Abstract
The internalizing spectrum of psychiatric disorders—depression and anxiety—are common, highly comorbid, and challenging to treat. Individuals with childhood onset depression have a particularly poor prognosis. There is compelling evidence that individuals with depression display reduced resting-state EEG activity at sensors overlying the left prefrontal cortex, even during periods of remission, but it remains unknown whether this asymmetry is evident among individuals with a comorbid anxiety disorder. Here, we demonstrate that women with a history of childhood onset depression and no anxiety disorder (n = 37) show reduced left lateral frontal activity compared to psychiatrically healthy controls (n = 69). In contrast, women with a history of childhood onset depression and pathological levels of anxious apprehension (n = 18)—as indexed by a current generalized anxiety disorder, obsessive compulsive disorder, or separation anxiety disorder diagnosis—were statistically indistinguishable from healthy controls. Collectively, these observations suggest that anxious apprehension can mask the relationship between prefrontal EEG asymmetry and depression. These findings have implications for understanding (a) prefrontal EEG asymmetry as a neurophysiological marker of depression, (b) the comorbidity of depression and anxiety, and (c) failures to replicate the relationship between prefrontal EEG asymmetry and depression. More broadly, they set the stage for developing refined interventions for internalizing psychopathology.
Keywords: anxiety, comorbidity, depression, frontal EEG asymmetry
1 | INTRODUCTION
Major depressive disorder (MDD) is the single largest burden on global public health (Collins et al., 2011; DiLuca & Olesen, 2014; Whiteford et al., 2013), generating significant hardship and accounting for nearly $40 billion in lost productivity annually in the United States (Kessler et al., 2006). These facts underscore the need for a deeper understanding of the neurobiology of depression. One approach has involved the identification of endophenotypic markers of depression risk. A well-replicated finding in this area is that individuals with depression display reduced resting-state EEG activity at sensors overlying the left prefrontal cortex during both depressive and remitted states (Stewart, Bismark, Towers, Coan, & Allen, 2010; Thibodeau, Jorgensen, & Kim, 2006), suggesting that this asymmetric pattern of activity represents a state-independent or traitlike marker of depression.1 Decreased left prefrontal activity prospectively predicts first onset of depression (Nusslock et al., 2011) and treatment response (Bruder et al., 2001). It is associated with genetic risk for depression (Smit, Posthuma, Boomsma, & De Geus, 2007), and has been observed in psychiatrically healthy offspring of individuals with depression (Dawson, Frey, Panagiotides, Osterling, & Hessl, 1997). Other work indicates that decreased left prefrontal activity reflects attenuated approach motivation, anhedonia, and reduced reward sensitivity (Coan & Allen, 2004; Davidson, 1998; Harmon-Jones, 2003; Shankman & Klein, 2003), all characteristic of depressive episodes.
Although MDD frequently co-occurs with anxiety disorders (Maser & Cloninger, 1990; Zimmerman, McDermut, & Mattia, 2000), few studies have examined whether comorbid anxiety moderates the relationship between a history of depression and prefrontal EEG asymmetry (although see Bruder et al., 1997; Kentgen et al., 2000). In the present study, we therefore examined resting-state prefrontal EEG activity in a relatively large sample of women with a history of childhood-onset depressive (COD) disorder (i.e., MDD and/or dysthymic disorder) who either had a current anxious-apprehension diagnosis (n = 18) or no current anxiety diagnosis (n = 37), and psychiatrically healthy controls (n = 69). Consistent with previous research (Barlow, 1991; Heller, Nitschke, Etienne, & Miller, 1997; Nitschke, Heller, Palmieri, & Miller, 1999; Watson, 2005), we defined anxious apprehension as excessive worry for the future and verbal rumination about negative expectations, reflected in generalized anxiety disorder (GAD), obsessive compulsive disorder (OCD), or separation anxiety disorder (SAD). We focused on COD, given its strong association with resting-state prefrontal EEG activity (Shankman & Klein, 2003) and because individuals with COD are at very high risk for recurrent depression and lifetime anxiety disorders (Kovacs, Obrosky, & George, 2016; Maser & Cloninger, 1990).
Using these data, we tested two competing hypotheses regarding the influence of comorbid anxiety on prefrontal EEG asymmetry. The first hypothesis is that individuals with COD with or without a comorbid anxious-apprehension diagnosis will both display reduced left prefrontal activity compared to controls. Among the anxiety disorders, GAD has the greatest phenomenological or structural affinity for depressive disorders (Krueger, 1999; Prenoveau et al., 2010; Vollebergh et al., 2001). Likewise, MDD and GAD are genetically correlated (Kendler, Neale, Kessler, Heath, & Eaves, 1992; Kendler, Prescott, Myers, & Neale, 2003). These observations suggest that MDD and GAD are more alike than different and should exhibit a similar profile of decreased left prefrontal activity, and that the co-occurrence of depression and GAD will tend to enhance this profile. Confirmatory findings would provide neurophysiological support for the comparability between depression and anxious-apprehension disorders, such as GAD, and structural models of depressive and anxiety symptoms, more generally (Kruger, 1999).
