Abstract
The plasmacytoma variant translocation 1 gene (PVT1) plays an oncogenic role in the initiation and progression of multiple cancers. In this study, by using deep-sequencing data and follow-up data in the Cancer Genome Atlas-Uveal melanomas (TCGA-UVM), we assessed the association between the expression of PVT1 and clinicopathological characteristics of patients with uveal melanoma, the mechanism of its dysregulation and its prognostic value. Results showed that high PVT1 expression group had a higher proportion of epithelioid cell dominant disease (a more malignant histological subtype than spindle cell dominant disease) and more cases of extrascleral extension (a risk factor for metastasis) compared with the low PVT1 expression group. 61 out of 80 cases (76.3%) of primary uveal melanoma had PVT1 amplification in TCGA-UVM. In addition, PVT1 expression was strongly and negatively correlated with its methylation status (Pearson's r = -0.712, Spearman’s r = -0.806). By performing univariate and multivariate analysis, we found that high PVT1 expression was an independent predictor of poor OS in patients with uveal melanoma (HR: 12.015, 95%CI: 1.854–77.876, p = 0.009). Based on these findings, we infer that PVT1 expression is modulated by both DNA amplification and methylation and its expression might serve as a valuable and specific prognostic biomarker in terms of OS in uveal melanoma.
Introduction
Long noncoding RNAs (lncRNAs) are a class of RNA that are longer than 200 nucleotides and do not code for proteins [1]. Previous studies found that this class of RNAs play a pivotal role in regulating gene expression at both transcriptional and post-transcriptional levels [1]. Uveal melanoma, which is also known as ocular melanoma is the most common primary intraocular cancer in adults. Some recent studies found that dysregulated lncRNAs are involved in the pathological development of uveal melanoma [2, 3]. For example, hypermethylated in cancer 1 (HIC1) can induce uveal melanoma progression by activating lncRNA-numb [4]. LncRNA CASC15-New-Transcript 1(CANT1) acts as a necessary suppressor of uveal melanoma via triggering the expression of lncRNA JPX and FTX and subsequently inducing the expression of lncRNA XIST [5]. HOXA11-AS can increase uveal melanoma cell growth and invasion by interacting with enhancer of zeste homolog 2 (EZH2) to suppress its target p21 protein expression and by sponging miR-124 [3].
The plasmacytoma variant translocation 1 gene (PVT1) has been demonstrated as an oncogenic lncRNA in multiple cancers, including ovarian cancer [6], breast cancer [7], prostate cancer [8], cervical cancer [9], gastric cancer [10] and non-small cell lung cancer [11]. In gastric cancer, high PVT1 expression is an independent prognostic marker for poor overall survival (OS) and disease-free survival (DFS) [10].
PVT1 overexpression promotes melanoma cells proliferation, cell cycle progression, and migration [12]. Mechanistically, PVT1 directly sponges miR-26b, which had been verified as a tumor suppressor in melanoma [13]. These findings suggest that PVT1 may also act as an oncogene in melanoma. However, its association with uveal melanoma, as well as its prognostic value and the mechanism of its dysregulation in uveal melanoma have not been explored. In this study, by using deep-sequencing data and follow-up data in the Cancer Genome Atlas-Uveal melanomas (TCGA-UVM), we found that PVT1 expression is modulated by both DNA amplification and methylation and its high expression independently predicts poor OS in patients with primary uveal melanoma.
Materials and methods
Data mining in the Cancer Genome Atlas-Uveal melanomas (UVM)
The level 3 data of patients with primary uveal melanoma in TCGA-UVM or with primary skin melanoma in TCGA-SKCM were downloaded by using the UCSC Xena browser (https://xenabrowser.net). Heatmap showing PVT1 copy number alterations, RNA expression and DNA methylation (450k) was generated. Regression analysis of the correlation between PVT1 RNA expression and its DNA methylation was examined by using cBioPortal for Cancer Genomics (http://www.cbioportal.org/) [14, 15].
Kaplan-Meier curves of overall survival (OS) were generated by GraphPad Prism v6.0 (GraphPad Software Inc.). Patients were grouped according to median PVT1 expression or median PVT1 DNA methylation.
