Abstract
Butyltins (BTs), tributyltin (TBT) and dibutyltin (DBT) are organotin compounds that have been used in a variety of industrial applications; as a result, these compounds have been found in human blood. IL-6 is a pro-inflammatory mediator that is produced by T lymphocytes and monocytes. It is responsible for immune response regulation as well as tissue repair and cellular growth. Both BTs decrease the ability of human natural killer (NK) cells to destroy tumor cells and alter the secretion of pro-inflammatory cytokines tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ), and IL-1 beta (β) from human lymphocytes ex vivo. Here, we show that BTs alter the secretion of IL-6 from increasingly reconstituted preparations of human immune cells. IL-6 secretion was examined after 24h, 48h, or 6 day exposures to TBT and DBT in highly enriched human NK cells, monocyte-depleted (MD) peripheral blood mononuclear cells (MD-PBMCs), PBMCs, granulocytes, and a preparation combining both PBMCs and granulocytes (PBMCs+granulocytes). The results indicated that both BTs altered IL-6 secretion from all cell preparations. Significant decreases of IL-6 secretion were seen at the highest concentration of TBT (200 nM) and DBT (5–2.5 µM) while the lower concentrations of DBT (0.05 and 0.1 µM) caused elevation of IL-6 secretion. The data indicates that BT-induced alterations of IL-6 secretion from immune cells may be a significant consequence of BT exposures that may potentially affect immune competence.
Keywords: NK cells, PBMCs, Granulocytes, Dibutyltin, Tributyltin, Interleukin 6
Introduction
Interleukin 6 (IL-6) is a glycosylated protein (22–27 kDA) (Kishimoto, 1989), and is mainly produced by monocytes/macrophages, fibroblasts, and vascular endothelial cells (Akira et al., 1993). It is also produced by T and NK lymphocytes (Li and He, 2006; Hall et al., 2010) and granulocytes (Riedemann et al., 2004; Zimmermann et al., 2014; 2016). MAP kinase and NFκB signaling pathways are utilized in the regulation of its production (Gaestel et al., 2009). IL-6 belongs to a family of cytokines that consists of IL-11, leukemia inhibitory factor, oncostatin M, ciliary neurotropic factor, cardiotrophin-1, cardiotrophin-like related cytokine, neuropoietin and IL-27. The similarity with IL-6 and this cytokine family is the utilization of the transmembrane glycoprotein gp130 in its signal transduction pathway (Gearing et al., 1992; Heinrich et al., 2003; Hirano et al., 1994; Ip et al., 1992; Liu et al., 1992; Pennica et al., 1995; Yin et al., 1993). Activation of JAK/STAT and MAPK pathways can occur through the activation of a membrane-bound IL-6 receptor (IL-6R) and gp130 expressed on the cell surface (termed classic signaling) (Heinrich et al., 2003; Scheller et al., 2006). Cells that express IL-6R on the cell surface include macrophages, neutrophils, some T-cells, and hepatocytes (Scheller et al., 2011). Those same pathways can also be activated by a soluble IL-6R in conjunction with gp130 in cells that have no endogenous IL-6R expressed on their surface (termed trans-signaling) (Scheller et al., 2006; Scheller et al., 2011; Rose-John, 2012). Trans-signaling has been demonstrated in chronic inflammatory diseases such as colon cancer, colitis, and peritonitis (Atreya et al., 2000; Hurst et al., 2001). IL-6 regulates endometrial tissue growth, bone formation, and hormone synthesis (Naitoh et al., 1988; Spangelo et al., 1989). Elevation of IL-6 can be seen in inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, psoriasis, and Crohn’s disease (Gabay, 2006). High IL-6 levels have been shown in patients with carcinomas such as breast, lung, and lymphoma (Hong et al., 2007). Elevation of IL-6 levels correlates with tumor progression (Culig et al., 2005; Nilsson et al., 2005).