The second hypothesis is that only COD individuals without a comorbid anxious-apprehension diagnosis will show reduced left prefrontal activity at rest, and that COD individuals with a comorbid anxious-apprehension diagnosis will not show this profile. Support for this perspective comes from work by Heller, Nitschke, and colleagues (Heller et al., 1997; Nitschke et al., 1999) who examined frontal EEG activity and two dimensions of anxiety: anxious apprehension and anxious arousal. Whereas anxious apprehension involves excessive worry for the future as often reflected in GAD, OCD, or SAD, anxious arousal is characterized by increased physiological activation and somatic tension and is most evident among individuals with panic or phobic disorders. Results revealed that participants high in self-reported anxious apprehension display increased, rather than decreased, left prefrontal activity (Heller et al., 1997) or no asymmetry (Nitschke et al., 1999). Furthermore, individuals with subclinical symptoms of both depression and anxious apprehension failed to display decreased relative left prefrontal activity (Nitschke et al., 1999). In short, research based on self-reported anxious-apprehension symptoms suggests that MDD and anxious apprehension should be associated with dissimilar profiles of prefrontal asymmetry, and that the co-occurrence of depression and an anxious-apprehension disorder (GAD, OCD, or SAD) should mask the relationship between depression and prefrontal EEG asymmetry. Results in line with this hypothesis would suggest an important disconnect between an endophenotype (frontal EEG asymmetry) and structural and genotypic models of depression and anxiety.
A second aim of our study was to clarify the nature of the relationship between anxious apprehension and left pre-frontal activity. Heller, Nitschke, and colleagues proposed that elevated relative left prefrontal activity in anxious apprehension may reflect ruminative activity and cognitive chatter in left prefrontal verbal processing circuits that presumably may not be active in certain variants of depression (Heller et al., 1997; Nitschke et al., 1999). Here, we therefore examined the relationship between left prefrontal activity and self-reported rumination (Nolen-Hoeksema & Morrow, 1991) and whether variation in rumination accounted for the impact of anxious apprehension on prefrontal EEG asymmetry.
2 | METHOD
2.1 | Participants
Participants were a subset of adult women who provided EEG data as part of a large multidisciplinary program project examining risk factors in childhood onset mood disorders (Forbes et al., 2006; Miller et al., 2002). As detailed in Table 1, the sample included 18 women with COD and at least one anxious-apprehension diagnosis on the day of EEG recording [GAD (n = 14), OCD (n = 6), or SAD (n = 2)], 37 women with COD and no comorbid anxiety diagnosis at the time of EEG acquisition, and 69 psychiatrically healthy controls who had no current or lifetime internalizing psychopathology. Five participants in the COD and anxious-apprehension group had a secondary diagnosis of either panic disorder or specific phobia at EEG recording. Participants were right-handed and at least 18 years old. Exclusionary criteria for the present analyses included current alcohol/substance abuse/dependence, a preexisting major medical disorder, or intellectual deficits. Participants also had to provide sufficient artifact-free EEG data and sufficient diagnostic data to establish their clinical status on the day of EEG recording. Informed consent was obtained prior to the first evaluation.
TABLE 1.
Demographic and clinical variables
| COD with anxious apprehension | COD without anxious apprehension | Healthy control | Contrast p value < .05 | ||||
|---|---|---|---|---|---|---|---|
| Mean or percentage | SD | Mean or Percentage | SD | Mean or Percentage | SD | ||
| Age at EEG | 25.26 | 3.59 | 25.25 | 4.03 | 29.01 | 5.82 | b, c |
| BDI | 20.70 | 10.78 | 8.84 | 6.37 | 2.67 | 3.88 | a, b, c |
| RDQ-R | 53.53 | 9.42 | 38.84 | 11.84 | 27.02 | 5.93 | a, b |
| Age at first MDE | 13.54 | 4.60 | 12.65 | 2.82 | |||
| Lifetime number of MDE | 3.33 | 1.94 | 2.43 | 1.55 | |||
| Psychotropic medication use, current | 33% | 14% | 0% | b, c | |||
| Psychotropic medication use, lifetime | 78% | 89% | 4% | b, c | |||
| Psychiatric hospitalization lifetime | 61% | 54% | 0% | b, c | |||
| MDE, current | 39% | 11% | 0% | a, b, c | |||
| Alcohol disorder, lifetime | 44% | 35% | 6% | b, c | |||
| Substance disorder, lifetime | 6% | 19% | 0% | c | |||
Note. a= COD with anxious apprehension significantly different from COD without anxious apprehension, p < .05; b= COD with anxious apprehension significantly different from healthy controls, p < .05; c= COD without anxious apprehension significantly different from healthy controls, p < .05. BDI = Beck Depression Inventory; RDQ-R = Response to Depression Questionnaire–Rumination; MDE = major depressive episode.
