Abstract
Stress research in youth typically considers either the autonomic nervous system or HPA axis. However, these systems are highly coordinated and physically interconnected. We examined whether the interrelation between cardio-autonomic and HPA axis measures was better associated with perceived stress than their singular associations. Children and adolescents (N = 201) collected saliva samples to measure cortisol (AUCAG, AUCI, maximum), wore an electrocardiogram monitor for 24 h to derive heart rate variability (HRV; LF, HF, LF/HF ratio), and completed the Perceived Stress Scale. The interaction between sympathovagal modulation (LF, LF/HF ratio) and cortisol awakening response (AUCAG, AUCI, maximum) explained significantly greater variance in perceived stress than either stress system alone. Higher sympathovagal modulation combined with higher cortisol awakening response was associated with greater perceived stress. Findings suggest that the inter-relation between cardio-autonomic and HPA axis activity may advance our understanding of how stress impacts health.
Keywords: Autonomic nervous system, Heart rate variability, Hypothalamic–pituitary–adrenal axis, Diurnal cortisol profile, Children, Adolescents, Perceived stress
1. Introduction
The stress response system is comprised of the autonomic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis. Stress and the repeated activation of the stress response system have been associated with adverse health outcomes in children and adolescents. Specifically, using prospective (Adam et al., 2010; Fuligni et al., 2009; Slopen, Kubzansky, McLaughlin, & Koenen, 2013) and cross-sectional designs (Dreger, Kozyrskyj, HayGlass, Becker, MacNeil, 2010; Kazuma, Otsuka, Matsuoka, & Murata, 1997; Nagai, Matsumoto, Kita, & Moritani, 2003; Sen, Aygun, Yilmaz, & Ayar, 2008; Van den Bergh & Van Calster, 2009), dysregulation of the stress response system has been associated with obesity, asthma, inflammation, and depression among children and adolescents. Studies examining the physiological mechanism by which stress “gets under the skin” to affect health outcomes typically consider either the autonomic nervous system or the HPA axis (Lovell, Moss, & Wetherell, 2011; Lucini, Di Fede, Parati, & Pagani, 2005; Sloan et al., 1994). Yet, the autonomic nervous system and HPA axis are highly coordinated and physically interconnected. Investigating the inter-relation between the autonomic nervous system and HPA axis may provide a more thorough understanding of the association between stress and health. Among children and adolescents, few studies have considered how stress is related to the inter-relation between the autonomic nervous system and HPA axis.
Activation of the autonomic nervous system and HPA axis in response to a stressor follows a coordinated, temporal sequence. The autonomic nervous system quickly promotes physiological changes through synaptic transmissions by its two branches: the sympathetic and parasympathetic nervous system. The parasympathetic system facilitates the sympathetic response to stress, commonly referred to as the “fight or flight” response, by withdrawing its inhibitory effects (Porges, 1995, 2007). This, in turn, promotes physiological changes including the release of noradrenaline from the locus coeruleus (Curtis, Lechner, Pavcovich, & Valentino, 1997; Jedema & Grace, 2004) and the stimulation of sympathetic preganglionic neurons to increase heart rate (Bengel & Schwaiger, 2004; Engelnad & Arnhold, 2005; Shahar & Palkovits, 2007). Conversely, the HPA axis is a hormonal system; thus, physiological changes associated with its activation occurs minutes after activation. The HPA axis is initiated by the release of the corticotrophin-releasing hormone from the paraventricular nucleus of the hypothalamus, which results in a series of endocrine events that culminates with the release of cortisol from the adrenal cortex (Egliston, McMahon, & Austin, 2007). Cortisol impacts many different physiological systems (e.g., immunity, metabolism) and plays a role in augmenting the activity of the autonomic nervous system, such as enhancing the sympathetically mediated cardiovascular response to stress (e.g., increased heart rate; Sapolsky, Romero, & Munck, 2000). Together, the autonomic nervous system and HPA axis work in concert to produce a state of biological and behavioral preparedness.
1.1. Interaction between stress systems: animal studies
Animal studies examining the physiological link between the autonomic nervous system and HPA axis provide multiple lines of evidence to support their inter-relation. First, the autonomic nervous system and the HPA axis are reciprocally innervated. Corticotrophin-releasing hormone neuronal afferents from the paraventricular nucleus project to the locus coeruleus (Reyes, Valentino, Xu, & Van Bockstaele, 2005) and noradrenergic neurons from the locus coeruleus project to the paraventricular nucleus (Itoi, Jiang, Iwasaki, & Watson, 2004; Ma & Morilak, 2005). Second, there is a feed forward mechanism between the autonomic nervous system and the HPA axis. Corticotrophin-releasing hormone increases the firing rate of locus coeruleus neurons and stimulates the release of noradrenaline (Jedema & Grace, 2004; Reyes, Valentino, & Van Bockstaele, 2008; Valentino & Van Bockstaele, 2008). In turn, noradrenaline promotes corticotrophin-releasing hormone mRNA expression in the paraventricular nucleus (Itoi et al., 2004; Ma and Morilak, 2005). Moreover, lesions to the locus coeruleus attenuate the HPA axis response to a stressor (Zeigler, Cass, & Herman, 1999). Third, animal studies suggest that the autonomic nervous system and the HPA axis are both under tonic inhibitory control by the Central Autonomic Network, which includes the prefrontal cortex and limbic structures (Benarroch, 1993; Ulrich-Lai and Herman, 2009); these findings have also been observed in adult human studies (Gianaros, Van der Veen, & Jennings, 2004; Herman, Ostrander, Mueller, & Figueriredo, 2005; Radley, Arais, & Sawchenko, 2006). Thus, studies of the structural and functional connectivity between the autonomic nervous system and the HPA axis highlight their interconnection.
1.2. Stress systems in humans: measurement of autonomic nervous system and HPA axis
Autonomic nervous system activity can be measured in humans using heart rate variability (HRV), an indicator of cardio-autonomic control (Pumprla, Howorka, Grove, Chester, & Nolan, 2002). Both the parasympathetic and sympathetic nervous systems innervate the sinoatrial node, the pacemaker of the heart (Berntson et al., 1997; Task Force, 1996), and modulate heart rate. Noradrenaline from sympathetic postganglionic receptors increases heart rate, while acetylcholine from parasympathetic postganglionic receptors decreases heart rate (Berntson et al., 1997). HRV is commonly quantified by frequency domain measures, which describe how power is distributed as a function of frequency. Two frequencies are predominantly considered: low frequency (LF, 0.04–0.15 Hz) and high frequency (HF, 0.15–0.40 Hz; Task Force, 1996). Pharmacological blockade studies indicate that HF is strongly associated with parasympathetic modulation, and LF is associated with both sympathetic and parasympathetic modulation (Cacioppo et al., 1994; Polanczyk et al., 1998). Some suggest that the LF/HF ratio reflects the balance of sympathetic and parasympathetic nervous systems, and is an indicator of sympathovagal modulation (Lahiri, Kannankeril, & Goldberger, 2008; Malliani, 2005; Sztajzel, 2004; Task Force, 1996); however, the physiological underpinning of LF remains debated in the literature (de Geus, Montano, Sloan, & Thayer, 2014). For instance, the LF/HF ratio has been associated with some measures of sympathetic modulation, such as ortho-static changes (Malliani, 2005), but not others (Heathers, 2014). Nevertheless, Heathers (2014, p. 3) notes that “a metric [such as LF/HF ratio] may be useful before it appears meaningful”.
