Abstract
Perilipin 2 (PLIN2) is a lipid-droplet protein that is up-regulated in alcoholic steatosis and associated with hepatic accumulation of ceramides, bioactive lipids implicated in alcoholic liver disease pathogenesis. The specific role of ceramide synthetic enzymes in the regulation of PLIN2 and promotion of hepatocellular lipid accumulation is not well understood. We examined the effects of pharmacologic ceramide synthesis inhibition on hepatic PLIN2 expression, steatosis, and glucose and lipid homeostasis in mice with alcoholic steatosis and in ethanol-incubated human hepatoma VL17A cells. In cells, pharmacologic inhibition of ceramide synthase reduced lipid accumulation by reducing PLIN2 RNA stability. The subtype ceramide synthase (CerS)6 was specifically up-regulated in experimental alcoholic steatosis in vivo and in vitro and was up-regulated in zone 3 hepatocytes in human alcoholic steatosis. In vivo ceramide reduction by inhibition of de novo ceramide synthesis reduced PLIN2 and hepatic steatosis in alcohol-fed mice, but only de novo synthesis inhibition, not sphingomyelin hydrolysis, improved glucose tolerance and dyslipidemia. These findings implicate CerS6 as a novel regulator of PLIN2 and suggest that ceramide synthetic enzymes may promote the earliest stage of alcoholic liver disease, alcoholic steatosis.—Williams, B., Correnti, J., Oranu, A., Lin, A., Scott, V., Annoh, M., Beck, J., Furth, E., Mitchell, V., Senkal, C. E., Obeid, L., Carr, R. M. A novel role for ceramide synthase 6 in mouse and human alcoholic steatosis.
Keywords: PLIN2, glucose tolerance, CerS6, lipid droplet, alcohol, liver
Alcoholic liver disease (ALD) is a major public health issue and accounts for nearly 50% of deaths caused by cirrhosis (1). The earliest stage of ALD is alcoholic steatosis, characterized by the excessive accumulation of lipid droplets (LDs) in the liver. The onset of alcoholic steatosis is marked by impaired lipid metabolism, glucose intolerance, and insulin resistance (2) and the presence of steatosis is associated with risk of steatohepatitis, fibrosis, and cirrhosis (3).
Hepatic LDs comprise a core of triglyceride and cholesterol ester neutral lipids, coated by a phospholipid monolayer of predominantly perilipin proteins. The perilipin family of proteins has roles in lipid and glucose homeostasis and includes perilipins (PLINs) 1–5. PLIN2 is expressed abundantly in the liver, is a marker of steatosis in alcohol-fed rats, and promotes lipid storage by restricting access of lipases to LDs [reviewed in Carr et al. (4)]. We demonstrated that PLIN2 is a marker of hepatic steatosis in adults and children (5). Moreover, the discovery of a human PLIN2 polymorphism that causes increased lipid accumulation and reduced lipolysis in macrophages and embryonic kidney cells (6) provides evidence of the PLIN2 role as a LD gatekeeper. In addition, PLIN2 has a role in cellular glucose homeostasis. PLIN2 overexpression inhibits cellular glucose uptake in L-cell fibroblasts, and small interfering RNA (siRNA)–mediated knockdown of PLIN2 increases glucose uptake in both L-cell and 3T3-L1 cells (7). Furthermore, absence of PLIN2 not only prevents hepatic steatosis and hepatic inflammation but also improves glucose tolerance in diet-induced non–alcoholic fatty liver disease and alcoholic steatosis mouse models (8–11).
The regulation of PLIN2 is incompletely understood. PLIN2 is regulated transcriptionally by peroxisome proliferator-activated receptors α, δ, and γ (12). However, limited data exist regarding the lipid signals that regulate hepatic PLIN2. Free fatty acids increase Plin2 transcript levels in rat hepatocytes (13), but the regulation of PLIN2 by ceramides—bioactive lipids suspected to have a pathogenic role in ALD (14)—is unknown.
We and others (2, 15) have demonstrated that hepatic ceramide levels are increased in alcoholic steatosis. Additionally, we have demonstrated a positive relationship between hepatic LD accumulation and hepatic ceramide content in alcohol-fed mice (2). Alcohol-fed mice with a genetic deficiency of PLIN2 are protected from hepatic steatosis, glucose intolerance, and hepatic ceramide accumulation compared with alcohol-fed wild-type mice, despite similar energy intake and body weight (10). These observations suggest that hepatic ceramide synthesis and PLIN2 may be linked mechanistically. Indeed, there is a temporal relationship between the increase in hepatic ceramide production and PLIN2 induction in alcohol-fed mice (2). However, it is unknown whether ceramides regulate PLIN2.
Ceramides are key intermediates in sphingolipid metabolism with roles in apoptosis, differentiation, cell cycle arrest, cell senescence, and insulin resistance (16). There are 3 pathways by which ceramides are generated (de novo synthesis, sphingomyelin hydrolysis, and lysosomal salvage). Two studies have implicated the de novo ceramide synthetic pathway in the pathogenesis of ALD in experimental models (17, 18). Namely, inhibition of de novo ceramide synthesis with myriocin (Myr) increases insulin signaling (17) and improves alcohol-induced steatohepatitis in mice (18). Ceramides are de novo synthesized through the serial actions of several enzymes. Serine palmitoyl-transferase (SPT) catalyzes the rate-limiting step of serine and palmitate condensation to produce 3-ketodihydrosphingosine, and the enzymes ceramide synthase (CerS) and dihydroceramide desaturase (DES) catalyze sequentially the final 2 steps of de novo ceramide synthesis.
We sought to examine the individual effects of de novo ceramide synthetic pathway enzymes in an in vitro model of alcoholic steatosis. We further elucidated the effects of ceramide synthetic pathways on PLIN2, hepatic steatosis, and glucose homeostasis in vivo in mice with similar energy balance. We report for the first time, to our knowledge, that de novo ceramide synthetic enzymes have differential effects on alcoholic steatosis and PLIN2 regulation and describe a novel role of the enzyme CerS in the promotion of alcoholic steatosis in mice and humans.
MATERIALS AND METHODS
Animal studies
Experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. All efforts were made to minimize animal discomfort, and animals were treated with humane care. Eight- to 10-wk-old, male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were pair-fed a liquid diet containing either 15% ethanol (Etoh)-derived calories or an isocaloric liquid control (Ctrl) diet for 4 wk (12% fat, 70% carbohydrate, 18% protein; Dyets Inc., Bethlehem, PA, USA). Mice received intraperitoneal injections of Myr 0.3 mg/kg, q.o.d. (Sigma-Aldrich, St. Louis, MO, USA), imipramine hydrochloride (10 mg/kg daily, Imip; Sigma-Aldrich), or saline (Sal) daily starting on the day the mice began consuming the diet. Food intake and body weight were measured. Body composition was measured with nuclear magnetic resonance spectroscopy (EchoMRI, Houston, TX, USA). Energy expenditure and glucose tolerance testing were performed as described previously (10). Hepatic LDs were isolated from mice who were similarly pair-fed a liquid Etoh or Ctrl diet for 4 wk. For all experiments, mice were euthanized by CO2 inhalation, and blood and liver tissue were collected for biochemistry and histology. For experiments with PLIN2-null mice, we previously (10) characterized 8–10-wk-old, male mice were pair-fed the aforementioned Etoh and Ctrl liquid diets for 4 wk. At euthanization, hepatic LDs were isolated for further analysis.
Cell culture
VL17A cells were a kind gift from Dr. Dahn Clemens (Omaha, NE, USA). Cells were maintained under standard cell culture conditions at 37°C in DMEM (GE Healthcare, Little Chalfont, United Kingdom) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All in vitro data represent analysis of triplicate values, and all experiments were repeated 3 times.