Statistical analysis
Statistical analysis was performed by using SPSS 19.0 (SPSS Inc.) and GraphPad Prism v6.0. Comparison of the clinicopathological features between high and low PVT1 expression groups was performed using χ2 tests. Log-rank test was performed to assess the difference between the survival curves. Prognostic values were analyzed by univariate and multivariate Cox regression models. Welch’s t-test was conducted to compare PVT1 expression between different copy number alterations. P < 0.05 was considered to be statistically significant.
Results
High expression of lncRNA PVT1 is associated with malignant behaviors of uveal melanoma
The association between PVT1 expression and the clinicopathological parameters was summarized in Table 1. Compared with the low PVT1 expression group, the high PVT1 expression was associated with older age (66.80 ± 11.55 vs. 56.50 ± 14.36, p = 0.0007), a higher proportion of epithelioid cell dominant disease (22/40 vs. 12/40, p = 0.024), more cases of distant metastasis (4/28 vs. 0/27, p = 0.043) and extrascleral extension (6/37 vs. 1/38, p = 0.043) and a higher death rate (20/40 vs. 3/40, p<0.0001) (Table 1).
Table 1. The association between PVT1 expression and the clinicopathological parameters in patients with primary uveal melanoma in TCGA.
| Parameters | PVT1 expression RNAseq | χ2 | p Value | ||
|---|---|---|---|---|---|
| High (N = 40) | Low (N = 40) | ||||
| Age (Mean ± SD) | 66.80 ± 11.55 | 56.50 ± 14.36 | 0.0007 | ||
| Gender | Female | 18 | 17 | 0.051 | 0.82 |
| Male | 22 | 23 | |||
| Histological type | Epithelioid cell dominant | 22 | 12 | 5.12 | 0.024 |
| Spindle Cell dominant | 18 | 28 | |||
| Pathological T | II | 6 | 8 | 0.35 | 0.56 |
| III/IV | 34 | 32 | |||
| Pathological N | N0 | 25 | 27 | N.A. | N.A |
| NX/null | 15 | 13 | |||
| Pathological M | M0 | 24 | 27 | 4.16 | 0.041 |
| M1+ | 4 | 0 | |||
| MX/null | 12 | 13 | |||
| Pathological stages | II | 18 | 21 | 0.32 | 0.57 |
| III/IV | 21 | 19 | |||
| Null | 1 | 0 | |||
| Tumor diameter (mm) | ≤16 | 16 | 18 | 0.13 | 0.72 |
| >16 | 23 | 22 | |||
| Null | 1 | 0 | |||
| Tumor thickness (mm) | ≤10 | 15 | 22 | 2.46 | 0.12 |
| >10 | 25 | 18 | |||
| Extrascleral extension | No | 31 | 37 | 4.09 | 0.043 |
| Yes | 6 | 1 | |||
| Null | 3 | 2 | |||
| Living Status | Living | 20 | 37 | 17.64 | <0.0001 |
| Dead | 20 | 3 | |||
Extrascleral extension: extension occurring outside the sclera of the orbit; M1+: M1a/M1b/M1c; NX: Nearby (regional) lymph nodes cannot be assessed; MX: Metastasis cannot be measured; Null: data was not available; N/A.: not applicable.
