Abstract
During the infection process, Apicomplexa discharge their secretory organelles called micronemes, rhoptries and dense granules to sustain host cell invasion, intracellular replication and to modulate host cell pathways and immune responses. Herein, we describe the Toxoplasma gondii Deg-like serine protein (TgDegP), a rhoptry protein homologous to High temperature requirement A (HtrA) or Deg-like family of serine proteases. TgDegP undergoes processing in both types I and II strains as most of the rhoptries proteins. We show that genetic disruption of the degP gene does not impact the parasite lytic cycle in vitro but affects virulence in mice. While in a type I strain DegPI appears dispensable for the establishment of an infection, removal of DegPII in a type II strain dramatically impairs the virulence. Finally, we show that KO-DegPII parasites kill immunodeficient mice as efficiently as the wild-type strain indicating that the protease might be involved in the complex crosstalk that the parasite engaged with the host immune response. Thus, this study unravels a novel rhoptry protein in T. gondii important for the establishment of lethal infection.
Introduction
The protozoan parasite Toxoplasma gondii is an obligate intracellular parasite responsible for toxoplasmosis. It belongs to the phylum Apicomplexa, which includes other significant human pathogens such as Plasmodium falciparum, the causative agent of malaria. Toxoplasmosis is usually asymptomatic and well controlled by the immune system of immunocompetent individuals. Following a short acute phase, achronic infection is established by the persistence of latent intracellular bradyzoite stages primarily encysted in the central nervous system and in striated muscles. This latency is well recognized as a strategy for efficient transmission and relies on several factors including host responses, parasite replication and genotypes.
T. gondii strains fall at least in six major clonal lineages which differ by less than 1% at the DNA level [1–4]. However, each strain harbors its own strategy to modulate the host immune signaling pathways resulting in strong phenotypic differences in the laboratory mouse models (for a review see [5,6]). Infections by type I parasites typified by RH strain is fatal (LD100 = 1) in most laboratory mouse strains whereas infections with type II such as Prugniaud (Pru) and ME49 (LD50 ~102) or type III such as NED (LD50~103) strains generally results in lifelong latent infections characterized by dormant tissue cyst formation [7].
The control of host cell protection against Toxoplasma requires an active type 1 cytokine response typified by the production of pro-inflammatory cytokines. However, as a pervasive pathogen, Toxoplasma has developed strategies to compromise host mechanisms, avoiding parasite killing. Opposite effects in the immune modulation adopted by the parasites are observed between different strains of Toxoplasma [5]. For instance, virulent type I strains down regulate the inflammatory response and control the mechanisms of parasite killing, while avirulent type II and III strains have developed strategies to limit infection and premature death of the host to favor transmission [5]. To do so, a fine balance between the parasite replication and the host immune response is required to preserve the host from the immunopathology effect of pro-inflammatory cytokines induced by type II effectors and to sustain moderate type II strains persistence in animals.
The underlying genetic loci responsible for the most striking phenotypic differences between the three main clonal lineages of Toxoplasma have been mapped using classical genetics of pairwise genetic crosses between strains [8–12]. Most of the effectors identified so far originate from the secretory organelles, i.e rhoptries and dense granules. In type I strains, proteins of both organelles co-opt to dampen host responses and favor parasite replication and high virulence. In type II strains, those effectors are mostly inactive due to polymorphism and/or differential expression in a way that control parasite dissemination and persistence in the host. Furthermore, type II strains also express dense granule proteins which participate in the modulation of host cell functions [10,13,14].
Here, we describe a rhoptry protein, TgDegP that plays a pivotal contribution to the virulence of T. gondii. TgDegP is a serine protease belonging to the HtrA (High Temperature Requirement A) or Deg-like family of serine proteases [15]. While DegP is dispensable for the lytic cycle of both RH type I and Pru type II strains, a marked decrease of acute virulence in Swiss mice is observed in DegP knock-out type II strain (KO-DegPII). In contrast, KO-DegPII parasites kill immunodeficient mice as efficiently as the wild-type strain. In conclusion, genetic ablation of TgDegP suggests a role for this serine protease in immune evasion.
Materials and methods
Ethics statement
The recommendations of the European Union guidelines for the handling of laboratory animals was followed for this study. Production of DegP and DegP* antibodies via rat and mouse immunizations was conducted at the CRBM animal facility (Montpellier) and approved by the Committee on the Ethics of Animal Experiments (Languedoc-Roussillon, Montpellier) (Permit Number: D34-172-4, delivered on 20/09/2009).
All mice protocols were approved by the Institutional Animal Care and Utilization Committee (IACUC) of the American University of Beirut (IACUC Permit Number IACUC#14-3-295). Mice were housed in dedicate pathogen free facilities and humane endpoints were used as requested by the AUB IACUC according to AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) guidelines and guide of animal care use book (Guide, NRC 2011). Mice were monitored on a daily basis. Eye pricks were done following deep anesthesia with isoflurane by inhalation. One eye per animal was pricked allowing to collect 50uL of blood. Mice were sacrificed if any of the following abnormal ethical features are noticed as described previously [16]. Animals were deeply anesthetized before cervical dislocation and no unexpected death was observed.
Mammalian cells and parasite cultures
Tachyzoites from RH, RHΔKu80 strain or PruΔKu80 (deleted for the ku80 gene [17,18]) were used throughout this study. Parasites were grown in human foreskin fibroblasts (HFFs) (American Type Culture Collection-CRL 1634) in Dulbecco’s Modified Eagle’s Medium (DMEM) (GIBCO, Invitrogen) supplemented with 5% of fetal calf serum (FCS), 1% penicillin-streptomycin and 1% glutamine. BHK-21 cells (American Type Culture Collection-CCL 10) were grown in BHK-21 medium (Gibco-BRL) supplemented with 5% FCS, 2 mM tryptose, 100 U/ml penicillin and 100 μg/ml streptomycin.
Transient transfection of mammalian cells and generation of transgenic parasites
For BHK-21, 3×105 BHK-21 cells were grown on coverslips for 24 h in 6-well plate prior transfection using Lipofectamine reagent (Gibco-BRL) as instructed by the manufacturer. Cells were grown for an additional 24 h before western blot analysis (see below). Parasite transfection and selection was performed as previously described [19]. Parasites were selected in presence of mycophenolic acid (20μg/mL) and xanthine (50μg/mL) for HXGPRT selection or pyrimethamine (1μM) for DHFR-TS selection. The selection of revertant parasites was made in the presence of 300μg/ml of 6-thioxanthine and their sensitivity to mycophenolic acid (20μg/mL) and xanthine (50μg/mL) was assessed. The isolation of clonal transgenic populations was performed using limiting dilution in 96-well plates.
Antibodies
The antibodies used and their dilution for western blot (WB) and immunofluorescence (IFA) were as follows:
mouse monoclonal antibodies (MAb) T4 1E5 anti-SAG1, 1:2000 (IFA), 1:2000 (WB) [20]
rabbit anti-ROP1, 1:3000 (IFA) [21]
rabbit anti-ROP2 1:500 (IFA) [21]
mouse anti-Ty MAb, 1:200 (WB) [22]
rat serum anti-DegP, 1:1000 (IFA), 1:1000 (WB) (this study)
mouse serum anti-DegP* 1:200 (WB) (this study)
rabbit anti-RON4, 1:500 (IFA) [23]
For IFA studies, the secondary antibodies used were AlexaFluor 488 (Sigma), AlexaFluor 594 (Sigma), and AlexaFluor 546 (Invitrogen), as recommended by the manufacturer. For immunoblots, the secondary rat, mouse or rabbit antibodies used were coupled to alkaline phosphatase (Promega).
Molecular cloning
To verify the predicted cDNA of DegP in RHΔKu80 (type I) and in PruΔKu80 (type II), total RNA was extracted from RHΔKu80 or PruΔKu80 tachyzoites using the NucleoSpin RNAII extraction kit (MACHEREY-NAGEL GmbH & Co.). Following RT-PCR using an oligo-dT and the Superscript first strand synthesis kit (Invitrogen), the specific full-length cDNA of DegP gene was amplified using primers ML220 and ML221 using the Phusion HF DNA polymerase (New England Biolabs). PCR amplification at the predicted size (2868bp) was cloned in the pCR-Blunt II-TOPO vector (Invitrogen) to generate TOPO-DegPI (type I) and TOPO-DegPII (type II). The ML220 and ML221 primers were designed to add the restriction sites BamHI and MfeI at the 5’ end and NsiI and NotI at the 3’ end upon PCR amplification.