Lipid analyses
Serum triglycerides (Stanbio Laboratory, St. Boerne, TX, USA) and nonesterified free fatty acids (Wako Diagnostics, Mountain View, CA, USA) were measured using enzymatic colorimetric assays. Liver triglycerides were measured by ethanolic:KOH lipid extraction, and ceramide analysis of mouse whole liver and cellular extracts were performed by the Children’s Hospital of Philadelphia Michael Palmieri Metabolic Disease Laboratory (Philadelphia, PA, USA) as described previously (10). For sphingolipid analysis of mouse LDs, LDs were submitted to Stony Brook University School of Medicine (Stony Brook, NY, USA) metabolomics core for analysis by mass spectrometry. Results are normalized per milligram protein in the LD lysate.
Gene expression
Total RNA was extracted using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA), and expression of mRNA levels of PLIN2 and CerS1–6 enzymes were measured by real-time PCR (Thermo Fisher Scientific) using TaqMan primers from Thermo Fisher Scientific. mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 18s rRNA, or 36B4.
Immunoblotting
Protein (20–40 µg) was separated, transferred, then incubated overnight (4°C) with an anti-rabbit mAb against PLIN2 (Abcam, Cambridge, MA, USA) at 1:1000 dilution; CerS6 (Abcam) at 1:500 dilution; CerS5 (Abcam) at 1:1000 dilution; CerS2 (Abcam) at 1:1000; or GAPDH 1:1000 (Cell Signaling Technology, Danvers, MA, USA). Secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied at 1:2000 or 1:5000 dilutions. Immunoblots were visualized with enhanced chemiluminescence (GE Healthcare) or SuperSignal West Femto maximum sensitivity substrate (Thermo Fisher Scientific).
Cellular ceramide synthesis inhibition
Cells were incubated for 48 h in 0 or 100 mM Etoh, with and without 100 nM Myr (Sigma-Aldrich); 0.35 mM fumonisin B1 (FB1; Cayman Chemicals, Ann Arbor, MI, USA), an inhibitor of CerS; or 0.5 µM GT-11, an inhibitor of DES; Avanti Polar Lipids, Alabaster, AL, USA). Fresh medium with inhibitors was added at 24-h intervals. To quantify cytotoxicity, cell viability was quantified by dye-exclusion assay. In addition, cells were incubated with the MTT substrate for 4 h, according to manufacturer protocol (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, G3582; Promega, Madison, WI, USA). Absorbance was measured at 490 nm.
CerS6 siRNA
VL17A cells were transfected with CerS6-silencing RNA (Qiagen, Germantown, MD, USA) and incubated in either 0 or 100 mM Etoh medium for 48 h. RNA was then isolated for gene expression analysis.
MG132 studies
Cells were incubated for 43 h in 0 or 100 mM Etoh medium, with or without 0.35 mM FB1. MG-132 proteasome inhibitor was added to a final concentration of 20 µM for the final 5 h before harvesting cells for protein analysis as described previously (19).
Actinomycin
Cells were incubated with 10 µg/ml actinomycin for a total of 8 h (20). RNA was isolated as above, and PLIN2 gene transcripts were measured, normalized to 18S rRNA. A repeated-measures model was used for analysis.
Oil-Red-O staining and Nile Red staining
Cells were washed and fixed with 10% formalin and stained with 100 nm Nile Red fluorescent stain. LDs were visualized and photographed with Nikon Eclipse Ti-U confocal microscope (Minato, Tokyo, Japan).
LD isolation
Cells were centrifuged, washed, resuspended in 100 mM K2HPO4/KH2PO4; 5 mM MgCl2 with protease inhibitors (F. Hoffmann-La Roche, Basel, Switzerland) and homogenized. Lysate was centrifuged and postnuclear supernatant (PNS) was loaded into a sucrose step gradient made in 100-mM K2HPO4/KH2PO4; 5 mM MgCl2 (top to bottom: 0.25 M sucrose, PNS, 0.86 M sucrose, 1.3 M sucrose) and ultracentrifuged for 1 h, 100,000 g at 4°C. LDs were collected from the top layer and washed 3 times in 0.25 M sucrose in 100 mM K2HPO4/KH2PO4; 5 mM MgCl2, by centrifugation at 20,000 g, 5 min each. Four times the LDS loading buffer with 10 times 2-ME was added to the LD fraction, boiled, and loaded for immunoblotting.
Immunohistochemistry
Paraffin-embedded liver tissue was used for immunohistochemistry (IHC) detection of CerS6 proteins. Tissue sections were deparaffinized with xylene and decreasing concentrations of Etoh. Mouse antigen retrieval was performed with microwaves, and IHC was performed with rabbit anti-CerS6 pAb 1:500 (Ab79509; Abcam) as published previously (21). Human institutional review board–exempt status was received to use archived human liver tissue. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. IHC staining of formalin-fixed, paraffin-embedded tissue was performed on a Bond instrument (Leica Microsystems, Mannheim, Germany) using the Bond Polymer Refine Detection System (Leica Microsystems). IHC was performed using anti-CerS6 antibodies (5H7, H00253782-M01, 1:125 dilution; Abnova, Taipei City, Taiwan). Heat-induced epitope retrieval was performed for 20 min with ER1 solution (AR9961; Leica Microsystems).
Statistics
With the exception of the actinomycin study, statistical analysis was performed with a Student’s t test or ANOVA with post hoc Newman Keuls multiple-comparison test (GraphPad Software, La Jolla, CA, USA). For the actinomycin data, statistical analysis was performed with R 3.2.2. (R Foundation for Statistical Computing, Vienna, Austria) and SAS 9.4 software (SAS Institute, Cary, NC, USA). For all analyses, P < 0.05 was considered significant.
RESULTS
VL17A human hepatoma cells as a model of alcoholic steatosis
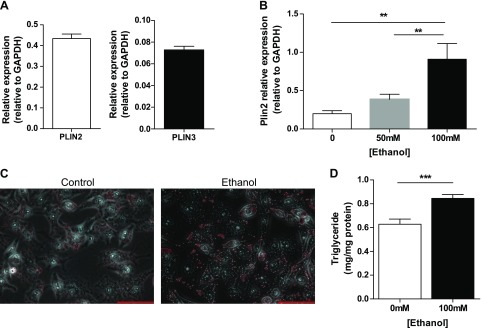
We used an in vitro model to examine the mechanistic contribution of de novo ceramide synthetic enzymes to alcohol-induced hepatic triglyceride accumulation. VL17A cells are human hepatoma HepG2 cells stably transfected with recombinant plasmids carrying the murine alcohol dehydrogenase gene and the human cytochrome P450 2E1 gene (22), but few lipid metabolic studies have been performed with those cells. First, we measured baseline perilipin gene expression in those cells. Similar to mouse experimental models (21), VL17A cells express both PLIN2 and PLIN3 at baseline (24 h incubation), but PLIN1 is undetectable (Fig. 1A). Because increased hepatic PLIN2 expression is a feature of experimental alcoholic steatosis in vivo (23), we measured PLIN2 in VL17A cells in response to 48-h incubation with Etoh. We observed that PLIN2 gene expression increased in a dose response manner with 0-, 50-, and 100-mM Etoh concentrations (Fig. 1B). Because PLIN2 expression is greatest at 100 mM, we chose the 100 mM Etoh dose for all experiments.
Figure 1.