The expression of PVT1 is modulated by DNA amplification and methylation in uveal melanoma
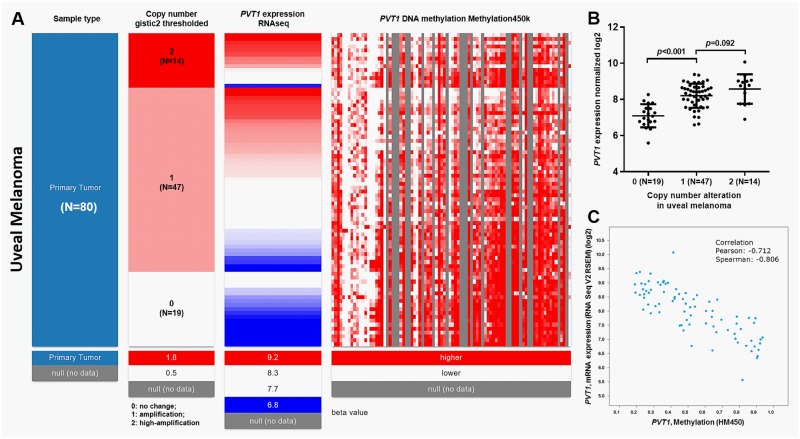
By using the deep sequencing data in TCGA, we tried to explore the mechanisms of PVT1 dysregulation in uveal melanoma. Gene-level thresholded GISTIC2-processed copy-number data, which defines genetic changes as homozygous deletion (-2), heterozygous loss (-1), copy-neutral (0), low-level copy gain (+1), high-level amplification (+2) were downloaded from the Xena browser. Among the 80 cases of primary uveal melanoma, 14 cases (17.5%) had PVT1 high-amplification (+2) and 47 cases (58.8%) had amplification (+1) (Fig 1A). The amplification was associated with significantly higher expression of PVT1 RNA (Fig 1B). However, no significant difference was observed between the +2 and +1 group (Fig 1B). Then, we characterized the correlation between PVT1 expression and its DNA methylation (Fig 1A and 1C). Heatmap and following regression analysis revealed a strong negative correlation between PVT1 expression and DNA methylation (Pearson's r = -0.712, Spearman’s r = -0.806) (Fig 1A and 1C).
Fig 1. The expression of PVT1 is modulated by DNA amplification and methylation in uveal melanoma.
A. Heatmap of PVT1 copy number alterations, PVT1 RNA expression and PVT1 DNA methylation in 80 cases of primary uveal melanoma. B. Bar chart of PVT1 expression in high-amplification (2), amplification (1) and no change (0) groups. C. Regression analysis of the correlation between PVT1 RNA expression and DNA methylation.
High expression of PVT1 independently predicts poor OS in patients with primary uveal melanoma
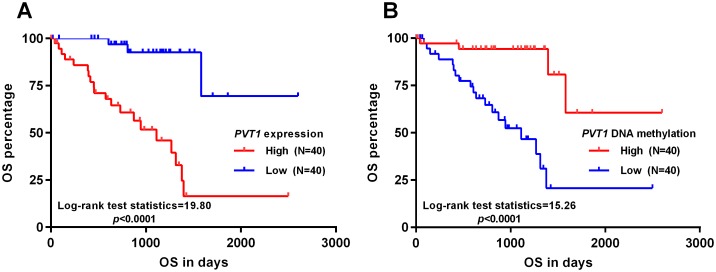
Since the expression of PVT1 was associated with malignant behaviors of uveal melanoma, we determine to assess its prognostic value. By generating Kaplan-Meier curves of OS, we found that high PVT1 expression was associated with significantly shorter OS (p<0.0001) (Fig 2A). Interestingly, as a mechanism of PVT1 dysregulation, low PVT1 DNA methylation was also associated with unfavorable OS (p<0.0001) (Fig 2B). In univariate analysis, we found that epithelioid cell dominant uveal melanoma, extrascleral extension, high PVT1 expression and low PVT1 DNA methylation were associated with unfavorable OS (Table 2). Multivariate analysis showed that older age (>60) (HR: 2.599, 95%CI: 1.049–6.437, p = 0.039), epithelioid cell dominant uveal melanoma (HR: 4.385, 95%CI: 1.514–12.703, p = 0.006) and high PVT1 expression (HR: 12.015, 95%CI: 1.854–77.876, p = 0.009) were independent predictors for poor OS (Table 2).
Fig 2. High expression of PVT1 is associated with poor OS in patients with primary uveal melanoma.
A-B. Kaplan-Meier curves of OS in uveal melanoma patients grouped by the median PVT1 expression (A) or PVT1 methylation (B).