The pGEX-DegP-GST plasmid was designed to produce a recombinant GST-tagged DegP protein. The TOPO-DegPI plasmid was digested by BamHI and NotI and the full-length cDNA sequence of TgDegP gene (2868bp) was cloned into the BamHI and NotI restriction sites of pGEX-4T-3 vector (GE healthcare).
The pET24a-DegP-His plasmidwas designed to produce a recombinant His-tagged DegP protein. An internal sequence of DegP cDNA was amplified using primers ML247 and ML248, digested by Nde1 and Xho1 and cloned into the Nde1 and Xho1 restriction sites of pET24a vector (Novagen).
The pdegP-DegP-Ty plasmid was designed to express under the control of degp promotor a second copy of DegPI fused with a Ty tag at the C-terminal. It was based on plasmid ptub-DegP-Ty, in which the tubulin promotor was replaced by the endogenous promotor of degP. The ptub-DegP-Ty plasmid was first designed to overexpress a second copy of DegP with a Ty tag at the C-terminal. To do so, the TOPO-DegPI plasmid was digested by MfeI and NsiI and the full-length cDNA sequence of TgDegP gene (2868bp) was cloned into the EcoRI and NsiI restriction sites of the pTUB8mycGFPPftailTY vector (a gift from Dominique Soldati-Favre), where it replaces the mycGFPPftail. pDegP-DegP-Ty was constructed by PCR amplification of ptub-DegP-Ty with primers ML234 and ML235 to create the deletion of the tubulin promotor as well as of the 108 first nucleotides encoding for the N-terminal 37 amino acids of DegP. The ML234 and ML235 primers were designed to add the restriction sites KpnI and AvrII at the 5’and 3’ ends respectively upon PCR amplification. The template plasmids were eliminated by digestion with DpnI, and a fragment corresponding to 1 kb of the 5’ non-coding region of DegP plus the 108 first nucleotides of the coding sequence of DegP was PCR amplified using primers ML232 and ML233, and then ligated with the PCR product obtained using ML234 and ML235 primers. This added an AvrII site six residues after the signal sequence of DegP.
The pDegP-V5 plasmid was designed to transfect mammalian cells with the DegP cDNA without the sequence signal. It was constructed by PCR amplification of the DegP cDNA from TOPO-DegPI using primers ML277 and ML278, and cloning into KpnI and XbaI sites of pTRACER-A (Invitrogen).
The KO-DegP-HXGPRT plasmid was used to disrupt the DegP endogenous locus in both RHΔKu80 and PruΔKu80 strains. The primers ML981 and ML2048 were used to PCR amplify a 627bp fragment corresponding to the 5’ of the RHΔKu80 DegP gene containing the ATG, the first exon and part of the first intronic region (88 nucleotides). The PCR product was sub-cloned into the pCR-Blunt II-TOPO vector (Invitrogen), sequenced and then cloned KpnI/NsiI in the 3TY-HXGPRT vector [24]. The plasmid was linearized by PstI prior to transfection. Correct integration of the disrupting plasmid was controlled by PCR using primers ML871/ML673. ML673 hybridizes to the TY tag present in the vector while ML871 is located 48bp upstream the DegP sequence cloned in KO-DegP-HXGPRT.
To generate KO-DegPII revertant parasites, the chiRNA targeting the sequence GCTTCAGCATTGAAGACGTC from the HXGPRT cassette was cloned using the primers ML2248 and ML2249 in the pU6-universal plasmid [25].
All the plasmids were sequenced prior to transfection.
Fluorescence staining of cells
For IFAs of transfected BHK-21 cells or intracellular parasites grown in host cells (HFF), cells were fixed with 4% formaldehyde in PBS and permeabilized with 0.1% Triton X-100 in PBS/3% BSA. Coverslips were blocked in PBS 10% FCS and proceeded further for IFA as previously described [26]. Samples were observed with a Zeiss Axioimager epifluorescence microscope equipped with an apotome and a Zeiss Axiocam MRmCCD camera driven by the Axiovision software (Zeiss), at the Montpellier RIO imaging facility. Images were collected and processed using Zeiss Zen software.
Production of a recombinant DegP protein fused to GST and of a specific serum
Escherichia coli C41RIG competent cells were transformed with pGEX-DegP-GST or pGEX-DegP*-GST. The expression of DegP-GST or DegP*-His recombinant proteins were induced by adding 0.2mM IPTG at 37°C for 4h. Cells from 500mL of culture were harvested at 4000g for 20 min at 4°C and the pellet was resuspended in 25mL of PBS. The bacteria were lysed by French press (2 times with 2 tonnes / cm2) and the total lysate centrifuged at 9000 r.p.m during 20 minutes at 4°C. The TgDegP-GST recombinant protein was abundantly recovered in the insoluble fraction. The DegP-GST protein was thus separated by SDS-PAGE and electro-eluted from the gel and used to raise polyclonal antibodies in rat as previously described [23].
Immunoblots
Freshly released tachyzoites were harvested, washed in PBS and resuspended directly in SDS sample buffer. All gels were run under non-reducing conditions except otherwise stated in the figures legends. 5.106 parasites were loaded in each lane. Transfer and immunodetection were done as previously described [19]. DegP*-His recombinant protein was purified using a nickel-agarose column in native or denaturing condition and according to the QIAGEN protein purification handbook.
Plaque and intracellular growth assays
Plaque and intracellular growth assays were performed as previously described [19]. To assess intracellular growth, only the vacuoles containing 2 parasites or more were scored.
Two-color invasion assays
Two-color invasion assays were performed as previously described [27]. Briefly, 5.106 freshly released tachyzoites from the control strain (RHΔKu80 or PruΔKu80) or from the mutant strains (KO-DegPI or KO-DegPII) were added to HFF grown on glass coverslips, synchronized on ice during 20 min and subsequently allowed to invade for 5 min in invasion buffer. Invasion was stopped by fixation in 4% PAF in HBSS and parasites were further processed for IFA. A first immunodetection with the mouse mAb T4 1E5 anti-SAG1 in 2% FCS/HBSS was performed to detect extracellular parasites. After permeabilization with 0.1% saponin for 15 min, a second IFA was performed using rabbit anti-ROP1 antibodies to label the parasitophorous vacuole of intracellular parasites. Extracellular and intracellular parasites were counted on 40–50 fields per coverslip from three coverslips.
In vivo experiments
To assess the KODegPI virulence in vivo, 100 tachyzoites from the KO-DegPI or RHΔKu80 were intraperitoneally injected (i.p) into 20 females 8 weeks-old Swiss mice. To assess the KO-DegPII virulence in vivo, 12–16 week-old Swiss mice (Charles River, France) were infected by i.p. injection of 105 or 1 million tachyzoites freshly harvested from cell culture. To test the virulence of PruΔKu80 or KO-DegPII in NOD/Shi-scid/IL-2Rγnull (NOG) Severe Combined Immunodeficiency), 12 weeks mice were infected with 105 parasites freshly harvested from cell culture. Invasiveness of the parasites was evaluated by simultaneous plaque assay of a similar dose of parasites on HFFs. Mouse survival was monitored daily until their death, end-point of all experiments. The immune response of surviving animals (except for NOG) was tested following eye pricks performed on day 7 post infection. Sera were tested by Western blotting tachyzoite lysates. Data were represented as Kaplan and Meier plots using GraphPad Prism version 4.00 for Windows (GraphPad Software).
Bioinformatic domain prediction
SignalP software (http://www.cbs.dtu.dk/services/SignalP/) was used to predict signal peptide. The catalytic domain was predicted by using Pfam software (http://pfam.xfam.org/) and the PDZ domains were mapped using supfam software (http://supfam.org/SUPERFAMILY/). Alignment was performed using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Statistical analysis
All results are presented as mean values with standard deviations shown as error bars. Two-tailed unpaired Student’s t tests (for plaque assays, invasion and replication assays) were used appropriately to determine statistical significance using GraphPad software (version 7) and a P value 0.05 was considered significant. For in vivo experiments, levels of significance were determined with the Logrank test using GraphPad.