COD was operationalized as the onset of major depressive disorder and/or dysthymic disorder by age 14, meeting DSM-IV (American Psychiatric Association, 1994) criteria. Participants were recruited through prior research studies or community media advertisements. Some healthy controls were recruited using the Cole Directory of households focusing on neighborhoods comparable in socioeconomic status to that of COD participants.
Control women were slightly older (M = 29.01, SD = 5.82) than both COD participants with (M = 25.27, SD = 3.60; t(85) = 2.60, p = .01) and without (M = 25.24, SD = 4.03; t(104) = 3.51, p = .001) an anxious-apprehension diagnosis (Table 1). No control was taking psychotropic medication at EEG recording. There was a weak trend for more COD participants with anxious apprehension (6/18) to be taking psychotropic medication than COD participants without anxious apprehension (5/37; χ2(1) = 2.97, p = .15). Accordingly, we conducted follow-up analyses adjusting for age and psychotropic medication use on the day of EEG recording for all significant effects in the present study.
As shown in Table 1, COD participants with an anxious-apprehension diagnosis were more likely to be in a major depressive episode (MDE) at EEG recording; they also self-reported greater depression severity, as indexed by the Beck Depression Inventory (BDI; Beck, Steer, & Garbin, 1988). The majority of COD participants, however, were not clinically depressed at EEG recording (39% of COD participants with, and 11% of COD participants without an anxious-apprehension diagnosis had a current MDE at EEG recording). COD participants with and without anxious apprehension did not differ on the various indices of clinical and diagnostic history that we examined (Table 1).
2.2 | Procedures
All participants were part of a multidisciplinary longitudinal investigation (Forbes et al., 2006; Miller et al., 2002). Diagnostic assessments included a psychiatric interview and the completion of self-report questionnaires, as detailed below. Resting-state EEG data were acquired in a separate laboratory session.
2.2.1 | Psychiatric diagnoses and self-report measures
Diagnoses were made by highly experienced professional-level clinical evaluators and independent best-estimate psychiatrists. Diagnostic information was obtained through one of several means. For patients who had participated in a longitudinal naturalistic follow-up study since they were children (Kovacs, Feinberg, Crouse-Novak, Paulauskas, & Finkelstein, 1984), prior diagnoses had been derived through annual assessments with the semistructured Interview Schedule for Children and Adolescents and its follow-up version for adults (Sherrill & Kovacs, 2000), involving both the proband and a parent informant. All other participants were assessed via the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID; First, Spitzer, Gibbon, & Williams, 1995), modified to include selected childhood diagnoses. For the SCID, a second informant also was required, as well as supporting clinical or medical records to verify childhood onset. Based on all data, two independent senior psychiatrists blind to EEG status provided final lifetime and current DSM best-estimate diagnoses. Disagreements were resolved by consensus.
Research indicates that diagnostic reliability (interrater, retest) is more strongly determined by underreporting (i.e., due to forgetting or social desirability) than fabrication (Kessler & Wethington, 1991). To combat this, multiple salient private and public events (e.g., Halloween, a cousin’s wedding) were used as markers to graphically chart clinical course during the diagnostic interviews, similar to approaches used by others (Warshaw, Keller, & Stout, 1994). Such graphical methods yield data with good to excellent 1-year retest reliability (Warshaw et al., 1994). The diagnostic assessment that most closely followed the EEG recording date was used to determine diagnostic status at the time of EEG recording (median interval = 299 days; SD = 278). The 21-item BDI (Beck et al., 1988) was used to assess state-related depressive symptoms (α = .89), and the 21-item rumination subscale of the Response to Depression Questionnaire (RDQ-R; Nolen-Hoeksema & Morrow, 1991) was used to assess trait rumination in response to sadness (α = .88).