Table 2. Univariate and multivariate analyses of OS in patients with primary uveal melanoma.
| Parameters | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| p | HR | 95%CI (lower/upper) | p | HR | 95%CI (lower/upper) | |||
| Age > 60 vs.≤ 60 |
0.080 | 2.123 | 0.914 | 4.933 | 0.039 | 2.599 | 1.049 | 6.437 |
| Female vs. Male | 0.325 | 0.649 | 0.274 | 1.536 | ||||
| Histological type Epithelioid cell dominant vs. Spindle Cell dominant | 0.001 | 4.551 | 1.814 | 11.418 | 0.006 | 4.385 | 1.514 | 12.703 |
| Pathological stage III/IV vs. II |
0.358 | 1.504 | 0.630 | 3.589 | ||||
| Tumor diameter (mm) >16 vs. ≤16 |
0.192 | 1.831 | 0.738 | 4.541 | ||||
| Tumor thickness (mm) >10 vs. ≤10 |
0.106 | 2.106 | 0.854 | 5.191 | ||||
| Extrascleral extension No vs. Yes |
0.008 | 0.219 | 0.071 | 0.675 | 0.125 | 0.392 | 0.118 | 1.297 |
|
PVT1 expression High vs. Low |
0.0003 | 9.748 | 2.872 | 33.080 | 0.009 | 12.015 | 1.854 | 77.876 |
|
PVT1 DNA methylation High vs. Low |
0.0006 | 0.148 | 0.050 | 0.443 | 0.751 | 1.317 | 0.240 | 7.231 |
PVT1 expression is not associated with OS in skin cutaneous melanoma
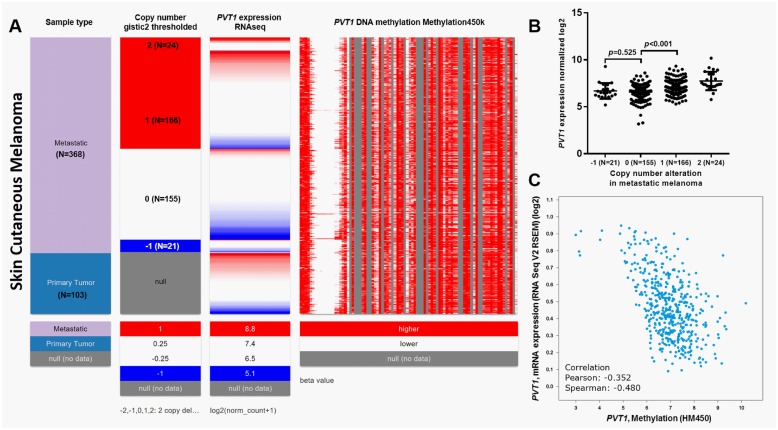
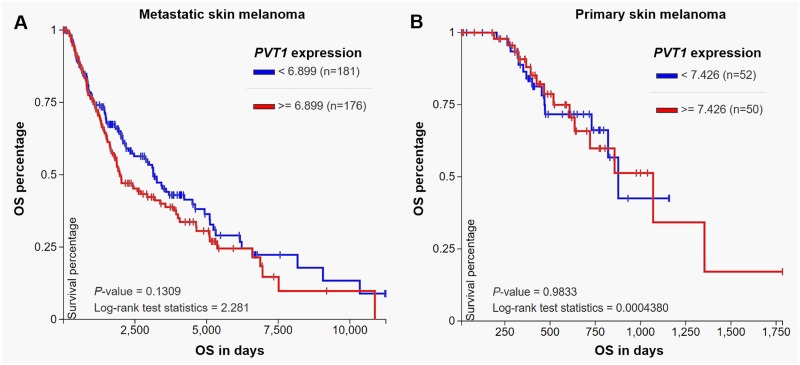
To further verify the specificity of the prognostic value of PVT1 in uveal melanoma, we also examined its expression profile and the association with OS in patients with primary skin cutaneous melanoma in TCGA. DNA copy number alteration data (N = 366) were only available in patients with metastatic skin melanoma (N = 368). PVT1 DNA amplification was less common in metastatic skin melanoma (190/366, 51.9%) than in uveal melanoma (61/80, 76.3%) (Fig 3A). 21 cases even had PVT1 heterozygous loss (Fig 3A). Elevated PVT1 transcription was also observed in DNA amplification group (Fig 3B). In comparison, heterozygous loss did not necessarily result in PVT1 decrease (Fig 3B). DNA methylation was weakly and negatively correlated with PVT1 expression in skin melanoma (Pearson's r = -0.352, Spearman’s r = -0.480) (Fig 3C). 357 metastatic patients and 102 primary patients with intact OS data were included in Table 3. According to the best cut-off of PVT1 expression, these patients were divided into high PVT1 (N = 230, which include 163 metastatic cases and 67 primary cases) and low PVT1 (N = 229, which include 194 metastatic cases and 35 primary cases) expression groups. The association between PVT1 expression and the clinicopathological parameters in this group of patients was summarized in Table 3. The high PVT1 expression group had a higher ratio of primary tumor (67/230) compared with the low PVT1 expression group (35/229) (p = 0.0004) (Table 3). No significant difference in the other parameters was observed between the high and low PVT1 expression groups (Table 3). Log-rank test of OS curves indicated that PVT1 expression was not related to OS, no matter in metastatic melanoma (p = 0.13, Fig 4A) or in primary melanoma (p = 0.98, Fig 4B).