Oligonucleotides used in this study
- ML220
5’-GGATCCCAATTGATGTTGCTCCTTCTGTCACT-3’
- ML221
5’-GCGGCCGCATGCATTGAGGAAGTAAGAGCGGTCTCTCA-3’
- ML232
5’-GGGGTACCCCGAAGACCACAGCCCCAGA-3’
- ML233
5’-ATGCATCCTAGGTAACAGAGACGAAGCCTCCG-3’
- ML234
5’-GGGGTACCCAATTCGCCCTATAGTGAAGTC-3’
- ML235
5’-ATGCATCCTAGGACTCCTGAACCTTCTGAGACC-3’
- ML247
5’-CATATGCAGAGTGTGTGCAGGCTCAAGA-3’
- ML248
5’-CTCGAGCAACAGGAGTTCTTCTTCCGA-3’
- ML277
5’-GGGGTACCATGGAGCAGAAGCTCATCTCCGAGGAGGAC-3’
- ML278
5’- GCGGTACCGAGGAAGTAAGAGCGGTCTC-3’
- ML673
5’-ATCGAGCGGGTCCTGGTTCGTGTGGACCTC-3’
- ML871
5’-TCTAGAATGGCGGCGTTCCACCGG-3’
- ML981
5’-GGTACCATGTTGCTCCTTCTGTCACTG-3’
- ML1022
5’-CTCGGGTTGTTTCACCAACGACG-3’
- ML2048
5’-ATGCATGTCTCTACTTTACTAACTTCAC-3’
- ML2248
5’-AAGTTGCTTCAGCATTGAAGACGTCG-3’
- ML2249
5’- AAAACGACGTCTTCAATGCTGAAGCA-3’
Results
TgDegP is a rhoptry protein
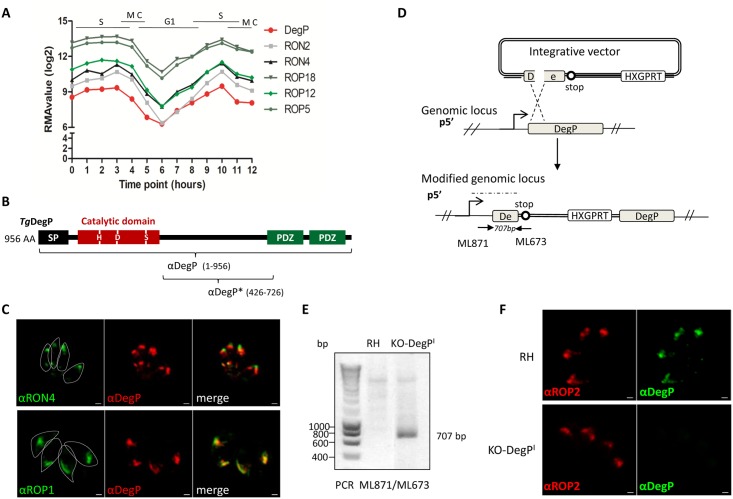
A previous proteomic analysis of a highly purified fraction of rhoptries from Toxoplasma revealed peptides corresponding to a protease (TGME49_262920), that is close to chymotrypsin [28]. However, its presence in the rhoptry compartment has not been yet validated experimentally. TGME49_262920 encodes a 956 amino acid protein, which displays a conserved protease domain in the N-terminal part of the protein, with the critical catalytic triad (aspartic acid, histidine, and serine residues) typical for serine proteases (Fig 1A). In addition, two PDZ domains are present in the C-terminal end of the protein. According to this modular architecture combining a protease domain with one or more C-terminal PDZ domains characteristic of Deg-like serine proteases, this protein was named TgDegP [15]. TgDegP harbors a predicted signal peptidase cleavage site after Ala30, indicative of trafficking along the secretory pathway. Moreover, its cell cycle time course transcription (ToxoDB.org) shows a maximum in S to M phase and then dramatically decreases in early G1 before picking again in the next S phase; a mRNA periodicity typical of rhoptry proteins [29] (Fig 1A). To validate TgDegP as a rhoptry protein, we experimentally assessed the location of TgDegP in RHΔKu80 type I strain by producing a specific serum (named anti-DegP) directed against the full length of TgDegP recombinant protein fused to GST and produced in Escherichia coli (Fig 1B). By IFA, the anti-DegP serum recognizes an antigen co-localizing with the rhoptry bulbous protein ROP1 but distinct from the rhoptry neck marker RON4 in intracellular parasites (Fig 1C). The specificity of the serum was confirmed using a TgDegP knock-out parasite line, named KO-DegPI (I, for KO in type I strain) (Fig 2A). KO-DegPI was engineered using a disruption strategy by single homologous recombination as previously described [21](Fig 1D). Following vector integration, a truncated version of DegP that does not contain the full catalytic domain but containing only the first 178 residues might be expressed. The correct integration of the vector in the DegP locus was verified by PCR (Fig 1E) and the subsequent loss of DegP protein in KO-DegPI parasites was verified by IFA (Fig 1F) and Western Blot analysis (Fig 2A).
Fig 1. TgDegP is a rhoptry bulb protein.
(A) Graph representing microarray data of transcripts encoding some known rhoptry proteins (RON2, RON4, ROP5, ROP12, ROP18) along with DegP transcript hourly following thymidine synchronization (from [29]). (B) Primary structure of TgDegP. TgDegP is a putative serine protease of 956 amino acids belonging to HtrA family. Signal peptide is shaded in black (SP), the catalytic domain is represented in red and H, D and S indicate the positions of the catalytic triad. The two PDZ domains are represented in green. The rat anti-DegP has been made against the complete DegP recombinant protein and the mouse anti-DegP* has been generated against a recombinant protein encompassing residues 426 to 726. (C) Immunofluorescence performed on intracellular parasites using the anti-DegP serum, anti-RON4 and anti-ROP1. On parasites fixed with methanol (upper panel), the rabbit polyclonal anti-RON4 antibodies revealed the neck of the rhoptry. On paraformaldehyde-fixed parasites (lower panel), the DegP protein co-localizes with the bulbous ROP1 protein (labeled with the rabbit polyclonal anti-ROP1) in the bulb of the rhoptry. Parasite boundaries are represented by dashed lines. Scale bar = 1μm. (D) Generation of a knock-out DegP parasites in type I strain RHΔKu80. Scheme depicting the strategy used to obtain a KO-DegPI cell line. The promoter of TgDegP is represented by an arrow. Integration of the plasmid by a single homologous recombination at the DegP locus results in a truncated version of DegP driven by the endogenous DegP promoter while the DegP coding sequence is now promoter less. HXGPRT: hypoxanthine guanine phosphoribosyl transferase selection marker. The solid arrows represent the primers used to verify the integration of the integrative vector and the expected size of the fragment is shown in italics. ML673 hybridized to a sequence specific of the vector, while ML871 is located in the 5’UTR of DegP outside the cloned fragment. (E) PCR verification of the correct integration of the vector at the endogenous DegP locus. The primers ML673 and ML871 are used in this PCR. The recombined locus was detectable only in transgenic parasites KO-DegPI, as shown by a specific amplification of a 707 bp fragment that is not amplified in the parental strain. (F) Immunofluorescence performed on KO-DegPI or RHΔKu80 cell lines with the rat anti-TgDegP antibodies and rabbit anti-ROP2 antibodies as control for rhoptry staining. The DegP labelling is absent in the KO-DegPI transgenic parasites. ROP2 staining remains unchanged in the mutant. Scale bar = 1 μm.
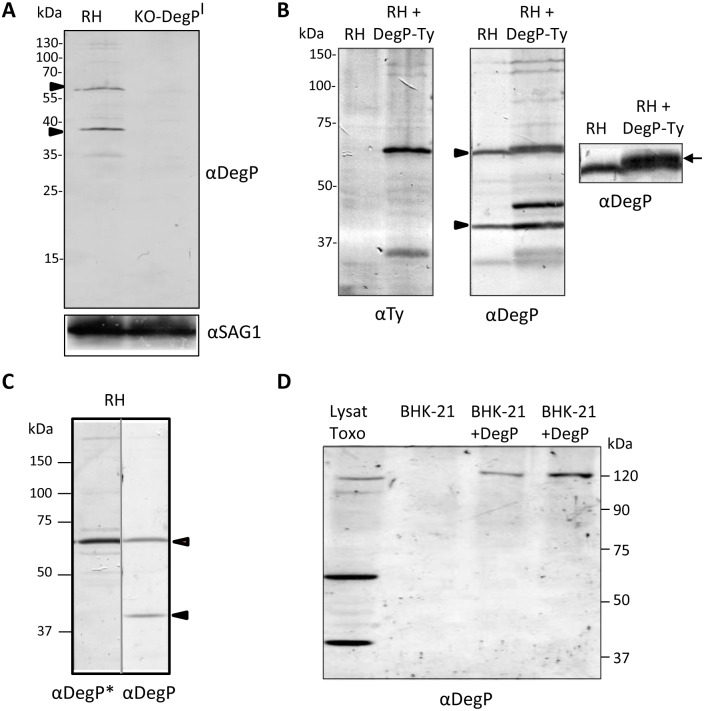
Fig 2. DegP is subjected to proteolytic maturation.
