Abstract
Purpose
To compare patient and Medicare savings from the use of optical coherence tomography (OCT) in guiding therapy for neovascular age-related macular degeneration (nvAMD) to the research investments made in developing OCT by the National Institutes of Health (NIH) and the National Science Foundation (NSF).
Design
Observational cohort study.
Methods
Main outcome measures were spending by Medicare as tracked by Current Procedural Terminology codes on intravitreal injections (67028), retinal OCT imaging (92134) and anti-VEGF treatment-specific J-codes (J0178, J2778, J9035, J3490 and J3590). These claims were identified from the Medicare Provider Utilization and Payment Data from the Centers for Medicare and Medicaid Services among fee-for-service (FFS) Medicare beneficiaries from 2012 – 2015; 2008 claims were acquired from the 100% FFS Part B Medicare Claims File. OCT research costs were determined by searching for grants awarded by NIH and NSF from inception to 2015. All costs and savings were discounted by 3% annually and adjusted for inflation to 2015 dollars.
Results
From 2008 – 2015, the United States government and nvAMD patients have accrued an estimated savings of $9.0 billion and $2.2 billion, respectively, from the use of OCT to guide personalized anti-VEGF treatment. The $9.0 billion represents a 21-fold return on government investment into developing the technology through NIH and NSF grants.
Conclusions
Although an overall cost-benefit ratio of government-sponsored research is difficult to estimate because the benefit may be diffuse and delayed, the investment in OCT over two decades has been recouped many times over in just a few years through better personalized therapy.
TOC image
We compared patient and Medicare savings from the use of optical coherence tomography (OCT) in guiding therapy for neovascular age-related macular degeneration (nvAMD) to the research investments made in developing OCT by the National Institutes of Health (NIH) and the National Science Foundation (NSF). We found that the investment in OCT over two decades has been recouped many times over in just a few years through better personalized therapy.
Introduction
Healthcare innovation is often associated with expensive new medical devices and drugs. For example, in 2000, new cancer drugs were priced from $5,000 – $10,000 for a year of therapy. By 2012, prices averaged more than $100,000.1 With drug prices increasing, technologies that can limit their use through appropriate personalization of care are becoming increasingly important.
Concurrent with the arrival of costly anti-vascular endothelial growth factor (anti-VEGF) biologics to treat neovascular (“wet”) age-related macular degeneration (nvAMD), ophthalmologists adopted optical coherence tomography (OCT) to more efficiently use these effective, but expensive, drugs. OCT is the most frequently used imaging technique within the field and aids in the diagnosis and monitoring of diseases such as glaucoma, diabetic macular edema and nvAMD.2 The technology uses low-coherence interferometry to rapidly and non-invasively obtain three-dimensional, micron-resolution images of the retina and choroid.3, 4 First popularized in ophthalmology, the use of OCT is expanding to other medical fields such as cardiology, neurology, gastroenterology and dermatology, as well as non-medical applications like industrial non-destructive testing and even art conservation.5–10
OCT is used frequently for the diagnosis and management of nvAMD, a potentially blinding condition in which abnormal blood vessels grow and leak fluid into the macula – the central part of the retina responsible for high-acuity vision.11 Anti-VEGF drugs injected directly into the eye have successfully treated this condition.12–16 There are two commonly used anti-VEGF drugs that are approved by the Food and Drug Administration (FDA) for this indication: ranibizumab (Lucentis, Genentech/Roche, South San Francisco, CA) and aflibercept (Eylea, Regeneron, Tarrytown, NY). Both of these protein drugs are effective, but are very expensive at approximately $2,000 per dose. A third anti-VEGF drug, bevacizumab (Avastin, Genentech/Roche, South San Francisco, CA) is more economical at ~$70 per dose, but bevacizumab is not FDA approved for this purpose despite being the most frequently used drug for nvAMD.17, 18 With FDA-approved monthly injections for ranibizumab and bimonthly injections for aflibercept, the cost for treatment per year, per patient is $24,000 and $12,000, respectively. Due to the high cost of these two drugs and the increasing incidence of nvAMD as the population ages, ranibizumab and aflibercept accounted for over 16% of Medicare Part B (drug) spending in 2013, at roughly $2.4 billion.19
Before OCT was part of routine clinical practice, physicians were limited to following a fixed, FDA-approved treatment schedule for anti-VEGF drugs to treat nvAMD. For example, the first, now rarely used, anti-VEGF drug to treat nvAMD known as pegaptanib (Macugen, OSI Pharmaceuticals, Melville, NY) required one injection every six weeks. Now, OCT has enabled ophthalmologists to personalize anti-VEGF therapy. A patient’s anti-VEGF schedule can be individually tailored based on the presence or absence of excess macular fluid, which can be detected with OCT imaging.