Butyltins (BTs) are man-made organotins that include tributyltin (TBT) and its metabolites dibutyltin (DBT) and monobutyltin (MBT). TBT and DBT have been used in a range of industrial and consumer applications, thus leading to environmental contamination (Kimbrough, 1976; Tanabe et al., 1998; Roper, 1992; Gipperth, 2009; Takahashi et al., 1999; Kannan et al., 1995; Forsyth et al., 1992; Forsyth and Jay, 1997; Sadiki et al., 1996). TBT has been used for wood preservation, slime control in paper mills, and antifungal agent in industrial cooling water systems and breweries (Antizar-Ladislao, 2008; Gipperth, 2009; Kannan et al., 1995; Kannan et al., 1999). It has contaminated the marine and fresh water environment due to antifouling paint usage on boats and ships. Although TBT use as a marine antifouling agent was restricted in the United States in the 1980s (Loganathan et al., 2001; Loganathan, 2016) and banned by the International Convention on the Control of Harmful Antifouling Systems on Ships in 2008 (Gipperth, 2009), it is still contaminating the environment due to its chemical stability (Gipperth, 2009) and use by those who do not acknowledge the ban. As a result, TBT has been found in human blood samples (ranging as high as 261 nM) and other tissues through the consumption of fish and other foods (Antizar-Ladislao, 2008; Kannan et al., 1995; Kannan et al., 1999; Whalen et al., 1999). It is also found in some household goods such as siliconized-paper baking parchments and shower curtains (Yamada et al., 1993). Mammals with TBT exposure show increased incidences of tumors (Wester et al., 1990).
DBT is used as a stabilizer in polyvinyl chloride (PVC) plastics such as pipes that distribute drinking water (Forsyth et al., 1992; Forsyth and Jay, 1997; Sadiki et al., 1996) and some plastic food containers (Nakashima et al., 1990; Yamada et al., 1993). DBT can leach from PVC plastics (Fent, 1996); as a result, it has been found in water samples (Sadiki and Williams, 1999). DBT is also used as de-worming agent in certain poultry and is found in some poultry products (Epstein et al., 1991). Due to these routes of exposure, DBT has been found in human blood samples ranging as high as 300 nM (Kannan et al., 1999; Whalen et al., 1999).
Both BTs decrease the ability of human NK cells to destroy tumor cells with accompanying decreases in cytotoxic and cell surface protein expression (Dudimah et al., 2007a; Dudimah et al., 2007b; Thomas et al., 2004; Whalen et al., 2002). In addition, both TBT and DBT inhibit and stimulate the secretion of tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ), and IL-1β from human lymphocytes (depending on the exposure concentration) (Hurt et al., 2013; Lawrence et al., 2015; Brown and Whalen, 2015; Brown et. al, 2017). TBT is more potent than DBT in causing these immunomodulatory alterations. The importance of introducing TBT and DBT as stress inducers to the cell environment is to understand whether exposure to BTs disrupt the secretion interleukin 6 (IL-6). Based on the effect of both BTs on TNFα, IFNγ, and IL-1β (Hurt et al., 2013; Lawrence et al., 2015; Brown and Whalen, 2015; Brown et. al, 2017), we hypothesize that exposure to TBT and DBT will alter the ability of human lymphocytes to secrete IL-6.
In this study, increasingly complex preparations of immune cells were examined for the effects of BT exposures on the secretion of IL-6. The preparations studied included: human NK cells, human monocyte-depleted (MD) peripheral blood mononuclear cells (PBMCs) (MD-PBMCs), PBMCs, PBMCs combined with granulocytes (PBMCs+granulocytes), and granulocytes. The use of increasingly reconstituted immune cell preparations allowed us to investigate the influence that various immune cell types may have on the ability of BTs to induce alteration in secretion of IL-6 which gives a better account for physiological circumstances. This work is significant because any disruption of the cytokines may indicate that TBT and DBT exposures may potentially affect immune competence as well as diseases that are influenced by increased levels of this pro-inflammatory cytokine as mentioned above.
