10–8 M, 10–7 M, and 10–6 M diluted in DMSO), ETA (BQ-123 10–8 M, 10–7 M, and 10–6 M diluted in water) or ETB (BQ-788 10–8 M, 10–7 M, and 10–6 M diluted in DMSO) receptor antagonists during 48 h at 37℃, 5% CO2 atmosphere. Conversely, control PAEC and PASMC were exposed to ET-1 (10–9 M, 10–8 M, and 10–7 M diluted in water) or its vehicle (water).
Tube formation assay: Matrigel assays were performed in 24-well plates previously coated with 50 µL/cm2 of Corning Matrigel Basement Membrane Matrix (Corning #354234; 9 mg/mL, Tewksbury, MA, USA). Cells were seed (40,000 cells/well, passage 2–5) and let adhered for 4–6 h. Four pictures/well were taken using the Cell Imaging System EVOS and automatic quantification of tubing formation was performed with the Image J Angiogenesis Analyzer. Briefly, the sum of nodes and isolated segments were calculated in order to quantify the capacity of PAECs for tube formation.
Proliferation and apoptosis measurements: Cultured control and PAH–PAEC cells were exposed to EBM-2 media + growth factors supplementation for 48 h, or EBM-2 media without supplementation (serum and growth factor starvation for 48 h) to assess the in vitro effect of ET receptor antagonists on human PAECs’ proliferation and apoptosis, respectively. Proliferation was measured using Ki67 immunostaning (Sigma-Aldrich, AB9260, St. Louis, MO, USA) and apoptosis by AnnexinV assay (Roche, 12156792910, Indianapolis, IN, USA). Two independent sets of experiments were performed in each cell lines. In each experiment, a minimum of 150 cells were evaluated. Cultured control and PAH–PASMC cells were cultivated in supplemented cell application smooth muscle media for 48 h, or media without supplementation (serum and growth factor starvation for 48 h) to assess the in vitro effect of ET receptor antagonists on human PASMC’s proliferation and apoptosis, respectively. Proliferation and apoptosis were measured and analyzed as for PAEC cells lines
Analysis of mitochondrial bioenergetics: Real-time measurements of oxygen consumption rate (OCR) were performed using Seahorse XFe24 extracellular flux analyzer (Seahorse Biosciences, North Billerica, MA, USA), as previously described.30 The spare respiratory capacity (SRC) was calculated as the difference between basal OCR and that obtained in the presence of carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP). To obtain the maximal respiration for each cell types, 1 µm and 5 µm of FCCP were injected for PAEC and PASMC, respectively.30,31 All experiments were performed in triplicate.
Statistical analysis
Statistical analyses were performed by the biostatistician of the CRIUCPQ. Values were expressed as mean ± SEM, as they followed a normal distribution. Unpaired Student’s t-tests were used for comparisons between two groups and one-way ANOVA followed by a Holm-Sidak’s multiple comparisons test was used for more than two groups. P values < 0.0001 (****), 0.001 (***), 0.01 (**), and 0.05 (*) were considered statistically significant. Statistical analyses were performed using Prism 6 (GraphPad Software Inc.).
Results
Macitentan treatment improves hemodynamics in the Sugen-hypoxia rat model of PAH
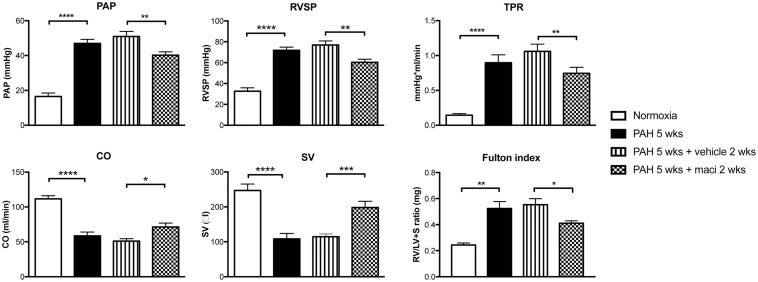
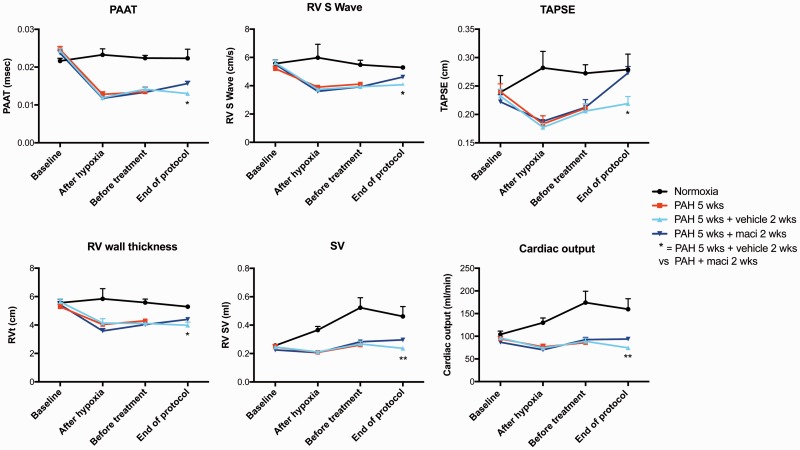
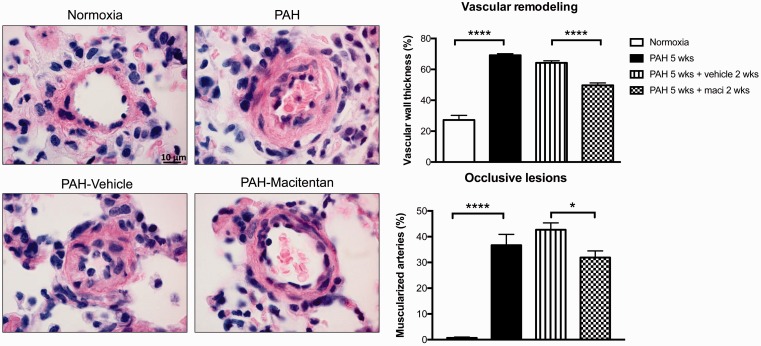
Daily treatment with macitentan was associated with significant improvements in pulmonary hemodynamics, including PA and systolic RV pressures as well as total pulmonary resistance (or TPR) measured by closed chest RHC (Fig. 1). Dual ETA/ETB blockade also improved cardiac output, RV hypertrophy, and function, as evaluated respectively by catheterization (Fig. 1), the Fulton index (Fig. 1), and echocardiography (Fig. 2). Macitentan-treated rats also exhibited less remodeling of distal PA (Fig. 3), which likely explains the significant decrease in total total pulmonary resistance (or TPR).