Fig 3. The association between DNA amplification/methylation and PVT1 expression in skin cutaneous melanoma.
A. Heatmap of PVT1 copy number alterations, PVT1 RNA expression and PVT1 DNA methylation in 368 metastatic skin melanoma cases and in 103 primary skin melanoma cases. B. Bar chart of PVT1 expression in high-amplification (2), amplification (1), no change (0) and heterozygous loss (-1) groups. C. Regression analysis of the correlation between PVT1 RNA expression and DNA methylation.
Table 3. The association between PVT1 expression and the clinicopathological parameters in patients with primary skin cutaneous melanoma in TCGA.
| Parameters | PVT1 expression RNAseq | χ2 | p-value | ||
|---|---|---|---|---|---|
| High (N = 230) | Low (N = 229) | ||||
| Age (Mean ± SD) | 58.32±12.15 | 57.88±16.31 | 0.76 | ||
| Sample type | Metastatic | 163 | 194 | 12.73 | 0.0004 |
| Primary Tumor | 67 | 35 | |||
| Gender | Female | 79 | 95 | 2.48 | 0.12 |
| Male | 151 | 134 | |||
| pathological T | T0 | 16 | 7 | 3.99 | 0.26 |
| Tis | 3 | 4 | |||
| T1/T2 | 56 | 62 | |||
| T3/T4 | 119 | 121 | |||
| TX/null | 36 | 35 | |||
| Pathological N | N0 | 115 | 114 | 0.016 | 0.90 |
| N1+ | 90 | 87 | |||
| NX/null | 25 | 28 | |||
| Pathological M | M0 | 205 | 204 | 0.15 | 0.70 |
| M1+ | 13 | 11 | |||
| Null | 12 | 14 | |||
| Pathological stages | 0 | 2 | 4 | 0.86 | 0.65 |
| I/II | 117 | 108 | |||
| III/IV | 97 | 95 | |||
| Null | 14 | 22 | |||
| Living Status | Living | 120 | 117 | 0.054 | 0.82 |
| Dead | 110 | 112 | |||
TX: Primary tumor cannot be assessed; T0: No evidence of primary tumor; NX: Nearby (regional) lymph nodes cannot be assessed; N1+: N1/N2/N3; M1+: M1a/M1b/M1c; null: no data.
Fig 4. Kaplan-Meier curves of OS in metastatic skin melanoma (A) and in primary skin melanoma (B).
Patients were divided into two groups according to the median PVT1 expression.
Discussion
PVT1 has been demonstrated as an oncogenic lncRNA that is usually upregulated in cancer tissues compared with normal tissues. It exerts a carcinogenetic effect via various epigenetic mechanisms such as regulating transcription activity and via acting as miRNAs sponges. For example, it can promote non-small cell lung cancer cell proliferation by recruiting EZH2 to the large tumor suppressor kinase 2 (LATS2) promoter and represses LATS2 transcription [11]. It suppresses miR-146a expression by inducing the methylation of CpG island in its promoter in prostate cancer cells [16]. It can also sponge miR-186 in gastric cancer cells [17], miR-448 in pancreatic cancer cells [18], miR-26b in melanoma cancer cells [13], miR-203 in esophageal squamous cell carcinoma cells [19], thereby contributing to malignant behaviors of these cancers, such as enhanced proliferation, migration, and metastasis.