Materials and Methods
Preparation of PBMCs, MD- PBMCs, and Granulocytes
PBMCs were isolated from Leukocyte filters (PALL- RCPL or RC2D) obtained from the Red Cross Blood Bank Facility (Nashville, TN) as described in Meyer et al., 2005. Leukocytes were retrieved from the filters by back-flushing them with an elution medium (sterile PBS containing 5 mM disodium EDTA and 2.5% [w/v] sucrose) and collecting the eluent. The eluent was layered onto Ficoll-Hypaque (1.077g/mL) and centrifuged at 1200g for 30–50 min. Granulocytes and red blood cells pelleted at the bottom of the tube while the PBMCs floated on the Ficoll-Hypaque. Mononuclear cells were collected and washed with PBS (500g, 10min). Following washing, the cells were layered on bovine calf serum for platelet removal. The cells were then suspended in RPMI-1640 complete medium which consisted of RPMI-1640 supplemented with 10% heat-inactivated BCS, 2 mM L-glutamine and 50 U penicillin G with 50 µg streptomycin/mL. This preparation constituted PBMCs. PBMCs are composed of 70–90% lymphocytes and 10–30% monocytes. Monocyte-depleted PBMCs (10–20% CD16+, 10–20 % CD56+, 70–80% CD3+, 3–5% CD19+, 2–20% CD14+) were prepared by incubating the cells in glass Petri dishes (150 × 15 mm) at 37 °C and air/CO2, 19:1 for 1.5 h. This cell preparation is referred to as MD-PBMCS cells. Granulocytes were isolated with the removal of mononuclear and red blood cells as described in Kuijpers et al., 2013. Granulocytes were collected and washed with PBS (2500 rpm, 15min). Red blood cells were lysed with NH4CL isotonic solution (155mM NH4Cl, 10mM KHCO3, 0.1 mM EDTA) for 10 min. Cells were then washed with PBS and centrifuged at 800g for 10 min. This preparation was greater than 90% granulocytes. The cells were then suspended in RPMI-1640 complete medium supplemented with 10% heat-inactivated BCS, 2 mM L-glutamine and 50 U penicillin G with 50 µg streptomycin/mL.
Preparation of NK cells
NK cells were prepared from buffy coats (from healthy adult donors) purchased from Key Biologics, LLC (Memphis, TN). Highly purified NK cells were prepared using a rosetting procedure. RosetteSep human NK cell enrichment antibody cocktail (0.6–0.8 mL) (StemCell Technologies, Vancouver, British Columbia, Canada) was added to 45 mL of buffy coat. The mixture was incubated for 20 min at room temperature (~ 25° C). Approximately 8 mL of the mixture was layered onto 4 mL of Ficoll-Hypaque (1.077 g/mL) (MP Biomedicals, Irvine, CA) and centrifuged at 1200 g for 30–50 min. NK cells were collected and washed twice with phosphate buffered saline (PBS) pH 7.2 and stored in complete media (RPMI-1640 supplemented with 10% heat-inactivated bovine calf serum (BCS), 2 mM L-glutamine and 50 U penicillin G with 50 µg streptomycin/ml) at 1 million cells/mL at 37 °C and air/CO2, 19:1. The preparation was greater than 85% NK cells.
Chemical Preparation
The organotin compounds tributyltin chloride (TBT) and dibutyltin dicholoride (DBT) were purchased from Sigma-Aldrich (96%) (St. Louis, MO). A DBT stock solution was prepared by dissolving DBT in dimethylsulfoxide (DMSO). Desired concentrations of DBT were prepared by dilution of the stock into cell culture media. The final concentration of DMSO for DBT exposures did not exceed 0.01%. Appropriate DMSO controls were run. Desired concentrations of TBT were prepared by dilution of the stock into cell culture media. TBT was a neat standard, dissolved initially in deionized water to give a 1 mM solution.
Cell Treatments
NK cells, MD-PBMCs, PBMCs, granulocytes (at a concentration of 1.5 million cells/ mL), or PBMCs+granulocytes (at a concentration of 1.5 million PBMCs/mL + 1.5 million granulocytes/mL) were treated with TBT at concentrations of 2.5–200 nM TBT for 24 h, 48 h, or 6 days. Following the incubations, the cells were pelleted and supernatants were collected and stored at −70° C until assaying for IL-6.
NK cells, MD-PBMCs, PBMCs, granulocytes (at a concentration of 1.5 million cells/ mL), or PBMCs+granulocytes (at a concentration of 1.5 million PBMCs/mL + 1.5 million granulocytes/mL) were treated with DBT at concentrations of 0.05–5 µM for 24 h, 48 h, or 6 days. Following the incubations, the cells were pelleted and supernatants were collected and stored at −70° C until assaying for IL-6.