Fig. 1.
Dual ETA/ETB receptor blockade improves hemodynamic parameters in the Sugen/hypoxia rat model of PAH. Macitentan treatment significantly decreased mean pulmonary arterial pressure (PAP), right ventricle systolic pressure (RVSP), and total pulmonary resistance (TPR) while it increased cardiac output (CO) and stroke volume (SV). Groups: Normoxia (n = 7–8), PAH 5 weeks (before treatment) (n = 7–10), PAH 5 weeks + vehicle 2 weeks (n = 17), and PAH 5 weeks + maci (macitentan) 2 weeks (n = 20). RV hypertrophy, as assessed by the Fulton Index (RV/left ventricle and septum [LV + S]), was decreased in PAH macitentan-treated rats. Groups: Normoxia (n = 5), PAH 5 weeks (before treatment) (n = 5), PAH 5 weeks + vehicle 2 weeks (n = 10) and PAH 5 weeks + maci (macitentan) 2 weeks (n = 10). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Fig. 2.
Measurements of PAH progression by non-invasive two-dimensional Doppler echocardiography dual ETA/ETB receptor blockade. Dual ETA/ETB receptor antagonist (macitentan) in Sugen/hypoxia rat significantly improved cardiac function at the end of the protocol, as assessed by the pulmonary artery acceleration time (PAAT), RV S wave, tricuspid annular plane systolic excursion (TAPSE), RV wall thickness, SV, and CO. Groups: Normoxia (n = 5), PAH 5 weeks (before treatment, n = 5), PAH 5 weeks + vehicle 2 weeks (n = 10) and PAH 5 weeks + macitentan 2 weeks (n = 10). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Fig. 3.
Dual ETA/ETB receptor blockade decreases distal pulmonary vascular remodeling in Sugen/hypoxia rat model of PAH. The observed improvements in pulmonary hemodynamics following macitentan treatment were associated with decreased vascular remodeling. Pulmonary arterial wall thickness was measured by H&E staining and calculated by the percentage of the PA wall area on total artery area. A minimum of five arteries/animal were measured. Pulmonary arterial muscularization was also assessed on (H&E) stained sections. The proportion of muscularized arteries was expressed as a percentage of total arteries counted. Groups: Normoxia (n = 5), PAH 5 weeks (before treatment) (n = 5), PAH 5 weeks + vehicle 2 weeks (n = 10), and PAH 5 weeks + maci (macitentan) 2 weeks (n = 10). Scale bar: 10 µm. ****P < 0.0001.
Macitentan prevents the Warburg effect and the pro-proliferative phenotype of PAH–PASMCs
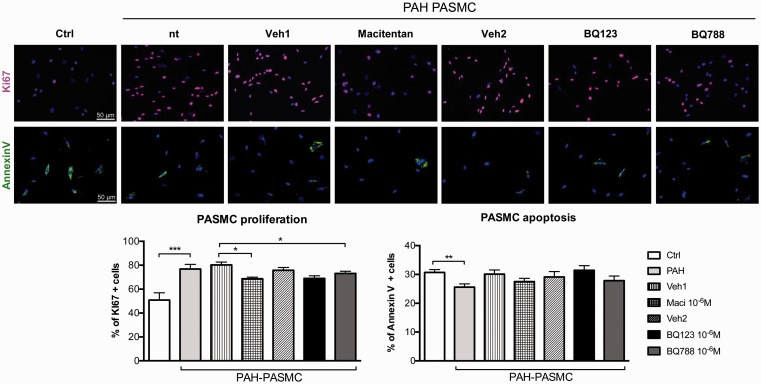
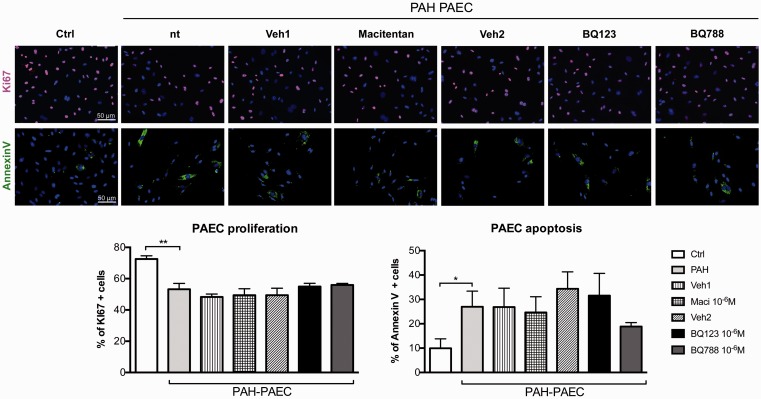
The proliferation rate of PAH–PASMCs was significantly reduced after dual ETA/ETB or selective ETB (BQ788) receptor antagonism at maximal dose (10–6 M) (Fig. 4). Conversely, ETA receptor blockade (BQ123) had no effects on PAH–PASMC proliferation. In addition, no effects on the apoptosis rate were observed after ET receptors blockade.
Fig. 4.
Dual ETA/ETB and selective ETB receptor antagonism decreases proliferation in isolated PASMCs of patients with PAH. Treatments with dual ETA/ETB receptor antagonist (macitentan) or selective ETB receptor antagonist (BQ788) at maximal dose (10–6 M) reduced the proliferation of PAH–PASMCs when compared to vehicle-treated cells. KI67 immunofluorescence (in red) and DAPI (in blue) was performed to quantify proliferation. Note that we observed no statistical changed in proliferation after selective ETA receptor antagonist (BQ123) treatment in PAH–PASMCs. While apoptosis rate (AnnexinV in green) was decreased in PAH–PASMCs compared to control PASMCs, no significant changes in apoptosis rate were observed after treatment with dual or selective ET-1 receptor antagonists. Two independent sets of experiments were performed in each cell lines. In each experiment, a minimum of 150 cells were evaluated (n = 3 cell lines/treatment, passage 4–6). Scale bar: 50 µm. *P < 0.05; **P < 0.01; ***P < 0.001.
