Abstract
Objective
The purpose of this study was to assess the intra- and interexaminer reliability of the upper trapezius muscle and fascia thickness measured by ultrasonography imaging and strain ratio by sonoelastography in participants with myofascial pain syndrome.
Methods
Thirty-two upper trapezius muscles were assessed. Two examiners measured the upper trapezius thickness and strain ratio 3 times by ultrasonography and sonoelastography independently in the test session. The retest session was completed 6 to 8 days later.
Results
A total of 87.5% of participants had trigger points on the right side, and 22.5% had trigger points on the left side. For the test session, the average upper trapezius thickness, fascia thickness, and strain ratio measured by first and second examiners were 11.86 mm and 11.56 mm, 1.23 mm and 1.25 mm, and 0.94 and 0.99, respectively. For the retest session, the previously mentioned parameters obtained by first and second examiners were 11.76 mm and 11.39 mm, 1.27 mm and 1.29 mm, and 0.96 and 0.99, respectively. The intraclass correlation coefficients indicated good to excellent reliability for both within-intraexaminer (0.78-0.96) and between-intraexaminer (0.75-0.98) measurements. Also, the intraclass correlation coefficients and standard errors of measurement of interexaminer reliability ranged between 0.88 to 0.93 and 0.05 to 0.44 for both muscle and fascia thickness and 0.70 to 0.75 and 0.04 to 0.20 for strain ratio of upper trapezius, respectively.
Conclusion
Upper trapezius thickness measurements by ultrasonography and strain ratio by sonoelastography are reliable methods in participants with myofascial pain syndrome.
Key Indexing Terms: Trigger Points, Ultrasonography: Diagnostic Imaging, Elasticity Imaging Techniques, Myofascial Pain Syndromes
Introduction
Myofascial pain syndrome (MPS) is one of the leading causes of chronic pain and imposes large financial costs to societies.1 Tenderness to palpation, referral pain, autonomic disorders, taut bands in muscles, and trigger points (TrPs) are introduced as main symptoms of MPS.2 TrPs are closely associated with pathophysiology and clinical manifestation of MPS that may be active or latent.3 Active TrPs provide spontaneous pain and are responsible for MPS. Latent TrPs are tender to palpation but do not provoke the specific pattern of referral pain in related muscles.4 However, many studies have focused on active TrPs.5
Assessment tools such as thermography,6 pressure algometry,7 microanalytical system,3 electromyography,8 magnetic resonance elastography (MRE),9 ultrasonography imaging (USI),10 and sonoelastography (SE)11 have been used to provide versatile information about the MPS. Notably, the effectiveness of therapeutic modalities on pain relief is unclear because of lack of objective, repeatable, and reliable clinical outcome measures.12
USI is a noninvasive, real-time, and low-risk instrument that is commonly used in musculoskeletal injuries to visualize soft tissues such as muscle, nerve, tendon, ligament, and fascia.13 In addition, SE is an ultrasound-based imaging technique that shows the stiffness of soft tissues.14 The tissue stiffness can be calculated through tissue strain induced by probe compression that is lower in softer tissues.15 Although SE is not currently used in routine clinical practice, it may be useful for differentiating many musculoskeletal conditions such as congenital muscular dystrophy,16 myositis,17 chronic low back pain,18 plantar fasciitis,19 cervical stiffness,20 neck muscle hardness,21 and MPS.11
Evidently, both USI and SE are useful imaging methods to identify pathologic conditions of muscles in MPS.22 Changes in muscle function and stiffness are key clinical outcomes in patients with MPS.23, 24 Assessment of muscle thickness changes by USI and mechanical properties of muscle using SE have been considered in many clinical trials.25, 26, 27, 28 The previously mentioned measurements quantify the changes in myofascial structures after treatments, especially dry needling.29, 30
On one hand, the reliability of USI, as an instrument for measuring muscle thickness, has been reported in some studies.31, 32, 33, 34, 35 However, the reliability of the upper trapezius muscle and fascia thickness in participants with MPS using USI were not reported in previous research.11 On the other hand, muscle stiffness is an important factor that should be evaluated in participants with MPS.22 There are limited methodological studies about the reliability of SE in participants with MPS.36 Nevertheless, measurements of muscle and fascia thickness and strain ratio for the upper trapezius muscle have not been established in participants with MPS.10, 11 Therefore, the aim of the present study was to assess intra- and interexaminer reliability of USI and SE measures of the thickness and strain ratio of the upper trapezius in participants with MPS.