(A) Western blot analysis of RHΔKu80 lysate with anti-DegP serum reveals a complex profile. The specificity of the bands was assessed using KO-DegPI parasite lysate. Two major bands are observed around 60 kDa and 40 kDa respectively and are indicated by black arrows. Molecular weights are indicated. Anti-SAG1 antibody was used as loading control. (B) Western blots analysis of RH strain expressing an ecto-copy of DegP fused to a Ty tag at the C-terminal end. Right panel: anti-DegP antibodies. Left panel: anti-Ty antibodies. An additional band around 60 kDa with a slight shift is visible by staining the RH strain expressing DegP-Ty with anti-DegP antibodies (see arrow on enlarged panel on the right). (C) Western blot profiles of DegP under reduced conditions using anti-DegP* (426–726) serum. Anti-DegP* reveals only the 60kDa band. (D) Exogenous expression of TgDegP in mammalian cells. Western blot analysis of BHK-21 cells transfected with the cDNA of DegP under the control of promotor EF-1α. Lane 1 correspond to T. gondii lysate; lane 2 was control BHK-21 cells transfected with empty plasmid pTRACER-A, lane 3 was lysate of BHK-21 cells transfected with 1μg of plasmid pDegP-V5 and lane 4 was lysate of BHK-21 cells transfected with 2 μg of plasmid pDegP-V5. The profile of TgDegP expressed in mammalian cells is restricted to a unique form corresponding to the full-length protein.
TgDegP undergoes proteolytic maturation
TgDegP has a predicted molecular mass of 104 kDa. Immuno-detection with the anti-DegP serum on a T. gondii tachyzoite lysate of the RHΔKu80 strain under non-reduced (Fig 2A) and reduced conditions (Fig 2C) revealed two major products migrating around 60 kDa and 40 kDa; additional weaker bands with inconsistent intensities depending on immunoblots were also obtained. Western-blot on KO-DegPI parasite lysate with anti-DegP antibodies showed the concomitant extinction of the 60 kDa and 40 kDa bands (Fig 2A), confirming the specificity of these products. Therefore, the observed profile suggests that TgDegP undergoes post-translational maturation, a characteristic shared by most of T. gondii rhoptry proteins described so far [30]. We then expressed an ectocopy of DegP fused in C-terminal with a sequence encoding a Ty epitope. A major band at 60 kDa and a faint band around 120 kDa were detected with anti-Ty antibody (Fig 2B), indicating that the 60 kDa fragment corresponds to the C-terminal part of DegP whereas the 120 kDa corresponds likely to the full length protein. Interestingly, in cells overexpressing DegP-Ty, the intensity of the additional bands increases compare to the parental strain (like the one observed around 45kDa) (Fig 2B) that might be attributed to a decrease in stability of DegP following tag addition. We further produced a serum against a recombinant protein encompassing residues 426 to 726 (anti-DegP*) (Fig 1A), which does not include the catalytic domain of DegP (corresponding to residues 147 to 379). The anti-DegP* labeled the 60 kDa band but failed to detect the 40 kDa (Fig 2C). This result is consistent with the results obtained with the Ty-tagged version of DegP and confirms that the 60 kDa corresponds to the C-terminal part of the protein whereas the 40 kDa band likely corresponds to its N-terminal region, encompassing the catalytic domain.
Interestingly, when the full length cDNA of DegP is expressed in mammalian cells, a single band migrating above 120 kDa is observed (Fig 2D). The absence of DegP processing in mammalian cells indicates that the post-translational maturation depends on its expression in Toxoplasma.
KO–DegP parasites in type I RH strain exhibit normal growth and virulence in mice
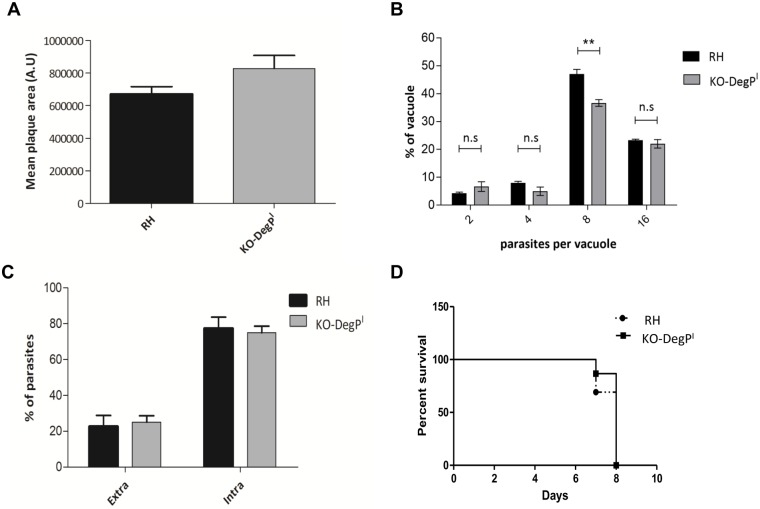
Isolation of stable parasite clone that does not express functional DegP (Fig 1) demonstrates that the protein is not strictly essential for the parasite in vitro. The dispensability of DegP was further confirmed by plaque assay in which KO-DegPI parasites displayed no significantly smaller plaques than the parental RHΔKu80 (Fig 3A). In order to highlight potential more subtle defects, we then assessed the ability of the KO-DegPI parasites to invade and replicate in HFF cells. Results presented in Fig 3B and 3C show no significant difference in the replication rate and invasion between the parental and the mutant cell lines. Considering the role of some rhoptry bulb proteins in the modulation of host immune responses, we then assessed the effect of DegP depletion on in vivo virulence in a murine model. After i.p injection of 100 tachyzoites into BALB/c mice, we observed a similar survival pattern between mice infected with the KO-DegPI parasites or those infected with the parental RHΔKu80 strain.
Fig 3. TgDegP is dispensable for in vitro and in vivo survival of the type I strain.
(A) Size of the plaques resulting from the lysis of host cells infected with RHΔKu80 or KO-DegPI parasites and grown for seven days. A.U.: arbitrary units. Values represent means ± SEM, n = 3, from a representative experiment out of 3 independent assays (p = 0.095; unpaired t-test). (B) Intracellular growth rate was assessed by counting the numbers of parasites per vacuole after 20 h infection of HFF cells with RHΔKu80 or KO-DegPI parasites. Data Values represent means ± SEM, n = 3, from a representative experiment out of 3 independent assays (0.001<**<0.005; unpaired t-test). (C) Host cell invasion efficiencies of RHΔKu80 or KO-DegPI strains determined by a two-color staining protocol that distinguishes intracellular from extracellular parasites. Data are mean values ± SEM determined by triplicate assays, performed in three separate experiments (p = 0.75; unpaired t-test). (D) Mouse survival after i.p injection of 100 tachyzoites of RHΔKu80 or KO-DegPI parasites was monitored daily for 10 days. N = 13 mice for RHΔKu80 and N = 15 mice for KO-DegPI strains. Representative data out of 3 experiments. KO-DegPI strain is not statistically less virulent than RHΔKu80 (p = 0.27, by Logrank test).
Overall, our data show that the loss of TgDegP does not impede the intracellular development of the type I RH strain in HFF cells or its virulence in a mouse model.