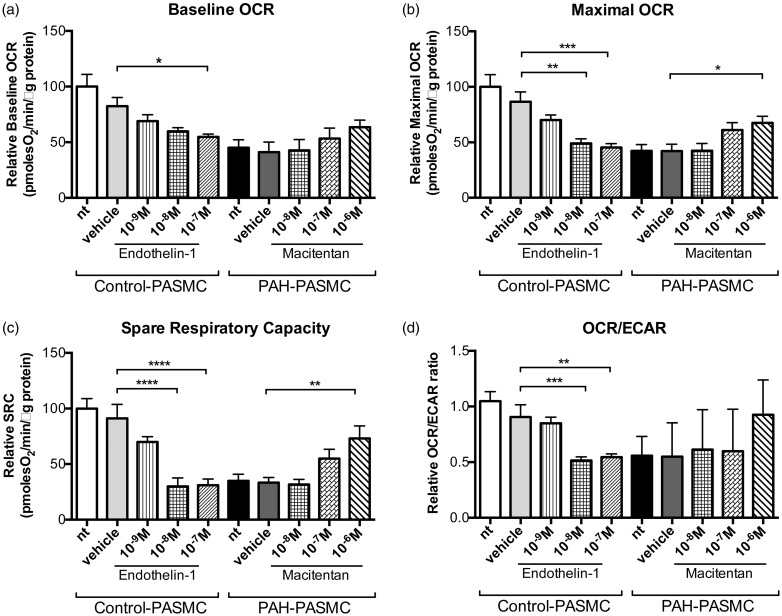
To explore the mechanism by which ET receptors blockade can affect proliferation rate of PAH–PASMCs, we assessed the influence of ET-1 on PASMC mitochondrial bioenergetics. We observed that ET-1 dose-dependently reduced basal and maximal respiration in control PASMCs (Fig. 5), resulting in reduced SRC and OCR/extracellular acidification rate (ECAR) ratio. Similar to ET-1-treated PASMCs, PAH–PASMCs had decreased basal and maximal respiration resulting in reduced SRC. Both maximal OCR and SRC were restored to levels comparable with control cells when pre-incubated with macitentan for 48 h.
Fig. 5.
Endothelin-1 influences the Warburg effect in PASMCs isolated from patients with PAH and controls. Seahorse XF24 analyzer experiments showed that endothelin-1 treatment in control PASMCs reduced mitochondrial respiration, whereas it was partly restored in PAH-PASMCs after dual ETA/ETB receptor antagonist (macitentan). (a) Basal mitochondrial respiration was significantly decreased after endothelin-1 (10–7 M) treatment in control PASMCs, as evaluated by the relative OCR. (b, c) Both the maximal respiratory capacity and the SRC were significantly and dose-dependently decreased after endothelin-1 treatment in control PASMCs. In PAH-PASMCs, an increase in maximal respiratory capacity and SRC was observed when compared to vehicle-treated cells, which was partially reversed by macitentan. (d) The decrease in OCR/ECAR ratio after endothelin-1 treatment in control PASMCs cells suggested a glycolic switch. All experiments were performed in triplicate (n = 3 cell lines/treatment, passage 4–6). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Macitentan increases perfusion and microvessels density in the lungs, RV, and skeletal muscles of Sugen/hypoxia PAH rats
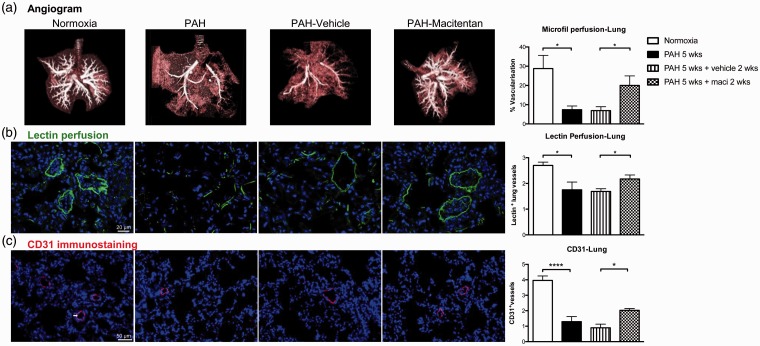
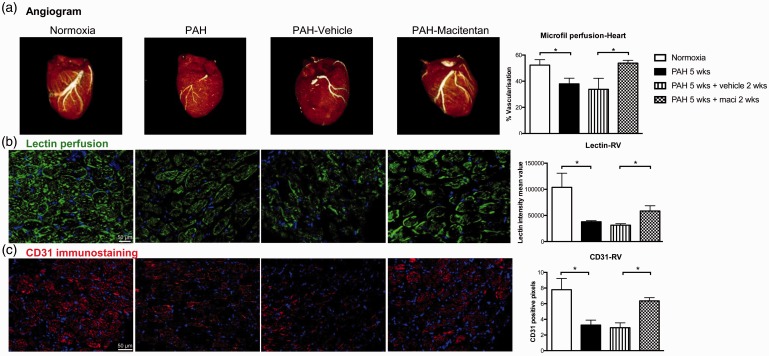
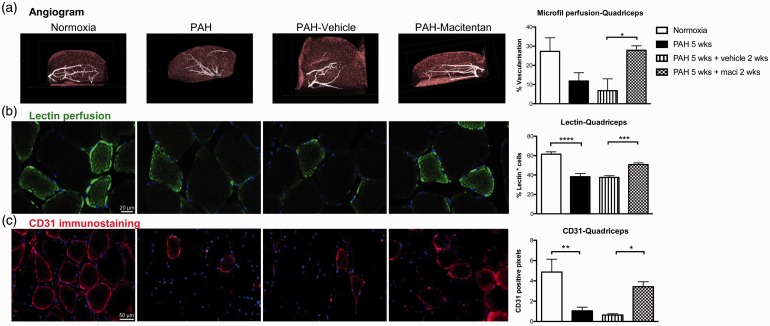
PH was associated with a dramatic decrease in perfusion as assessed by lung CT angiogram and lectin perfusion (Fig. 6a, b). PH was also associated with decreased lung microvascular density assessed by CD31 immunostaining (Fig. 6c). As previously documented in patients with PAH and monocrotaline rats,12,32 Sugen/hypoxia-induced PAH was also associated with a significant decrease in RV and skeletal muscle perfusion and microvascular density (Figs. 7 and 8). Dual ETA/ETB receptor blockade significantly improved lung, RV, and skeletal muscle perfusion (Figs. 6(–8). CD31 immunostaining also confirmed that macitentan increased the detection of vessels within the lungs, the RV, and the quadriceps, suggesting that dual ETA/ETB receptor blockade could enhance vessel formation.