2.2.2 | EEG acquisition and reduction
Six 60-s trials (half eyes open/half eyes closed; order counterbalanced) were collected using 21 electrodes (AF3/AF4, F3/F4, F7/F8, FC1/FC2, FC5/FC6, C3/C4, T7/T8, P3/P4, P7/P8, O1/O2, Fz) referenced to Cz and grounded at AFz (impedances < 10 kΩ; homologs ± 0.5 kΩ). Data were filtered (0.01–100 Hz; 60 Hz), amplified, and digitized (512 Hz).
Artifacts (high/low variance; deviations ± 65 μV) were rejected using code adapted from EEGLAB (http://sccn.ucsd.edu/eeglab; Delorme, Sejnowski, & Makeig, 2007). Unusable channels were spline-interpolated if a nearest neighbor was usable. Data were then rereferenced to an average montage, and power density (μV2/Hz) was estimated for the alpha-1 (8–10 Hz) band using Hanning-windowed 1.024-s epochs (50% overlap). Alpha-1 was employed in accord with prior work by our laboratory (Davidson, Marshall, Tomarken, & Henriques, 2000; Shackman, McMenamin, Maxwell, Greischar, & Davidson, 2009). Asymmetry analyses employing broadband alpha (8–13 Hz) yielded similar results (not reported). Power densities were log10 transformed, and mean power was computed. Because alpha is an inverse measure of cerebral activity (Allen, Coan, & Nazarian, 2004), negative asymmetry scores (right—left) were interpreted as relatively less left hemisphere activity or relatively more right hemisphere activity. In line with existing research (Allen et al., 2004), hypothesis testing focused on midfrontal (F3/F4) and lateral frontal (F7/F8) asymmetry indices. We expected no differences in parietal asymmetries given these regions’ primary involvement in anxious arousal, as opposed to anxious apprehension (Heller et al., 1997; Nitschke et al., 1999).
2.3 | Hypothesis testing strategy
2.3.1 | Group mean differences in relative left prefrontal activity
Analyses of variance (ANOVAs) were used to examine differences in mid- and lateral relative left prefrontal activity for the three groups of subjects. Fisher’s protected t tests (Cohen, Cohen, West, & Aiken, 2003) served to minimize familywise error rate, which requires a significant omnibus ANOVA F test in order to proceed to pairwise comparisons and follow-up analyses. We conducted follow-up analyses of covariance (ANCOVAs) for all significant effects adjusting for age and psychotropic medication status on the day of EEG recording, as well as current MDE status, BDI scores, and lifetime alcohol and substance disorder given evidence that a history of addiction modulates prefrontal EEG asymmetry (Knott et al., 2008; Zinser, Fiore, Davidson, & Baker, 1999). We also conducted follow-up analyses on nonprefrontal asymmetry indices to assess the extent to which findings were specific to the mid- and/or lateral prefrontal region.
2.3.2 | Rumination and relative left prefrontal activity
In the case of a significant omnibus, we examined correlations between relative left prefrontal activity and rumination (RDQ-R) scores among COD participants. Correlations were computed using all COD participants, as well as separately for COD participants with and without an anxious-apprehension diagnosis. Next, we reran the analyses of group mean differences on left prefrontal activity adjusting for RDQ-R scores to assess whether variation in self-reported rumination accounted for the impact of anxious apprehension on prefrontal EEG asymmetry.
2.3.3 | Hemispheric specificity
Hypothesis testing focused on prefrontal EEG asymmetry at mid- and lateral frontal sensors. When a significant relationship was observed between an outcome variable and either the mid- or lateral frontal asymmetry index, follow-up analyses examined the relationship between the particular outcome variable and alpha power at both the right and left mid-or lateral frontal electrodes separately. To accomplish this, we separately regressed left and right mid- or lateral frontal alpha power onto the arithmetic average of alpha power at all recording sites, and saved the unstandardized residuals (Allen et al., 2004). We then ran the aforementioned analyses, replacing the relevant asymmetry index with the appropriate left or right mid- or lateral alpha power residuals.