VL17A cells, an in vitro model of alcoholic steatosis. A) VL17A cells PLIN2 and PLIN3 gene expression at baseline (24 h of plating). B) PLIN2 gene expression in response to 0, 50, or 100 mM Etoh incubation. C) Photomicrograph of Oil-Red-O staining of VL17A cells incubated in Ctrl or Etoh medium (40 times). Scale bars, 100 µm. D) Cellular triglyceride content in response to Ctrl or Etoh medium. **P < 0.01, ***P < 0.0001.
When incubated in 100 mM Etoh for 48 h, VL17A cells develop an increase in perinuclear LDs and in total cellular triglyceride content (Fig. 1C, D). These results suggest that VL17A cells are a suitable model for the examination of early effects of Etoh on hepatic lipid accumulation.
Inhibition of SPT and CerS, but not DES, reduces cellular triglyceride and PLIN2 protein
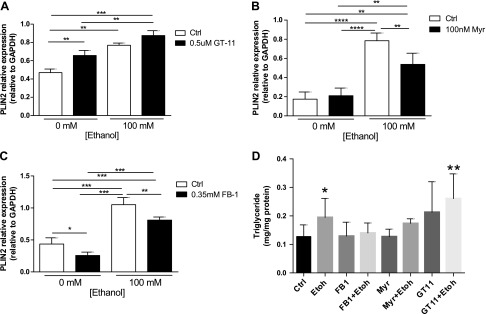
The de novo ceramide synthetic pathway results from the serial action of 4 enzymes and in vitro pharmacologic inhibitors are available for 3 of the enzymes (24). SPT is inhibited by Myr; CerS is inhibited by FB1, and DES is inhibited by GT-11 (Supplemental Fig. 1A). Based on an absence of toxicity demonstrated using the MTT assay and quantification of cell viability (Supplemental Fig. 1B), VL17A cells were cultured for 48 h with 0 or 100 mM Etoh and 100 nM Myr, 0.5 µM GT-11, or 0.35 mM FB1.
Etoh incubation caused a 2–4 times elevation of PLIN2 protein levels in VL17A cells compared with Ctrl-treated cells. Moreover, although GT-11 had no effect on PLIN2 protein levels in Etoh-incubated cells, both Myr and FB1 incubation reduced PLIN2 levels in Etoh-incubated cells (P < 0.01) and prevented cellular triglyceride accumulation in response to Etoh. FB1 additionally reduced PLIN2 protein in Ctrl cells suggesting a broader role of ceramide synthase in LD biology (Fig. 2A–D). These results imply that de novo ceramide synthetic enzymes have differential effects and that the enzyme CerS may be specifically implicated in the regulation of PLIN2 under both Ctrl and Etoh conditions.
Figure 2.
FB1 ceramide synthase inhibition reduces PLIN2 in Ctrl and Etoh cells. A–C) Densitometry quantification of PLIN2 protein expression relative to GAPDH in Ctrl and Etoh cells incubated with GT-11 (A), Myr (B), or FB1 (C). D) Triglyceride content after treatment with ceramide synthetic inhibitors. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
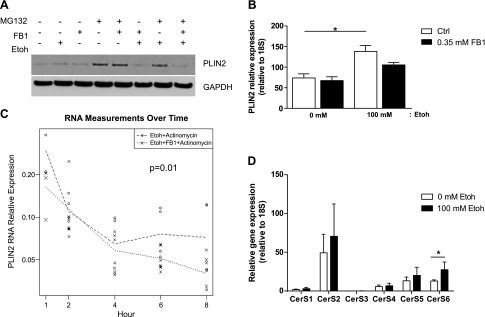
Because ceramide synthase regulates PLIN2 protein expression, we examined whether the mechanism of this regulation is transcriptional or posttranslational. PLIN2 is posttranslationally modified by ubiquitination and targeted to the proteasome for degradation (25–27). Therefore, we examined the effects of CerS inhibition on PLIN2 proteasomal degradation. Ctrl- and Etoh-incubated cells were coincubated with FB1 and the proteasomal inhibitor MG132. In the presence of MG132, PLIN2 protein was not increased in FB1-incubated cells, suggesting that the effects were independent of effects on proteasomal degradation (Fig. 3A).
Figure 3.
Transcriptional and posttranslational regulation of PLIN2 by FB1. A) Immunoblot of PLIN2 protein isolated from VL17A cells incubated with Ctrl or Etoh medium, with or without FB1, and with or without the proteasomal inhibitor MG132. B) PLIN2 mRNA level relative to 18S in Ctrl and Etoh cells incubated with FB1. C) PLIN2 degradation. Data are presented as PLIN2 mRNA level relative to 18S in Etoh cells incubated with or without FB1 and actinomycin over time. D) CerS1–6 gene expression relative to 18S in VL17A cells incubated 48 h in Ctrl or Etoh medium. *P ≤ 0.05, **P ≤ 0.01.
We next measured PLIN2 gene transcript levels in Etoh cells incubated with FB1. Because there was a trend toward reduction of PLIN2 total gene transcription by FB1 (Fig. 3B), we measured effects of FB1 on PLIN2 gene stability in Etoh-incubated cells. FB1-incubated Ctrl and Etoh VL17A cells were coincubated with the transcription inhibitor actinomycin D (20). PLIN2 mRNA was measured for a total of 8 h, and RNA decay over time was quantified. We observed that Etoh cells incubated with FB1 increased the rate of RNA decay (Fig. 3B, C). These results suggest that FB1 reduces PLIN2 in Etoh-incubated cells by reducing PLIN2 RNA gene stability.
CerS6 is up-regulated in response to Etoh and regulates PLIN2 expression in Etoh-incubated cells
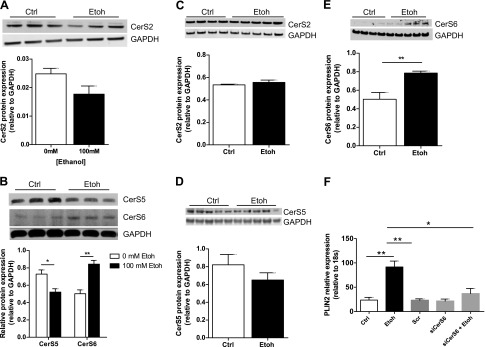
There are 6 isoforms of CerS (CerS1–6), which are differentiated by their tissue distribution and substrate specificity [CerS1 (C18), CerS2 (C20–26), CerS3 (C22–26), CerS4 (C18–20), CerS5 (C16), and CerS6 (C14 and C16)] (28, 29). Of these CerS isoforms, CerS2 and CerS6 have been shown previously with Northern blot analysis to have the highest hepatic expression in mice (30). CerS6 is responsible for synthesis of long-chain ceramide species associated with programmed cell death and insulin resistance (31, 32), and CerS5 has the greatest homology to CerS6 (29). We, therefore, compared CerS2, CerS5, and CerS6 expression in Ctrl- and Etoh-incubated VL17A cells and in Ctrl- and alcohol-fed mice. In VL17A cells, CerS2, CerS5, and CerS6 had the highest gene expression, and CerS6 gene expression increased significantly in response to Etoh treatment (P = 0.01; Fig. 3D). CerS2 protein expression was similar between Ctrl- and Etoh-incubated cells, and CerS5 protein was reduced in response to Etoh incubation (P = 0.03; Fig. 4A, B). In mice, CerS2 and CerS5 protein levels were similar in alcohol- and Ctrl-fed mice (Fig. 4C, D). In both cells and mice, CerS6 protein was up-regulated by 50% in response to Etoh (P ≤ 0.01; Fig. 4B, E).
Figure 4.