Methods
Participants
Participants with MPS were eligible and met inclusion criteria if found to have TrPs in the upper trapezius muscle unilaterally or bilaterally. One examiner did a thorough musculoskeletal evaluation to rule out other causes of muscle pain. Participants were included in the study if they fulfilled the following criteria: presence of at least 1 active trigger point in the central region of upper trapezius, age between 20 and 40 years, and pain duration ≥3 months. Participants were excluded if they had concomitant fibromyalgia, degenerative joint disease, cervical nerve root irritation, thoracic outlet syndrome, upper extremity entrapment syndromes, bursitis, severe joints immobility, and torticoli. Moreover, participants with history of rheumatoid arthritis, pregnancy, abnormal laboratory results, facial neuralgia, fracture, dislocation, neck and shoulder myopathy, neuropathy, myelopathy, cancer, infection, pulmonary diseases, HIV, and surgical interventions in the neck, shoulder, and other regions of the trunk were also excluded. Additionally, participants who had received a physical therapy program or any local injection therapy within the last 3 months and those with history of dry needling; nonsteroidal anti-inflammatory drug, opioid, or alcohol use; addiction; psychological problems; and athletics were ruled out too.10, 11 The Ethics Committee of the University of Social Welfare and Rehabilitation Sciences approved this study, and all participants were asked to read and sign a consent form.
Clinical Examination
The standard clinical criteria for diagnosing MPS were (1) palpable taut bands, (2) local tenderness in the taut bands (TrPs), and (3) pain recognition.2 Undoubtedly, local twitch response and referral pain were confirmatory findings.2, 11 The examiners determined the presence or absence of TrPs in the upper trapezius muscle. Palpation was made in the central region of the upper trapezius muscle between the C7 spinous process and the acromion process that coincided with the presence of active TrP.11 Active TrPs are associated with spontaneous pain, acutely tender to palpation and referral pain, but latent TrPs are painful only when palpated and don’t produce referral pain. Consequently, the examiners recorded the number of TrPs and marked the key and active trigger point in central region of the muscle for measurements. Finally, they did all the procedures and measurements independently. The examiners were experienced, certified, and trained in diagnosis of MPS.
Measurement of the Upper Trapezius Muscle and Fascia Thickness by USI
All participants were placed in the relaxed prone position with their elbows on the examination bed and the head midway on a special pillow.37 The C7 spinous process was found through flexion-extension method of the cervical spine.38 The examiner palpated the most prominent 2 cervical spinous processes with the index and middle fingers in the seated participant’s cervical spine during flexion. Consequently, through an assisted extension of the cervical spine, if the upper palpated cervical spinous process moved anteriorly while the lower one remained fixed, the latter would be marked C7. If both of the palpated spinous processes remained fixed, the upper cervical spinous process would be considered to be C7 and marked.38 Eventually, the examiner drew a line between C7 and acromion process and marked the midpoint of this line.
A diagnostic ultrasound instrument (Ultrasonix, Sonix MDP, Richmond, BC, Canada) with a linear array frequency of 5 to 14 MHz was used. The probe was rotated parallel to the muscle fibers and the middle portion of it was placed on the marked region of the muscle.37 When the examiner identified the longitudinal view of upper trapezius clearly, the closest vertical distance between superior and inferior high echogenic borders of the muscle, in the center of the image, was calculated as muscle thickness (Fig 1). As a result, the distance between the lower to upper part of the fascia was estimated as the fascial thickness of the upper trapezius.13, 39, 40
Fig 1.
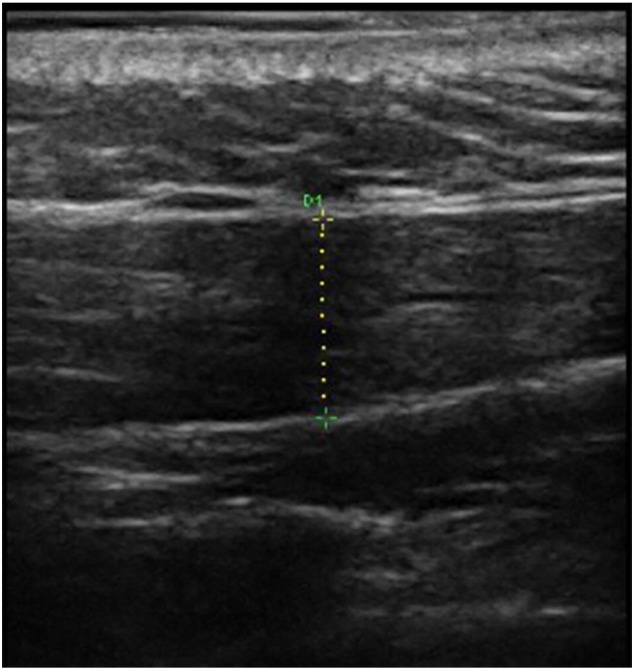
Thickness measurement technique for the upper trapezius.