3 | RESULTS
3.1 | Group mean differences in relative left prefrontal activity
An ANOVA revealed a significant difference in resting left lateral frontal cortical activity (F7/F8) across the three groups of participants, F(2, 121) = 5.29, p = .006, (Figure 1). Consistent with our second competing hypothesis, pairwise comparisons revealed that only those COD participants without an anxious-apprehension diagnosis showed lower left lateral frontal activity (M = −.04, SD = .08), which was significantly less than the extent of left lateralization for COD participants with an anxious-apprehension diagnosis (M = .01, SD = .07), F(1, 53) = 5.37, p = .03, , and controls (M = .01, SD = .07), F(1, 104) = 9.03, p = .003, . These mean differences remained significant after adjusting for age and psychotropic medication status, as well as MDE status at EEG recording, BDI scores, and lifetime alcohol and substance use disorder, F(2, 114) = 4.18, p = .02, . Also consistent with our second competing hypothesis, COD participants with an anxious-apprehension diagnosis and healthy controls did not differ on relative left lateral frontal cortical activity, F(1, 85) = .10, p = .75, , suggesting that clinical levels of anxious apprehension mask the relationship between depression and prefrontal EEG asymmetry. The three participant groups did not significantly differ at midfrontal sensors, F(2, 121) = .83, p = .44, . Exploratory analyses failed to uncover significant group mean differences in other regions (e.g., P3/4; ps > .32, ). Finally, there were no significant correlations between relative left lateral frontal activity and either MDE status or BDI scores when the two COD groups were combined, or separately among COD participants with and without an anxious-apprehension diagnosis (rs < .18; ps > .30).
FIGURE 1.
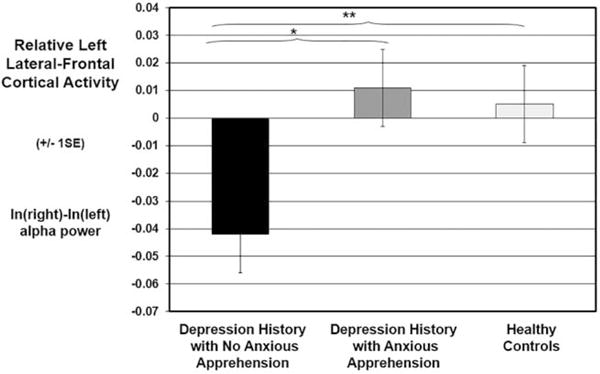
Relative left lateral frontal cortical activity (F7/F8 asymmetry index) as a function of diagnosis. Depression refers to lifetime history of childhood onset depression. Anxious apprehension refers to a current diagnosis of generalized anxiety disorder, obsessive compulsive disorder, or separation anxiety disorder. Error bars depict SE. *p < .05; **p < .01
3.2 | Rumination and relative left prefrontal activity
There were no significant correlations between relative left lateral frontal activity and RDQ-R scores when the two COD groups were combined, or separately among COD participants with and without an anxious-apprehension diagnosis (ps > .10). Furthermore, adjusting for rumination did not attenuate the aforementioned across-group differences on resting left lateral frontal cortical activity (F7/F8), F(2, 110) = 4.80, p = .01, .
3.3 | Hemispheric specificity
The three participant groups did not differ on alpha power at either the right, F(2, 121) = 1.98, p = .14, , or left, F(2, 121) = 1.44, p = .24, , lateral frontal electrodes, separately. This suggests that diagnosis is more closely related to the difference in activity between right and left lateral frontal activity (i.e., the asymmetry index).
4 | DISCUSSION
The present study is the first investigation of whether a comorbid anxiety disorder characterized by high levels of anxious apprehension (GAD, OCD, or SAD) moderates the relationship between a lifetime history of depression and prefrontal EEG asymmetry. Consistent with our second hypothesis, COD participants who were free of a comorbid anxious-apprehension diagnosis had significantly lower levels of left prefrontal activity compared to both COD participants with an anxious-apprehension diagnosis and healthy controls. These effects remained significant after adjusting for a range of variables, including age, current psychotropic medication use, current MDE status, self-reported depression symptom severity (as indexed by the BDI), and lifetime alcohol and substance disorder status. The fact that the majority of COD participants free of an anxious-apprehension diagnosis were not experiencing a MDE at EEG recording, and the fact that mean differences remained significant after adjusting for both MDE status at EEG recording and self-reported depression severity, highlight the traitlike quality of reduced relative left frontal EEG activity. These findings are consistent with previous research that frontal EEG asymmetry is a state-independent marker of depression (see Thibodeau et al., 2006, for meta-analytic review).
In contrast, COD participants with a comorbid anxious-apprehension diagnosis did not differ from healthy controls on prefrontal EEG asymmetry. There were no significant differences between any of these groups at nonfrontal asymmetry indices, suggesting anatomical specificity to the pre-frontal cortex. Overall, these findings indicate that decreased left frontal activity may be specific to a variant of depression that does not co-occur with disorders of anxious apprehension, and that comorbid anxious apprehension suppresses or masks the relationship between depression and prefrontal asymmetry. Relatedly, these findings also suggest a potential disconnect between frontal EEG asymmetry and both structural (e.g., Kendler et al., 2003; Krueger, 1999; Prenoveau et al., 2010) and genetic (e.g., Kendler et al., 1992, 2003) models of internalizing disorders, as these models predict reduced relative left frontal activity among COD participants with an anxious-apprehension diagnosis. This disconnect has potential implications for the recently launched NIMH Research Domain Criteria (RDoC) initiative, which aims to examine the relationship between mechanistic dimensions and symptom profiles that either cut across traditional disorder categories or that are unique to specific clinical phenomenon (Insel et al., 2010; Nusslock & Alloy, 2017; Nusslock, Walden, & Harmon-Jones, 2015). Our results suggest that a particular symptom profile (e.g., anxious apprehension) may have a distinct relationship with biological indices at different levels of analysis (e.g., genetic vs. neurophysiological).