Deletion of DegP in type II Prugniaud strain dramatically affects the parasite virulence in mice
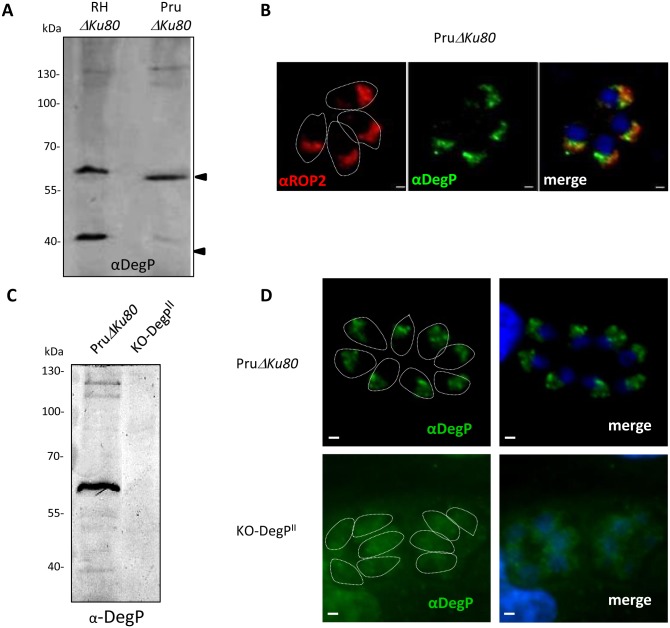
As rhoptry proteins are pivotal players in the strategies developed by type I and II strains to establish an infection, we further investigate the role of DegP in a type II strain (Pru). DegP is conserved in type II strain. The gene shows low level of polymorphisms between strains with a strong conservation in the sequences encoding the predicted functional domains (protease and PDZ) while few SNPs are found both in the 5’end of the gene and in the fragment between the sequences coding for protease and PDZ domains. We show that DegPII is well expressed in type II PruΔKu80 strain (Fig 4A) and harbors the same localization as in type I strain i.e in the bulb of the rhoptries (Fig 4B). Western blot analysis showed that TgDegPII undergoes a similar maturation profile than in the type I RH strain with the notable difference that the intensity of signal for the 40 kDa product was consistently lower in PruΔKu80 compareto RHΔKu80 strain (Fig 4A).
Fig 4. Expression of TgDegP in the type II PruΔKu80 strain.
(A) Western blot detection of TgDegP in the Prugnaud strain (Pru), a representative member of the Type II Toxoplasma gondii genotype. Blot was revealed using the polyclonal anti-DegP antibody. Molecular weights are indicated. Note that the intensity of the 40kDa band is faint the PruΔKu80 strain. (B) Immunofluorescence performed on PruΔKu80 intracellular parasites using the anti-DegP and anti-ROP2 antibodies. As observed in the type I strain, TgDegPII colocalizes with ROP2 in the bulb of the rhoptry. Parasite boundaries are represented by dashed lines. Scale bar = 1μm. Western blot (C) and immunofluorescence (D) performed on PruΔKu80 and KO-DegPII cell lines with the rat anti-TgDegP. The DegP labelling is absent in the KO-DegPII transgenic parasites. Parasite boundaries are represented by dashed lines. Scale bar = 1 μm.
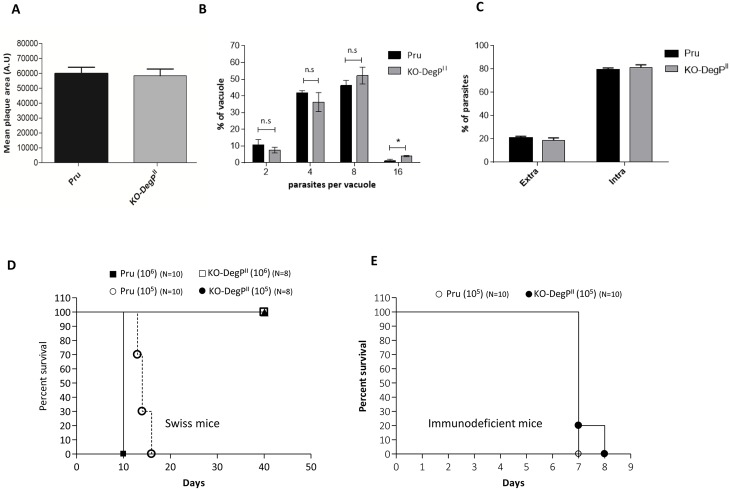
To further expand our comparative approach in both types of strains, we generated a KO-DegP in the PruΔKu80 strain (KO-DegPII) (Fig 4C and 4D; S1A and S1B Fig) using the same genetic disruption approach as in type I strain. The disruption of the DegP gene in the type II strain does not affect the size of the lysis plaques in HFF cells, or the replication rate and the ability of the parasites to invade HFF cells (Fig 5A–5C). However, a clear impact of DegPII removal was observed on the virulence in vivo (Fig 5D). All the Swiss mice survived i.p infection with 105 tachyzoites of the KO-DegPII strain for at least 40 days, while mice infected with the wild-type PruΔKu80 strain died within around 15 days. Inoculation of mice with a higher dose of parasites (106) confirmed the defect in virulence of KODegPII parasites (Fig 5D).
Fig 5. Deletion of DegP in type II strain affects the in vivo virulence of the parasite.
(A) Confluent monolayers of human fibroblasts were infected with PruΔKu80 or KO-DegPII parasites and grown for seven days. Quantification of the size of the lysis plaques is shown in the graph. The size of the lysis plaques is similar between control (PruΔKu80) and KO-DegPII strains. A.U.: arbitrary units. Values represent means ± SEM, n = 3, from a representative experiment out of two independent assays (p = 0.82; unpaired t-test). (B) Intracellular growth rate was assessed by counting the numbers of parasites per vacuole after 20 hours infection of HFF cells with PruΔKu80 or KO-DegPII parasites. Data Values represent means ± SEM, n = 3, from a representative experiment out of two independent assays (0.05<*<0.1; unpaired t-test). (C) Host cell invasion efficiencies of RHΔKu80 or KO-DegPII strains determined by a two-color staining protocol that distinguishes intracellular from extracellular parasites. Data are mean values ± SEM determined by triplicate assays, performed in two separate experiments (p = 0.48; unpaired t-test). (D) Mouse survival was monitored daily for 40 days after i.p injection of 105 or 106 parasites of PruΔKu80 or KO-DegPII parasites. N = 10 mice per group. Representative data out of 2 experiments. The immune response of surviving animals was tested by Western blotting against PruΔKu80 tachyzoite lysates. In Swiss mice, KO-DegPII strain is statistically less virulent than RHΔKu80 (p<0.001, by Logrank test for105 and 106 i.p injections). (E) Mouse survival in immunodeficient NOG mice after i.p. injection of 105 parasites PruΔKu80 or KO-DegPII parasites. N = 10 mice per group. Representative data out of 2 experiments. In NOG mice, the virulence of KO-DegPII and RHΔKu80 was not statistically different (p = 0.146, by Logrank test).
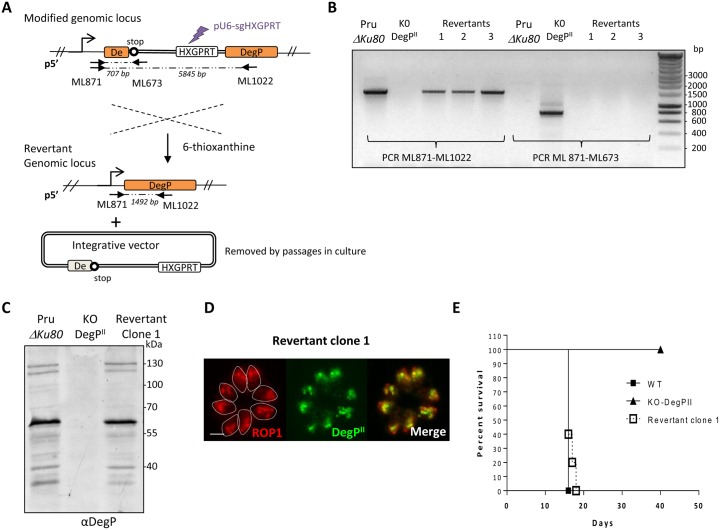
To ascertain that the observed in vivo phenotype is solely due to the disruption of the DegP locus, we tried to complement the KO-DegPII strain with an additional copy of the coding sequence. As all attempts to genetically complement the mutant, using different strategies (random integration into the genome, targeting the UPRT locus) and different selection markers failed, we opted for an alternative strategy. As previously mentioned, disruption of the DegP locus was obtained by single recombination and insertion of a linear plasmid containing the HXGPRT gene (Fig 1C). Thus, another strategy was adopted consisting in the generation of revertant parasites that have lost the integrative plasmid (i.e reversion) and restored the DegP locus. Expression of HXGPRT in parasites confers resistance to mycophenolic acid (MPA) but becomes lethal for the parasite in presence of 6-thioxanthine (Fig 6A), allowing to select revertant in presence of 6-thioxanthine. To increase the efficiency of removing the plasmid and HXGPRT cassette, parasites were transfected with a pU6-Universal plasmid [25] containing the Cas9-YFP and a 20 bp protospacer sequence targeting the coding sequence of HXGPRT (Fig 6A). Revertant clones were selected in presence of 300 μg/ml of 6-thioxanthine and their sensitivity to MPA-xanthine was confirmed. The restoration of the DegP locus was verified by PCR (Fig 6B) and the expression of DegP was confirmed by immunofluorescence (Fig 6D) and western blot (Fig 6C) using anti-DegP antibodies. In mouse model, we then successfully showed that the in vivo virulence of revertant was fully restored (Fig 6E).