Fig. 6.
Dual ETA/ETB receptor blockade improves lung perfusion in Sugen/hypoxia rat model of PAH. (a) CT angiogram with image reconstruction of the lung after microfil perfusion. Vascularization was quantified in silico using Microview software in normoxic rats, 5 weeks after sugen injection, as well as 5 weeks after sugen injection + 2 weeks of treatment with vehicle and macitentan (n = 6/group). (b) Lectin perfusion (in green) and DAPI (in blue) were also used to visualize lung vascularization in lungs of normoxia (n = 4), PAH 5 weeks (n = 4), PAH 5 weeks +vehicle 2 weeks (n = 10), and PAH 5 weeks + maci (macitentan) 2 weeks (n = 10). The number of lectin-positive vessels was manually calculated in ten different fields per specimen. (c) CD31 immunostaining (in red; arrow) and DAPI (in blue) were used to assess lung microcirculation density of normoxia, PAH 5 weeks, PAH weeks + vehicle 2 weeks, and PAH 5 weeks + maci (macitentan) 2 weeks (n = 4–5/group). The number of CD31 positive vessels was manually calculated in ten different fields per specimen. *P < 0.05; ****P < 0.0001. Scale bars: 20 µm in (b); 50 µm in (c).
Fig. 7.
Dual ETA/ETB receptor blockade improves heart and RV perfusion in Sugen/hypoxia rat model of PAH. (a) CT image reconstruction of the heart after microfil perfusion. Vascularization was quantified in silico using Microview software in normoxic rats, 5 weeks after Sugen injection, as well as 5 weeks after Sugen injection + 2 weeks of treatment with vehicle and macitentan (n = 5–6/group). (b) Lectin perfusion (in green) and DAPI (in blue) were also used to evaluate vascularization in the RV of normoxia (n = 5), PAH 5 weeks (n = 4), PAH weeks + vehicle 2 weeks (n = 9), and PAH 5 weeks + maci (macitentan) 2 weeks (n = 10). (c) CD31 immunostaining (in red) and DAPI (in blue) were used to assess the RV microcirculation density of normoxia, PAH 5 weeks, PAH weeks + vehicle 2 weeks, and PAH 5 weeks + maci (macitentan) 2 weeks (n = 4–5/group). For the lectin and CD31 analyses, ImageJ software automatically quantified signal intensity as the percentage of positive pixels in five fields per specimen. *P < 0.05. Scale bars: 20 µm.
Fig. 8.
Dual ETA/ETB receptor blockade improves quadriceps perfusion in Sugen/hypoxia rat model of PAH. (a) CT reconstruction of the quadriceps after microfil perfusion in normoxic rats, 5 weeks after Sugen injection, as well as 5 weeks after Sugen injection + 2 weeks of treatment with vehicle and macitentan (n = 5–7/group). (b) Lectin perfusion (in green) and DAPI (in blue) were used to evaluate vascularization in quadriceps of normoxia (n = 4), PAH 5 weeks (n = 4), PAH weeks + vehicle 2 weeks (n = 10), and PAH 5 weeks + maci (macitentan) 2 weeks (n = 9). (c) Finally, CD31 immunostaining (in red) and DAPI (in blue) were used to assess microcirculation density in quadriceps (n = 4–5/group). The number of CD31 or lectin-positive cells under the total number of cells was manually calculated in ten different fields per specimen. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Scale bars: 20 µm in (b); 50 µm in (c).
Macitentan treatment promotes PAH–PAECs angiogenic capacity
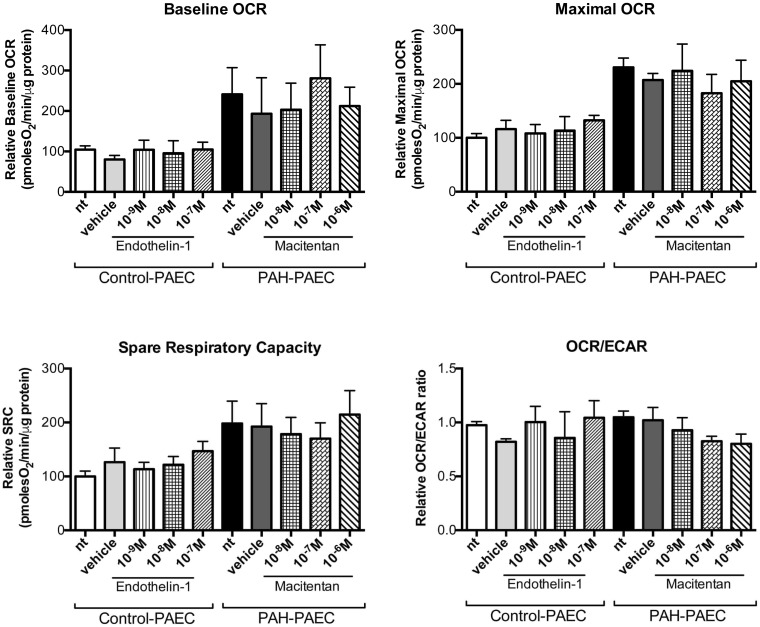
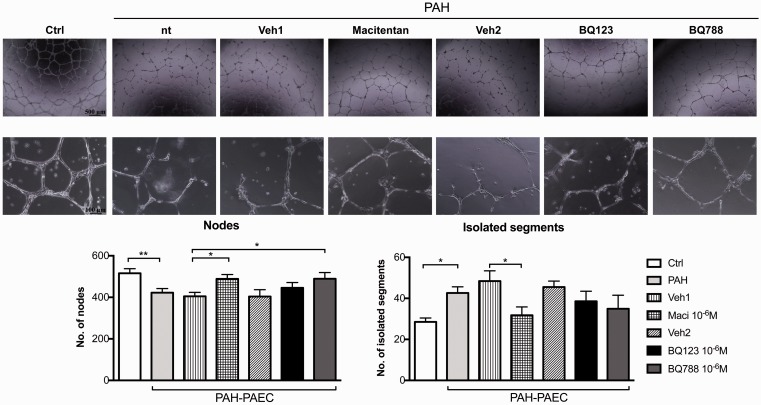
As no significant changes in proliferation/apoptosis rates (Fig. 9) and metabolism (Fig. 10) of PAH–PAEC were observed after ETA/ETB receptor blockade, we thus hypothesized that ET-1 could influence the angiogenic potential of PAEC through changes in PAECs’ migration and tube formation capacity. We showed that PAH–PAECs had significantly lower angiogenic potential as confirmed by a significant decrease in effective nodes and an increased isolated segment compared to control PAEC on matrigel assay (Fig. 11). Conversely, macitentan partially restored their angiogenic potential, whereas ETB receptor blockade increased node formation.