CerS6 reduction ameliorates Etoh-induced PLIN2 up-regulation in VL17A cells. A, B) Immunoblot and densitometry quantification of CerS2 (A) and CerS5 and CerS6 (B) protein in VL17A cells. C–E) Immunoblot and densitometry quantification of CerS2 (C), CerS5 (D), and CerS6 (E) protein in mouse liver. F) PLIN2 gene expression relative to 18S after siRNA knockdown of CerS6 in VL17A cells incubated in Ctrl or Etoh medium. *P ≤ 0.05, **P ≤ 0.01.
To examine directly whether the CerS6 isoform regulates alcohol-mediated PLIN2 up-regulation, we performed siRNA knockdown of CerS6 in VL17A cells incubated for 48 h with either Ctrl or Etoh medium. We demonstrated that CerS6 deficiency prevents Etoh-mediated PLIN2 gene up-regulation (Fig. 4F), and those findings are associated with inhibition of Etoh-mediated induction of fatty acid synthase (FASN) and diacylglycerol O-acyltransferase 2 (DGAT2), 2 enzymes important for triglyceride synthesis (Table 1). We, also, observed a nonstatistically significant up-regulation of the nuclear receptor peroxisome proliferator-activated receptor γ (PPAR-γ) in CerS6-deficient cells treated with Etoh (Table 1).
TABLE 1.
Gene expression in CerS6-deficient cells exposed to Etoh
| Gene name | Ctrl | Etoh | Scr | CerS6si | Etoh + CerS6si | P |
|---|---|---|---|---|---|---|
| FASN | 254.5 ± 107.7 | 1998 ± 327.4 | 182.0 ± 28.16 | 144.6 ± 4.81 | 270.5 ± 77.44 | ≤ 0.0001 |
| DGAT2 | 57.16 ± 23.78 | 439.4 ± 103.20 | 40.38 ± 4.68 | 54.10 ± 4.35 | 105.3 ± 30.33 | ≤ 0.001 |
| PPARγ | 21.66 ± 7.13 | 0.1517 ± 0.06 | 15.92 ± 1.36 | 16.23 ± 1.04 | 34.23 ± 11.28 | ns |
Data are means ± sem expression relative to 18S. P values are Etoh vs. Etoh + CerS6si. si, siRNA.
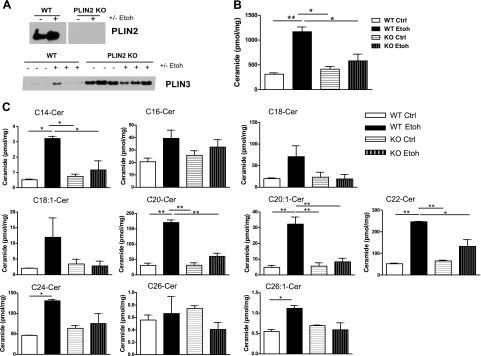
Long-chain ceramides are up-regulated in the hepatocellular LD fraction of Etoh-fed mice and dependent on PLIN2
CerSs have been localized previously to both the endoplasmic reticulum and mitochondria in rodents (28, 29). We and others (2, 10, 33) have also demonstrated an increase of long-chain ceramide species in whole-liver extracts of mice chronically fed alcohol. In addition, our group reported that alcohol-mediated, whole-liver ceramide accumulation requires PLIN2 (10). Recently, this study’s coauthors reported the presence of ceramides within hepatic LDs in a high-fat diet mouse model of hepatic steatosis (34). In the present study, we examined whether LD long-chain ceramides are regulated by Etoh and PLIN2.
Wild-type and PLIN2-null mice were pair fed Ctrl or Etoh liquid diets for 4 wk; after which, LD isolation by sucrose gradient was performed. Our results demonstrate that PLIN2 is up-regulated in the LD fraction of wild-type mice in response to Etoh and is absent from the LD fraction of PLIN2-null mice. In PLIN2-null mice, PLIN3 compensates for the loss of PLIN2 (Fig. 5A). Similar to findings reported by Senkal et al. (34), we observed that long-chain ceramides are present in hepatic LD fractions of both wild-type and PLIN2-null mice and that alcohol significantly increases LD total long-chain ceramide content in wild-type mice (P ≤ 0.01) but not in PLIN2-null mice (Fig. 5B). Specifically, Etoh significantly increases C14, C20, C20:1, C22, C24, and C26:1 and causes a nonstatistically significant elevation of C16 and C18. In PLIN2-null mice, Etoh failed to increase most ceramide species in LDs, despite the compensation by PLIN3 (Fig. 5C). These results demonstrate that ceramides and PLIN2 are mechanistically linked either directly or indirectly and suggest a unique role of ceramides in Etoh-regulated LD biology.
Figure 5.
Absence of PLIN2 prevents Etoh-induced, long-chain ceramide up-regulation in LDs. A) PLIN2 and PLIN3 immunoblots of LDs isolated from wild-type (WT) or PLIN2-null mice fed Ctrl or Etoh diets for 4 wk. B, C) Mass spectrometric analysis of total long-chain ceramides (B) and individual ceramide species (C) in LDs isolated from WT and PLIN2-null mice fed Ctrl or Etoh diets; n = 5 mice/group. KO, knockout. *P ≤ 0.05, **P ≤ 0.01.
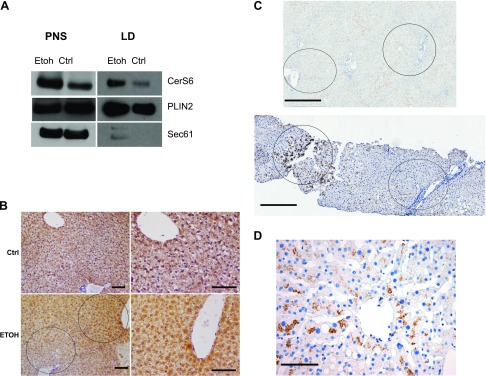
CerS6 is up-regulated in mouse and human alcoholic steatosis
Although the presence of CerS6 in LDs was published recently by Senkal et al. (34), the regulation of LD CerS6 by Etoh has not, to our knowledge, been reported previously. Immunoblot of isolated LDs demonstrates that CerS6 is up-regulated in the LD and PNS fractions in response to Etoh (Fig. 6A).
Figure 6.
CerS6 localizes to the LD fraction of VL17A cells and is up-regulated in zone 3 hepatocytes in murine and human alcoholic steatosis. A) Sucrose gradient fractionation immunoblots of VL17A LD and PNS fractions incubated with Ctrl or Etoh medium. B) CerS6 IHC of Ctrl and Etoh-fed wild-type mouse livers. Original magnification, ×20. Scale bars, 100 μm. C) CerS6 IHC in nonsteatotic human liver (upper) and human alcoholic steatosis (lower) showing hepatic zones 1–3. Scale bars, 500 μm. D) CerS6 IHC of zone 3 in a patient with mild alcoholic steatosis. Original magnification, ×40. Scale bar, 100 μm. Dashed circle, zone 1; solid circle, zone 3. Sec61, endoplasmic reticulum membrane protein.
In addition to the immunoblot findings, IHC demonstrates that hepatic CerS6 is up-regulated in the livers of alcohol-fed mice compared with Ctrl-fed mice concomitant with the development of mild steatosis. That up-regulation is most prominent in zone-3 hepatocytes (Fig. 6B).