HPA axis activity can be assessed using salivary cortisol sampling. Cortisol is released in a circadian fashion characterized by cortisol levels that peak within the first hour post-awakening and gradually decline throughout the day (Fries, Dettenborn, & Kirschbaum, 2009). The collection of multiple samples across the day are used to derive aggregate measures that describe the diurnal cortisol profile, including the awakening response and diurnal slope (Rotenberg, McGrath, Roy-Gagnon, & Thanh Tu, 2012). The awakening response refers to the rise in cortisol by 50–75% (approximately 4–15 nmol/L) during the first hour post-awakening, and is measured by total amount of cortisol released during the awakening response (AUCAG; area under the awakening response relative to ground or zero) and dynamic increase in the amount of cortisol secreted following awakening (AUCI; area under the curve relative to increase; Clow, Thorn, Evans, & Hucklebridge, 2004; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Diurnal slope is characterized as the decline in cortisol over the day. Single sample cortisol measures are commonly reported as well, including the maximum or specific time of day (e.g., morning, afternoon, bedtime; cf., Blair, Peters, & Granger, 2004; Cohen et al., 2006; El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008; Lupien, King, Meaney, & McEwen, 2001).
1.3. Interaction between stress systems: human studies
Human studies examining the concurrent functioning of cardio-autonomic and HPA axis activity provide further evidence in support of their inter-relation. Specifically, higher morning and afternoon cortisol levels have been associated with low HF among adolescents in ambulatory settings (El-Sheikh, Arsiwalla, Hinnant, & Erath, 2011); although, no association has been reported as well (Oldehinkel et al., 2010). Additionally, elevated cortisol awakening response (AUCI) has been associated with reduced LF and HF among young adults (Stadler, Evans, Hucklebridge, & Clow, 2011). These findings, taken together with those from the animal literature, converge to suggest that autonomic and HPA axis activity are coordinated and work together to respond to stress across the lifespan.
1.4. Theoretical rationale for considering the inter-relation
Extant psychophysiology theories highlight the importance of considering the role of the inter-relation between the autonomic nervous system and HPA axis. Bauer, Quas, and Boyce (2002) hypothesized that the coordination of the autonomic and HPA axis response to stress is related to an individual’s risk for adverse outcomes. Bauer et al. proposed two competing inter-relation models: Additive or Interactive. The Additive model contends that symmetrical activation of both systems (hyper-arousal: high autonomic and HPA axis activity; or hypo-arousal: low autonomic and HPA axis activity) increases risk. In contrast, the Interactive model contends that asymmetric activation increases risk, as the most adaptive physiological response may be when there is a balance between autonomic and HPA axis activity. Del Giudice, Ellis, and Shirtcliff (2010) extended Bauer et al.’s model by suggesting that distinct response patterns between the autonomic nervous system and HPA axis emerge due to early life experiences (e.g., exposure to chronic stress) and that the match or mismatch between environmental context and stress response patterns is vital for determining risk.
The Polyvagal Theory (Porges, 1995, 2007) and Neurovisceral Integration Model (Thayer & Lane, 2000 Thayer & Sternberg, 2006) also support examining the inter-relation and provide greater insight into how the autonomic nervous system and HPA axis may be inter-related. Grounded in Porges’ work on emotion regulation, both theories suggest that the parasympathetic nervous system plays an integral role in regulating the stress response system. Specifically, the parasympathetic nervous system is thought to moderate an individual’s stress response by either inhibiting or disinhibiting the sympathetic nervous system and HPA axis. Parasympathetic inhibition promotes a calm or restorative state, while parasympathetic disinhibition promotes the mobilization of energy and the “fight or flight” response (Porges, 2007). Adult findings from laboratory-based stress studies partially support these theories as both sympathetic and parasympathetic activity have been found to moderate the HPA axis response to stress (Cacioppo et al., 1995; Sgoutas-Emch et al., 1994; Uchino, Cacioppo, Malarkey, & Glaser, 1995; Weber et al., 2010). Similar studies with children and adolescents could not be identified; thus, it remains to be determined whether measures of parasympathetic modulation (e.g., HF) moderate HPA axis activity in youth. Examining the association between stress and the inter-relation of cardio-autonomic control and HPA axis activity in children is particularly opportune given that stressful experiences in childhood shape disease trajectories later in life (Hertzman, 1999).
1.5. Perceived stress and stress systems in children and adolescents
Studies examining the association between perceived stress with either cardio-autonomic or HPA axis activity in children and adolescents yield inconsistent results. Levels of perceived stress over the past year have been positively (Bevans, Cerbone, & Overstreet, 2008), negatively (Maldonado et al., 2008), and unassociated (Maldonado et al., 2008; Williamson, Birmaher, Dahl, & Ryan, 2005) with cortisol levels in children and adolescents. Adolescents’ momentary analysis of perceived stress has also been positively associated with cortisol levels, in a single study (Adam, 2006). Previous studies relating perceived stress to HRV in children and adolescents could not be identified; however, in the adult literature, findings have been mixed. Among adults, chronic perceived stress has been positively associated with LF and LF/HF ratio (Lucini et al., 2005); momentary levels of perceived stress have been negatively associated with HF (Looser et al., 2010); and, perceived stress levels over the past month have been unassociated with HRV (Stadler et al., 2011). Thus, the association between perceived stress and the stress response system remains unclear.
Two possible explanations may account for these inconsistencies. First, measurement limitations may contribute to the mixed findings, as the majority of studies with HPA axis activity (Bevans et al., 2008; Maldonado et al., 2008; Williamson et al., 2005) included only two cortisol samples collected on one day, which has been shown to yield poor reliability (Rotenberg et al., 2012). Timing of perceived stress measurement also varied across studies, ranging from momentary assessment to past year. The specific measures of cardio-autonomic control and HPA axis activity have also varied across studies, making synthesis difficult due to measurement differences. Second, the analytical approach may account for the mixed findings. Specifically, previous studies did not test the interaction between the autonomic nervous system and HPA axis, and instead only analyzed cardio-autonomic control or HPA axis activity alone. In the context of Bauer’s Additive Model (Bauer et al., 2002), individuals with greater perceived stress may have asymmetrical stress response systems, yet this finding would be masked if only HRV or cortisol measures are considered singularly. Thus, it is plausible that these measurement limitations and constrained analytical methods may account for the observed inconsistences in the literature. Comprehensive and concurrent assessment of both cardio-autonomic and HPA axis activity is required to tease apart and address these questions.
The overarching aim of the current study was to examine whether the inter-relation between cardio-autonomic and HPA axis activity was a better indicator of the association between perceived stress and the stress response system in children and adolescents, than either system alone. Informed by previous theoretical models, we first hypothesized that inter-relation models, which include the interaction between HRV and cortisol, would better explain perceived stress compared to models with HRV or cortisol singularly. The secondary aim was to further examine the patterning of the cardio-autonomic and HPA axis inter-relations. We hypothesized that altered cardio-autonomic activity (low parasympathetic, high sympathovagal modulation), combined with elevated cortisol awakening response, would be associated with higher perceived stress. Given the inconsistencies reported in the literature, we explored the patterning of the inter-relations across measures of cardio-autonomic and HPA axis activity.