Fig. 9.
Dual or selective blockade of endothelin receptors does not influence proliferation and apoptosis of isolated PAECs isolated from patients with PAH. Neither dual ETA/ETB (macitentan) nor ETA- (BQ123) or ETB (BQ788)-selective receptor antagonists affected proliferation or apoptosis of PAH–PAECs as evaluated by ki67 (in red) immunostaining and annexin V (in green) assay. Two independent sets of experiments were performed in each cell lines. In each experiment, a minimum of 150 cells were evaluated (n = 3 cell lines/treatment, passage 2–5).
Fig. 10.
Endothelin-1 does not affect the metabolic status of PAECs isolated from distal pulmonary arteries. Endothelin-1 and macitentan did not influence the mitochondrial respiration of control and PAH–PAECs, respectively. The basal and maximal OCR, as well as the SRC and OCR/ECAR ratio were unchanged after endothelin-1 modulation as evaluated by Seahorse XF24 analyzer. All experiments were performed in triplicate (n = 3 cell lines/treatment, passage 2–5).
Fig. 11.
Dual ETA/ETB and selective ETB receptor antagonism increases the angiogenic potential of PAECs isolated from the distal pulmonary artery of patients with PAH. Treatments with dual ETA/ETB receptor antagonist (macitentan, 10–6 M) or selective ETB receptor antagonist (BQ788, 10–6 M) improved the angiogenic capacity of PAH–PAECs as evaluated by Matrigel assays. Macitentan treatment significantly reduced the number of isolated segments while it improved the number of nodes formation, which reflects an increased capacity of PAH–PAECs to do tube formation. ETB receptor antagonism also significantly increased the number of nodes formation in PAH–PAECs when compared to vehicle-treated cells. Four pictures/well were taken and automatic quantification of tubing formation was performed with the Image J Angiogenesis Analyzer (n = 3 cell lines/treatment, passage 2–5). *P < 0.05; **P < 0.01 (n = 3). Scale bars: 500 µm in upper panel; 100 µm in lower panel.
Discussion
Our investigation of the role of ET receptor antagonism on angiogenesis in PAH led us to the following key observations: (1) dual ETA/ETB receptor blockade with macitentan improved RV systolic and PA pressures of Sugen/hypoxia-induced PH through decreases in distal PA remodeling and increases in perfusion and microvessel density within the lungs; (2) in vitro, ET-1 is involved in PAH-PASMCs metabolism changes and dual ETA/ETB antagonism decreased proliferation and partially reversed the Warburg effect of human PAH-PASMC; (3) dual ETA/ETB receptor blockade also improved perfusion and microvessel density within the RV and quadriceps in Sugen/hypoxia-induced PH; and 4) in vitro, dual ETA/ETB receptor antagonism increased the angiogenetic capacity of human PAH–PAEC.