Fig 6. The repair of the DegP locus in KO-DegPII restores virulence in mice.
(A) Scheme depicting the strategy used to select parasites that have removed by single recombination the knock-out plasmid in KO-DegPI cell line. The purple arrow indicates the site targeted to induce a double strand break in HXGPRT locus using plasmid pU6-sgHXGPRT. In absence of donor fragment containing HXGPRT homology regions for double strand break repair and culture in presence of 6-thioxanthine (selection for loss of HXGPRT gene), this strategy fosters the selection of single recombination event at the DegP homology regions (in orange), and reconstitution of wild-type DegP locus. The promoter of TgDegP is represented by an arrow. Primers ML871 and ML673 are used to check the interrupted DegP locus. Primers ML871 and ML1022 are used to control reversion of the mutation. ML673 hybridized to a sequence specific of the vector, ML871 is located in the 5’UTR of DegP (outside the cloned fragment in the integrative plasmid) and ML1022 hybridized in exon 2 of DegP (outside the cloned fragment in the integrative plasmid). The solid arrows and the expected sizes of the fragments are shown in italics. (B) PCR verification of the correct DegP locus in revertant. The recombined locus (specific amplification of a 707 bp fragment with the primers ML673 and ML871) was detectable only in transgenic parasites KO-DegPII, as shown by amplification of a 707 bp fragment with primers ML871 and ML673. A fragment of 1492bp corresponding to the wild-type genomic locus is amplified in the parental strain PruΔKu80 and in the selected revertant clone, but is absent in KO-DegPII strain. (C) Western blot and (D) Immunofluorescence performed on PruΔKu80, KO-DegPII and revertant cell lines with the rat anti-TgDegP antibodies and rabbit anti-ROP1 antibodies confirm the DegP labelling in revertant clone. Parasite boundaries are represented by dashed lines. Scale bar = 5 μm. (E) Mouse survival after i.p injection of 105 parasites of PruΔKu80, KO-DegPII and revertant parasites was monitored daily for 40 days. N = 9 mice for PruΔKu80, N = 11 mice for KO-DegPII and N = 10 for revertant strains. Representative data out of 2 experiments. The revertant parasites kill mice similar to wild-type PruΔKu80 strain.
All together these results clearly pointed on a specific role of DegP in virulence of the Prugniaud type II strain.
Infection of immuno-deficient mice suggests a role for DegPII in immune evasion
During infection, Toxoplasma is known to modulate numerous host cell processes, including lipid metabolism, protein synthesis, cell signaling, amino acid metabolism, apoptosis as well as the immune response by secreting parasite effectors [31]. To further discriminate whether DegPII might play a role in the subversion of host cell protecting immune processes, we used immunodeficient mice NOG. These mice are homozygous for the SCID mutation and were generated by targeted disruption of the interleukin (IL)-2Rγ gene. Since the γ-chain is common to the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, NOG mice lack B- and T-cell development, have impaired macrophage and NK-cell functions and have a severe reduction in interferon-γ production from dendritic cells [32]. Following infection of NOG mice with 105 parasites, we observed the same rate of mortality of mice whether they were infected with the wild-type or KO-DegPII parasites (Fig 5E). In conclusion, these results showed that, in the absence of an efficient immune response setting, KO-DegPII parasites show the same ability to cause lethal infection than WT parasites suggesting that DegPII is important for virulence in a fully immune competent environment.
Discussion
To date, two distinct type of proteases have been discovered in the rhoptry bulb: a subtilisin-like serine protease (TgSUB2) [33] and an insulinase-like protein named Toxolysin-1 (TLN1) [34] that belongs to the M16 metalloprotease family. As most of the subtilisins, TgSUB2 is autocatalytically processed [33] and has been proposed to be the maturase of rhoptry proteins [33,35], while the function of TLN1 is unknown. A third protease belonging to Deg-like serine proteases had been found in the rhoptry proteome [28], but its formal association with the organelle and its precise sub-compartment localization were missing. Here, we show that TgDegP is a rhoptry bulb protein and perform a comparative functional analysis by generating mutant harboring a truncated version of DegP (lacking the protease and PDZ domains) in both type I and type II strains. We notice any defect in lytic cycle in absence of the protease in both strains, indicating that DegP does not play any role for invasion, replication and egress in vitro as predicted by a genome-wide CRISPR screen in which DegP deletion was found neutral for in vitro growth in fibroblasts [36]. In contrast, a marked defect in virulence for KO-DegP parasites in a type II strain was observed in immune-competent mice where KO-DegPII parasites failed to establish a lethal infection in vivo. Whereas we were unable to detect the truncated version of DegP by western blot in KO-DegPI parasites (Fig 2A) suggesting that it is not stably expressed, it remains possible that a small proportion of truncated DegP participates to the observed in vivo phenotype. In the method adopted to generate the KO-DegP, the insertion of the integrative vector is susceptible to disturbs DegP neighboring genes. However, it seems unlikely to be the case as DegP proximal genes are reported to strongly impair in vitro growth of T. gondii in fibroblasts [36], a phenotype that we do not observed in both KO-DegP parasites. Interestingly, the lack of virulence of KO-DegPII parasites was not observed in SCID mice where all immunocompromised mice were killed by KO-DegPII at a similar rate as WT parasites. Rather than a defect in virulence due to nutrient limitation (as observed for uracil phosphoribosyltransferase mutant [37]), the ability of KO-DegPII parasites to establish a lethal infection in a permissive immune environment (SCID mice) suggests a role for DegPII in the complex mechanism of resistance to the immune response. How DegP exerts its functions remains to be determined and several scenarios might be envisaged. Similar to most of the rhoptry proteins studied so far, DegP might be secreted into the host cell during invasion and target a host cell pathway. We were unable to detect DegP post-secretion in the host cell either by immunofluorescence or by using a reporter system adapted for the detection of Toxoplasma secreted proteins [38], precluding to know the fate of DegP following invasion. The possible secretion of DegP into the host cells to act as an effector per se requires further investigations. We cannot exclude that DegP might also play an indirect role on rhoptry effectors. It could be important for processing, functions and trafficking of other rhoptry proteins or effectors. Since DegP proteins are reported to function both as molecular proteases and chaperones (see Discussion below), a third hypothesis could be that TgDegP functions as a chaperone specifically in the stress conditions encountered in vivo or in specific cell type. Supporting this hypothesis, P. falciparum homologous DegP, PfDegP, has been recently proposed to confer protection against thermal/oxidative stress [39]. PfDegP exists as a complex with parasite-encoded heat shock protein 70, iron superoxide dismutase and enolase and its expression is significantly induced in parasite culture upon heat shock/oxidative stress. More broadly, in intracellular bacteria such as S. typhimurium, L. monocytogenes, and Y. enterocolitica, htrA mutants show increase sensitivity to oxidative agents leading to reduced survival in macrophages. Hence, attenuated virulence of these pathogens will be attributed to a decreased ability to persist in the hostile environment of macrophages, which like for Toxoplasma is fundamental to trigger successful infection [40,41].