Measurement of the Upper Trapezius Strain Ratio by SE
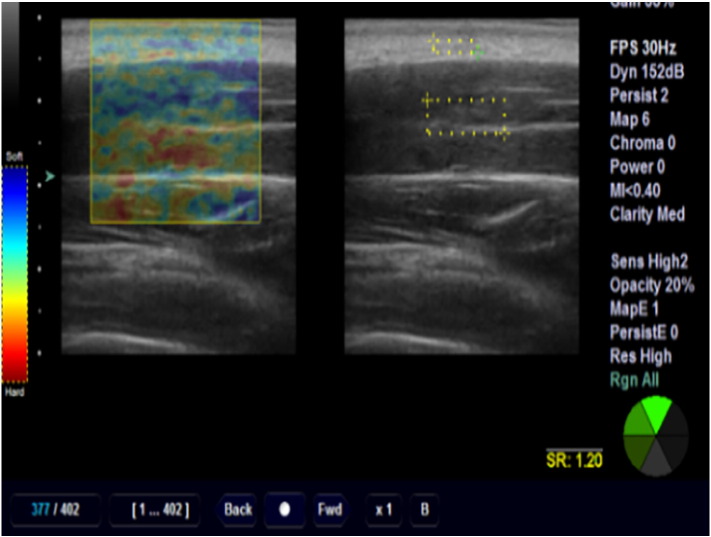
After measuring the thickness of upper trapezius muscle and fascia, the USI system was changed to elastography mode, which was the combination of 2 images: elastogram on the left side and B-mode image on the right side of the monitor. The elastographic image was color coded and superimposed on the B-mode scan.36 All probe placements procedures were similar to the description given in the previous section for muscle thickness.
The examiner tried to apply constant mild pressure on the skin by monitoring the pressure indicator feedback incorporated into the scanner.41 The pressure indicator feedback displayed in a scale from 1 to 6 levels, detecting the mean changes in the strain within the region of interest (ROI) per frame.15, 41 Initial pressure on the structure is an important factor in strain measurements.42 The pressure that was applied to the surface remained constant on level 4, which in turn was the optimal strain and best frame for measurements.41 A square ROI of approximately 2 × 5 mm was placed onto the fat and a square ROI with an approximate diameter of 3 × 8 mm was placed inside the muscle belly.21 The relative elasticity of the muscle compared with that of the fat was indicated by a strain ratio; it means that the ROI of the muscle was divided by the ROI of the fat (Fig 2).42 Three trials were performed by examiners during each session, and the retest session was performed 6 to 8 days later. Measurements were recorded at the end of expiration phase of breathing of both participant and examiner.36
Fig 2.
Sonoelastography image of upper trapezius muscle.
Statistical Analysis
Using STATA SE12 software (Statacorp LP, College Station, Texas), 0 by the command “sampsi_rho, p(0.9) one sided" for 1-sided α = 0.05, power = 0.90, null coefficient = 0.00, and alternative coefficient = 0.50, the sample size of 32.381 804 was calculated.43, 44 Therefore, a total number of 32 muscles were examined in this study.
Paired t test analysis was used to show any systematic bias between scores in test and retest sessions. For within- and between-session intra- and interexaminer reliability, the 3 trials of measuring muscle and fascia thickness and strain ratio by 2 examiners were averaged (in each session).45 Two-way random model intraclass correlation coefficients (ICCs) were calculated to examine the within- (ICC 2, 3) and between-session (ICC 2, 3) reliability of the measures of upper trapezius muscle and fascia thickness and strain ratio. The measured variables were ratio-scaled in nature, and their distributions did not differ significantly from normal by Shapiro-Wilk tests results. Therefore, paired t test and ICCs were assumed to be appropriate for the studies variables.44
To assess absolute reliability, the standard error of measurement (SEM; the square root of the error variance) was computed to estimate measurement error.45 The interpretations of reliability coefficients were based on a general rule: ICCs ≥0.75 are considered good, and ICCs ≥0.90 are considered excellent.46 To evaluate the clinically significant changes between 2 times of measurements, the minimal detectable change (MDC) was determined as 95% confidence interval (CI) of SEM (1.96 SEM).46 Furthermore, the coefficient of variation (CV) was calculated to indicate the similarities and differences of absolute reliability between measures ([standard deviation/mean] × 100). Significance levels were set at P < .05 for all measurements.45
Results
A total of 22 participants (15 women, 7 men; mean age: 25.7 ± 4.7 years; mean weight: 62.6 kg ± 8.5 kg; mean height: 169 cm ± 7.5 cm) with 32 upper trapezius MPS, for which 87.5% had TrPs on the right side and 22.5% had TrPs on the left side, were assessed. The mean values of upper trapezius muscle and fascia thickness and strain ratio for 2 examiners were not significantly different between test and retest sessions, indicating absence of systematic bias in measurements (Table 1).
Table 1.
Descriptive Data for Test-Retest Measures of the Upper Trapezius Muscle and Fascia Thickness and Strain Ratio in 32 Upper Trapezius Muscles
| Sonographic Measures | First Examiner |
Second Examiner |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test |
Retest |
P | Test |
Retest |
P | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| UTMT | 11.86 | 2.1 | 11.76 | 2.16 | .45 | 11.56 | 2.01 | 11.39 | 2.05 | .37 |
| UTFT | 1.23 | 0.38 | 1.27 | 0.38 | .31 | 1.25 | 0.47 | 1.29 | 0.41 | .42 |
| UTSR | 0.94 | 0.09 | 0.96 | 0.1 | .26 | 0.99 | 0.09 | 0.99 | 0.08 | .83 |
SD, standard deviation; UTFT, upper trapezius fascia thickness; UTMT, upper trapezius muscle thickness; UTSR, upper trapezius strain ratio.