Over the past decade, PAH-specific therapies were shown to improve overall quality of life33 and delay clinical worsening.18 Macitentan was the first dual ETA/ETB receptor antagonist associated with reduced morbidity events in a long-term randomized clinical trial.19 Although its mode of action was expected to mainly rely on vasodilatation, its anti-remodeling and pro-angiogeneic remained elusive. Importantly, the hyperproliferation and anti-apoptotic phenotype of PAH–PASMC is recognized as a major component of distal PA remodeling in both human and experimental PAH.4 Similarly, improving vessels formation and repair by restoring PAEC angiogenic functions are currently being explored clinically in PAH.34
In the present study, dual ETA/ETB receptor blockade significantly improved pulmonary hemodynamics and vascular remodeling in the Sugen/hypoxia rat model, as previously described in other experimental models.35 In vitro, macitentan reversed the glycolytic metabolic switch of PAH–PASMC and decreased PAH–PASMC proliferation. This observation is consistent with the metabolic theory of PAH,3 and could thus explain in part reversal of PA remodeling, as previously described.36,37 Although not previously described, the impact of dual ETA/ETB receptor blockade on PAH–PASMC metabolism is not surprising since ET-1 was shown to modulate the expression of numerous transcription factors at the origin of the Warburg effect and the reprogramming of metabolism within PASMC mitochondria, including STAT329 and survivin.38
In addition to this “cancer-like” phenotype of PAH–PASMC,39,40 the exact role of dysregulated angiogenesis in the pathogenesis of PAH has been largely debated over the last decades.41 Early studies suggested that the enhanced PAEC and myofibroblast proliferation contributed to the obliterative arteriolar and multi-channeled “plexiform” lesions observed in PAH.5 This was supported by the observations showing that VEGF plasma levels are elevated,24,42–44 whereas both VEGF and VEGF receptor 2 are markedly expressed in the complex vascular lesions of patients with severe PAH.4,5,45,46 Paradoxically, Sugen, a VEGFR1 and VEGFR2 inhibitor, induces lung endothelial cell apoptosis, loss of small lung vessels, and severe angio-obliterative PAH when combined with chronic hypoxia.6 Similarly, experimental overexpression of VEGF blunts the development of hypoxia-induced PH47 and improves PH in a rat model of pulmonary fibrosis, whereas VEGF blockade worsens it.48 These preclinical observations are supported by the recent validation of blood levels of anti-angiogenic modulatory proteins as biomarkers for PAH.15,49
While most studies have focused on the effects of ET-1 on vascular smooth muscle cells, recent in vitro studies have demonstrated that ET-1 increases tumor vascularity in lung cancer50 and has pro-angiogenic effects in isolated human umbilical vein endothelial cells51 through ETA and ETB receptors activation. In striking contrast, angiogenesis and lung vascular density is decreased in experimental PH of the newborn,52 and increased ET-1 production impairs PAEC angiogenic capacity in vitro, which is restored by dual ETA/ETB receptor blockade.20 It is noteworthy that the discrepant effects of ET-1 on tumor vs. PH angiogenesis may be related to differences in cell type, developmental timing, or perhaps differential receptor activation. As previously described, we observed marked reduction in perfusion and capillary density in pulmonary hypertensive lungs in our experimental model, which was partly reversed by macitentan. While a glycolytic phenotype of PAH–PAEC has also been described,53 we did not observe changes in PAEC proliferation or metabolism with macientan in our in vitro experimental model. This could suggest that changes in capillary density in PAH lungs were not mediated through changes in endothelial mitotic state by macitentan, although we cannot exclude that this observation can reflect differences between species used in vivo and in vitro. Conversely, dual ETA/ETB and selective ETB receptor antagonism increased tube formation on matrigel assay. Consistently with our results, previous studies reported that ET-1 had no effect on PAEC growth, but significantly decreased tube formation in normal PAECs and PAECs isolated from pulmonary hypertensive distal PA,20 whereas their angiogenic capacity was restored following treatment with ET-1 SiRNA or a dual ETA/ETB receptor antagonist, but not an ETA receptor antagonist. These results suggest that the effects of ET-1 on pulmonary angiogenesis are ETB-mediated. This is not surprising since ETA receptors are normally not expressed on PAECs.54,55
Interestingly, we, as others, have shown that significant microcirculation loss was also observed within the quadriceps and RV of human and experimental PAH.11,12 These angiogenic defects contributed to reduced skeletal muscle oxygenation,56 exercise intolerance,12 and RV failure.11 The similarities in microcirculation loss within these organs raised questions on whether the pulmonary and systemic vascular defects could be connected by similar molecular mechanisms.12,14 Given the tight coupling between angiogenesis and metabolism,13 the metabolic dysregulation observed within the distal PA, RV,9 and skeletal muscles10 in PAH has been hypothesized to contribute to this capillary rarefaction in PAH.9 Interestingly, macitentan was recently associated with metabolic changes in the RV of Sugen-induced PAH.57 Consistent with this hypothesis, dual ETA/ETB receptor blockade also impacted on capillary density and perfusion within the RV and skeletal muscles in our PH model. However, part of the increases in perfusion (i.e. CT angiogram) may reflect improved pulmonary hemodynamics parameters as well as systemic vasodilation rather than enhanced angiogenesis per se, since increases in ET-1 levels influence peripheral endothelium-dependent flow-mediated vascular tone.58–60 Notwithstanding this possibility, increases in microcirculation density (lectin and CD31 staining) suggested that dual ETA/ETB receptor blockade partially reversed the microvessels loss and enhanced microcirculation integrity in our PAH model. This was also supported by in vitro studies that confirmed that both dual ETA/ETB and ETB receptor blockade restored the angiogenic capacity of human PAEC. Taken together, these observations suggest that clinical improvements observed with dual ETA/ETB receptor antagonists might be related to their multifaceted effects in human PAH, including modulation of the vasomotor tone, PASMC metabolism, and proliferation, as well as pulmonary and systemic perfusion and angiogenesis.
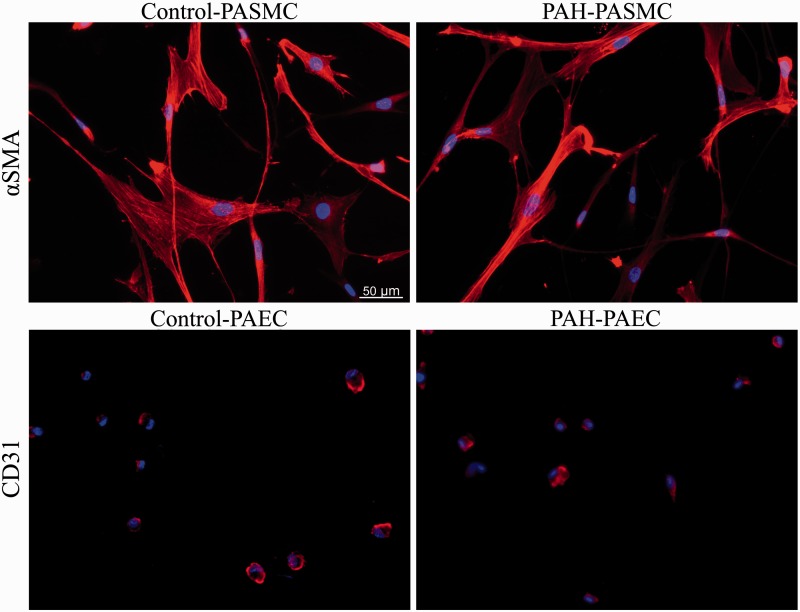
Our study has several limitations. Although cell lines were characterized morphologically and by specific immununolabeling (Fig. 12), technical isolation differences between the purchased controls and isolated PAH cell lines may have influenced our results. Moreover, the Sugen/hypoxia rat model was used for this study since it recapitulates many key features of PAH.1–4 Thus, whether our findings are representative of pulmonary and systemic vessel loss in other experimental models and in human PAH remains to be confirmed.