To determine whether the findings in the experimental models are relevant to human alcoholic steatosis, we next compared CerS6 IHC staining in human Ctrl specimens without steatosis and in liver biopsies from patients with mild alcoholic steatosis. Figure 6C demonstrates that CerS6 is increased in human alcoholic steatosis compared with control and is especially prominent in zone 3 (similar to the distribution seen in mice). Notably, that zonal distribution mirrors that of the zone-3 predominance of ALD pathology (35). One of those patients is represented in Fig. 6D, and clinical features of that patient at the time of liver biopsy are listed in Table 2. Those results suggest that CerS6 may be a novel mediator of the earliest stage of ALD, alcoholic steatosis.
TABLE 2.
Clinical characteristics of patient with alcohol use disorder
| Parameter | Result |
|---|---|
| Age (yr) | 64 |
| BMI (kg/m2) | 22 |
| AST (U/L) | 26 |
| ALT (U/L) | 24 |
| Alkaline phosphatase (U/L) | 51 |
| Bilirubin (mg/dl) | 1.0 |
| Albumin (g/dl) | 4.8 |
| Platelets (103/µl) | 114 |
| Serum TG (mg/dl) | 89 |
| Glucose (mg/dl) | 160 |
| Creatinine (mg/dl) | 1.10 |
| INR | 1.0 |
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body–mass index; INR, international normalized ratio; TG, triglyceride.
Inhibition of ceramide synthesis during alcohol feeding has minimal effect on body composition, food intake, and energy expenditure
Understanding the functional relevance of CerS6 in alcoholic steatosis in vivo is limited by the lack of nontoxic pharmacologic agents that target CerS systemically. Moreover, although prior studies have demonstrated a reduction of hepatic steatosis in alcohol-feeding rodent models of ceramide inhibition (17, 18, 33), differences in body weight and energy homeostasis may have contributed to the improvements seen in lipid and glucose homeostasis in those models. We, therefore, used Myr to inhibit de novo ceramide synthesis upstream of CerS to examine effects of ceramide reduction on alcoholic steatosis and PLIN2 regulation in mice with similar energy homeostasis. Of note, we were unable to use fumonisin (the direct CerS inhibitor) in these studies because it is very toxic. On average, mice consumed 11–12 kcal/d and gained ∼2 g. Weight gain and lean mass were similar among all groups. De novo synthesis inhibition slightly lowered the percentage of body fat, but that difference was not statistically significant. Indirect calorimetry showed no differences in oxygen consumption or carbon dioxide production. All treatment groups had mildly lower respiratory quotients (RQs) compared with Sal:Ctrl, but each group had an overall RQ of ∼0.7, indicating that fat, rather than carbohydrate, was the primary source of energy used (Table 3). Those data demonstrate that the treatment groups had similar body composition and energy expenditure, reducing the chance that the effects of ceramide synthesis inhibition were due to alterations in energy balance.
TABLE 3.
Effects of myriocin and imipramine on energy balance, serum lipids and hepatic ceramides
| Metabolic parameter | Sal:Ctrl | Sal:Etoh | Myr:Ctrl | Myr:Etoh | Imip:Ctrl | Imip:Etoh | P |
|---|---|---|---|---|---|---|---|
| Food intake (kcal/mouse/d) | 11.91 ± 0.18 | 11.75 ± 0.17 | 11.99 ± 0.18 | 11.87 ± 0.15 | 11.18 ± 0.26 | 10.89 ± 0.32**,§,##,Ŧ | 0.002 |
| Baseline body weight (g) | 24.3 ± 0.42 | 24.2 ± 0.40 | 24.6 ± 0.36 | 24.3 ± 0.50 | 23.6 ± 0.40 | 24.1 ± 0.32 | 0.74 |
| Week 4 body weight, (g) | 26.8 ± 0.48 | 26.5 ± 0.49 | 26.5 ± 0.51 | 25.8 ± 0.55 | 26.0 ± 0.54 | 26.7 ± 0.59 | 0.68 |
| Week 4 fat mass (%) | 16.46 ± 1.13 | 15.70 ± 1.13 | 14.70 ± 0.99 | 13.87 ± 1.12 | 16.6 ± 1.27 | 16.7 ± 1.33 | 0.43 |
| Week 4 lean mass (%) | 80.68 ± 1.26 | 81.28 ± 1.15 | 83.43 ± 1.21 | 80.71 ± 1.30 | 78.8 ± 1.10 | 78.8 ± 1.03 | 0.11 |
| Vo2 (ml/h) | 78.82 ± 3.61 | 84.70 ± 5.17 | 81.63 ± 4.10 | 87.54 ± 6.12 | 86.92 ± 4.61 | 84.87 ± 5.48 | 0.81 |
| Vco2 (ml/h) | 58.81 ± 2.68 | 62.57 ± 3.87 | 60.00 ± 2.94 | 64.77 ± 4.60 | 63.92 ± 3.59 | 61.88 ± 3.98 | 0.85 |
| RQ | 0.743 ± 0.0013 | 0.735 ± 0.0019* | 0.734 ± 0.0012** | 0.739 ± 0.0022 | 0.733 ± 0.0023** | 0.727 ± 0.0007***,§,#,ŦŦ,ŦŦŦ | <0.0001 |
| Serum TG (mg/dl) | 100.3 ± 10.02 | 146.0 ± 9.95** | 125.9 ± 14.72 | 91.19 ± 8.06§§,# | 111.9 ± 6.20§ | 120.0 ± 9.36 | 0.01 |
| NEFA (mEq/L) | 2.88 ± 0.19 | 3.42 ± 0.13* | 3.69 ± 0.32** | 2.79 ± 0.16§ | 2.91 ± 0.12*,§§ | 3.0 ± 0.14*,§§ | 0.02 |
| Serum insulin (ng/ml) | 1.25 ± 0.08 | 1.60 ± 0.27 | 1.29 ± 0.34 | 1.06 ± 0.19 | 1.14 ± 0.31 | 1.71 ± 0.23 | 0.48 |
| Total hepatic ceramides (nmol/mg protein) | 16.44 ± 2.7 | 35.08 ± 6.6** | 18.91§ ± 4.7 | 17.29 ± 2.9§§ | 15.88 ± 1.2§§ | 18.80 ± 3.3§§ | 0.02 |
Data are expressed as means ± sem. TG, triglyceride; Vo2, oxygen consumption; Vco2, carbon dioxide production. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.0001 vs. Sal:Ctrl; §P ≤ 0.05, §§P ≤ 0.01 vs. Sal:Etoh; #P ≤ 0.05, ##P ≤ 0.01 vs. Myr:Ctrl; ŦP ≤ 0.01, ŦŦP ≤ 0.0001, ŦŦŦP ≤ 0.05 vs. Imip:Ctrl; n = 3–5 mice/group; mRNA is relative to 36B4.
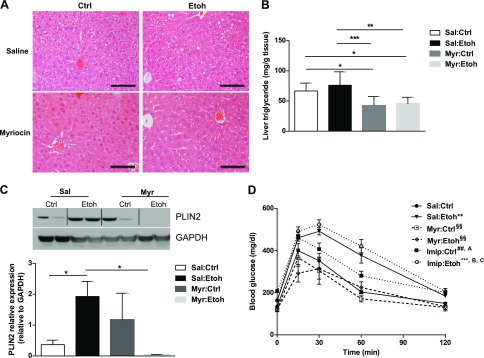
Inhibition of de novo ceramide synthesis reduces hepatic steatosis and improves serum lipids in alcohol-fed mice
The 4 wk of chronic alcohol feeding resulted in a statistically significant elevation of total ceramides in Sal-injected mice (P ≤ 0.01). Myr treatment prevented alcohol-feeding–induced, total hepatic ceramide accumulation (Table 3).