2. Method
2.1. Participants
Children and adolescents aged 9–18 years were recruited to take part in the larger Healthy Heart Project, a longitudinal study examining early cardiovascular risk factors, at Concordia University, Montreal, QC. Flyers, postcards, and bookmarks were distributed throughout the community and in schools approved by the Montreal English School Board. Children and adolescents with serious psychopathology or medication use known to interfere with cardiovascular or endocrine functioning were excluded. This study was approved by the Concordia University Ethics Review Committee (UH2005-077 & 10000088).
Participants (N = 201) included children and adolescents (Mage = 12.69, SD = 2.05, see Table 1). About half of the sample was female (n = 90, 45%) and in the early to mid stages of pubertal adrenarche (stages I–III, n = 91, 45.2%). The majority of participants were of normal body mass (5–85th BMI percentile: n = 136; 67.7%). On average, parents were university educated (Meducation = 16.37 years, SD = 3.37) and had a yearly household income of $78.9K Canadian (SD = $52.4K). Youth had a mean wake-time of 7:31 am (SD = 1.29 h) and typically slept for 8.95 h (SD = 1.22). The majority of the physiological measures were collected while school was in session (78.1%), and most participants (85.1%) indicated that the collection of measures easily fit into their typical routine.
Table 1.
Descriptive statistics: demographics, cortisol, and HRV measures.
| M (n) | SD (%) | |
|---|---|---|
| Demographics | ||
| Age (8–18) | 12.69 | 2.05 |
| Female | (90) | (44.8%) |
| Adrenarche | ||
| Stage 0 | (14) | (7.0%) |
| Stage 1 | (33) | (16.4%) |
| Stage 2 | (23) | (11.4%) |
| Stage 3 | (37) | (18.4%) |
| Stage 4 | (49) | (24.4%) |
| Stage 5 | (45) | (22.4%) |
| BMI Z-score (−3.00 to 2.60) | 0.49 | 0.97 |
| Normal weight | (136) | (67.7%) |
| Overweight | (39) | (19.4%) |
| Obese | (22) | (10.9%) |
| Parental education (0–22 years) | 16.37 | 3.37 |
| Household income ($5K–210K CAN) | 78.9K | 52.4K |
| Perceived stress score (0–40) | 15.56 | 6.87 |
| Cortisol measures | ||
| AUCAG | 11.25 | 5.40 |
| AUCI | 2.31 | 5.38 |
| SlopeMax. | −1.05 | 0.51 |
| Maximum | 18.38 | 8.04 |
| HRV measures | ||
| LF (ms2) | 2061.99 | 1083.13 |
| HF (ms2) | 1656.49 | 1189.40 |
| LF/HF ratio | 1.63 | 0.79 |
Note: N = 201. AUCAG = area under curve relative to ground. AUCI = area under curve relative to increase. SlopeMax = diurnal slope anchored to max sample using regression. LF = low frequency. HF = high frequency.
2.2. Measures
2.2.1. Cortisol
Saliva samples were collected six times per day for two consecutive weekdays. Samples were collected at awakening (awake0), +30 min post-awakening (awake30), +45 min post-awakening (awake45), before lunch, before dinner, and at bedtime.
Saliva samples were collected using the Salivette sampling device. Participants were instructed to place the cotton swab under their tongue for at least 30 s. When saturated, it was placed back in the Salivette tube and refrigerated until returned at the second visit. Participants were instructed not to eat, drink, or brush their teeth 10 min before taking a sample. Participants recorded the date and time each sample was taken in a daily log, which was initialed by parents or teachers as a marker of compliance (97% of log entries initialed by parent/teacher). Compliance was also verified for the awakening (awake0) sample using accelerometry data (supine to sitting; Rotenberg & McGrath, 2014). Consistent with previous adult findings, most participants (88.1%) collected the awake0 sample within 15 min of the accelerometer-based wake-time (Dockray et al., 2008; DeSantis et al., 2011) and 91% of the participants collected the awake0, awake30, and awake45 samples within one hour post awakening; further details on compliance can be found in Rotenberg & McGrath (2014).
Upon receipt at the laboratory, saliva samples were stored in a sub-zero freezer until they were packaged in dry ice and couriered to the University of Trier, Germany for cortisol assaying. Cortisol levels are robust to environmental conditions associated with the shipping process (Clements & Parker, 1998). Cortisol levels were determined in duplicate using a competitive solid phase time-resolved fluorescence immunoassay with fluorometric end point detection (DELFIA; Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). The intra-assay coefficients of variation were less than 11%.
Untransformed cortisol values were used to derive four cortisol measures per day: area under the awakening response relative to ground (AUCAG), dynamic increase of the awakening response (AUCI), diurnal slope, and maximum. The diurnal slope was determined by standard linear regression and was anchored to the maximum sample (SlopeMax; for formulae see Rotenberg et al., 2012). Maximum was identified as the highest morning cortisol value identified each day. The two-day average of each cortisol measure was used in the analyses. All cortisol measures were normally distributed.
2.2.2. Heart rate variability (HRV)
Continuous electrocardiogram (ECG) recordings were collected with ambulatory monitors (Vivometrics, San Diego, California, USA) worn at home and school over 24 h. ECG data acquisition began during the first laboratory visit when participants were fitted with the ambulatory monitor and continued for 24 h. Using self-report measures and accelerometry, ECG recordings corresponding to waking hours were selected and only daytime ECG data were analyzed.
Raw ECG data were processed according to Task Force Guidelines (1996). The ECG signal was imported, converted, and formatted into MindWare files using Biolab 3.0 acquisition software (MindWare Technologies Ltd., Columbus, Ohio, USA). The MindWare file was then converted into an ASCII text file which was processed in Kubios® HRV v.2.0 (University of Eastern Finland, Kuopio, Finland; Niskanen, Tarvainen, Rantaaho, & Karjalainen, 2004). Artifact correction was conducted manually in 5 min epochs based on Task Force Guidelines (1996), and by visual inspection of the software graphical interface. Using a window width of 256 s, data were tapered using a Hanning window.
Frequency-domain measures of HRV were calculated for each 5 min epoch, and averaged across the entire daytime recording period. The two-day average of each HRV measure was used in analyses. Frequency domain measures were based on power spectral analysis (ms2) and were derived using the Fast Fourier Transformation. Frequency domain measures included: low frequency (LF; 0.04–0.15 Hz), high frequency (HF; 0.15–0.40 Hz), and the LF/HF ratio. All frequency measures were normally distributed.
2.2.3. Perceived stress
Perceived stress was assessed using the 10-item Perceived Stress Scale, which assesses how unpredictable, uncontrollable, and overwhelming an individual considers their life to be over the past month (Cohen, Kamarck, & Mermelstein, 1983; Cohen & Williamson, 1988). The 10-item Perceived Stress Scale has been shown to be valid and reliable in children and adolescents aged 11–20 (α = 0.72–0.76; Finkelstein, Kubzansky, Capitman, & Goodman, 2007; Seller, Copeland-Linder, Martin, & L’Heureux Lewis, 2006; α = 0.81 current study).