Personalized therapy is typically separated into two phases. The induction phase of treatment requires monthly injections until the excess macular fluid is resorbed and usually lasts two to three months. Then, in the much lengthier maintenance phase, the injection frequency can be reduced as long as OCT imaging shows that macular fluid has not re-accumulated. Two common OCT-guided treatment regimens are known as pro re nata (PRN) or “as needed,” and “treat and extend” (TAE).20–22 While there is still some controversy over whether TAE and PRN regimens are as effective as monthly dosing, most studies have shown non-inferiority for these maintenance protocols.23–26 In the PRN regimen, a patient returns to the clinic once a month for OCT evaluation. If the OCT image indicates the absence of macular fluid, then the patient does not receive an injection; if fluid is present, then an injection is given. In the TAE regimen, when OCT imaging demonstrates resolution of macular fluid, an injection is still given and the visit interval is extended (e.g., two weeks). For example, if the patient returns after four weeks and the fluid has resorbed, then an injection is given and the next appointment is scheduled for six weeks. At the six-week visit, if no fluid is detected by OCT imaging, then an injection is given and the interval is extended to eight weeks. If at any time OCT imaging identifies the recurrence of macular fluid, then an injection is given and the next appointment interval is decreased (e.g. from eight weeks to six weeks). The treatment interval continues to be decreased until the macula is fluid-free once again. TAE and PRN regimens were identified as the preferred practice pattern by 91% of surveyed retina specialists in 2015.27
By reducing the number of expensive anti-VEGF injections a nvAMD patient receives, these personalized-treatment protocols cut costs in the healthcare system and reduce the treatment burden on patients and clinicians.28 Without the high-resolution, non-invasive macular imaging by OCT, these personalized regimens would be significantly more difficult to accomplish because physicians would have to treat according to the fixed dosing regimen used in the pivotal trials or possibly rely on more invasive and expensive angiography to direct therapy. Here, we examine the cost savings enjoyed by patients and Medicare in treating nvAMD with OCT-enabled personalized-treatment protocols compared with a fixed-treatment schedule. We estimate the return on investment from this single application of the technology relative to the government funding of research in this area and reimbursement for use.
Methods
Definitions
Fixed-regimen spending: Defined as total Medicare spending on anti-VEGF drugs and their delivery (i.e., intravitreal injection) for the treatment of nvAMD in the absence of OCT. Assumes physicians would need to inject patients at the fixed monthly (ranibizumab) or bimonthly (aflibercept) schedule on the FDA label; bevacizumab is assumed to be delivered on a monthly schedule.
Personalized-regimen spending: The estimated Medicare drug and delivery spending on OCT-guided anti-VEGF treatment for nvAMD, assuming that physicians use PRN and TAE regimens according to practice pattern surveys, and assuming that PRN and TAE regimens requires fewer injections according to our meta-analysis of major published studies.
Actual Medicare spending: The actual Medicare drug and delivery spending on anti-VEGF therapy for nvAMD by Medicare. This is expected to be lower than the above hypothetical calculations (see Discussion).
OCT-related government investment: Limited to the cost of reimbursing clinicians for each OCT image on nvAMD patients, and funding for the development of OCT technology and its clinical applications by the National Institutes of Health (NIH) and National Science Foundation (NSF).
Neovascular-AMD patient savings: Savings nvAMD patients experience from the use of OCT in managing their disease, stemming from the 20% copay for each anti-VEGF injection and OCT image under Medicare Part B. For simplicity, assumes the absence of supplementary Medigap insurance.