Values are mean ± SD.
The average thickness of muscle and fascia and strain ratio in the test session were 11.86 mm and 11.56 mm, 1.23 mm and 1.25 mm, and 0.94 and 0.99 for the first and second examiners, respectively. Additionally, in retest session the average of these parameters was calculated as 11.76 mm and 11.39 mm, 1.27 mm and 1.29 mm, and 0.96 and 0.99 for the first and second examiners, respectively.
The within- and between-session intraexaminer reliability of upper trapezius muscle and fascia thickness and strain ratio are presented in Table 2. The ICC values had good to excellent reliability for both within- and between-intraexaminer measurements (ICC, first examiner: 0.92-0.96; ICC, second examiner: 0.86-0.98). Also, the ICC values for within- and between-session intraexaminer strain ratio measurements were good (ICC, first examiner: 0.78-0.81; ICC, second examiner: 0.75-0.80). The ICC, SEM, MDC, and CV values for interexaminer reliability are indicated in Table 3. The ICC and SEM of interexaminer reliability ranged between 0.88 to 0.93 and 0.05 to 0.44 for upper trapezius muscle and fascia thickness (MDC: 0.27-0.71; CV: 17.39%- 37.6%). Finally, the ICC and SEM of interexaminer reliability ranged between 0.70 to 0.75 and 0.04 to 0.20 for strain ratio of upper trapezius (MDC: 0.1-0.39; CV: 8.08%-10.24%).
Table 2.
Within and Between Session Reliability of the Upper Trapezius Muscle and Fascia Thickness and Strain Ratio in 32 Upper Trapezius Muscles With Myofascial Pain Syndrome
| Examiner | Sonographic Measures | Within-Session Intraexaminer |
Between-Session Intraexaminer |
||||
|---|---|---|---|---|---|---|---|
| ICC, Test | ICC, Retest | ICC | SEM | MDC | CV | ||
| First examiner | UTMT | 0.93 | 0.95 | 0.92 | 0.17 | 0.33 | 18.37 |
| UTFT | 0.96 | 0.95 | 0.93 | 0.10 | 0.20 | 29.92 | |
| UTSR | 0.81 | 0.78 | 0.79 | 0.05 | 0.10 | 10.49 | |
| Second examiner | UTMT | 0.91 | 0.95 | 0.90 | 0.18 | 0.35 | 18.01 |
| UTFT | 0.98 | 0.92 | 0.86 | 0.20 | 0.39 | 31.78 | |
| UTSR | 0.79 | 0.80 | 0.75 | 0.05 | 0.10 | 8.08 | |
CV, coefficient of variation; ICC, intraclass correlation coefficients; MDC, minimal detectable change; SEM, standard error of measurement; UTFT, upper trapezius fascia thickness; UTMT, upper trapezius muscle thickness; UTSR, upper trapezius strain ratio.
Table 3.
Interexaminer Reliability of the Upper Trapezius Muscle and Fascia Thickness and Strain Ratio in 32 Upper Trapezius Muscles With Myofascial Pain Syndrome
| Reliability | Measures | ICC | SEM | MDC | CV |
|---|---|---|---|---|---|
| Interexaminer (test session) | UTMT | 0.89 | 0.36 | 0.71 | 17.39 |
| UTFT | 0.88 | 0.17 | 0.33 | 37.60 | |
| UTSR | 0.71 | 0.05 | 0.10 | 9.09 | |
| Interexaminer (retest session) | UTMT | 0.92 | 0.44 | 0.86 | 18.00 |
| UTFT | 0.92 | 0.14 | 0.27 | 31.78 | |
| UTSR | 0.70 | 0.20 | 0.39 | 8.08 | |
| Interexaminer (test and retest sessions) | UTMT | 0.93 | 0.33 | 0.60 | 18.37 |
| UTFT | 0.91 | 0.14 | 0.27 | 29.92 | |
| UTSR | 0.75 | 0.04 | 0.10 | 10.42 |
CV, coefficient of variation; ICC, intraclass correlation coefficients; MDC, minimal detectable change; SEM, standard error of measurement; UTFT, upper trapezius fascia thickness; UTMT, upper trapezius muscle thickness; UTSR, upper trapezius strain ratio.
Discussion
The present study had good to excellent intraexaminer reliability for upper trapezius muscle and fascia thickness measurements. Moreover, the interexaminer reliability of the mentioned variables was good to excellent.