We examined liver histology to determine whether treatment of mice with myriocin ameliorated the steatotic effects of alcohol on the liver. In this study, Sal:Etoh mice developed mild steatosis, relative to Ctrl mice. Myr reduced hepatic steatosis and liver triglycerides by 40% compared with Sal:Ctrl and Sal:Etoh mice (Fig. 7A, B). Moreover, Myr caused a nonstatistically significant reduction in hepatic mRNA PLIN2 levels (Supplemental Fig. 2) and reduced PLIN2 protein to nearly undetectable levels in alcohol-fed mice (Fig. 7C).
Figure 7.
Myr reduces hepatic steatosis and PLIN2 and improves glucose tolerance in alcohol-fed mice. A) Hematoxylin and eosin stain of liver sections from Ctrl or alcohol-fed wild-type mice injected with Sal or Myr. Original magnification, ×40. Scale bars, 100 μm. B) Liver triglyceride content. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. C) Immunoblot and densitometry quantification of mouse liver PLIN2 protein. *P ≤ 0.05. D) Glucose tolerance test, n = 3–5 mice/group. **P ≤ 0.01, ***P ≤ 0.0001 vs. Sal:Ctrl; §§P ≤ 0.01 vs. Sal:Etoh; ##P < 0.05 vs. Myr:Ctrl; AP ≤ 0.05 vs. Myr:Etoh; BP ≤ 0.001 vs. Myr:Ctrl; CP ≤ 0.001 vs. Myr:Etoh (D).
Hepatic triglycerides can accumulate from both fatty acid esterification and reduced hepatic triglyceride secretion. We, therefore, measured serum triglyceride and nonesterified fatty acids (NEFA). Alcohol significantly increased both serum triglyceride and NEFA levels in Sal-treated mice. Compared with Sal:Etoh mice, Myr:Etoh mice had reduced serum triglycerides. Myr:Etoh mice also had reduced NEFA levels compared with Sal:Etoh mice (Table 3). Together, those data suggest that de novo ceramide inhibition prevents hepatic LD accumulation, down-regulates PLIN2, and improves serum lipids in experimental alcoholic steatosis.
Inhibition of de novo synthesis, but not sphingomyelinase inhibition, improves glucose homeostasis in Ctrl and Etoh mice
Both ceramides and increased fat mass are associated with impaired insulin signaling (36), and the development of insulin resistance is associated with progression from alcoholic steatosis to advanced liver disease (3). Therefore, we sought to examine whether inhibition of de novo ceramide synthesis could ameliorate the effects of alcohol on glucose tolerance, independent of changes in weight or energy expenditure. As observed previously (2), alcohol-fed mice are glucose intolerant compared with Ctrl-fed mice despite similar food intake, energy expenditure, and body mass. In both alcohol- and Ctrl-fed mice, Myr significantly improved glucose tolerance to Ctrl levels (Fig. 7D). There was no difference in serum insulin levels at the time of sacrifice (Table 3). We further investigated whether that improvement of glucose tolerance was specific to enzymes involved in de novo ceramide synthesis by comparing results to mice that had ceramide reduction by sphingomyelinase (SMASE) inhibition with imipramine. Despite similar reduction of hepatic ceramides and similar effects on energy homeostasis (Table 3), SMASE inhibition worsened glucose tolerance in both Ctrl- and alcohol-fed mice compared with Sal:Ctrl and Myr-treated mice and worsened serum lipids (Fig. 7D).
Together, these data indicate that ceramide pathways, and perhaps, ceramide pools, differentially modulate lipid and glucose homeostasis in alcoholic steatosis. Reduction of ceramides through interventions that inhibit CerS6 or enzymes upstream of CerS6 may be novel strategies for treatment of patients with alcoholic steatosis.
DISCUSSION
PLIN2 is required for the development of alcoholic steatosis, and we have previously reported a temporal relationship among PLIN2 up-regulation, hepatic ceramide accumulation, and glucose intolerance in alcohol-fed mice (2). In addition, we and others (10, 17, 33, 37, 38) have reported an inverse relationship between hepatic ceramide content and glucose tolerance in alcohol-fed rodents. Here, we provide evidence that PLIN2 is regulated either directly or indirectly by CerS and that CerS6, in particular, may have an important role in experimental and human alcoholic steatosis. Our in vitro studies demonstrate that pharmacologic inhibition of CerSs reduces cellular LD accumulation and PLIN2 protein expression in both Ctrl- and Etoh-incubated cells, suggesting CerSs have important roles in cellular LD biology. This effect is due to increased PLIN2 RNA degradation and is not due to enhanced PLIN2 protein lysosomal degradation.
In our in vitro model of alcoholic steatosis, using human hepatoma VL17A cells, we have established that nonspecific CerS reduction with FB1 and SPT inhibition from Myr reduces PLIN2 protein. The differential effect of CerS inhibition on PLIN2 protein expression compared with inhibition of the downstream de novo ceramide synthetic enzyme DES may result from the dual action of CerS in both the de novo and ceramide salvage pathways. To date, the salvage pathway has been implicated in several biologic processes (39) but is less well studied compared with the de novo and sphingomyelin hydrolysis pathways. Studies specifically targeting ceramide salvage are needed to better understand the contribution of this pathway in alcoholic steatosis.
Little is known about the role of the CerS isoform CerS6 in human gastrointestinal disease. In vivo, CerS6 liver-specific knockout mice are protected against high-fat diet-induced hepatic-insulin resistance (40). In the colon cancer cell line SW620, lower expression of CerS6 protein and C16 ceramide confers resistance to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), a ligand involved in the selective killing of cancer cells (41). In hepatoma cells, CerS6 knockdown reduces CD95 activation, thereby, inhibiting apoptosis (42).
We now add to this growing body of literature and demonstrate that CerS6 regulates PLIN2 expression. CerS6 is up-regulated in the livers of alcohol-fed mice and in LD fractions of Etoh-incubated VL17A cells. Perhaps most striking, CerS6 is up-regulated in human alcoholic steatosis and has zone-3 distribution, the zone in which alcoholic steatosis and lipid peroxidation initially manifest in patients (35). Although our studies reported here are unable to establish whether that regulation is direct or indirect (e.g., via a primary effect on hepatic triglycerides), we have demonstrated that CerS6 knockdown prevents Etoh-mediated PLIN2 gene up-regulation. To our knowledge, this is the first report describing a role for CerS6 in human ALD.
In further support of a role for CerS in alcoholic steatosis, we demonstrate an increase in several long-chain ceramides in the LD fraction of Etoh-fed mice. Ceramides have been localized to several cellular compartments, including the ER, Golgi apparatus, lysosomes, and membranes. Here, we suggest that, in hepatocytes, ceramides are present in the LD itself and are regulated by Etoh. The presence of ceramides in LDs was, also reported recently in a study by Senkal et al. (34), in which the authors reported a mechanistic link between LD ceramides and the enzyme DGAT2 in an obesity model. Congruent with their findings, here, we demonstrated that CerS6 inhibition prevents Etoh-mediated up-regulation of DGAT2. Because DGAT2 is also required for triglyceride synthesis, this finding may provide a potential mechanistic link between ceramide and triglyceride biochemical pathways.
Although ceramides accumulate from several synthetic pathways, our data suggest that ceramide pathways differentially affect lipid and glucose homeostasis in alcoholic steatosis independent of differences in body weight, fat mass, and energy expenditure. Specifically, imipramine-mediated SMASE inhibition worsens serum lipids and glucose tolerance, whereas de novo ceramide synthesis inhibition with Myr prevents alcoholic steatosis, dyslipidemia, and glucose intolerance in alcohol-fed mice. Reduced serum triglyceride can result from either less hepatic triglyceride synthesis available for secretion as very LDL or greater storage of triglycerides in hepatic LDs. Because we did not observe the latter in Myr-treated mice and observed lower NEFAs in alcohol-fed Myr mice, a unifying explanation is that there is reduced synthesis of triglyceride from NEFA precursors. Because the major source of NEFAs is from adipose lipolysis, it is also possible that systemic de novo ceramide inhibition has effects on both liver and white adipose tissue. In contrast, we suspect that the elevated triglycerides observed in Imip:Etoh mice resulted from increased hepatic export of very LDL, reduced peripheral clearance, or a combination of the two.