Study Design
We used the free-to-access Medicare Provider Utilization and Payment Data 2012 – 2015: Physician and Other Supplier Public Use Files (Physician and other Supplier PUF), and previously published data from the 2008 100% fee-for-service (FFS) Part B Medicare Claims File, both from the Center for Medicare and Medicaid Services (CMS), as our primary sources of Medicare data.29, 30 Similar databases from 2009 – 2011 were not used in this analysis because they are not publicly available. The files contained information on all Medicare Part B claims provided to FFS Medicare beneficiaries, representing ~70% of the Medicare beneficiary population. Medicare Advantage beneficiaries are not included in these data sets.31
All anti-VEGF drug claims were identified by treatment-specific J code: J9035, J3490 and J3590 for bevacizumab; J2778 for ranibizumab; J0178 for aflibercept. While J3490 and J3590 are unclassified drug and biologic J codes, respectively, previous work has shown that these codes are overwhelmingly paired with International Classification of Diseases (ICD-9) code 362.52 – exudative senile macular degeneration.18, 29 Because all bevacizumab codes are used in reimbursement for other indications (e.g., cancer), codes for 2012 – 2015 were limited by provider type (i.e., ophthalmology). Codes J3490 and J3590 were further limited to providers with an average Medicare allowed payment amount of less than two times the Medicare reimbursement rate – equal to the Average Sales Price (ASP) + 6%.17, 32 The total number of patients receiving each drug in 2012 – 2015 was found by summing each provider’s count of unique beneficiaries paired with an anti-VEGF J code. The number of patients using ranibizumab and bevacizumab in 2008 was found in the literature and assumed to increase linearly to 2012 (see Table S1 in the Supplementary Materials).29
Spending on intravitreal injection (delivery) of anti-VEGF drugs was identified by Current Procedural Terminology (CPT) code 67028. Previous work has shown that CPT code 67028 is overwhelmingly paired with ICD-9 code 362.52 – exudative senile macular degeneration.18
We obtained consumer price index data to adjust government research expenditures and medical care consumer price index data to adjust Medicare spending for inflation to 2015 dollars.33 Starting from the earliest investment in OCT (1995), we discounted all future expenditures and savings by 3% annually to account for the time value of money.34
Fixed-regimen spending
Fixed-regimen Medicare spending on anti-VEGF drugs and their delivery were calculated using the number of nvAMD patients on each drug in a given year, the Medicare reimbursement rate for each drug (ASP + 6%) and injection, and the FDA-approved number of injections per year (12 for bevacizumab and ranibizumab, six for aflibercept). Due to sequestration, Medicare reimbursement was ASP + 4% in 2013.17, 32, 35 Total fixed-regimen spending on each anti-VEGF drug and its delivery was found by summing all years.
Personalized-regimen spending
We performed a meta-analysis of the literature to determine the number of injections per year an average nvAMD patient would receive on the PRN or TAE treatment protocols during the maintenance phase of their therapy. The maintenance phase is defined as the period after the induction phase, when newly diagnosed patients receive a series of 1–3 monthly anti-VEGF injections to bring the disease under control. The meta-analysis was performed by a PubMed search using the terms “((“PRN” or “treat and extend” or “inject and extend” or “as needed”) AND (ranibizumab or aflibercept or bevacizumab) AND AMD).” Articles that had patients undergoing intravitreal injections of 0.5 mg ranibizumab, 1.25 mg bevacizumab or 2.0 mg aflibercept using TAE or PRN regimens for treatment of nvAMD were included. All evaluated papers were in English and had their references to related articles examined. Manuscripts with non-treatment naïve patients, or with patients suffering nvAMD secondary to another condition or receiving anti-VEGF treatment in conjunction with another procedure were excluded. Of the initial 121 papers that met our search criteria, 38 were included in the meta-analysis (see page four and Table S3 of the Supplementary Materials).
We estimated the percentage of Medicare patients treated with TAE or PRN protocols by using survey data from retinal specialists (see Table S4 of the Supplementary Materials).27, 36, 37 We calculated the protocol-independent number of injections a Medicare beneficiary received of a drug in a given year using the meta-analysis and survey data (see page 11 of the Supplementary Materials). The protocol-independent number of injections were then applied to determine the personalized-regimen Medicare spending on anti-VEGF drugs and their delivery by multiplying by the fixed-regimen drug and delivery spending total, and dividing by the percentage of patients treated under PRN or TAE protocols.
Actual Medicare spending
Actual Medicare spending for ranibizumab (J2778), aflibercept (J0178) and intravitreal injection of anti-VEGF drugs (67028) was found using the Medicare Part B National Summary Data File.38 Medicare spending on bevacizumab provided in this dataset could not be used because the codes are not limited to the treatment of nvAMD. Instead, the Physician and other Supplier PUF dataset was used to find the actual Medicare spending for ophthalmic use of bevacizumab from 2012 – 2015 by multiplying a provider’s line service count by the average Medicare allowed payment and summing. Actual Medicare spending on ophthalmic use of bevacizumab in 2008 across all J codes was previously published and assumed to increase linearly to 2012 (see Table S2 in the Supplementary Materials).29 Injection costs were distributed based on the percentage of patients using each anti-VEGF drug.