One recent meta-analysis based on 1,443 patients from 15 previous studies found that increased PVT1 expression was significantly associated with positive lymph node metastasis, positive distant metastasis, advanced tumor-node-metastasis stage and poor differentiation grade, but was not related to tumor size in some cancers [20]. In this study, we found that high PVT1 expression was associated with a higher proportion of epithelioid cell dominant disease (a more malignant histological subtype than spindle cell dominant disease) and more cases of extrascleral extension (a risk factor for metastasis), suggesting that high PVT1 expression may confer some malignant phenotypes to uveal melanoma. However, we did not find a significant difference in tumor size and thickness between the high and low PVT1 expression groups, which were in consistent with the findings in the meta-analysis.
The mechanisms of PVT1 dysregulation in these cancers are quite complex and far from being fully understood. PVT1 locates at 8q24 in the human genome, a region that is usually amplified in some cancers [8, 21]. In gastric cancer, FOXM1 can bind to the promoter region of PVT1 and enhance its transcription [22]. Upregulation of SOX2 can activate PVT1 expression in breast cancer cells via binding to its promoter and promote breast cancer cell growth and invasion [23]. These findings indicate that dysregulated PVT1 may be caused by both genetic and epigenetic alterations. By examining copy number alterations in TCGA-UVM, we found that 61 out of 80 cases (76.3%) of primary uveal melanoma had PVT1 amplification. In addition, the amplification was associated with significantly higher PVT1 RNA expression. These findings supported our hypothesis that genetic amplification is a mechanism of aberrant PVT1 expression in uveal melanoma. Interestingly, by analyzing the DNA methylation status of PVT1, we observed a strong negative correlation between PVT1 expression and its methylation (Pearson's r = -0.712, Spearman’s r = -0.806), suggesting that DNA methylation status can also influence PVT1 expression in uveal melanoma. In addition, we also observed different levels of copy number alterations and methylation status between uveal melanoma and skin cutaneous melanoma, which indicate that PVT1 dysregulation might be cancer-specific.
Several previous studies also observed the promising prognostic value of PVT1 in multiple types of cancer. In gastrointestinal cancers, elevated PVT1 expression was significantly related to poor OS, DFS, disease-specific survival (DSS) and relapse-free survival (RFS) [10, 24]. Its overexpression also serves as an independent prognostic indicator for the OS of patients with small cell lung cancer [25]. By performing univariate and multivariate analysis, we found that high PVT1 expression was an independent predictor of poor OS in patients with uveal melanoma (HR: 12.015, 95%CI: 1.854–77.876, p = 0.009). In comparison, although it acts as an oncogene in skin melanoma, it had no prognostic value in terms of OS. Therefore, the expression of PVT1 might serve as a valuable and specific prognostic biomarker in uveal melanoma.
This study also has some limitations. The most important one is that some patients’ characteristics (such as pigmentation) and treatment information was not recorded in the database. In fact, pigmentation has a critical role in melanoma biology [26]. It affects melanoma patients’ survival, radiotherapy, chemotherapy and immune therapy [27–30]. Melanogenesis could simulate HIF-1α expression, thereby conferring malignant behaviors of melanoma cells [30, 31]. Inhibition of melanogenesis might enhance the efficacy of radiotherapy and chemotherapy in advanced melanomas [32, 33]. Another limitation is the relatively small sample size in the database (N = 80). In addition, due to insufficient data, we were unable to assess the association between PVT1 and DFS among the patients. Therefore, it is meaningful to assess its independent prognostic value in uveal melanoma in a larger cohort in the future.
Conclusion
Aberrant PVT1 expression is associated with malignant behaviors of uveal melanoma and might independently predict poor OS.
Data Availability
All relevant data are included in the paper.