2.2.4. Pubertal stage
Pubertal stage was based on adrenarche (pubic hair growth) and was assessed using sex-specific illustrations corresponding to Tanner stages I–V (Growing and Changing Questionnaire; Golding, Pembray, & Jones, 2001). While visual examination performed by physician to assess sexual maturation status is the gold standard, it is often not conducted due to concerns about privacy and the sensitivity of the physical examination. Pubertal illustrations have demonstrated good reliability and validity (r = 0.77–0.91; Morris & Udry, 1980; Netherton, Goodyer, Tamplin, & Herbert, 2004).
2.2.5. Sleep duration
The quantity of sleep on the night preceding saliva sampling was recorded in the child’s daily logs. Sleep duration was calculated as the time elapsed between self-reported bedtime and wake-time. Wake-time was defined as the time children and adolescents woke-up and collected the awake0 saliva sample. Sleep duration is an established covariate of both HRV and cortisol (Jarrin et al., 2015; Rotenberg et al., 2012)
2.3. Procedure
Participants and their parents were scheduled for two visits to the laboratory. During the first visit, participants and their parents completed demographic and health questionnaires. Participants also completed the Perceived Stress Scale during the first visit. Participants were instructed how to use the Salivette sampling device (Salimetrics, Inc.) and provided saliva collection kits for home and school. They were also fitted with an ambulatory heart rate monitor. Salivary cortisol samples and the ECG recording began the day after the first visit. During the second visit, participants returned their saliva samples and the heart rate monitor. Informed consent and participant assent were obtained prior to the study. Participants received monetary compensation for their time.
2.4. Sample exclusion criteria
Of the initial 241 participants who were included in the larger Healthy Heart Project, participants who did not return any saliva samples (n = 15), collected saliva samples only on weekend days (n = 10), or did not have ECG recording due to equipment malfunction (n = 15) were excluded from the sample. Thus, the final sample included 201 participants. Excluded participants did not differ from the final sample on sex, age, pubertal stage, parental education, household income, cortisol measures, HRV measures, or perceived stress (results not shown for parsimony).
2.5. Statistical analyses
Variables were inspected for normality and outliers to ensure the assumptions of the analytic methods were met. Missing data were imputed using multiple imputation (McKnight, McKnight, Sidani, & Figueredo, 2007). Imputation of missing cortisol values (single samples not returned, 10%; insufficient saliva for assaying, 0.01%) was informed by data from the larger Healthy Heart Project (e.g., subsequent cortisol samples, day of sampling, puberty); missing values were imputed 20 times with re-sampling techniques. Box plots and scatterplots were visually inspected to check the distributions and linearity. Descriptive data (means, standard deviations, minimum, maximum, skewness, kurtosis) were reviewed for all variables. All analyses were conducted using IBM SPSS Statistics software (version 20).
To test the first hypothesis that the inter-relation models, which include interactions between HRV and cortisol, would better explain perceived stress compared to HRV or cortisol singularly, we conducted multivariate regression analyses (SPSS General Linear Model, weighted least squares estimation method). Due to concerns regarding multicollinearity (see correlations between stress system measures, top of Table 2), singular models tested the association between each stress system measure (HRV: HF, LF, LF/HR ratio; Cortisol: AUCI, AUCAG, SlopeMax, Maximum) with perceived stress. Inter-relation models included the main-effects and two-way interaction of HRV and cortisol measures. Both singular and inter-relation models adjusted for developmentally relevant covariates of HRV and cortisol that have been previously established in the literature (Jarrin et al., 2015; Rotenberg et al., 2012), including age, sex, pubertal stage, BMI Z-score, household income, parental education, sleep duration, and wake time. (Inclusion of compliance with cortisol sampling as a covariate did not alter results; data not shown for parsimony). Test statistics (ΔF and ΔR2) were used to compare the singular and inter-relation models to determine whether the inter-relation models accounted for significantly greater variance in perceived stress.
Table 2.
Correlations among HRV, cortisol, and covariate measures.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| HRV measures | |||||||
| 1. LF | – | ||||||
| 2. HF | .81** | – | |||||
| 3. LF/HF ratio | −.29** | −.62** | – | ||||
| Cortisol measures | |||||||
| 4. AUCAG | .05 | .05 | −.07 | – | |||
| 5. AUCI | .01 | .03 | .01 | .02 | – | ||
| 6. SlopeMax | −.11 | −.09 | −.08 | −.73** | −.38** | – | |
| 7. Maximum | .08 | .07 | −.06 | .81** | .39** | −.92** | – |
| Covariates | |||||||
| Age | .07 | −.06 | .15* | −.00 | .13 | .02 | .03 |
| Sex | −.30** | −.23** | .09 | .10 | .13 | −.08 | .15* |
| Adrenarche | .08 | −.01 | .02 | −.02 | .09 | .04 | .00 |
| BMI Z-score | −.07 | −.05 | −.06 | −.06 | −.12 | .13 | −.07 |
| Parent education | −.04 | .00 | −.04 | .03 | .08 | .06 | −.04 |
| Household income | .01 | .03 | −.03 | .08 | .09 | −.07 | .08 |
| Sleep duration | .07 | .11 | −.03 | −.05 | −.18* | −.01 | −.09 |
| Wake-time | .18* | .15* | −.03 | −.19** | −.11 | .14* | −.19* |
| Compliance | .07 | .07 | −.04 | .01 | −.02 | −.04 | .00 |
Note: AUCAG = area under curve relative to ground. AUCI = area under curve relative to increase. SlopeMax = diurnal slope anchored to max sample using regression. LF = low frequency. HF = high frequency. Bold values indicate significance:
p < 0.05;
p < 0.01.
To test the second hypothesis that altered cardio-autonomic activity (low parasympathetic, high sympathovagal modulation), combined with elevated cortisol awakening response, would be associated with higher perceived stress, we examined the interactions of the inter-relation regression models. Simple slopes analyses were used to interpret significant interactions (Aiken & West, 1991). We anticipated the interactions of low HF and high LF/HF ratio with high AUCAG or AUCI would be associated with higher perceived stress. Exploratory analyses were conducted for the interactions with LF HRV and maximum cortisol.
3. Results
Descriptive statistics are presented in Table 1. Correlations among HRV, cortisol, and covariate measures are presented in Table 2.
3.1. Singular versus inter-relation models
The first hypothesis that inter-relation models would be associated with perceived stress, above and beyond the singular HRV or cortisol models, was partially supported (see Table 3). Interrelation models with the interactions of LF*AUCAG, LF*Maximum, as well as LF/HF ratio*AUCI, and LF/HF ratio*Maximum accounted for significantly greater variance in perceived stress, than either the singular HRV or cortisol models. No interactions with HF were significant. The inter-relation of HRV and cortisol accounted for an additional 2–4% of the variance in perceived stress. Interestingly, AUCI was unassociated with perceived stress in the singular cortisol model, but its interaction with LF/HF ratio accounted for 4% of the variance in perceived stress.
Table 3.