OCT-related government investment
Research spending by NIH and NSF on OCT was determined via a NIH RePORTER and NSF Award search using the term “optical coherence tomography” in the title and abstract of every grant from inception to 2015.39, 40 Spending on OCT imaging reimbursement was determined by calculating the number of patients under PRN and assuming they would receive imaging with OCT once a month. Patients under TAE were assumed to receive imaging every time they received an injection. The total number of images was then multiplied by the allowed charge for OCT, which was found using the Healthcare Common Procedure Coding System (HCPCS) code 92135 for 2008 – 2010 and 92134 for 2011 – 2015.35
Neovascular-AMD patient savings
Copay by nvAMD patients for their anti-VEGF therapy on a fixed-injection schedule was calculated for each drug using the FDA-approved number of injections per year.41 Copay by nvAMD patients on a personalized-injection schedule was determined for each drug using the average number of injections and OCT images a patient received. The average number of injections was found by averaging the protocol-independent number of injections across all years (see personalized-regimen spending section). The average number of OCT images was determined by assuming patients under a PRN protocol received one scan per month, while patients under a TAE protocol received one scan per injection. Patient savings was found by subtracting the cost of treatment on a personalized-injection schedule from the cost on a fixed-injection schedule.
Results
Hypothetical vs. Actual Medicare Spending
Our analysis is based on FFS Medicare claims, which represents ~70% of the Medicare beneficiary population (~37.4 million people in 2015), from 2008 and 2012 – 2015.29–31 Where necessary, we assumed linear increases in patient totals and anti-VEGF drug spending from 2008 – 2012 (see Methodology). Figure 1 consists of data for ranibizumab and bevacizumab from 2008 – 2015, and aflibercept from 2013 – 2015. Fixed-regimen spending over these time frames for ranibizumab, bevacizumab and aflibercept was calculated to be $17.8, $3.2 and $3.4 billion, respectively.
Figure 1.
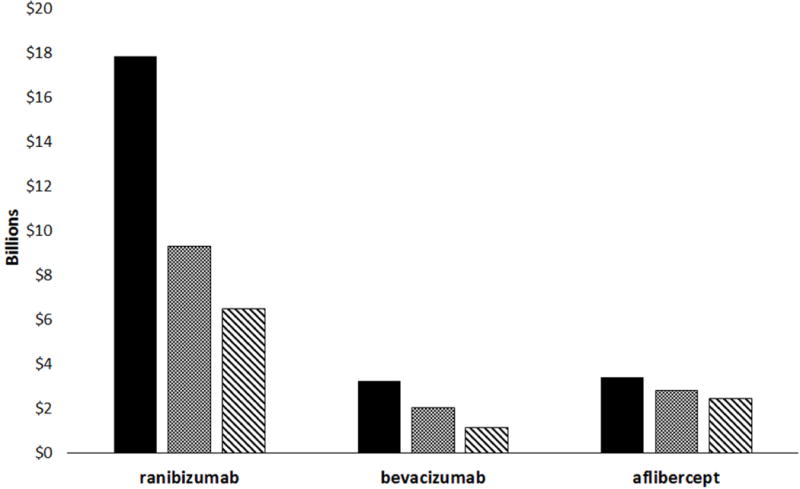
Hypothetical vs. actual drug and delivery expenditures on anti-VEGF therapy for nvAMD, 2008 – 2015. Fixed-regimen spending is shown in black, personalized-regimen spending is depicted with a cross-hatch pattern and actual spending is shown in a striped pattern. Aflibercept spending is limited to 2013 – 2015.
Based on our meta-analysis of the literature and practice pattern survey data of retinal specialists (see Tables S3 and S4 in the Supplementary Materials), we calculated personalized-regimen spending by Medicare to be $9.3, $2.0 billion and $2.8 billion for ranibizumab, bevacizumab and aflibercept, respectively.27, 36, 37 Compared to the fixed-regimen spending totals, OCT-guided personalized-treatment regimens enabled Medicare to save $10.3 billion dollars on anti-VEGF therapy costs for nvAMD from 2008 – 2015. Fewer injections of ranibizumab are responsible for 83% of the calculated savings.
Actual Medicare spending was $6.5 billion for ranibizumab, $1.1 billion for bevacizumab and $2.4 billion for aflibercept, for a total of $10.0 billion. This is $4.1 billion (29%) less than our calculated personalized-regimen spending total (Figure 1).
Government Investment vs. Savings
Figure 2 highlights three government budget categories related to OCT and its use in anti-VEGF therapy for nvAMD: Medicare savings from fewer drug injections ($10.3 billion, Figure 1); reimbursement on OCT imaging used to monitor nvAMD; and the investment made by NIH and NSF to develop OCT from an academic lab curiosity to an effective clinical tool. Using the meta-analysis and survey data, we calculated the cost of using OCT imaging to guide anti-VEGF treatment decisions from 2008– 2015 was $0.8 billion. NIH and NSF spent ~$0.4 billion on basic and clinical research toward the development of OCT from 1995 – 2015.39, 40 After summing these three budget categories, the United States government has saved $9.0 billion, a return on its investment in OCT research of ~2,100%.
Figure 2.