Funding Statement
This study was supported by Experimental Animal Science Project of Science Technology Department of Zhejiang, Province of China (no. 2015C37132) Recipient: Haiming Xu; Chinese Medicine Scientific Research Foundation of Zhejiang Province of China (no. 2016ZA033). Recipient: Hui Liu; Medical scientific Research Foundation of Zhejiang Province of China (no.2018233958). Recipient: Hui Liu; and Medical scientific Research Foundation of Zhejiang Province of China, (no.2018273482). Recipient: Haiming Xu.
References
- 1.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407. doi: 10.1158/2159-8290.CD-11-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding X, Wang X, Lin M, Xing Y, Ge S, Jia R, et al. PAUPAR lncRNA suppresses tumourigenesis by H3K4 demethylation in uveal melanoma. FEBS Lett. 2016;590(12):1729–38. doi: 10.1002/1873-3468.12220 . [DOI] [PubMed] [Google Scholar]
- 3.Lu Q, Zhao N, Zha G, Wang H, Tong Q, Xin S. LncRNA HOXA11-AS Exerts Oncogenic Functions by Repressing p21 and miR-124 in Uveal Melanoma. DNA Cell Biol. 2017. doi: 10.1089/dna.2017.3808 . [DOI] [PubMed] [Google Scholar]
- 4.Cheng G, He J, Zhang L, Ge S, Zhang H, Fan X. HIC1 modulates uveal melanoma progression by activating lncRNA-numb. Tumour Biol. 2016;37(9):12779–89. doi: 10.1007/s13277-016-5243-3 . [DOI] [PubMed] [Google Scholar]
- 5.Xing Y, Wen X, Ding X, Fan J, Chai P, Jia R, et al. CANT1 lncRNA Triggers Efficient Therapeutic Efficacy by Correcting Aberrant lncing Cascade in Malignant Uveal Melanoma. Mol Ther. 2017;25(5):1209–21. doi: 10.1016/j.ymthe.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13(19):5745–55. doi: 10.1158/1078-0432.CCR-06-2882 . [DOI] [PubMed] [Google Scholar]
- 7.Conte F, Fiscon G, Chiara M, Colombo T, Farina L, Paci P. Role of the long non-coding RNA PVT1 in the dysregulation of the ceRNA-ceRNA network in human breast cancer. PLoS One. 2017;12(2):e0171661 doi: 10.1371/journal.pone.0171661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer KB, Maia AT, O'Reilly M, Ghoussaini M, Prathalingam R, Porter-Gill P, et al. A functional variant at a prostate cancer predisposition locus at 8q24 is associated with PVT1 expression. PLoS Genet. 2011;7(7):e1002165 doi: 10.1371/journal.pgen.1002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iden M, Fye S, Li K, Chowdhury T, Ramchandran R, Rader JS. The lncRNA PVT1 Contributes to the Cervical Cancer Phenotype and Associates with Poor Patient Prognosis. PLoS One. 2016;11(5):e0156274 doi: 10.1371/journal.pone.0156274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan CL, Li H, Zhu L, Liu Z, Zhou J, Shu Y. Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma. 2016;63(3):442–9. doi: 10.4149/314_150825N45 . [DOI] [PubMed] [Google Scholar]
- 11.Wan L, Sun M, Liu GJ, Wei CC, Zhang EB, Kong R, et al. Long Noncoding RNA PVT1 Promotes Non-Small Cell Lung Cancer Cell Proliferation through Epigenetically Regulating LATS2 Expression. Mol Cancer Ther. 2016;15(5):1082–94. doi: 10.1158/1535-7163.MCT-15-0707 . [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Gao G, Liu S, Yu L, Yan D, Yao X, et al. Long Noncoding RNA PVT1 as a Novel Diagnostic Biomarker and Therapeutic Target for Melanoma. Biomed Res Int. 2017;2017:7038579 doi: 10.1155/2017/7038579 publication of this paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang BJ, Ding HW, Ma GA. Long Noncoding RNA PVT1 Promotes Melanoma Progression Via Endogenous Sponging MiR-26b. Oncol Res. 2017. doi: 10.3727/096504017X14920318811730 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1 doi: 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu HT, Fang L, Cheng YX, Sun Q. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Med. 2016;5(12):3512–9. doi: 10.1002/cam4.900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang T, Liu HW, Chen JQ, Wang SH, Hao LQ, Liu M, et al. The long noncoding RNA PVT1 functions as a competing endogenous RNA by sponging miR-186 in gastric cancer. Biomed Pharmacother. 2017;88:302–8. doi: 10.1016/j.biopha.2017.01.049 . [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Kong H, Sun H, Chen Z, Chen B, Zhou M. LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J Cell Physiol. 2017. doi: 10.1002/jcp.26072 . [DOI] [PubMed] [Google Scholar]