Fig. 12.
Confirmation of the PASMC and PAEC phenotypes using α-smooth muscle actin (SMA) and CD31 staining. (a, b) α-smooth muscle actin (SMA) immunofluorescence in control and PAH–PASMC (passage 4–6). (c, d) CD31 immunofluorescence in control and PAH–PAEC (passage 2–5).
In conclusion, we identified two novel modes of action by which dual ETA/ETB receptor blockade could impact on PAH pathophysiology. It reversed the metabolic changes involved in PASMC proliferation and it improved the angiogenic capacity of diseased endothelial cells. Thus, in addition to improved pulmonary hemodynamics, dual ETA/ETB receptor blockade resulted in improvements in perfusion and angiogenesis within the lungs, RV, and skeletal muscles in the Sugen/hypoxia-induced PAH rat model, suggesting that clinical improvements observed with dual ETA/ETB receptor antagonists might be multifaceted. It is noteworthy, however, that even though ET receptor antagonists were associated with vascular repair and endothelial regeneration within the pulmonary, RV, and skeletal muscle vasculatures, these effects are clearly insufficient to fully offset the PAH-related angiogenic defects. Therefore, novel therapeutic strategies are urgently required, in addition to the current ones, to address the underlying structural and functional abnormalities driving the relentless progression of this disease.
Conflict of interest
MI works within the Drug Discovery Department of Actelion Pharmaceuticals Ltd., Allschwil, Switzerland. SB holds a Canadian Research Chair in translational research in pulmonary vascular diseases at Université Laval and has received research grants from Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline. He has also received speaker fees from Actelion Pharmaceuticals. SP is clinician–scientist of the Fonds de Recherche en Santé du Québec and has received research grants from Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, and has received speaker fees from Actelion Pharmaceuticals. The Pulmonary Hypertension Research Group is also supported by the “Réseau en Santé Respiratoire” of the FRSQ.
Funding
The present work was supported by an unrestricted grant from Actelion Pharmaceuticals Ltd., Allschwil, Switzerland, which has had access to the manuscript before submission. However, the study was independently designed, results were analyzed, and the manuscript was entirely written by members of the Pulmonary Hypertension Research Group (www.hypertensionsrteriellepulmonaire.ca) only.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D34–41. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46(4): 903–975. [DOI] [PubMed] [Google Scholar]
- 3.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 2014; 115(1): 148–164. [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM, Archer SL, Dorfmuller P, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuder RM, Chacon M, Alger L, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 2001; 195(3): 367–374. [DOI] [PubMed] [Google Scholar]
- 6.Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001; 15(2): 427–438. [DOI] [PubMed] [Google Scholar]
- 7.Vilar J, Waeckel L, Bonnin P, et al. Chronic hypoxia-induced angiogenesis normalizes blood pressure in spontaneously hypertensive rats. Circ Res 2008; 103(7): 761–769. [DOI] [PubMed] [Google Scholar]
- 8.Jurasz P, Courtman D, Babaie S, et al. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther 2010; 126(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014; 115(1): 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malenfant S, Potus F, Fournier F, et al. Skeletal muscle proteomic signature and metabolic impairment in pulmonary hypertension. J Mol Med (Berl) 2015; 93(5): 573–584. [DOI] [PubMed] [Google Scholar]
- 11.Potus F, Ruffenach G, Dahou A, et al. Downregulation of miR-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation 2015; 132: 932–943. [DOI] [PubMed] [Google Scholar]
- 12.Potus F, Malenfant S, Graydon C, et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med 2014; 190(3): 318–328. [DOI] [PubMed] [Google Scholar]
- 13.De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab 2013; 18(5): 634–647. [DOI] [PubMed] [Google Scholar]
- 14.de Jesus Perez VA. Pumping it up! Angiogenesis and muscle deconditioning in pulmonary hypertension. Am J Respir Crit Care Med 2014; 190(3): 250–251. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra R, Paskin-Flerlage S, Zamanian RT, et al. Circulating angiogenic modulatory factors predict survival and functional class in pulmonary arterial hypertension. Pulm Circ 2013; 3(2): 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumpers P, Nickel N, Lukasz A, et al. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J 2010; 31(18): 2291–2300. [DOI] [PubMed] [Google Scholar]
- 17.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993; 328(24): 1732–1739. [DOI] [PubMed] [Google Scholar]
- 18.Lajoie AC, Lauziere G, Lega JC, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med 2016; 4: 291–305. [DOI] [PubMed] [Google Scholar]
- 19.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369(9): 809–818. [DOI] [PubMed] [Google Scholar]
- 20.Gien J, Tseng N, Seedorf G, et al. Endothelin-1 impairs angiogenesis in vitro through Rho-kinase activation after chronic intrauterine pulmonary hypertension in fetal sheep. Pediatr Res 2013; 73(3): 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gien J, Tseng N, Seedorf G, et al. Peroxisome proliferator activated receptor-gamma-Rho-kinase interactions contribute to vascular remodeling after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014; 306(3): L299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf D, Tseng N, Seedorf G, et al. Endothelin-1 decreases endothelial PPARgamma signaling and impairs angiogenesis after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014; 306(4): L361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iglarz M, Silvestre JS, Duriez M, et al. Chronic blockade of endothelin receptors improves ischemia-induced angiogenesis in rat hindlimbs through activation of vascular endothelial growth factor-no pathway. Arterioscler Thromb Vasc Biol 2001; 21(10): 1598–1603. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet S, Provencher S, Guignabert C, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med 2017; 195: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meloche J, Pflieger A, Vaillancourt M, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation 2014; 129(7): 786–797. [DOI] [PubMed] [Google Scholar]
- 26.Ruffenach G, Chabot S, Tanguay VF, et al. Role for RUNX2 in proliferative and calcified vascular lesions in pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 194: 1273–1285. [DOI] [PubMed] [Google Scholar]