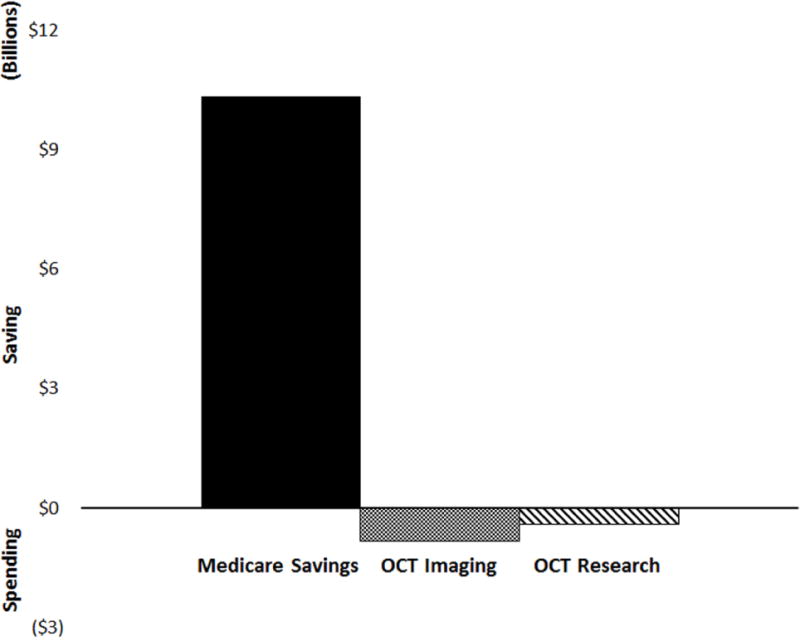
Government investment in OCT vs. savings from OCT-guided anti-VEGF therapy for nvAMD. Medicare savings is from 2008 – 2015 due to reduced drug and delivery costs. Medicare spending on reimbursing clinicians for OCT imaging is from 2008 – 2015, while research spending on OCT by the NIH and NSF is from 1995 – 2015.
Patient savings
Anti-VEGF drugs, their injection and OCT imaging are covered under Medicare Part B, which requires beneficiaries to copay 20% of the Medicare-approved amount in the absence of supplementary Medigap insurance.41, 42 On a fixed-injection schedule, patients on ranibizumab, bevacizumab and aflibercept would have an average copay of $3,693, $342 and $1,568, respectively, per year for their anti-VEGF therapy from 2008 – 2015 (see Table 1). On an OCT-guided, personalized-injection schedule, annual patient copay spending on treatment was reduced by $1,918 for ranibizumab, $94 for bevacizumab and $306 for aflibercept. Taken together, OCT has enabled nvAMD patients to save over $2.2 billion by avoiding 17.7 million anti-VEGF injections from 2008 – 2015.
Table 1.
Medicare beneficiary copay spending for anti-VEGF therapy on fixed- or personalized-injection schedules
| Drug | ranibizumab | bevacizumab | aflibercept |
|---|---|---|---|
| Number of patients | 995,396 | 2,020,091 | 446,915 |
| Average number of annual injections on personalized-injection schedule | 5.6 | 6.7 | 4.7 |
| Drug copay cost | $288 | $9 | $242 |
| OCT imaging copay cost | $6 | $6 | $6 |
| Injection copay cost | $20 | $20 | $20 |
| Fixed-injection schedule – annual patient copay | $3,693 | $342 | $1,568 |
| Personalized-injection schedule – annual patient copay | $1,776 | $247 | $1,262 |
| Annual patient savings | $1,918 | $94 | $306 |
| Total cohort savings | $1,908,804,551 | $190,892,790 | $136,910,501 |
| Total injections avoided | 6,370,534 | 10,706,482 | 580,990 |
Discussion
Our analysis shows that innovative technology can significantly reduce healthcare costs and improve patient care. OCT-enabled personalized-treatment protocols allow clinicians to manage their patients’ nvAMD using significantly fewer injections of anti-VEGF agents than approved by the FDA, which saves patients and taxpayers billions of dollars. In addition, patient burden is reduced through fewer trips to the clinic and needles into the eye, while physicians benefit from having timely information on treatment efficacy with which to advise the patient and then personalize management decisions.