- 19.Li PD, Hu JL, Ma C, Ma H, Yao J, Chen LL, et al. Upregulation of the long non-coding RNA PVT1 promotes esophageal squamous cell carcinoma progression by acting as a molecular sponge of miR-203 and LASP1. Oncotarget. 2017;8(21):34164–76. doi: 10.18632/oncotarget.15878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu D, Luo P, Wang Q, Ye Y, Wang B. lncRNA PVT1 in cancer: A review and meta-analysis. Clin Chim Acta. 2017;474:1–7. doi: 10.1016/j.cca.2017.08.038 . [DOI] [PubMed] [Google Scholar]
- 21.Nagoshi H, Taki T, Hanamura I, Nitta M, Otsuki T, Nishida K, et al. Frequent PVT1 rearrangement and novel chimeric genes PVT1-NBEA and PVT1-WWOX occur in multiple myeloma with 8q24 abnormality. Cancer Res. 2012;72(19):4954–62. doi: 10.1158/0008-5472.CAN-12-0213 . [DOI] [PubMed] [Google Scholar]
- 22.Xu MD, Wang Y, Weng W, Wei P, Qi P, Zhang Q, et al. A Positive Feedback Loop of lncRNA-PVT1 and FOXM1 Facilitates Gastric Cancer Growth and Invasion. Clin Cancer Res. 2017;23(8):2071–80. doi: 10.1158/1078-0432.CCR-16-0742 . [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhou J, Wang Z, Wang P, Li S. Upregulation of SOX2 activated LncRNA PVT1 expression promotes breast cancer cell growth and invasion. Biochem Biophys Res Commun. 2017. doi: 10.1016/j.bbrc.2017.09.005 . [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Dong Q, Huang J. Overexpression of LncRNA PVT1 Predicts Advanced Clinicopathological Features and Serves as an Unfavorable Risk Factor for Survival of Patients with Gastrointestinal Cancers. Cell Physiol Biochem. 2017;43(3):1077–89. doi: 10.1159/000481719 . [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Liu S, Wang H, Zhang Z, Yang Q, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8(11):5025–34. [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–228. doi: 10.1152/physrev.00044.2003 . [DOI] [PubMed] [Google Scholar]
- 27.Brozyna AA, Jozwicki W, Carlson JA, Slominski AT. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum Pathol. 2013;44(10):2071–4. doi: 10.1016/j.humpath.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harbour JW, Brantley MA Jr., Hollingsworth H, Gordon M. Association between choroidal pigmentation and posterior uveal melanoma in a white population. Br J Ophthalmol. 2004;88(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brozyna AA, Jozwicki W, Roszkowski K, Filipiak J, Slominski AT. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7(14):17844–53. doi: 10.18632/oncotarget.7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slominski RM, Zmijewski MA, Slominski AT. The role of melanin pigment in melanoma. Exp Dermatol. 2015;24(4):258–9. doi: 10.1111/exd.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slominski A, Kim TK, Brozyna AA, Janjetovic Z, Brooks DL, Schwab LP, et al. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1alpha expression and HIF-dependent attendant pathways. Arch Biochem Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brozyna AA, VanMiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123(6):1448–56. doi: 10.1002/ijc.23664 . [DOI] [PubMed] [Google Scholar]
- 33.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124(6):1470–7. doi: 10.1002/ijc.24005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the paper.