Singular versus inter-relation model comparisons.
| HRV | Cortisol | Singular models HRV | Singular models cortisol | Inter-relation models | Model comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||
| βhrv | F | R2 | βcort | F | R2 | βcort | βhrv | βint | F | R2 | ΔFb | ΔR2 | ||
| LF | −.01 | 4.22 | .18 | – | – | – | – | – | – | – | – | – | – | |
| AUCAG | – | – | – | .17* | 4.82 | .20 | −.21 | −.54** | .67** | 4.96 | .25 | 4.72* | .04 | |
| AUCI | – | – | – | .08 | 4.23 | .18 | .07 | −.03 | .08 | 3.86 | .20 | 0.15 | .00 | |
| SlopeMax a | – | – | – | .15 | 4.52 | .21 | .27 | −.56** | −.24 | 4.81 | .26 | 5.56** | .04 | |
| Maximum | – | – | – | .19* | 4.96 | .21 | −.17 | −.49** | .63** | 5.05 | .25 | 3.76* | .03 | |
| HF | −.05 | 4.29 | .19 | – | – | – | – | – | – | – | – | – | – | |
| AUCAG | – | – | – | – | – | – | .08 | −.23 | .19 | 4.10 | .21 | 0.94 | .01 | |
| AUCI | – | – | – | – | – | – | .21 | −.04 | −.07 | 3.95 | .21 | 0.54 | .00 | |
| SlopeMax a | – | – | – | – | – | – | .17 | −.17 | .01 | 3.72 | .23 | 0.39 | .00 | |
| Maximum | – | – | – | – | – | – | .16 | −.15 | .08 | 4.34 | .22 | 0.74 | .01 | |
| LF/HF ratio | .01 | 4.22 | .18 | – | – | – | – | – | – | – | – | – | – | |
| AUCAG | – | – | – | – | – | – | −.10 | −.23 | .37 | 4.15 | .22 | 1.19 | .01 | |
| AUCI | – | – | – | – | – | – | −.33 | −.12 | .52* | 4.50 | .23 | 2.96* | .04 | |
| SlopeMax a | – | – | – | – | – | – | .82 | −.02 | −.79 | 3.93 | .24 | 1.12 | .01 | |
| Maximum | – | – | – | – | – | – | −.22 | −0.39± | .59* | 4.79 | .24 | 2.65± | .02 | |
Note: All analyses controlled for age, sex, BMI Z-score, adrenarche, parental education, household income, sleep duration, & wake-time. βcort = standardized beta coefficient for cortisol measure. βhrv = standardized beta coefficient for HRV measure. βint = standardized beta coefficient for interaction term. AUCAG = area under curve relative to ground. AUCI = area under curve relative to increase. SlopeMax = diurnal slope anchored to max sample using regression. LF = low frequency. HF = high frequency. Bold values indicate significance:
p < .07
p < .05
p < .01.
Analyses also controlled for maximum cortisol sample, anchor point for calculation of SlopeMax.
Change in F test-statistic conservatively based on higher F test-statistic of either singular model.
3.2. Patterning of inter-relations across cardio-autonomic and HPA axis activity
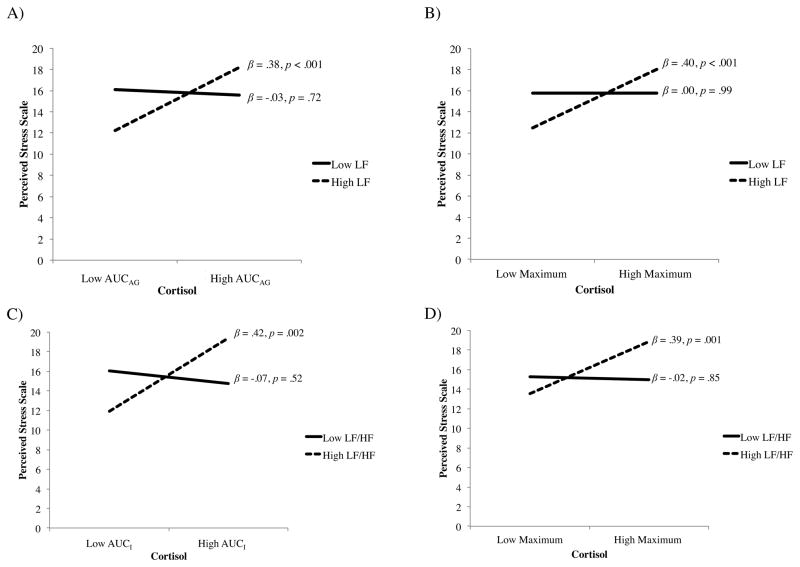
The second hypothesis that altered cardio-autonomic activity (low parasympathetic, high sympathovagal modulation), combined with elevated cortisol awakening response, would be associated with higher perceived stress was partly supported. Simple slopes analyses indicated that LF and LF/HF ratio moderated the association between the cortisol awakening response and perceived stress (Fig. 1A & C). Specifically, higher LF combined with greater AUCAG was associated with higher perceived stress; this inter-relation was not significant for AUCI. Higher LF/HF ratio combined with greater AUCI, but not AUCAG, was associated with higher perceived stress. Contrary to expectations, no inter-relations of HF, the most robust measure of parasympathetic activity, with the cortisol awakening measures were significant.
Fig. 1.
Simple slopes interpretation of significant inter-relations. Higher sympathovagal modulation and higher cortisol awakening response associated with greater perceived stress. In the panels, (A) higher LF and higher AUCAG ; (B) higher LF and higher maximum cortisol; (C) higher LF/HF ratio and higher AUCI ; and, (D) higher LF/HF ratio and higher maximum cortisol, associated with higher perceived stress. Data were dichotomized (mean ± 1 SD) for interpretation purposes only; continuous data were retained in analyses.
Exploratory analyses were conducted to consider the patterning of the remaining inter-relations across measures of cardio-autonomic and HPA axis activity. Higher LF combined with higher maximum cortisol was associated with higher perceived stress (Fig. 1B). Similarly, higher LF/HF ratio combined with higher maximum cortisol was associated with higher perceived stress (Fig. 1D). No patterns emerged between HF with any cortisol measures. Finally, no patterns emerged between SlopeMax with any HRV measures.
4. Discussion
Previous studies that have examined the association between perceived stress and the stress response system have typically investigated the autonomic nervous system or the HPA axis (Lovell et al., 2011; Lucini et al., 2005; Sloan et al., 1994). Yet, the autonomic nervous system and HPA axis are highly coordinated and physically interconnected (Ma & Morilak, 2005; Reyes et al., 2008; Ulrich-Lai & Herman, 2009). The current study is one of the first to test whether the inter-relation between cardio-autonomic control and HPA axis activity was better associated with perceived stress than the singular effect of either cardio-autonomic or HPA axis activity. We first hypothesized that the inter-relation models would be related to perceived stress, above and beyond the singular HRV or cortisol models. The patterning of the inter-relations was more closely examined as a secondary aim. We further hypothesized that altered cardio-autonomic activity (low parasympathetic, high sympathovagal modulation), combined with elevated cortisol awakening response, would be associated with higher perceived stress. The results partially supported the first hypothesis, as the inter-relation between measures of sympathovagal modulation (LF, LF/HF ratio) and the cortisol awakening response was significantly associated with perceived stress above and beyond the singular association of either stress response system alone. The second hypothesis was also partly supported, as cardio-autonomic activity moderated the association between the cortisol awakening response and perceived stress. Namely, greater sympathovagal modulation (high LF, high LF/HF ratio) combined with higher cortisol awakening response (AUCAG, AUCI) was associated with greater perceived stress. Exploratory analyses revealed these patterns were largely similar to the maximum cortisol measure, but no inter-relations with low parasympathetic activity (low HF) or diurnal cortisol slope were significant.