Our findings are compatible with some previous studies about the reliability of thickness measurements with USI.31, 32, 33, 34, 35 To date, the reliability of USI measures of the upper trapezius muscle in participants with MPS has not been reported.11 Day and Uhl33 examined the reliability for measuring scapular muscle thickness in asymptomatic participants and reported good within- and between-session reliability for the lower trapezius (ICC = 0.86-0.99) and serratus anterior muscles (ICC = 0.88-0.99). O’Sullivan et al46 established intra- and inter-rater reliability of lower trapezius thickness in 16 asymptomatic participants and reported moderate to high reliability (ICC = 0.88-0.99). Bentman et al31 assessed the test-retest reliability of middle trapezius in 16 asymptomatic participants and reported moderate between-session (ICC = 0.67) and good inter-rater (ICC = 0.81) reliability for the middle trapezius thickness measurement. Furthermore, Im Yi et al47 indicated that the intra- and inter-rater reliability of the supraspinatus thickness by USI were 0.91 and 0.88, respectively. Similar to the present study, they emphasized surface anatomy locations for better imaging measurements. In the present study, the line that was drawn between C7 and acromion process was important for high reliability of USI.31, 47
The strain ratio of the upper trapezius muscle was low (0.94 ± 0.09) in the present study. It means that the stiffness of upper trapezius is high in participants with MPS because the strain ratio is inversely related to stiffness.15 Ballyns et al48 indicated that stiffness of active myofascial trigger points and the surrounding muscle tissues were significantly higher than healthy ones. In addition, Muraki et al15 reported that the strain ratio of supraspinatus muscle may decrease after isometric contraction that indicated more stiffness in contraction state of a muscle such as MPS.2, 15
The intra- and interexaminer reliabilities of strain ratio measurement in the present study were moderate to good (ICC = 0.70-0.81). Muraki et al15 assessed the reliability of supraspinatus muscle and tendon strain ratio in 23 healthy individuals. The intraobserver reliability of strain ratio scores was high (ICC = 0.93-0.98). They used an acoustic coupler as a reference material and claimed that using an acoustic coupler in measurements can allow precise detection of the strain ratio of different tissues.15 In the present study, the fat layer was used as reference area. In many musculoskeletal studies of SE, the fat layer is selected as reference zone to measure strain ratio of the muscles because the stiffness of fat is almost stable and constant.49, 50 Leong et al36 reported excellent within and between-session intraoperator (ICC = 0.87-0.97) and interoperator (ICC = 0.78-0.83) reliability for the upper trapezius elasticity, measured by shear wave imaging, with the position of arm at rest and at 30° abduction. They suggested that the scanning site of the upper trapezius muscle with reference to body landmarks, the imaging parameters, and the location and size of the ROI may result in a more repeatable method for assessing muscle elasticity.36 Although some studies have reported interexaminer variability as a limitation of SE, many studies have described higher interexaminer agreement in real-time elastography.51, 52 SE can estimate the muscle stiffness in participants with MPS.11 The use of SE appears to be more suitable for measuring muscle stiffness than other methods such as MRE. Chen et al53 reported that patients with MPS had higher stiffness in the taut band region than its surrounding muscle tissues, as well as the normal tissue in healthy participants, using MRE. The sample size in that study was small and the reliability of stiffness scores was not reported.53 Compared with SE, environmental factors such as participant positioning and space may greatly influence MRE.15
The results of the present study indicated that interexaminer reliability was lower than intraexaminer reliability in measuring thickness or elasticity. Ozkan et al54 indicated that the intraexaminer reliability of elastography was excellent but the interexaminer reliability was low (0.46) in transplant kidneys. These findings may indicate the inconsistency in manual compression and probe placement as a result of different skin and fat elasticity of the participants.52, 54
It seems that the reliability of SE depends on many factors. One of them is the location and depth of the muscles. Interestingly, the superficial muscles, such as the trapezius, have greater reliability than deeper structures such as the gastrocnemius muscle because the applied pressure may not have reached to deeper tissues.42 In addition, the exact selection of the ROI should be considered. The extent and location of the ROI of the muscle and reference point (fat layer in this study) also affect the stiffness scores.15
The amount of manual pressure and monitoring of the device are other important factors that should be considered in reliability studies.22 In the present study, the amount of pressure was observed on the device as the green color on the middle side of the elastogram that ranged from 1 to 6. In some studies that had lower reliabilities, the amount of pressure was unclear or the device had not been equipped with pressure feedback.22
The positioning of the examiner and participants may affect the accuracy of imaging.36 Maher et al37 reported that the stiffness of the upper trapezius muscle was significantly greater in sitting position relative to prone position. All participants in the present study were located in prone position during the test trials with the head relaxed on the pillow. Additionally, the examiners were in a seated position during measurements and the images were recorded at the end of their expiration for both examiner and participant.