The worldwide impact of OCT on human health and healthcare delivery would have been much slower to evolve, if at all, without long-term government research funding. While the first paper to use the term “optical coherence tomography” was published in 1991, widespread adoption of the technology in the clinic occurred only after an additional decade of government-supported development.4 Our calculations show that this investment over decades has been repaid 21-fold by savings to Medicare from fewer injections of anti-VEGF drugs over just eight years. Meanwhile, OCT manufacturing has grown to be a sizable industry on its own right, supporting thousands of private-sector jobs through an instrument market with a revenue of ~$750 million a year.2, 43
Our analysis has several limitations that could lead to over- or under-estimation of the cost savings from OCT-guided anti-VEGF treatment regimens. Our calculations assumed full compliance with either fixed or personalized regimens. Outside of clinical trial settings, studies have suggested that patients may be under-treated.25 Reasons why patients may miss injections include intolerance of discomfort, inconvenience, disappointment by failure to recover useful vision, other pressing medical issues and inability to secure transportation or afford the copay. Under-treatment likely contributes to why actual Medicare spending on anti-VEGF medication is lower than our calculated costs. Switching drugs may also lower actual Medicare spending compared to our calculations that assume patients stick with the same drug throughout the year. Our calculations used practice patterns based on a survey of retina specialists, which may not accurately represent the actual practice pattern on a per-patient basis. Our cost-saving calculations were limited to actual and estimated FFS Medicare claims data from 2008 – 2015; FFS Medicare claims data is not yet available for 2016. Furthermore, we did not have data for the 30% of Medicare beneficiaries using Medicare Advantage or any patient under private insurance. Aflibercept was approved by the FDA in late 2011, but did not receive its own J-code until 2013. Clinicians using the drug in 2012 likely filed it under the unclassified biologics code J3590, which is also used for bevacizumab. Because it was impossible separate usage of the two drugs under the same code, we limited our patient count to medical practices with an average Medicare reimbursement of two times the ASP + 6% of bevacizumab. Thus, injections of aflibercept in 2012 were not captured in this analysis. For these reasons, the full savings from OCT may be substantially higher. For simplicity, we assumed that all Medicare patients did not have Medigap insurance. As many patients do have supplementary insurance, our patient-savings totals are likely overstated. Finally, we restricted our analysis to OCT usage related to nvAMD only. OCT and anti-VEGF agents are also used in the treatment of retinal vein occlusion and diabetic macular edema, but FDA approval for these indications were recent.44, 45 There is not yet sufficient information on the evolving practice patterns for us to calculate the cost savings associated with the use of OCT in making treatment decisions for these conditions. In addition to anti-VEGF therapy, OCT is used to guide treatment in a wide range of retinal and optic nerve diseases with potential for cost savings by avoiding unnecessary surgery or drugs. OCT is also utilized outside the eye, such as the monitoring and placement of coronary stents.8 While evaluation of these examples and others is beyond the scope of this analysis, their exclusion suggests our calculations represent a lower boundary on the savings (and intangible benefits) attributable to OCT.
Contrary to many of today’s headlines that highlight the cost of high-tech medicine, there exist examples of innovation – like OCT – that make healthcare more affordable. Funded by modest investments in research by taxpayers over 20 years, such innovation has paid for itself and continues to yield billions of dollars in healthcare savings for patients and insurers. We hope that highlighting the impact of OCT on patient health and public spending encourages further government investment in biomedical research – even in these budgetary-constrained times.
Supplementary Material
Acknowledgments
Funding/Support: David Huang was supported by an unrestricted grant from Research to Prevent Blindness (New York, NY), National Institutes of Health (Bethesda, MD) P30 EY010572, and the Champalimaud Foundation (Lisbon, Portugal).
Financial Disclosures: Matthew Windsor: no financial disclosures. Sissi Sun: no financial disclosures. Kevin Frick is a consultant for Glaukos. Eric Swanson receives patent royalties from MIT owned OCT patents which are licensed to several companies and is on the board of directors and has financial interest in NinePoint Medical Incorporated. Philip Rosenfeld is a consultant for Achillion Pharmaceuticals, Acucela, Boehringer-Ingelheim, Carl Zeiss Meditec, Cell Cure Neurosciences, Chengdu Kanghong Biotech, Ocunexus Therapeutics, Genentech, Healios K.K, Hemera Biosciences, F. Hoffmann-La Roche Ltd., MacRegen Inc, Astellas Institute for Regenerative Medicine (AIRM), Regeneron, Tyrogenex, Ocudyne and Unity Biotechnology. He receives research support from Acucela, Apellis, Carl Zeiss Meditec, Genentech, GlaxoSmithKline, Astellas Institute for Regenerative Medicine (AIRM), Tyrogenex. Dr. Rosenfeld as has equity interest in Apellis, Digisight and Ocudyne. David Huang has a significant financial interest in Optovue, a company that may have a commercial interest in the results of this research and technology. Financial interests include patent royalty, stock ownership, research grant and material support.