We similarly demonstrate a divergence of ceramide synthetic pathways in their effect on glucose homeostasis, which suggests that there is an interaction between specific ceramide pools and glucose homeostatic pathways. Our results are consistent with those of Lizarazo et al. (17) who demonstrated Myr restores levels of hepatic insulin signaling proteins in Etoh-fed rats and with studies demonstrating that SMASE inhibition impairs glucose handling in humans (43) and in vitro (44). Our results differ from those observed by Liangpunsakul et al. (33), who observed both a modest reduction of hepatic steatosis and a modest improvement in glucose tolerance in imipramine-treated Etoh mice, compared with Etoh mice. Apparent discrepancies between our studies may be due to differences in experimental design or to off-target effects because imipramine is a nonspecific inhibitor of SMASE (45). In the cited study, mice were fed more Etoh and received imipramine for 2 wk. In addition, experimental mice had weight fluctuations and overall lower body weight compared with Ctrls, differences that may affect glucose tolerance.
In summary, ceramide synthetic pathways differentially affect LD accumulation, PLIN2 regulation, and glucose homeostasis in alcoholic steatosis. We introduce CerSs and the subtype CerS6 as potential novel regulators of LD biology in early ALD and mechanistically link CerS with PLIN2 in experimental alcoholic steatosis. This enzyme may serve as a novel therapeutic target or biomarker for patients with alcoholic steatosis at risk for advanced ALD.
ACKNOWLEDGMENTS
The authors thank the Lipidomics Core facility at the State University of New York (SUNY; Stony Brook, NY, USA), the Michael J. Palmieri Metabolic Laboratory (Philadelphia, PA, USA) for lipid analyses; the University of Pennsylvania Molecular Pathology/Imaging, Molecular Biology, and Mouse Phenotyping Cores (Philadelphia, PA, USA); and Dr. Dahn Clemens (University of Nebraska, Omaha, NE, USA) for VL17A cells. The authors acknowledge Amy Praestgaard (Biostatistics Analysis Center, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania), for her biostatistical analysis and support. This work was supported by the U.S. National Institutes of Health (NIH) National Institute on Alcohol Abuse and Alcoholism Grant K08-AA021424; the Robert Wood Johnson Foundation; Harold Amos Medical Faculty Development Award (Grant 7158); the Institute for Diabetes, Obesity and Metabolism Diabetes Research Center Pilot Award P30 DK019525 (to R.M.C.); Health Resources and Services Administration Grant D34HP24459, Center of Excellence for Diversity in Health Education and Research; NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant T32 DK007066 (to A.O.), NIH National Institute of General Medical Sciences Grant R01 GM062887 (to L.M.O.), and, in part, by NIH/NIDDK Grant P30-DK050306; its core facilities (University of Pennsylvania Molecular Pathology and Imaging Core, Molecular Biology/Gene Expression Core, Transgenic and Chimeric Mouse Core, and Cell Culture Core) and its pilot grant program; and NIH National Cancer Institute Grant P01-CA97132 (SUNY Stony Brook Lipidomics Core). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- ALD
alcoholic liver disease
- CerS
ceramide synthase
- Ctrl
control
- DES
dihydroceramide desaturase
- DGAT2
diacylglycerol O-acyltransferase
- Etoh
ethanol
- FASN
fatty acid synthase
- FB1
fumonisin B1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IHC
immunohistochemistry
- Imip
imipramine
- LD
lipid droplet
- Myr
myriocin
- NEFA
nonesterified fatty acid
- PLIN
perilipin
- PNS
postnuclear supernatant
- PPAR
peroxisome proliferator-activated receptor
- q.o.d
every other day
- RQ
respiratory quotient
- Sal
saline
- siRNA
small interfering RNA
- SMASE
sphingomyelinase
- SPT
serine palmitoyl transferase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. Williams, L. Obeid, and R. M. Carr planned and/or conducted the study; B. Williams, J. Correnti, A. Oranu, A. Lin, V. Scott, M. Annoh, J. Beck, E. Furth, V. Mitchell, C. E. Senkal, L. Obeid, and R. M. Carr collected and/or interpreted data; B. Williams and R. M. Carr drafted the manuscript; and all authors approved the final draft.
REFERENCES
- 1.Rehm J.,, Samokhvalov A. V.,, Shield K. D. (2013) Global burden of alcoholic liver diseases. J. Hepatol. 59, 160–168 [DOI] [PubMed] [Google Scholar]
- 2.Carr R. M.,, Dhir R.,, Yin X.,, Agarwal B.,, Ahima R. S. (2013) Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol. Clin. Exp. Res. 37, 1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raynard B.,, Balian A.,, Fallik D.,, Capron F.,, Bedossa P.,, Chaput J. C.,, Naveau S. (2002) Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 35, 635–638 [DOI] [PubMed] [Google Scholar]
- 4.Carr R. M.,, Ahima R. S. (2016) Pathophysiology of lipid droplet proteins in liver diseases. Exp. Cell Res. 340, 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr R. M.,, Dhir R.,, Mahadev K.,, Comerford M.,, Chalasani N. P.,, Ahima R. S. (2017) Perilipin staining distinguishes between steatosis and nonalcoholic steatohepatitis in adults and children. Clin. Gastroenterol. Hepatol. 15, 145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magné J.,, Aminoff A.,, Perman Sundelin J.,, Mannila M. N.,, Gustafsson P.,, Hultenby K.,, Wernerson A.,, Bauer G.,, Listenberger L.,, Neville M. J.,, Karpe F.,, Borén J.,, Ehrenborg E. (2013) The minor allele of the missense polymorphism Ser251Pro in perilipin 2 (PLIN2) disrupts an α-helix, affects lipolysis, and is associated with reduced plasma triglyceride concentration in humans. FASEB J. 27, 3090–3099 [DOI] [PubMed] [Google Scholar]
- 7.Senthivinayagam S.,, McIntosh A. L.,, Moon K. C.,, Atshaves B. P. (2013) Plin2 inhibits cellular glucose uptake through interactions with SNAP23, a SNARE complex protein. PLoS One 8, e73696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McManaman J. L.,, Bales E. S.,, Orlicky D. J.,, Jackman M.,, MacLean P. S.,, Cain S.,, Crunk A. E.,, Mansur A.,, Graham C. E.,, Bowman T. A.,, Greenberg A. S. (2013) Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J. Lipid Res. 54, 1346–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varela G. M.,, Antwi D. A.,, Dhir R.,, Yin X.,, Singhal N. S.,, Graham M. J.,, Crooke R. M.,, Ahima R. S. (2008) Inhibition of ADRP prevents diet-induced insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G621–G628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr R. M.,, Peralta G.,, Yin X.,, Ahima R. S. (2014) Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One 9, e97118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najt C. P.,, Senthivinayagam S.,, Aljazi M. B.,, Fader K. A.,, Olenic S. D.,, Brock J. R.,, Lydic T. A.,, Jones A. D.,, Atshaves B. P. (2016) Liver-specific loss of perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G726–G738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachibana K.,, Kobayashi Y.,, Tanaka T.,, Tagami M.,, Sugiyama A.,, Katayama T.,, Ueda C.,, Yamasaki D.,, Ishimoto K.,, Sumitomo M.,, Uchiyama Y.,, Kohro T.,, Sakai J.,, Hamakubo T.,, Kodama T.,, Doi T. (2005) Gene expression profiling of potential peroxisome proliferator-activated receptor (PPAR) target genes in human hepatoblastoma cell lines inducibly expressing different PPAR isoforms. Nucl. Recept. 