The current results were largely consistent with the few previous studies that considered the interaction between the autonomic nervous system and HPA axis among adults (Cacioppo et al., 1995; Uchino et al., 1995) and children and adolescents (El-Sheikh et al., 2008; Gordis, Granger, Susman, & Trickett, 2006). Similar to our findings, adult studies using laboratory stressors reported the HPA axis stress response was only evident among individuals with greater sympathetic activity, indexed by pre-ejection period; yet, HF was not associated with the HPA axis stress response (Cacioppo et al., 1995; Uchino et al., 1995). Ambulatory or laboratory-based stress studies with children and adolescents that examined the interaction between the autonomic nervous system and HPA axis could not be identified. However, sympathetic activity measured by alpha-amylase has been reported to influence the association between cortisol and aggressive behavior (Gordis et al., 2006), and internalizing and externalizing symptoms (El-Sheikh et al., 2008). Contrary to the current study, one study found that children with asymmetric cardio-autonomic and HPA axis activity (e.g., low HF, high cortisol) had the highest levels of anxiety and depression symptoms (El-Sheikh et al., 2011); however, three methodological differences likely account for these discrepancies: outcome variable, age of participants, and HPA axis measure. First, while the current study examined perceived stress as the outcome variable, El-Sheikh and colleagues measured symptoms of anxiety and depression. Second, participants were younger than those in the current sample (Mage = 9.06 versus Mage = 12.69, respectively). Third, only a single afternoon sample was used, which has been previously shown to have poor reliability (Rotenberg et al., 2012), compared to aggregate measures used in the current study. The current findings combined with previous results suggest that cardio-autonomic activity may moderate HPA axis activity. Prospective studies and experimental research are needed to further examine the patterning of cardio-autonomic and HPA axis activity.
In the present study, significant interactions between cardio-autonomic control and the HPA axis accounted for a unique portion of the variance (2–4%) in perceived stress. The magnitude of this effect is congruent with previous child studies that considered the inter-relation between autonomic and HPA axis activity. Gordis et al. (2006) found that the interaction of alpha-amylase and cortisol accounted for 7% of the variance in children’s aggressive behavior. El-Sheikh et al. (2008) reported that the interaction of HF and cortisol accounted for between 2–14% of the variance in anxiety and depressive symptoms. Overall, the current findings support emerging theories in the field of stress physiology that emphasize the importance of considering the inter-relation among physiological components of the stress response system (Bauer et al., 2002; Del Giudice et al., 2010). The interactions also suggest that existing analytical inconsistencies in the child literature regarding the relationship between stress and the stress response system (Bevans et al., 2008; Maldonado et al., 2008; Williamson et al., 2005) may be accounted for by the interaction between the stress systems. The significant contribution of the inter-relation in this study highlights the need for future research to consider the use of multi-dimensional models when examining how the stress response system is related to adverse outcomes.
Interpretations of the significant interactions in the current study indicate that children and adolescents with greater sympathovagal modulation and higher cortisol awakening response had greater perceived stress. This symmetrical activation may support Bauer et al.’s Additive model of risk, which suggests that children and adolescents are at a greater risk for adverse outcomes when there was hyper-arousal of cardio-autonomic and HPA axis activity; however, longitudinal studies testing this hypothesis are needed. Hyper-arousal of the stress response system is thought to exacerbate the development of disease (e.g., metabolic syndrome, cardiovascular disease) by promoting physiological changes, such as increased blood pressure and visceral fat accumulation (Bjorntorp, Holm, Rosmond, & Folkow, 2000).
Our observation that sympathovagal modulation interacts with cortisol awakening response coincides with anatomical studies regarding the regulation of the cortisol awakening response. In addition to the HPA axis, the cortisol awakening response is also regulated by direct sympathetic innervation via the splanchnic nerve (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2010; Engeland & Arnhold, 2005). Ulrich-Lai, Arnhold, and Engeland (2006) conducted splanchnic nerve transection in rats, which have a diurnal cortisol profile opposite that of humans (cortisol levels peak in evening and reach nadir in morning). The sphlanchnic nerve transection markedly reduced cortisol concentrations during the evening, but only modestly increased cortisol levels in the morning, evidencing that the sympathetic nervous system plays a prominent role in regulating the rise in cortisol post-awakening. We anticipated that parasympathetic modulation would have significantly interacted with the cortisol awakening response based on these findings and informed by the Polyvagal Theory and Neurovisceral model. However, contrary to expectations, inter-relations between HF and measures of the cortisol awakening response and diurnal slope did not emerge in our study. Further research examining the relationship between perceived stress and the inter-relation of cardio-autonomic control and HPA axis activity is warranted.
It is important to note that the physiological underpinning and interpretation of the LF and LF/HF ratio HRV measures remains actively debated in the field of psychophysiology (de Geus et al., 2014). Some suggest that LF is a measure of sympathetic modulation (Malliani, 1999; Montano et al., 2009); others argue that it is a measure of both sympathetic and parasympathetic modulation (Cacioppo et al., 1994; Reyes del Paso, Langewitz, Mulder, van Roon & Duschek, 2013); and still others suggest that it represents barore-flex activity (Heathers, 2014). Friedman (2007) suggests that in some contexts LF can serve as an index of sympathetic modulation, whereas in other contexts there can be extensive parasympathetic influence. Given that the current findings related to LF did not parallel HF, a known measure of parasympathetic modulation, it is possible that in the current study LF reflects sympathetic rather than parasympathetic modulation. This is consistent with several experimental and ambulatory studies, which contend that stress is associated with increased sympathetic control, as evidenced by increased LF (cf., Berntson & Cacioppo, 2004). Despite the lack of consensus on the interpretation of LF and LF/HF ratio, the results of the present study support the heuristic value of LF and LF/HF ratio, and their role as moderators of the association between stress and cortisol.
Investigating the association between the inter-relation of cardio-autonomic control and HPA axis activity with perceived stress during childhood and adolescence is particularly important given that this early timing in lifecourse development is considered a critical period when the effects of stress become biologically embedded (Danese & McEwen, 2012; Goodman, McEwen, Huang, Dolan, & Adler, 2005; Shokoff, Boyce, & McEwen, 2009). Children and adolescents experience numerous physiological changes (e.g., puberty, shift in sleep-wake cycle) and begin a vital stage of brain development (i.e., frontal cortex development; Goodman et al., 2005; Lupien, McEwen, Gunnar, & Heim, 2009; Shokoff et al., 2009). The importance of considering the impact of stress during childhood was recently highlighted by an animal study, which found that exposure to an acute stressor during childhood resulted in greater anxiety-like behavior and the highest basal cortisol levels later in life, compared to exposure to stress during the prenatal period, infancy, or adulthood (Cymberblit-Sabba et al., 2015). Identifying different patterns in stress responsivity, and relating these patterns to adverse outcomes, is important to advance the current understanding of how stress impacts children’s health. Examining the role of the inter-relation across different developmental periods is recommended for future research.