The present study also has implications for understanding inconsistencies in the literature on depression and prefrontal EEG asymmetry. Although a meta-analytic review (Thibodeau et al., 2006) documents a moderate effect size for the relationship between decreased left frontal activity and depression, some studies have failed to replicate this effect (e.g., Reid, Duke, & Allen, 1998). Our results suggest that studies that, either by design or chance, have a high percentage of depressed individuals with co-occurring anxious apprehension are likely to observe a weaker (or no) relationship between relative left frontal activity and depression because anxious apprehension may mask this relationship. This possibility should be taken into account by future research on frontal EEG asymmetry and depression.
As noted, Heller and colleagues (Heller et al., 1997; Nitschke et al., 1999) proposed that the strong verbal or ruminative component inherent in worry may be responsible for the elevated left prefrontal activity in anxious apprehension given the left hemisphere’s dominance for language in right-handed individuals. Although our COD participants with anxious apprehension did report elevated levels of rumination compared to both COD participants with no anxious apprehension and healthy controls (Table 1), left lateral frontal activity and rumination were unrelated in the former group. Furthermore, adjusting for rumination did not attenuate across-group differences on relative left frontal activity. Our reliance on one self-report measure of rumination, however, may limit our ability to assess the cognitive processes to which Heller and colleagues refer, and future research is needed to examine the mechanisms underlying elevated left prefrontal activity in anxious apprehension. This work may benefit from moving beyond self-report indices of rumination to using tasks designed to provoke left hemispheric language processes (e.g., verbal dichotic listening tasks; Wexler & Goodman, 1991). In conducting this research, it will be important to recognize that the profile of left frontal activation associated with the verbal rumination of anxiety, or language processes more generally, may be in a distinct left prefrontal cluster compared to the left frontal hypoactivation associated with depression and no comorbid anxiety. However, EEG measures are likely to have insufficient spatial resolution to disambiguate this, and it may be important to use imaging measures to separate these effects.
An additional limitation of the present study is that we did not include symptom measures directly assessing the presence and severity of anxious apprehension. It will be important for future research to include symptom measures to corroborate anxious-apprehension diagnoses and determine whether certain symptoms more strongly moderate the relationship between a lifetime history of depression and pre-frontal EEG asymmetry. The present study is also limited by the fact that we did not include COD participants with an anxious-arousal diagnosis. Growing evidence, however, indicates that disorders of anxious arousal, such as panic disorders and phobias, are associated with decreased left (or increased right) frontal activity (Davidson et al., 2000; Heller et al., 1997; Moscovitch et al., 2011; Wiedemann et al., 1999). Thus, despite significant symptom (Krueger, 1999) and genetic (Kendler et al., 1992, 2003) similarity between depression and disorders of anxious apprehension, the profile of frontal EEG asymmetry observed in depression appears to be more similar to that observed in disorders of anxious arousal. In line with this perspective, Bruder and colleagues (1997) reported that depressed individuals with a comorbid anxiety disorder characterized by anxious arousal (primarily social phobia or panic disorder) displayed the expected profile of decreased left prefrontal activity. Future research should test the predicted similarity in frontal EEG asymmetry between depression and disorders of anxious arousal and examine mechanisms underlying this proposed neurophysiological similarity.
4.1 | Conclusion
In sum, clinical levels of anxious apprehension (GAD, OCD, SAD) moderate the relationship between prefrontal EEG asymmetry and a lifetime history of depression risk, such that only COD participants free of an anxious-apprehension diagnosis displayed decreased left frontal activity. A key challenge for future research is to identify the mechanisms supporting heightened left prefrontal activity among individuals with elevated anxious apprehension, as well as neurophysiological similarities and differences between depression and disorders of anxious arousal. Finally, results from the present study may help refine neuromodulation techniques for treating internalizing disorders (e.g., Kalu, Sexton, Loo, & Ebmeier, 2012). Transcranial magnetic stimulation and/or transcranial direct current stimulation for normalizing pre-frontal asymmetry may be particularly useful for depressed individuals free of co-occurring anxious apprehension.