Deg proteases are a highly conserved family of proteins from bacteria to human composed of a N-terminal protease domain and one or two C-terminal PDZ domains. They can function as both a molecular protease and a chaperone, without any requirement for ATP hydrolysis [42]. The switch from molecular chaperone to protease activity is regulated by temperature and also by substrate recognition [43,44]. While the protease domain is required for both protease and chaperone activities, the role of the PDZ domains is far less understood. To date, they are proposed to play a role in substrate recognition [45]. TgDegP follows the same organization as Deg proteases with two PDZ domains. The PDZ and the protease domains are identical between type I and type II strains. However, we do observe changes at the protein level post-processing with the fragment corresponding to the catalytic domain (40 kDa fragment) underrepresented in the Prugniaud type II strain compared to the type I strain RHΔKu80. Unfortunately, despite multiple attempts we were unable to complement the strain either with a WT or a protease dead version of DegP precluding any conclusion regarding the importance of its protease function. As DegP/HtrA proteases harbors an allosteric activation mechanism in other organisms, it is possible that the stoichiometry between the PDZ and catalytic domains is an important parameter to regulate the activity of the protein with the possibility that the function of TgDegP in type II strain might reside in the ‘noncatalytic’ PDZ domains.
Genome mining and multiple sequence alignment identified homologs of TgDegP in most Apicomplexa, including Eimeria, Neospora, Hammondia, Babesia, Theileria and Plasmodium spp. with the notable exception of rodent malaria parasites [15]. Multiple copies of trypsin genes have been found in Toxoplasma and Plasmodium. For instance, Toxoplasma contains four DegP-like genes (TGME49_262920 (this study), TGME49_290840, TGME49_277850, TGME49_318290) and P. falciparum has two (Pf_MAL8P1.12 (named PfDegP [39]) and Pf_MAL8P1.98). Phylogenic analysis supports two different evolutionary origins for these trypsins. A group of apicomplexan trypsins clusters with plants, metazoan and cyanobacterial trypsins and contains TgDegP (TGME49_262920), TGME49_290840 and PfDegP (Pf_MAL8P1.12), the two copies in Toxoplasma arising probably by gene duplication [15]. Sequence alignment of Apicomplexa trypsins reveals the presence of a large insertion in the central region of TgDegP, between the protease and PDZ domains (S2A Fig). This insertion is only present in Coccidian parasites, and moreover is divergent between species (S2B Fig). For instance, the insertion domain of Eimeria and Neospora are very divergent whereas HhDegP and TgDegP share the most conserved insertion domain. We do not know the role of this domain and if it may impact the immune-regulatory function of TgDegP, but it is temptative to speculate that this insertion reflects a species’ functional adaptation for the host infected. So far, studies focusing on strain-dependent virulence factors have highlighted that the difference of virulence observed between strains is mainly due to single nucleotide polymorphism (SNP) or changes in the level of expression. By comparing the cDNA of TgDegP between type I (RH) and type II (Pru) we found that DegP harbors a high degree of conservation between strains with the protease and PDZ domains that remain strictly identical while SNP can be only found in the sequence encoding for signal peptide and in the insertion region of TgDegP. However, it remains possible that SNPs present in the insertion domain contributes to the strain-dependent function of DegP. Another hypothesis is that the high degree of virulence of type I strain maskes the in vivo phenotype of KO-DegPI in mice whereas the moderate virulence of the type II strain allows the visualization of this phenotype.
PfDegP is the closest trpysin-like protease found in P. falciparum; despite a predicted signal peptide, many features show that they are very divergent. First, they are not associated with the same compartment. While, Toxoplasma DegP is a rhoptry bulb protein, immunolocalization of PfDegP in the asexual blood stages of malaria parasite does not indicated an association with rhoptry but rather that the protease is synthetized during trophozoite stages (26-30h; before rhoptry biogenesis) and appears to be transported to the cytosol and membrane surface of infected RBCs during the late developmental stages of the parasite. Second, we showed that Toxoplasma DegP is subject to proteolytic cleavage that likely takes place in the rhoptry based on precedent for other rhoptry proteins. Proteolytic maturation of DegP proteases is unconventional, and seems to not undergo in the P. falciparum orthologue [39]. Finally, the insertion domain between the trypsin and PDZ domains does not exist in PfDegP (S2A Fig). Interestingly, this extension does not exist either in the duplicated Toxoplasma DegP-like gene TGME49_290840, supporting the idea that PfDegP might harbors a more similar architecture with TGME49_290840 than with TgDegP. In addition, the cell cycle time course transcription of TGME49_290840 does not show the typical periodicity of rhoptry proteins (ToxoDB.org) [29], dismissing a potential rhoptry localization. These observations reflect that the two closest DegP-like proteins of Toxoplasma have probably different locations and then functions, highlighting the versatility of roles played by this protease family.
Supporting information
(A) Strategy and (B) PCR verification of the correct integration of the vector by single homologous recombination at the endogenous DegP locus in PruΔKu80 strain. The primers ML673 and ML871 are used in this PCR. The recombined locus was detectable only in transgenic parasites KO-DegPII, as shown by a specific amplification of a 707 bp fragment that is not amplified in the parental strain.
(TIF)
(A) Local alignments generated between TgDegP and homologs found in Apicomplexa: TgDegP (T. gondii TGME49_262920), HhDegP (Hammondia hammondi HHA_262920), NcDegP (Neospora caninum NCLIV_025000), EtDegP (Eimeria tenella ETH_00028355), PfDegP (P. falciparum PF3D7_0807700), PvDegP (P. vivax PVX_088155), PcynDegP (P. cynomolgi PCYB_011950), TpDegP (Theileria parva TP01_0318), BbDegP (Babesia bovis BBOV_IV004330). (B) Local alignments generated between TgDegP and its closest homologs found in Coccidia Eimeria tenella, Neospora caninum and Hammondia hammondi. Identical amino acids are highlighted in black and similar amino acids are shaded in grey. The catalytic domain and the two PDZ domains are highly conserved among Coccidia whereas the central region of the protein is the most divergent part.
(PDF)
Acknowledgments
We thank Ali Hakimi for critical reading the manuscript. Thanks to the Montpellier Rio Imaging platform for providing access to their microscopes. ML is INSERM researcher.