Three study limitations merit discussion. First, we were unable to make inferences about causality because we employed a cross-sectional design. Future research should examine longitudinal, developmental trajectories of the inter-relation between autonomic and HPA axis functioning and its link with stress. Second, we used the Perceived Stress Scale to measure the construct of perceived stress over the past month (Cohen et al., 1983; Cohen & Williamson, 1988). In addition to perceived stress, chronic stress and stressful life events have also been associated with adverse health outcomes in children and adolescents (Slopen et al., 2013). Future research should consider measuring stress from a multidimensional perspective (i.e., longer duration, early life adversity). Third, we measured cardio-autonomic activity using HRV, which is the gold standard for measuring parasympathetic activity, but only an indirect measure of sympathetic activity (Lahiri et al., 2008). Future research should consider other autonomic measures (i.e., salivary alpha amylase, pre-ejection period) to more comprehensively characterize the autonomic nervous system. In summary, future research would benefit from extending the current findings by examining how the inter-relation between the autonomic nervous system and the HPA axis is associated with other constructs of stress (e.g., stressful life events), over a longer period of time (e.g., past year, childhood), in clinical populations (e.g., children and adolescents with obesity, depression), and using additional measures of autonomic activity (e.g., alpha amylase). Despite these limitations, our methodological protocol to comprehensively measure cardio-autonomic control and HPA axis activity was an important strength of this study. Previous studies relating the autonomic nervous system or HPA axis to perceived stress have yielded inconsistent results which may be partly attributable to measurement limitations (e.g., collecting a single cortisol sample). In the current study, we collected measures over multiple days using continuous ambulatory ECG monitoring and repeated salivary cortisol sampling to derive stable and reliable indices of cardio-autonomic and HPA axis activity, in accordance with existing methodological guidelines (Adam & Kumari, 2009; Rotenberg et al., 2012; Task Force, 1996).
Taken together, our results are among the first to provide evidence for the importance of considering the inter-relation between the autonomic nervous system and the HPA axis when investigating the association between stress and the stress response system. We found that the inter-relation between cardio-autonomic control and the HPA axis was more associated with perceived stress, than the singular association of either stress response system alone. Children with higher sympathovagal modulation in combination with a higher cortisol awakening response had greater perceived stress. These results contribute to the existing literature by providing empirical evidence suggesting that the interaction between the autonomic nervous system and HPA axis is uniquely associated with perceived stress. Future studies examining the association between stress and health should consider the interrelation between the autonomic nervous system and the HPA axis to gain a more thorough understanding of the mechanism by which psychological stress effects health.
Acknowledgments
We thank the participants and their families of the Healthy Heart Project, the teachers and principals of the Montreal English School Board, and the research assistants and study coordinators of the Pediatric Public Health Psychology Laboratory at Concordia University. Special thanks to Natasha Hunt, Sabrina Giovanniello, and Neressa Noel for their continued dedication. This work was made possible through funding support from the Canadian Institutes of Health Research (CIHR) Operating Grants (MOP89886 and OCO79897), New Investigator Award (J.J. McGrath MSH95353), Canada Graduate Scholarships Master’s Award (S. Rotenberg), and Health Professional Student Research Award (S. Rotenberg).
References
- Adam E. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale: epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Thousand Oaks: Sage; 1991. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clinic Proceedings. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Bengel F, Schwaiger M. Assessment of cardiac sympathetic neuronal function using PET imaging. Journal of Nuclear Cardiology. 2004;11:603–616. doi: 10.1016/j.nuclcard.2004.06.133. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Van der Molen MW. Heart rate variability: origins, methods: and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. Heart rate variability: stress and psychiatric conditions. In: Malik M, Camm AJ, editors. Dynamic electrocardiography. Elmsford, NY: Futura; 2004. pp. 56–63. [Google Scholar]
- Bevans K, Cerbone A, Overstreet S. Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Developmental Psychopathology. 2008;20:257–272. doi: 10.1017/S0954579408000126. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Holm G, Rosmond R, Folkow B. Hypertension and the metabolic syndrome: closely related central origin? Blood Pressure. 2000;9:71–82. doi: 10.1080/08037050050151762. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R, Granger D. Physiological and neuropsychological correlates of approach/withdrawal tendencies in preschool: further examination of the behavioral inhibition system/behavioral activation system scales for young children. Developmental Psychobiology. 2004;45:113–124. doi: 10.1002/dev.20022. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31(6):586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Malarkey WB, Kiecolt-Glaser JK, Uchino BN, Sgoutas-Emch SA, Sheridan JF, Glaser R. Heterogeneity in neuroendocrine and immune responses to brief psychological stressors as a function of autonomic cardiac activation. Psychosomatic Medicine. 1995;57:154–164. doi: 10.1097/00006842-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park: Sage; 1988. pp. 31–67. [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. Journal of Pharmacology & Experimental Therapeutics. 1997;281:163–172. [PubMed] [Google Scholar]
- Cymberblit-Sabba A, Zubedat S, Aga-Mizrachi S, Biady G, Nakhash B, Rubin Ganel S, Avital A. Mapping the developmental trajectory of stress effects: pubescence as the risk window. Psychoneuroendocrinology. 2015;52:168–175. doi: 10.1016/j.psyneuen.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2010;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Mendelsohn KA, Doane LD. Concordance between self-reported and objective wakeup times in ambulatory salivary cortisol research. International Journal of Behavioral Medicine. 2011;17:74–78. doi: 10.1007/s12529-009-9053-5. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Dreger LC, Kozyrskyj AL, HayGlass KT, Becker AB, MacNeil BJ. Lower cortisol levels in children with asthma exposed to recurrent maternal distress from birth. Journal of Allergy & Clinical Immunology. 2010;125:116–122. doi: 10.1016/j.jaci.2009.09.051. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Egliston KA, McMahon C, Austin MP. Stress in pregnancy and infant HPA axis function: conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology. 2007;32:1–13. doi: 10.1016/j.psyneuen.2006.10.003. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Arsiwalla DD, Hinnant JB, Erath SA. Children’s internalizing symptoms: the role of interactions between cortisol and respiratory sinus arrhythmia. Physiology & Behavior. 2011;103:225–232. doi: 10.1016/j.physbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: the moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- Engeland WC, Arnhold MM. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 2005;28:325–331. doi: 10.1385/ENDO:28:3:325. [DOI] [PubMed] [Google Scholar]
- Finkelstein DM, Kubzansky LD, Capitman J, Goodman E. Socioeconomic differences in adolescent stress: the role of psychological resources. Journal of Adolescent Health. 2007;40:127–134. doi: 10.1016/j.jadohealth.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;50:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosomatic Medicine. 2009;71:329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus E, Montano N, Sloan R, Thayer J. Debate on promise and pitfalls of heart rate variability in psychosomatic medicine research. Symposium conducted at the meeting of the American Psychosomatic Society.2014. [Google Scholar]
- Gianaros P, Van der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart rate variability during working-memory tasks: implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Pembray M, Jones R. ALSPAC: the Avon longitudinal study of parents and children. I. Study methodology. Paediatric and Perinatal Epidemiology. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Goodman E, McEwen BS, Huang B, Dolan LM, Adler N. Social inequalities in biomarkers of cardiovascular risk in adolescence. Psychosomatic Medicine. 2005;67:9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]