Other: none
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available at AJO.com
References
- 1.Kantarjian H, Rajkumar S. Why Are Cancer Drugs So Expensive in the United States, and What Are the Solutions? Mayo Clinic Proceedings. 2015;90(4):500–504. doi: 10.1016/j.mayocp.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Swanson E. Estimates of Ophthalmic OCT Market Size and the Dramatic Reduction in Reimbursement Payments. OCTnews.org; http://www.octnews.org/articles/4176266/estimates-of-ophthalmic-oct-market-size-and-the-dr/ Published 2012. Accessed March 1, 2016. [Google Scholar]
- 3.Izatt JA, Choma MA. Theory of Optical Coherence Tomography. In: Drexler W, Fujimoto JG, editors. Optical Coherence Tomography: Technology and Applications. Heidelberg, Germany: Springer; 2008. pp. 47–72. [Google Scholar]
- 4.Huang D, Swanson E, Lin C, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welzel J. Optical coherence tomography in dermatology: a review. Skin Research and Technology. 2001;7(1):1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi P, Balcer L, Frohman E. Optical Coherence Tomography in Neurologic Diseases. Cambridge, Massachusetts: Cambridge University Press; 2015. [Google Scholar]
- 7.Liang H, Cid M, Cucu R, et al. En-face optical coherence tomography - a novel application of non-invasive imaging to art conservation. Optics Express. 2005;13(16):6133. doi: 10.1364/opex.13.006133. [DOI] [PubMed] [Google Scholar]
- 8.Bezerra H, Costa M, Guagliumi G, Rollins A, Simon D. Intracoronary Optical Coherence Tomography: A Comprehensive Review. JACC: Cardiovascular Interventions. 2009;2(11):1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai T, Fujimoto J, Mashimo H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics. 2014;4(2):57–93. doi: 10.3390/diagnostics4020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth A, Hannesschlager G, Leiss-Holzinger E, Wiesauer K, Leitner M. Optical coherence tomography – applications in non-destructive testing and evaluation. In: Kawasaki M, editor. Optical Coherence Tomography. INTECH Open Access Publisher; 2013. pp. 163–85. [Google Scholar]
- 11.Ambati J, Fowler B. Mechanisms of Age-Related Macular Degeneration. Neuron. 2012;75(1):26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld P, Brown D, Heier J, et al. Ranibizumab for Neovascular Age-Related Macular Degeneration. New England Journal of Medicine. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Brown D, Kaiser P, Michels M, et al. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. New England Journal of Medicine. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 14.Avery R, Pieramici D, Rabena M, Castellarin A, Nasir M, Giust M. Intravitreal Bevacizumab (Avastin) for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2006;113(3):363–372.e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to agerelated macular degeneration. Retina. 2006;26(4):383–390. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 16.Heier JS, Brown DM, Chong V, et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Medicare Part B Drug Average Sales Price. Washington, DC: Centers for Medicare & Medicaid Services; 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html. Accessed on July 15, 2017. [Google Scholar]
- 18.Erie J, Barkmeier A, Hodge D, Mahr M. High Variation of Intravitreal Injection Rates and Medicare Anti–Vascular Endothelial Growth Factor Payments per Injection in the United States. Ophthalmology. 2016;123(6):1257–1262. doi: 10.1016/j.ophtha.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 19.June 2015 Databook: Health care spending and the Medicare Program. Washington, DC: Medicare Payment Advisory Commission; 2015. http://medpac.gov/documents/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed on October 8, 2015. [Google Scholar]
- 20.Fung A, Lalwani G, Rosenfeld P, et al. An Optical Coherence Tomography-Guided, Variable Dosing Regimen with Intravitreal Ranibizumab (Lucentis) for Neovascular Age-related Macular Degeneration. American Journal of Ophthalmology. 2007;143(4):566–583.e2. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Brown D, Regillo C. Anti-VEGF Agents in the Treatment of Neovascular Age-related Macular Degeneration: Applying Clinical Trial Results to the Treatment of Everyday Patients. American Journal of Ophthalmology. 2007;144(4):627–637.e2. doi: 10.1016/j.ajo.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 22.Berg K, Pedersen T, Sandvik L, Bragadóttir R. Comparison of Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration According to LUCAS Treat-and-Extend Protocol. Ophthalmology. 2015;122(1):146–152. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 23.Wykoff C, Croft D, Brown D, et al. Prospective Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2015;122(12):2514–2522. doi: 10.1016/j.ophtha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Ying G, Maguire M, Daniel E, et al. Association of Baseline Characteristics and Early Vision Response with 2-Year Vision Outcomes in the Comparison of AMD Treatments Trials (CATT) Ophthalmology. 