The type of muscle architecture and fiber orientation may affect the imaging of SE.55 In the present study, the strain ratio of the upper trapezius was obtained through scanning in the longitudinal view and the probe was placed parallel to the muscle fibers to avoid a muscle anisotropic artifact.47 In some studies, the probe placement for SE was in the longitudinal plane, whereas in other ones, the transverse plane over the ROI was selected for probe placement.11, 37
Limitations
Some limitations should be taken into account. First, the ROI of the present study was on the middle portion of the muscle belly (primary area of TrPs of the upper trapezius), which does not seem to exactly specify the region of trigger point relative to fat layer. Second, the strain ratio of other portions of the upper trapezius muscle has not been calculated. Third, the muscle activity has not been measured in the present study. Fourth, as in previous studies, the concerns about the amount of manual compression of the probe still remained unclear. Fifth, the correlation among biomechanics, structure, and local deformation of myofascial tissues is unknown in participants with MPS. Sixth, the findings of the present study can only be generalized to participants with MPS.
Conclusion
Measurement of the upper trapezius muscle and fascia thickness by USI is a good to excellent reliable intraexaminer and interexaminer method in participants with MPS. Additionally, an SE measurement of strain ratio in participants with MPS is highly reproducible with moderate to high intraexaminer and interexaminer reliability. Therefore, when measuring the upper trapezius thickness and strain ratio, if the surface anatomy and pressure of the probe proposed in this study are used, this will be helpful in evaluating the extent of the affected upper trapezius muscle regarding TrPs as well as in predicting functional evaluation and recovery in participants with MPS.
Acknowledgments
Acknowledgments
The authors thank Dr. Abdolhosein Emami Sigaroudi for help in revision.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): K.E., M.S., B.A., I.E.T., H.H.
Design (planned the methods to generate the results): K.E., M.S., B.A.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): K.E., M.S., B.A., I.E.T., H.H.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): K.E., M.S., B.A.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): K.E., M.S., B.A.
Literature search (performed the literature search): K.E., M.S., B.A., I.E.T., H.H.
Writing (responsible for writing a substantive part of the manuscript): K.E., M.S., B.A.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): K.E., M.S., B.A., I.E.T., H.H.
Practical Applications
-
•
Ultrasonography can be used to measure fascia thickness in MPS patients.
-
•
Sonoelastography can be used to measure muscle stiffness in MPS patients.
Alt-text: Image 1
References
- 1.Cummings M, Baldry P. Regional myofascial pain: diagnosis and management. Best Pract Res Clin Rheumatol. 2007;21(2):367–387. doi: 10.1016/j.berh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Simons DG, Travell JG, Simons LS. Lippincott Williams & Wilkins; Baltimore, MD: 1999. Travell & Simons' Myofascial Pain and Dysfunction: Upper Half of Body. [Google Scholar]
- 3.Shah JP, Danoff JV, Desai MJ. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653–660. [PubMed] [Google Scholar]
- 5.Fernández-de-las-Peñas C, Alonso-Blanco C, Fernández-Carnero J, Carlos Miangolarra-Page J. The immediate effect of ischemic compression technique and transverse friction massage on tenderness of active and latent myofascial trigger points: a pilot study. J Bodyw Mov Ther. 2006;10(1):3–9. [Google Scholar]
- 6.Diakow PR. Differentiation of active and latent trigger points by thermography. J Manipulative Physiol Ther. 1992;15(7):439–441. [PubMed] [Google Scholar]
- 7.Pöntinen PJ. Reliability, validity, reproducibility of algometry in diagnosis of active and latent tender spots and trigger points. J Musculoskelet Pain. 1998;6(1):61–71. [Google Scholar]
- 8.Giamberardino MA, Affaitati G, Fabrizio A, Costantini R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2011;25(2):185–198. doi: 10.1016/j.berh.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Bensamoun S, Basford JR, Thompson JM, An KN. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil. 2007;88(12):1658–1661. doi: 10.1016/j.apmr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30(10):1331–1340. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikdar S, Shah JP, Gebreab T. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90(11):1829–1838. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar H, Cummings C. Ultrasound imaging of embedded shrapnel facilitates diagnosis and management of myofascial pain syndrome. Pain Pract. 2013;13(5):405–408. doi: 10.1111/papr.12002. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker JL, Teyhen DS, Elliott JM. Rehabilitative ultrasound imaging: understanding the technology and its applications. J Orthop Sports Phys Ther. 2007;37(8):434–449. doi: 10.2519/jospt.2007.2350. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Andrade E, Hassan SS, Ahn H. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet Gynecol. 2013;41(2):152–161. doi: 10.1002/uog.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muraki T, Ishikawa H, Morise S. Ultrasound elastography–based assessment of the elasticity of the supraspinatus muscle and tendon during muscle contraction. J Shoulder Elbow Surg. 2015;24(1):120–126. doi: 10.1016/j.jse.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Drakonaki EE, Allen GM. Magnetic resonance imaging, ultrasound and real-time ultrasound elastography of the thigh muscles in congenital muscle dystrophy. Skeletal Radiol. 2010;39(4):391–396. doi: 10.1007/s00256-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 17.Drakonaki E, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012;85(1019):1435–1445. doi: 10.1259/bjr/93042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan ST, Fung PK, Ng NY. Dynamic changes of elasticity, cross-sectional area, and fat infiltration of multifidus at different postures in men with chronic low back pain. Spine J. 2012;12(5):381–388. doi: 10.1016/j.spinee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Wu CH, Chang KV, Mio S, Chen WS, Wang TG. Sonoelastography of the plantar fascia. Radiology. 2011;259(2):502–507. doi: 10.1148/radiol.11101665. [DOI] [PubMed] [Google Scholar]
- 20.Fruscalzo A, Londero A, Fröhlich C, Möllmann U, Schmitz R. Quantitative elastography for cervical stiffness assessment during pregnancy. Biomed Res Int. 2014;2014:826535. doi: 10.1155/2014/826535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo WH, Jian DW, Wang TG, Wang YC. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med Biol. 2013;39(8):1356–1361. doi: 10.1016/j.ultrasmedbio.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Park GY, Kwon DR. Application of real-time sonoelastography in musculoskeletal diseases related to physical medicine and rehabilitation. Am J Phys Med Rehabil. 2011;90(11):875–886. doi: 10.1097/PHM.0b013e31821a6f8d. [DOI] [PubMed] [Google Scholar]
- 23.Akhbari B, Salavati M, Ebarahimi I, Ezzati K, Haghighat KH. Association of ultrasonography findings with pain, range of motion, disability and pressure pain threshold in subjects with upper trapezius myofascial pain syndrome. PTJ. 2015;4(4):221–227. [Google Scholar]
- 24.Lisiński P, Huber J. Evolution of muscles dysfunction from myofascial pain syndrome through cervical disc-root conflict to degenerative spine disease. Spine (Phila Pa 1976) 2017;42(3):151–159. doi: 10.1097/BRS.0000000000001691. [DOI] [PubMed] [Google Scholar]
- 25.Koppenhaver SL, Walker MJ, Su J. Changes in lumbar multifidus muscle function and nociceptive sensitivity in low back pain patient responders versus non-responders after dry needling treatment. Man Ther. 2015;20(6):769–776. doi: 10.1016/j.math.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Koppenhaver S, Embry R, Ciccarello J. Effects of dry needling to the symptomatic versus control shoulder in patients with unilateral subacromial pain syndrome. Man Ther. 2016;26(1):62–69. doi: 10.1016/j.math.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Turo D, Otto P, Shah JP. Ultrasonic characterization of the upper trapezius muscle in patients with chronic neck pain. Ultrason Imaging. 2013;35(2):173–187. doi: 10.1177/0161734612472408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turo D, Otto P, Hossain M. Novel use of ultrasound elastography to quantify muscle tissue changes after dry needling of myofascial trigger points in patients with chronic myofascial pain. J Ultrasound Med. 2015;34(12):2149–2161. doi: 10.7863/ultra.14.08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koppenhaver SL, Walker MJ, Rettig C. The association between dry needling-induced twitch response and change in pain and muscle function in patients with low back pain: a quasi-experimental study. Physiotherapy. 2017;103(2):131–137. doi: 10.1016/j.physio.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Turo D, Otto P, Hossain M. Novel use of ultrasound elastography to quantify muscle tissue changes after dry needling of myofascial trigger points in patients with chronic myofascial pain. J Ultrasound Med. 2015;34(12):2149–2161. doi: 10.7863/ultra.14.08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentman S, O’Sullivan C, Stokes M. Thickness of the middle trapezius muscle measured by rehabilitative ultrasound imaging: description of the technique and reliability study. Clin Physiol Funct Imaging. 2010;30(6):426–431. doi: 10.1111/j.1475-097X.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 32.English CK, Thoirs KA, Fisher L, McLennan H, Bernhardt J. Ultrasound is a reliable measure of muscle thickness in acute stroke patients, for some, but not all anatomical sites: a study of the intra-rater reliability of muscle thickness measures in acute stroke patients. Ultrasound Med Biol. 2012;38(3):368–376. doi: 10.1016/j.ultrasmedbio.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Day JM, Uhl T. Thickness of the lower trapezius and serratus anterior using ultrasound imaging during a repeated arm lifting task. Man Ther. 2013;18(6):588–593. doi: 10.1016/j.math.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Sions JM, Velasco TO, Teyhen DS, Hicks GE. Ultrasound imaging: intraexaminer and interexaminer reliability for multifidus muscle thickness assessment in adults aged 60 to 85 years versus younger adults. J Orthop Sports Phys Ther. 2014;44(6):425–434. doi: 10.2519/jospt.2014.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talbott NR, Witt DW. Ultrasonographic measurements of lower trapezius muscle thickness at rest and during isometric contraction: a reliability study. Physiother Theory Pract. 2014;30(5):360–366. doi: 10.3109/09593985.2013.876693. [DOI] [PubMed] [Google Scholar]