- Gordis E, Granger D, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Heathers JAJ. Everything hertz: methodological issues in short-term frequency domain HRV. Frontiers in Physiology. 2014;5:1–15. doi: 10.3389/fphys.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueriredo H. Limbic system mechanisms of stress regulation: hypothalamo–pituitary–adrenocortical axis. Progress in Neuro-Psychopharamcology and Biological Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Science. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. Journal of Neuroendocrinology. 2004;16:348–355. doi: 10.1111/j.0953-8194.2004.01172.x. [DOI] [PubMed] [Google Scholar]
- Jarrin DC, McGrath JJ, Poirier P, Seguin L, Tremblay RE, Montplaisir JY, Seguin JR. Short-term heart rate variability in a population-based sample of 10-year-old children. Pediatric Cardiology. 2015;35:41–48. doi: 10.1007/s00246-014-0962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. Journal of Neuroscience. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuma N, Otsuka K, Matsuoka I, Murata M. Heart rate variability during 24 hours in asthmatic children. Chronobiology International. 1997;14:597–606. doi: 10.3109/07420529709001450. [DOI] [PubMed] [Google Scholar]
- Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease. Journal of the American College of Cardiology. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Looser RR, Metzenthin P, Helfricht S, Kudielka BM, Loerbroks A, Thayer JF, Fischer JE. Cortisol is significantly correlated with cardiovascular responses during high levels of stress in critical care personnel. Psychosomatic Medicine. 2010;72:281–289. doi: 10.1097/PSY.0b013e3181d35065. [DOI] [PubMed] [Google Scholar]
- Lovell B, Moss M, Wetherell MA. Perceived stress, common health complaints and diurnal patterns of cortisol secretion in young, otherwise healthy individuals. Hormones and Behavior. 2011;60:301–305. doi: 10.1016/j.yhbeh.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. 2005;46:1201–1206. doi: 10.1161/01.HYP.0000185147.32385.4b. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Developmental Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour: and cognition. Nature Review Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic–pituitary–adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. Journal of Neuroendocrinology. 2005;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Maldonado EF, Fernandez FJ, Traines MV, Wesnes K, Petrini O, Zangara A, Ambrosetti L. Cognitive performance and morning levels of salivary cortisol and alpha-amylase in children reporting high vs. low daily stress perception. Spanish Journal of Psychology. 2008;11:3–15. doi: 10.1017/s1138741600004066. [DOI] [PubMed] [Google Scholar]
- Malliani A. The pattern of sympathovagal balance explored in the frequency domain. Physiology. 1999;14:111–117. doi: 10.1152/physiologyonline.1999.14.3.111. [DOI] [PubMed] [Google Scholar]
- Malliani A. Heart rate variability: from bench to bedside. European Journal Of Internal Medicine. 2005;16:12–20. doi: 10.1016/j.ejim.2004.06.016. [DOI] [PubMed] [Google Scholar]
- McKnight PE, McKnight KM, Sidani S, Figueredo AJ. Missing data: a gentle introduction. New York: The Guilford Press; 2007. [Google Scholar]
- Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Rabello Casali K, Iellamo F. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neuroscience and Biobehavioral Reviews. 2009;33:71–80. doi: 10.1016/j.neubiorev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Nagai N, Matsumoto T, Kita H, Moritani T. Autonomic nervous system activity and the state and development of obesity in Japanese school children. Obesity Research. 2003;11:25–32. doi: 10.1038/oby.2003.6. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Niskanen JP, Tarvainen MP, Rantaaho PO, Karjalainen PA. Software for advanced HRV analysis. Computer Methods and Programs in Biomedicine. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Ormel J, Bosch NM, Bouma EMC, Van Roon AM, Rosmalen JGM, Riese H. Stressed out? Associations between perceived and physiological stress responses in adolescents: the TRAILS study. Psychophysiology. 2010;48:441–452. doi: 10.1111/j.1469-8986.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- Polanczyk CA, Rohde LE, Moraes RS, Ferlin EL, Leite C, Ribeiro JP. Sympathetic nervous system representation in time and frequency domain indices of heart rate variability. European Journal of Applied Physiology and Occupational Physiology. 1998;79(1):69–73. doi: 10.1007/s004210050475. [DOI] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995;19(2):225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represents measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applicatinons. International Journal of Cardiology. 2002;84:1–14. doi: 10.1016/s0167-5273(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arais CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. Journal of Neuroscience. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Valentino RJ, Van Bockstaele EJ. Stress-inducted intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. European Journal of Neuroscience. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- ReyesdelPaso G, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ, Roy-Gagnon MH, Thanh Tu M. Stability of the diurnal cortisol profile in children and adolescents. Psychoneuroendocrinology. 2012;37:1981–1989. doi: 10.1016/j.psyneuen.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ. Sampling compliance for cortisol upon awakening in children and adolescents. Psychoneuroendocrinology. 2014;40:69–75. doi: 10.1016/j.psyneuen.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seller RM, Copeland-Linder N, Martin PP, L’Heureux Lewis R. Racial identity matters: the relationship between racial discrimination and psychological functioning in African American adolescents. Journal of Research on Adolescence. 2006;16:187–216. [Google Scholar]
- Sen Y, Aygun D, Yilmaz E, Ayar A. Children and adolescents with obesity and metabolic syndrome have high circulating cortisol levels. Neuroendocrinology Letters. 2008;29:141–145. [PubMed] [Google Scholar]
- Sgoutas-Emch SA, Cacioppo JT, Uchino BN, Malarkey W, Pearl D, Kiecolt-Glaser JK, Glaser R. The effects of an acute psychological stressor on cardiovascular, endocrine, and cellular immune response: a prospective study of individuals high and low in heart rate reactivity. Psychophysiology. 1994;31:264–271. doi: 10.1111/j.1469-8986.1994.tb02215.x. [DOI] [PubMed] [Google Scholar]
- Shahar T, Palkovits M. Cross over of forebrain and brainstem neuronal projections to spinal cord sympathetic preganglionic neurons in the rat. Stress. 2007;10:145–152. doi: 10.1080/10253890701424712. [DOI] [PubMed] [Google Scholar]
- Shokoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Boni SM, Paik M, Bigger JT, Gorman JM. Effects of mental stress throughout the day on cardiac autonomic control. Biological Psychology. 1994;37:89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T, Evans P, Hucklebridge F, Clow A. Associations between the cortisol awakening response and heart rate variability. Psychoneuroendocrinology. 2011;36:454–462. doi: 10.1016/j.psyneuen.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Medical Weekly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–381. [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond hear rate variability: vagal regulation of allostatic systems. Annals of the New York Academy of Science. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Malarkey W, Glaser R. Individual differences in cardiac sympathetic control predict endocrine and immune responses to acute psychological stress. Journal of Personality and Social Psychology. 1995;69:736–743. doi: 10.1037//0022-3514.69.4.736. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2006;290(4):R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EV. Convergent regulation of locus coeruleus activity as an adaptive response to stress. European Journal of Pharmacology. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children’s Depression Inventory. Psychoneuroendocrinology. 2009;34:791–794. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Deter HC. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. European Journal of Applied Physiology. 2010;109:201–211. doi: 10.1007/s00421-009-1341-x. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Dahl RE, Ryan ND. Stressful life events in anxious and depressed children. Journal of Child and Adolescent Psychopharmacology. 2005;15:571–580. doi: 10.1089/cap.2005.15.571. [DOI] [PubMed] [Google Scholar]
- Zeigler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic–pituitary–adrenocortical axis responses to stress. Journal of Neuroendocrinology. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]