Abbreviations
- DegP
Deg-like serine protein
- HtrA
High temperature requirement A
- Pru
Prugniaud
- Tg
Toxoplasma gondii
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Laboratoire d’Excellence (LabEx) (ParaFrap ANR-11-LABX-0024), by the Fondation pour la Recherche Médicale (Equipe FRM DEQ20130326508) and The Cèdre France-Lebanon program (Project N2177) to M.L., by the Cèdre France-Lebanon program (Project N21774) and the American University of Beirut Medical Practice Plan and the National Council for Scientific Research (CNRS-L) to H.E.H and M.L. Hiba El Hajj (H.E.H.) is funded by the American University of Beirut Faculty of Medicine Medical Practice Plan and the Centre National de Recherche Scientifique Libanais (CNRS-L) and The Cèdre France-Lebanon program (Project N2177) and The Centre National de Recherche Scientifique Libanais (CNRS-L). Gaelle Lentini received a fellowship through the FRM FDT20140931002. Gamou Fall was supported by a fellowship from the Fonds Inkerman Fondation de France. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM, et al. (2011) Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol 41: 645–655. doi: 10.1016/j.ijpara.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172: 1561–1566. [DOI] [PubMed] [Google Scholar]
- 3.Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, et al. (2012) Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A 109: 5844–5849. doi: 10.1073/pnas.1203190109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, et al. (2016) Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun 7: 10147 doi: 10.1038/ncomms10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo MB, Jensen KD, Saeij JP (2011) Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol 27: 487–495. doi: 10.1016/j.pt.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter CA, Sibley LD (2012) Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol 10: 766–778. doi: 10.1038/nrmicro2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibley LD, Boothroyd JC (1992) Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359: 82–85. doi: 10.1038/359082a0 [DOI] [PubMed] [Google Scholar]
- 8.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, et al. (2007) Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445: 324–327. doi: 10.1038/nature05395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, et al. (2006) Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314: 1780–1783. doi: 10.1126/science.1133690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, et al. (2011) Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 208: 195–212. doi: 10.1084/jem.20100717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, et al. (2006) A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314: 1776–1780. doi: 10.1126/science.1133643 [DOI] [PubMed] [Google Scholar]
- 12.Shastri AJ, Marino ND, Franco M, Lodoen MB, Boothroyd JC (2014) GRA25 is a novel virulence factor of Toxoplasma gondii and influences the host immune response. Infect Immun 82: 2595–2605. doi: 10.1128/IAI.01339-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, et al. (2013) Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe 13: 489–500. doi: 10.1016/j.chom.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, et al. (2013) A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med 210: 2071–2086. doi: 10.1084/jem.20130103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arenas AF, Osorio-Mendez JF, Gutierrez AJ, Gomez-Marin JE (2010) Genome-wide survey and evolutionary analysis of trypsin proteases in apicomplexan parasites. Genomics Proteomics Bioinformatics 8: 103–112. doi: 10.1016/S1672-0229(10)60011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Hajj H, Ali J, Ghantous A, Hodroj D, Daher A, et al. (2013) Combination of arsenic and interferon-alpha inhibits expression of KSHV latent transcripts and synergistically improves survival of mice with primary effusion lymphomas. PLoS One 8: e79474 doi: 10.1371/journal.pone.0079474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox BA, Falla A, Rommereim LM, Tomita T, Gigley JP, et al. (2011) Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot Cell 10: 1193–1206. doi: 10.1128/EC.00297-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh MH, Carruthers VB (2009) Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell 8: 530–539. doi: 10.1128/EC.00358-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lentini G, Kong-Hap M, El Hajj H, Francia M, Claudet C, et al. (2015) Identification and characterization of Toxoplasma SIP, a conserved apicomplexan cytoskeleton protein involved in maintaining the shape, motility and virulence of the parasite. Cell Microbiol 17: 62–78. doi: 10.1111/cmi.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couvreur G, Sadak A, Fortier B, Dubremetz JF (1988) Surface antigens of Toxoplasma gondii. Parasitology 97 (Pt 1): 1–10. [DOI] [PubMed] [Google Scholar]
- 21.Lamarque MH, Roques M, Kong-Hap M, Tonkin ML, Rugarabamu G, et al. (2014) Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat Commun 5: 4098 doi: 10.1038/ncomms5098 [DOI] [PubMed] [Google Scholar]
- 22.Bastin P, Bagherzadeh Z, Matthews KR, Gull K (1996) A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol Biochem Parasitol 77: 235–239. [DOI] [PubMed] [Google Scholar]
- 23.Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M (2009) Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog 5: e1000309 doi: 10.1371/journal.ppat.1000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daher W, Klages N, Carlier MF, Soldati-Favre D (2012) Molecular characterization of Toxoplasma gondii formin 3, an actin nucleator dispensable for tachyzoite growth and motility. Eukaryot Cell 11: 343–352. doi: 10.1128/EC.05192-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S (2014) Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS One 9: e100450 doi: 10.1371/journal.pone.0100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Hajj H, Papoin J, Cerede O, Garcia-Reguet N, Soete M, et al. (2008) Molecular signals in the trafficking of Toxoplasma gondii protein MIC3 to the micronemes. Eukaryot Cell 7: 1019–1028. doi: 10.1128/EC.00413-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerede O, Dubremetz JF, Soete M, Deslee D, Vial H, et al. (2005) Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J Exp Med 201: 453–463. doi: 10.1084/jem.20041672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, et al. (2005) Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem 280: 34245–34258. doi: 10.1074/jbc.M504158200 [DOI] [PubMed] [Google Scholar]
- 29.Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, et al. (2010) Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One 5: e12354 doi: 10.1371/journal.pone.0012354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebrun M, Carruthers VB, Cesbron-Delauw MF (2012) Toxoplasma Secretory Proteins and Their Roles in Cell Invasion and Intracellular Survival in Toxoplasma gondii: The Model Apicomplexan-Perspectives and Methods Ed Weiss L M and Kim K: 389–453. [Google Scholar]
- 31.Blader IJ, Manger ID, Boothroyd JC (2001) Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem 276: 24223–24231. doi: 10.1074/jbc.M100951200 [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, et al. (2002) NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100: 3175–3182. doi: 10.1182/blood-2001-12-0207 [DOI] [PubMed] [Google Scholar]
- 33.Miller SA, Thathy V, Ajioka JW, Blackman MJ, Kim K (2003) TgSUB2 is a Toxoplasma gondii rhoptry organelle processing proteinase. Mol Microbiol 49: 883–894. [DOI] [PubMed] [Google Scholar]
- 34.Hajagos BE, Turetzky JM, Peng ED, Cheng SJ, Ryan CM, et al. (2012) Molecular dissection of novel trafficking and processing of the Toxoplasma gondii rhoptry metalloprotease toxolysin-1. Traffic 13: 292–304. doi: 10.1111/j.1600-0854.2011.01308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley PJ, Boothroyd JC (1999) Identification of the pro-mature processing site of Toxoplasma ROP1 by mass spectrometry. Mol Biochem Parasitol 100: 103–109. [DOI] [PubMed] [Google Scholar]
- 36.Sidik SM, Huet D, Ganesan SM, Huynh MH, Wang T, et al. (2016) A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 166: 1423–1435.e1412. doi: 10.1016/j.cell.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox BA, Bzik DJ (2002) De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415: 926–929. doi: 10.1038/415926a [DOI] [PubMed] [Google Scholar]
- 38.Lodoen MB, Gerke C, Boothroyd JC (2010) A highly sensitive FRET-based approach reveals secretion of the actin-binding protein toxofilin during Toxoplasma gondii infection. Cell Microbiol 12: 55–66. doi: 10.1111/j.1462-5822.2009.01378.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S, Jadli M, Singh A, Arora K, Malhotra P (2014) A secretory multifunctional serine protease, DegP of Plasmodium falciparum, plays an important role in thermo-oxidative stress, parasite growth and development. FEBS J 281: 1679–1699. doi: 10.1111/febs.12732 [DOI] [PubMed] [Google Scholar]
- 40.Wilson RL, Brown LL, Kirkwood-Watts D, Warren TK, Lund SA, et al. (2006) Listeria monocytogenes 10403S HtrA is necessary for resistance to cellular stress and virulence. Infect Immun 74: 765–768. doi: 10.1128/IAI.74.1.765-768.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto T, Hanawa T, Ogata S, Kamiya S (1997) The Yersinia enterocolitica GsrA stress protein, involved in intracellular survival, is induced by macrophage phagocytosis. Infect Immun 65: 2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clausen T, Southan C, Ehrmann M (2002) The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell 10: 443–455. [DOI] [PubMed] [Google Scholar]
- 43.Krojer T, Sawa J, Schafer E, Saibil HR, Ehrmann M, et al. (2008) Structural basis for the regulated protease and chaperone function of DegP. Nature 453: 885–890. doi: 10.1038/nature07004 [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, Zhang X, Chen Y, Wu Y, Zhou ZH, et al. (2008) Activation of DegP chaperone-protease via formation of large cage-like oligomers upon binding to substrate proteins. Proc Natl Acad Sci U S A 105: 11939–11944. doi: 10.1073/pnas.0805464105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chien J, Ota T, Aletti G, Shridhar R, Boccellino M, et al. (2009) Serine protease HtrA1 associates with microtubules and inhibits cell migration. Mol Cell Biol 29: 4177–4187. doi: 10.1128/MCB.00035-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Strategy and (B) PCR verification of the correct integration of the vector by single homologous recombination at the endogenous DegP locus in PruΔKu80 strain. The primers ML673 and ML871 are used in this PCR. The recombined locus was detectable only in transgenic parasites KO-DegPII, as shown by a specific amplification of a 707 bp fragment that is not amplified in the parental strain.
(TIF)
(A) Local alignments generated between TgDegP and homologs found in Apicomplexa: TgDegP (T. gondii TGME49_262920), HhDegP (Hammondia hammondi HHA_262920), NcDegP (Neospora caninum NCLIV_025000), EtDegP (Eimeria tenella ETH_00028355), PfDegP (P. falciparum PF3D7_0807700), PvDegP (P. vivax PVX_088155), PcynDegP (P. cynomolgi PCYB_011950), TpDegP (Theileria parva TP01_0318), BbDegP (Babesia bovis BBOV_IV004330). (B) Local alignments generated between TgDegP and its closest homologs found in Coccidia Eimeria tenella, Neospora caninum and Hammondia hammondi. Identical amino acids are highlighted in black and similar amino acids are shaded in grey. The catalytic domain and the two PDZ domains are highly conserved among Coccidia whereas the central region of the protein is the most divergent part.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.