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bickel P. E.,, Tansey J. T.,, Welte M. A. (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta 1791, 419–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longato L.,, Ripp K.,, Setshedi M.,, Dostalek M.,, Akhlaghi F.,, Branda M.,, Wands J. R.,, de la Monte S. M. (2012) Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid. Med. Cell. Longev. 2012, 479348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liangpunsakul S.,, Sozio M. S.,, Shin E.,, Zhao Z.,, Xu Y.,, Ross R. A.,, Zeng Y.,, Crabb D. W. (2010) Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G1004–G1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannun Y. A.,, Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 17.Lizarazo D.,, Zabala V.,, Tong M.,, Longato L.,, de la Monte S. M. (2013) Ceramide inhibitor myriocin restores insulin/insulin growth factor signaling for liver remodeling in experimental alcohol-related steatohepatitis. J. Gastroenterol. Hepatol. 28, 1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong , M.,, Longato L.,, Ramirez T.,, Zabala V.,, Wands J. R.,, de la Monte S. M. (2014) Therapeutic reversal of chronic alcohol-related steatohepatitis with the ceramide inhibitor myriocin. Int. J. Exp. Pathol. 95, 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osna N. A.,, Clemens D. L.,, Donohue T. M., Jr. (2005) Ethanol metabolism alters interferon γ signaling in recombinant HepG2 cells. Hepatology 42, 1109–1117 [DOI] [PubMed] [Google Scholar]
- 20.Chen C. Y.,, Ezzeddine N.,, Shyu A. B. (2008) Messenger RNA half-life measurements in mammalian cells. Methods Enzymol. 448, 335–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carr R. M.,, Patel R. T.,, Rao V.,, Dhir R.,, Graham M. J.,, Crooke R. M.,, Ahima R. S. (2012) Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R996–R1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donohue T. M.,, Osna N. A.,, Clemens D. L. (2006) Recombinant Hep G2 cells that express alcohol dehydrogenase and cytochrome P450 2E1 as a model of ethanol-elicited cytotoxicity. Int. J. Biochem. Cell Biol. 38, 92–101 [DOI] [PubMed] [Google Scholar]
- 23.Mak K. M.,, Ren C.,, Ponomarenko A.,, Cao Q.,, Lieber C. S. (2008) Adipose differentiation-related protein is a reliable lipid droplet marker in alcoholic fatty liver of rats. Alcohol. Clin. Exp. Res. 32, 683–689 [DOI] [PubMed] [Google Scholar]
- 24.Morad S. A.,, Cabot M. C. (2013) Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 13, 51–65 [DOI] [PubMed] [Google Scholar]
- 25.Kaushik S.,, Cuervo A. M. (2015) Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 17, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuda Y.,, Itabe H.,, Odaki M.,, Hama K.,, Fujimoto Y.,, Mori M.,, Sasabe N.,, Aoki J.,, Arai H.,, Takano T. (2006) ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J. Lipid Res. 47, 87–98 [DOI] [PubMed] [Google Scholar]
- 27.Xu G.,, Sztalryd C.,, Lu X.,, Tansey J. T.,, Gan J.,, Dorward H.,, Kimmel A. R.,, Londos C. (2005) Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J. Biol. Chem. 280, 42841–42847 [DOI] [PubMed] [Google Scholar]
- 28.Mullen T. D.,, Hannun Y. A.,, Obeid L. M. (2012) Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 441, 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy M.,, Futerman A. H. (2010) Mammalian ceramide synthases. IUBMB Life 62, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizutani Y.,, Kihara A.,, Igarashi Y. (2005) Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390, 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullen T. D.,, Jenkins R. W.,, Clarke C. J.,, Bielawski J.,, Hannun Y. A.,, Obeid L. M. (2011) Ceramide synthase-dependent ceramide generation and programmed cell death: involvement of salvage pathway in regulating postmitochondrial events. J. Biol. Chem. 286, 15929–15942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasumov T.,, Solomon T. P.,, Hwang C.,, Huang H.,, Haus J. M.,, Zhang R.,, Kirwan J. P. (2015) Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity (Silver Spring) 23, 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liangpunsakul S.,, Rahmini Y.,, Ross R. A.,, Zhao Z.,, Xu Y.,, Crabb D. W. (2012) Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G515–G523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senkal C. E., Salama M. F., Snider A. J., Allopenna J. J., Rana N. A., Koller A., Hannun Y. A., Obeid L. M. (2017) Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 25, 686–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald G. A.,, Bridle K. R.,, Ward P. J.,, Walker N. I.,, Houglum K.,, George D. K.,, Smith J. L.,, Powell L. W.,, Crawford D. H.,, Ramm G. A. (2001) Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J. Gastroenterol. Hepatol. 16, 599-606 [DOI] [PubMed] [Google Scholar]
- 36.Carr R. M., Correnti J. (2015) Insulin resistance in clinical and experimental alcoholic liver disease. Ann. N. Y. Acad. Sci. 1353, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correnti J. M., Juskeviciute E., Swarup A., Hoek J. B. (2014) Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G959–G973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez T., Longato L., Dostalek M., Tong M., Wands J. R., de la Monte S. M. (2013) Insulin resistance, ceramide accumulation and endoplasmic reticulum stress in experimental chronic alcohol-induced steatohepatitis. Alcohol Alcohol. 48, 39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitatani K., Idkowiak-Baldys J., Hannun Y. A. (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20, 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turpin S. M., Nicholls H. T., Willmes D. M., Mourier A., Brodesser S., Wunderlich C. M., Mauer J., Xu E., Hammerschmidt P., Brönneke H. S., Trifunovic A., LoSasso G., Wunderlich F. T., Kornfeld J. W., Blüher M., Krönke M., Brüning J. C. (2014) Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 [DOI] [PubMed] [Google Scholar]
- 41.White-Gilbertson S., Mullen T., Senkal C., Lu P., Ogretmen B., Obeid L., Voelkel-Johnson C. (2009) Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene 28, 1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker T., Mitchell C., Park M. A., Yacoub A., Graf M., Rahmani M., Houghton P. J., Voelkel-Johnson C., Grant S., Dent P. (2009) Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms. Mol. Pharmacol. 76, 342–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghaeli P., Shahsavand E., Mesbahi M., Kamkar M. Z., Sadeghi M., Dashti-Khavidaki S. (2004) Comparing the effects of 8-week treatment with fluoxetine and imipramine on fasting blood glucose of patients with major depressive disorder. J. Clin. Psychopharmacol. 24, 386–388 [DOI] [PubMed] [Google Scholar]
- 44.Osawa Y.,, Seki E.,, Kodama Y.,, Suetsugu A.,, Miura K.,, Adachi M.,, Ito H.,, Shiratori Y.,, Banno Y.,, Olefsky J. M.,, Nagaki M.,, Moriwaki H.,, Brenner D. A.,, Seishima M. (2011) Acid sphingomyelinase regulates glucose and lipid metabolism in hepatocytes through AKT activation and AMP-activated protein kinase suppression. FASEB J. 25, 1133–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckmann N.,, Sharma D.,, Gulbins E.,, Becker K. A.,, Edelmann B. (2014) Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front. Physiol. 5, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]