Acknowledgments
We thank C. George, B. Kumer, and M. Vuga of the Childhood Depression Research Program, WPIC, Pittsburgh, PA, and the staffs of the Laboratory for Affective Neuroscience and the Waisman Laboratory for Brain Imaging and Behavior at the University of Wisconsin-Madison for assistance. This work was supported by the National Institutes of Health (DA040717, MH069315, MH43454, MH056193, MH18931, HD007151, MH100117, MH077908, MH107444). The authors declare no conflicts of interest. This paper is dedicated to the memory of Lawrence L. Greischar.
Funding information
National Institutes of Health (DA040717, MH069315, MH43454, MH056193, MH18-931, HD007151, MH100117, MH077908, MH107444)
Footnotes
There are inconsistencies in how people describe negative values in pre-frontal EEG asymmetry research, with some studies referring to this as decreased relative left prefrontal activity and others increased relative right prefrontal activity. To maximize consistency with prior publications from our group, we use the term decreased left prefrontal activity.
References
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. https://doi.org/10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Barlow DH. Disorders of emotion. Psychological Inquiry. 1991;2:58–71. https://doi.org/10.1207/s15327965pli0201_15. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. https://doi.org/10.1016/0272-7358(88)90050-5. [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;9:939–948. doi: 10.1016/S0006-3223(96)00260-0. https://doi.org/10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattachrya N, Quitkin FM. Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biological Psychiatry. 2001;49:416–425. doi: 10.1016/s0006-3223(00)01016-7. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. https://doi.org/10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Stein DJ. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. https://doi.org/10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–320. https://doi.org/10.1080/026999398379628. [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47:85–95. doi: 10.1016/s0006-3223(99)00222-x. https://doi.org/10.1016/S0006-3223(99)00222-X. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Osterling J, Hessl D. Infants of depressed mothers exhibit atypical frontal brain activity: A replication and extension of previous findings. Journal of Child Psychology and Psychiatry. 1997;38:179–186. doi: 10.1111/j.1469-7610.1997.tb01852.x. https://doi.org/10.1111/j.1469-7610.1997.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. https://doi.org/10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLuca M, Olesen J. The cost of brain diseases: A burden or a challenge? Neuron. 2014;82:1205–1208. doi: 10.1016/j.neuron.2014.05.044. https://doi.org/10.1016/j.neuron.2014.05.044. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCIDP) (Version 2) New York, NY: Biometrics Research; 1995. [Google Scholar]
- Forbes EE, Shaw DS, Fox NA, Cohn JF, Silk JS, Kovacs M. Maternal depression, child frontal asymmetry, and child affective behavior as factors in child behavior problems. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:79–87. doi: 10.1111/j.1469-7610.2005.01442.x. https://doi.org/10.1111/j.1469-7610.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–848. doi: 10.1111/1469-8986.00121. https://doi.org/10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. https://doi.org/10.1037/0021-843X.106.3.376. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang PW. Research Domain Criteria (RDoC): Developing a valid diagnostic framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kalu UG, Sexton CE, Loo CK, Ebmeier KP. Transcranial direct current stimulation in the treatment of major depression: A meta-analysis. Psychological Medicine. 2012;42:1791–1800. doi: 10.1017/S0033291711003059. https://doi.org/10.1017/S0033291711003059. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder: Same genes, (partly) different environments? Archives of General Psychiatry. 1992;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. https://doi.org/10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. https://doi.org/10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: Influence of comorbidity with anxiety disorders. Journal of Abnormal Psychology. 2000;109:797–802. doi: 10.1037//0021-843x.109.4.797. https://doi.org/10.1037/0021-843X.109.4.797. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RA, Wang PS. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. American Journal of Psychiatry. 2006;163:1561–1568. doi: 10.1176/appi.ajp.163.9.1561. https://doi.org/10.1176/ajp.2006.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wethington E. The reliability of life event reports in a community survey. Psychological Medicine. 1991;21:723–738. doi: 10.1017/s0033291700022364. https://doi.org/10.1017/S0033291700022364. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Naccache L, Cyr E, Fisher DJ, McIntosh JF, Millar AM, Villeneuve CM. Craving-induced EEG reactivity in smokers: Effects of mood induction, nicotine, dependence and gender. Neuropsychobiology. 2008;58:187–199. doi: 10.1159/000201716. https://doi.org/10.1159/000201716. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas SL, Finkelstein R. Depressive disorders in childhood, I: A longitudinal prospective study of characteristics and recovery. Archives of General Psychiatry. 1984;41:229–237. doi: 10.1001/archpsyc.1984.01790140019002. https://doi.org/10.1001/archpsyc.1984.01790140019002. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Obrosky S, George C. The course of major depressive disorder from childhood to young adulthood: Recovery and recurrence in a longitudinal observational study. Journal of Affective Disorders. 2016;203:374–381. doi: 10.1016/j.jad.2016.05.042. https://doi.org/10.1016/j.jad.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. https://doi.org/10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Maser JD, Cloninger CR. Comorbidity of mood and anxiety disorders. Washington, DC: American Psychiatric Press, Inc.; 1990. [Google Scholar]
- Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, Kovacs M. Regional patterns of brain activity in adults with a history of childhood-onset depression: Gender differences and clinical variability. American Journal of Psychiatry. 2002;159:934–940. doi: 10.1176/appi.ajp.159.6.934. https://doi.org/10.1176/appi.ajp.159.6.934. [DOI] [PubMed] [Google Scholar]
- Moscovitch DA, Santesso DL, Miskovic V, McCabe RE, Antony MM, Schmidt LA. Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biological Psychology. 2011;87:379–385. doi: 10.1016/j.biopsycho.2011.04.009. https://doi.org/10.1016/j.biopsycho.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. https://doi.org/10.1111/1469-8986.3650628. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. https://doi.org/10.1037/0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Alloy LB. Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders. 2017;216:3–16. doi: 10.1016/j.jad.2017.02.001. https://doi.org/10.1016/j.jad.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Coan JA, Harmon-Jones E, Alloy LB, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120:497–503. doi: 10.1037/a0022940. https://doi.org/10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Walden K, Harmon-Jones E. Asymmetrical frontal cortical activity a marker of differential risk for mood and anxiety disorder symptoms: An RDoC perspective. International Journal of Psychophysiology. 2015;98:249–261. doi: 10.1016/j.ijpsycho.2015.06.004. https://doi.org/10.1016/j.ijpsycho.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Prenoveau J, Zinbarg RE, Craske M, Mineka S, Giffith J, Epstein A. Testing a hierarchical model of anxiety and depression in adolescents: A tri-level model. Journal of Anxiety Disorders. 2010;24:334–344. doi: 10.1016/j.janxdis.2010.01.006. https://doi.org/10.1016/j.janxdis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. https://doi.org/10.1111/1469-8986.3540389. [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science. 2009;20:1500–1506. doi: 10.1111/j.1467-9280.2009.02476.x. https://doi.org/10.1111/j.1467-9280.2009.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: An evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23:605–637. doi: 10.1016/s0272-7358(03)00038-2. https://doi.org/10.1016/S0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Sherrill JT, Kovacs M. Interview Schedule for Children and Adolescents (ISCA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:67–75. doi: 10.1097/00004583-200001000-00018. https://doi.org/10.1097/00004583-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, De Geus EJ. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biological Psychology. 2007;74:26–33. doi: 10.1016/j.biopsycho.2006.06.002. https://doi.org/10.1016/j.biopsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119:502–513. doi: 10.1037/a0019196. https://doi.org/10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. https://doi.org/10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Vollebergh WAM, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: The NEMESIS Study. Archives of General Psychiatry. 2001;58:597–603. doi: 10.1001/archpsyc.58.6.597. https://doi.org/10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- Warshaw MG, Keller MB, Stout RL. Reliability and validity of the longitudinal interval follow-up evaluation for assessing outcome of anxiety disorders. Journal of Psychiatric Research. 1994;28:531–545. doi: 10.1016/0022-3956(94)90043-4. https://doi.org/10.1016/0022-3956(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. https://doi.org/10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Goodman WK. Cerebral laterality, perception of emotion, and treatment response in obsessive-compulsive disorder. Biological Psychiatry. 1991;29:900–908. doi: 10.1016/0006-3223(91)90056-r. https://doi.org/10.1016/0006-3223(91)90056-R. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Vos T. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. https://doi.org/10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorder. Archives of General Psychiatry. 1999;56:78–84. doi: 10.1001/archpsyc.56.1.78. https://doi.org/10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, McDermut W, Mattia JI. Frequency of anxiety disorders in psychiatric outpatients with major depressive disorder. American Journal of Psychiatry. 2000;157:1337–1340. doi: 10.1176/appi.ajp.157.8.1337. https://doi.org/10.1176/appi.ajp.157.8.1337. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating smoking motivation: Impact on an electrophysiological index of approach motivation. Journal of Abnormal Psychology. 1999;108:240–254. doi: 10.1037//0021-843x.108.2.240. https://doi.org/10.1037/0021-843X.108.2.240. [DOI] [PubMed] [Google Scholar]