- 36.Leong HT, Ng GY, Leung VY, Fu SN. Quantitative estimation of muscle shear elastic modulus of the upper trapezius with supersonic shear imaging during arm positioning. PLoS One. 2013;8(6):e67199. doi: 10.1371/journal.pone.0067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maher RM, Hayes DM, Shinohara M. Quantification of dry needling and posture effects on myofascial trigger points using ultrasound shear-wave elastography. Arch Phys Med Rehabil. 2013;94(11):2146–2150. doi: 10.1016/j.apmr.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Shin S, Yoon DM, Yoon KB. Identification of the correct cervical level by palpation of spinous processes. Anesth Analg. 2011;112(5):1232–1235. doi: 10.1213/ANE.0b013e3182110f9f. [DOI] [PubMed] [Google Scholar]
- 39.O’Sullivan C, Meaney J, Boyle G, Gormley J, Stokes M. The validity of rehabilitative ultrasound imaging for measurement of trapezius muscle thickness. Man Ther. 2009;14(5):572–578. doi: 10.1016/j.math.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Stecco A, Meneghini A, Stern R, Stecco C, Imamura M. Ultrasonography in myofascial neck pain: randomized clinical trial for diagnosis and follow-up. Surg Radiol Anat. 2014;36(3):243–253. doi: 10.1007/s00276-013-1185-2. [DOI] [PubMed] [Google Scholar]
- 41.Niitsu M, Michizaki A, Endo A, Takei H, Yanagisawa O. Muscle hardness measurement by using ultrasound elastography: a feasibility study. Acta Radiol. 2011;52(1):99–105. doi: 10.1258/ar.2010.100190. [DOI] [PubMed] [Google Scholar]
- 42.Yoshii Y, Ishii T, Etou F, Sakai S, Tanaka T, Ochiai N. Reliability of automatic vibratory equipment for ultrasonic strain measurement of the median nerve. Ultrasound Med Biol. 2014;40(10):2352–2357. doi: 10.1016/j.ultrasmedbio.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Lexell JE, Downham DY. How to assess the reliability of measurements in rehabilitation. Am J Phys Med Rehabil. 2005;84(9):719–723. doi: 10.1097/01.phm.0000176452.17771.20. [DOI] [PubMed] [Google Scholar]
- 44.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 45.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 46.O'Sullivan C, Bentman S, Bennett K, Stokes M. Rehabilitative ultrasound imaging of the lower trapezius muscle: technical description and reliability. J Orthop Sports Phys Ther. 2007;37(10):620–626. doi: 10.2519/jospt.2007.2446. [DOI] [PubMed] [Google Scholar]
- 47.Yi TI, Han IS, Kim JS, Jin JR, Han JS. Reliability of the supraspinatus muscle thickness measurement by ultrasonography. Ann Rehabil Med. 2012;36(4):488–495. doi: 10.5535/arm.2012.36.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ballyns JJ, Turo D, Otto P. Office-based elastographic technique for quantifying mechanical properties of skeletal muscle. J Ultrasound Med. 2012;31(8):1209–1219. doi: 10.7863/jum.2012.31.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ariji Y, Katsumata A, Hiraiwa Y. Use of sonographic elastography of the masseter muscles for optimizing massage pressure: a preliminary study. J Oral Rehabil. 2009;36(9):627–635. doi: 10.1111/j.1365-2842.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- 50.Thomas A, Degenhardt F, Farrokh A, Wojcinski S, Slowinski T, Fischer T. Significant differentiation of focal breast lesions: calculation of strain ratio in breast sonoelastography. Acad Radiol. 2010;17(5):558–563. doi: 10.1016/j.acra.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Yoon JH, Kim MH, Kim EK, Moon HJ, Kwak JY, Kim MJ. Interobserver variability of ultrasound elastography: how it affects the diagnosis of breast lesions. AJR Am J Roentgenol. 2011;196(3):730–736. doi: 10.2214/AJR.10.4654. [DOI] [PubMed] [Google Scholar]
- 52.Ragazzoni F, Deandrea M, Mormile A. High diagnostic accuracy and interobserver reliability of real-time elastography in the evaluation of thyroid nodules. Ultrasound Med Biol. 2012;38(7):1154–1162. doi: 10.1016/j.ultrasmedbio.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 53.Chen Q, Basford J, An KN. Ability of magnetic resonance elastography to assess taut bands. Clin Biomech (Bristol, Avon) 2008;23(5):623–629. doi: 10.1016/j.clinbiomech.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozkan F, Yavuz YC, Inci MF. Interobserver variability of ultrasound elastography in transplant kidneys: correlations with clinical-doppler parameters. Ultrasound Med Biol. 2013;39(1):4–9. doi: 10.1016/j.ultrasmedbio.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Whittaker JL, Stokes M. Ultrasound imaging and muscle function. J Orthop Sports Phys Ther. 2011;41(8):572–580. doi: 10.2519/jospt.2011.3682. [DOI] [PubMed] [Google Scholar]