2015;122(12):2523–2531.e1. doi: 10.1016/j.ophtha.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguire M, Martin D, Ying G, et al. Five-Year Outcomes with Anti–Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2016;123(8):1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho A, Busbee B, Regillo C, et al. Twenty-four-Month Efficacy and Safety of 0.5 mg or 2.0 mg Ranibizumab in Patients with Subfoveal Neovascular Age-Related Macular Degeneration. Ophthalmology. 2014;121(11):2181–2192. doi: 10.1016/j.ophtha.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Stone TW, editor. ASRS 2015 Preferences and Trends Membership Survey. Chicago, IL: American Society of Retina Specialists; 2015. [Google Scholar]
- 28.Smiddy W. Economic Implications of Current Age-Related Macular Degeneration Treatments. Ophthalmology. 2009;116(3):481–487. doi: 10.1016/j.ophtha.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Brechner R, Rosenfeld P, Babish J, Caplan S. Pharmacotherapy for Neovascular Age-Related Macular Degeneration: An Analysis of the 100% 2008 Medicare Fee-For-Service Part B Claims File. American Journal of Ophthalmology. 2011;151(5):887–895.e1. doi: 10.1016/j.ajo.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Medicare Provider Utilization and Payment Data: Physician and Other Supplier. Washington, DC: Centers for Medicare & Medicaid Services; 2017. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Physician-and-Other-Supplier.html. Accessed July 10, 2017. [Google Scholar]
- 31.Gold M, Jacobson G, Damico A, Neuman T. Medicare Advantage 2015 Spotlight: Enrollment Market Update. The Henry J Kaiser Family Foundation. 2017 http://www.kff.org/medicare/issue-brief/medicare-advantage-2015-spotlight-enrollment-market-update/. Accessed July 24, 2017.
- 32.Centers for Medicare & Medicaid Services. Medicare and Medicaid Programs: Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs; Electronic Reporting Pilot; Inpatient Rehabilitation Facilities Quality Reporting Program; Revision to Quality Improvement Organization Regulations. Federal Regist. 2012;77:68210–565. [PubMed] [Google Scholar]
- 33.Consumer Price Index. Washington, DC: U.S. Bureau of Labor Statistics; 2017. https://www.bls.gov/. Accessed July 20, 2017. [Google Scholar]
- 34.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 35.Medicare Physician Fee Schedule Search. Washington, DC: Centers for Medicare & Medicaid Services; 2017. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx). Accessed July 20, 2017. [Google Scholar]
- 36.Mittra RA, Pollack JS, editors. ASRS 2008 Preferences and Trends Membership Survey. Chico, CA: American Society of Retina Specialists; 2008. [Google Scholar]
- 37.Jumper JM, Mittra RA, editors. ASRS 2012 Preferences and Trends Membership Survey. Chicago, IL: American Society of Retina Specialists; 2012. [Google Scholar]
- 38.Part B National Summary Data File (Previously known as BESS) Washington, DC: Centers for Medicare & Medicaid Services; 2017. https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Part-B-National-Summary-Data-File/Overview.html. Accessed July 10, 2017. [Google Scholar]
- 39.NIH Research Portfolio Online Reporting Tools (RePORTER) Washington, DC: National Institutes of Health; 2017. https://projectreporter.nih.gov/reporter.cfm. Accessed on July 21, 2017. [Google Scholar]
- 40.NSF Award Search. Washington, DC: National Science Foundation; 2017. http://www.nsf.gov/awardsearch/simpleSearch.jsp. Accessed on July 21, 2017. [Google Scholar]
- 41.Medicare 2016 costs at a glance. Washington, DC: Centers for Medicare & Medicaid Services; 2016. https://www.medicare.gov/your-medicare-costs/costs-at-a-glance/costs-at-glance.html#collapse-4809. Accessed May 14, 2016. [Google Scholar]
- 42.What’s Medicare Supplement Insurance (Medigap)? Washington, DC: Centers for Medicare & Medicaid Services; 2016. https://www.medicare.gov/supplement-other-insurance/medigap/whats-medigap.html. Accessed May 20, 2016. [Google Scholar]
- 43.Fujimoto J, Swanson E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investigative Opthalmology & Visual Science. 2016;57(9):OCT1–OCT13. doi: 10.1167/iovs.16-19963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown D, Nguyen Q, Marcus D, et al. Long-term Outcomes of Ranibizumab Therapy for Diabetic Macular Edema: The 36-Month Results from Two Phase III Trials. Ophthalmology. 2013;120(10):2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 45.Cheung N, Wong I, Wong T. Ocular Anti-VEGF Therapy for Diabetic Retinopathy: Overview of Clinical Efficacy and Evolving Applications. Diabetes Care. 2014;37(4):900–905. doi: 10.2337/dc13-1990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