- 27.Iglarz M, Landskroner K, Bauer Y, et al. Comparison of macitentan and bosentan on right ventricular remodeling in a rat model of non-vasoreactive pulmonary hypertension. J Cardiovasc Pharmacol 2015; 66(5): 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meloche J, Le Guen M, Potus F, et al. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol 2015; 309(6): C363–372. [DOI] [PubMed] [Google Scholar]
- 29.Paulin R, Courboulin A, Meloche J, et al. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 2011; 123(11): 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meloche J, Potus F, Vaillancourt M, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res 2015; 117(6): 525–535. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Kumar S, Sharma S, et al. Endothelin-1 induces a glycolytic switch in pulmonary arterial endothelial cells via the mitochondrial translocation of endothelial nitric oxide synthase. Am J Respir Cell Mol Biol 2014; 50(6): 1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potus F, Ruffenach G, Dahou A, et al. Downregulation of microRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation 2015; 132(10): 932–943. [DOI] [PubMed] [Google Scholar]
- 33.Rival G, Lacasse Y, Martin S, et al. Effect of pulmonary arterial hypertension-specific therapies on health-related quality of life: a systematic review. Chest 2014; 146(3): 686–708. [DOI] [PubMed] [Google Scholar]
- 34.Granton J, Langleben D, Kutryk MB, et al. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension: the PHACeT Trial. Circ Res 2015; 117(7): 645–654. [DOI] [PubMed] [Google Scholar]
- 35.Temple IP, Monfredi O, Quigley G, et al. Macitentan treatment retards the progression of established pulmonary arterial hypertension in an animal model. Int J Cardiol 2014; 177(2): 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulin R, Dromparis P, Sutendra G, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab 2014; 20(5): 827–839. [DOI] [PubMed] [Google Scholar]
- 37.Sutendra G, Bonnet S, Rochefort G, et al. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med 2010; 2(44): 44ra58. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara T, Sawada H, Otsuki S, et al. Macitentan reverses early obstructive pulmonary vasculopathy in rats: early intervention in overcoming the survivin-mediated resistance to apoptosis. Am J Physiol Lung Cell Mol Physiol 2015; 308(6): L523–538. [DOI] [PubMed] [Google Scholar]
- 39.Paulin R, Courboulin A, Barrier M, et al. From oncoproteins/tumor suppressors to microRNAs, the newest therapeutic targets for pulmonary arterial hypertension. J Mol Med (Berl) 2011; 89(11): 1089–1101. [DOI] [PubMed] [Google Scholar]
- 40.Boucherat O, Vitry G, Trinh I, et al. The cancer theory of pulmonary arterial hypertension. Pulm Circ 2017; 7: 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voelkel NF, Gomez-Arroyo J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol 2014; 51(4): 474–484. [DOI] [PubMed] [Google Scholar]
- 42.Eddahibi S, Humbert M, Sediame S, et al. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension. Effect of prostacyclin therapy. Am J Respir Crit Care Med 2000; 162(4 Pt 1): 1493–1499. [DOI] [PubMed] [Google Scholar]
- 43.Papaioannou AI, Zakynthinos E, Kostikas K, et al. Serum VEGF levels are related to the presence of pulmonary arterial hypertension in systemic sclerosis. BMC Pulm Med 2009; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selimovic N, Bergh CH, Andersson B, et al. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 2009; 34(3): 662–668. [DOI] [PubMed] [Google Scholar]
- 45.Hirose S, Hosoda Y, Furuya S, et al. Expression of vascular endothelial growth factor and its receptors correlates closely with formation of the plexiform lesion in human pulmonary hypertension. Pathol Int 2000; 50(6): 472–479. [DOI] [PubMed] [Google Scholar]
- 46.Tuder RM, Groves B, Badesch DB, et al. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994; 144(2): 275–285. [PMC free article] [PubMed] [Google Scholar]
- 47.Partovian C, Adnot S, Raffestin B, et al. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 2000; 23(6): 762–771. [DOI] [PubMed] [Google Scholar]
- 48.Farkas L, Farkas D, Ask K, et al. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 2009; 119(5): 1298–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki S, Yoshihisa A, Yokokawa T, et al. Association between levels of anti-angiogenic isoform of vascular endothelial growth factor A and pulmonary hypertension. Int J Cardiol 2016; 222: 416–420. [DOI] [PubMed] [Google Scholar]
- 50.Zhao YD, Springall DR, Hamid Q, et al. Localization and characterization of endothelin-1 receptor binding in the blood vessels of human pulmonary tumors. J Cardiovasc Pharmacol 1995; 26(Suppl. 3): S341–345. [PubMed] [Google Scholar]
- 51.Salani D, Taraboletti G, Rosano L, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol 2000; 157(5): 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grover TR, Parker TA, Balasubramaniam V, et al. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 2005; 288(4): L648–654. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, Koeck T, Lara AR, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A 2007; 104(4): 1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunita-Takanezawa M, Abe K, Hirooka Y, et al. Novel dual endothelin receptor antagonist macitentan reverses severe pulmonary arterial hypertension in rats. J Cardiovasc Pharmacol 2014; 64(5): 473–480. [DOI] [PubMed] [Google Scholar]
- 55.Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 2007; 47: 731–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malenfant S, Potus F, Mainguy V, et al. Impaired skeletal muscle oxygenation and exercise tolerance in pulmonary hypertension. Med Sci Sports Exerc 2015; 47: 2273–2282. [DOI] [PubMed] [Google Scholar]
- 57.Drozd K, Ahmadi A, Deng Y, et al. Effects of an endothelin receptor antagonist, Macitentan, on right ventricular substrate utilization and function in a Sugen 5416/hypoxia rat model of severe pulmonary arterial hypertension. J Nucl Cardiol 2016. DOI:10.1007/s12350-016-0663-4. [DOI] [PubMed] [Google Scholar]
- 58.Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54(1 Suppl): S20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz SD, Balidemaj K, Homma S, et al. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol 2000; 36(3): 845–851. [DOI] [PubMed] [Google Scholar]
- 60.Wray DW, Nishiyama SK, Donato AJ, et al. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 2007; 293(4): H2550–2556. [DOI] [PubMed] [Google Scholar]