TNFα and IL-1β were tested for their ability to stimulate secretion of IL-6. MD-PBMCs were treated with TNFα or IL-1β (10,000 pg/mL, 1000 pg/mL and 100 pg/mL) for 24 h. Following the incubations supernatants were collected and stored as described above until being tested for IL-6 secretion.
Antibodies to TNF, IL-1β, and an isotype control (BD-Pharmingen, San Diego, CA) were added to PBMCs 1 h prior to exposing the cells to DBT (0.25–0.05 µM) for 24 h. Following the incubations, supernatants were collected and stored as described above until assaying for IL-6.
Cell Viability
Cell viability was assessed at the beginning and end of each exposure period. Viability was determined using the trypan blue exclusion method. Briefly, cells were mixed with trypan blue and counted using a hemocytometer. The total number of cells and the total number of live cells were determined for both control and treated cells to determine the percent viable cells.
IL-6 Secretion Assay
IL-6 levels were measured using the BD OptEIA™ Human IL-6 enzyme-linked immunosorbent assay (ELISA) kit (BD-Pharmingen, San Diego, CA). Briefly, a 96-well micro well plate, designed for ELISA (Fisher, Pittsburgh, PA), was coated with a capture antibody for IL-6 diluted in coating buffer. The plate was incubated with the capture antibody overnight at 4°C. After incubation, the capture antibody was removed by washing the plate three times with wash buffer (PBS and 0.05% Tween-20). Assay diluent (PBS and bovine calf serum) was added to each well (blocking non-specific binding) and incubated at room temperature for 1h. The assay diluent was removed by washing the plate three times, and the cell supernatants and IL-6 standards were added to the coated plated and incubated for 2 h at room temperature. Following this incubation, the plate was thoroughly washed five times and then incubated for 1h with a detection antibody to IL-6 which was conjugated with horseradish peroxidase. The excess detection antibody was removed by washing seven times and a substrate solution was added for 30 min at room temperature to produce a colored product. The incubation with the substrate was ended by addition of acid and the absorbance was measured at 450 nm on a Thermo Labsystems Multiskan MCC/340 plate reader (Fisher Scientific).
Statistical Analysis
Statistical analysis of the data was performed by using ANOVA and Student’s t test. Data were initially compared within a given experimental setup by ANOVA. A significant ANOVA was followed by pair wise analysis of control versus exposed data using Student’s t test, a p value of less than 0.05 was considered significant.
Results
Viability of NK cells, MD-PBMCs, PBMCs, PBMCs+ Granulocytes, and Granulocytes exposed to TBT
Table 1 shows the effects of TBT exposures (2.5–200 nM) on the viability of highly purified NK cells, MD-PBMCs, PBMCs, PBMCs+granulocytes, and granulocytes. Exposure of NK cells to 2.5–100 nM TBT had no effect on their viability as compared to the control after 24 h length of exposure. A slight decrease in NK cell viability was noticed at the highest concentration of TBT (200 nM). Exposure to these same concentrations for 48 h caused some decrease (about 20%) only at the highest concentration of TBT while the remaining concentrations showed no decrease in viability compared to control cells. NK cells exposed to 2.5–50 nM TBT for 6 days showed no change in viability as compared to the control. A significant reduction in viability of 50% was seen with 200 nM TBT exposures. MD-PBMCs showed a very slight decrease in viability with exposure to 200 nM TBT while PBMCs showed significant decreases at 100 nM (about 18%) and 200 nM (about 38%) for 24 h. Both MD-PBMCs and PBMCs showed significant decreases in viability at the highest concentration after 48 h exposure. Significant decreases were also seen after 6 days for MD-PBMCs and PBMCs for TBT concentrations of 100, and 200 nM. Exposure of PBMCs+granulocytes to TBT did not significantly decrease viability compared to control cells after any length of incubation. Exposure to 200 nM TBT for 48 h and 6 days significantly diminished the viability of granulocytes.
Table 1.
Percent viability of NK cells, Monocyte-depleted PBMCs, PBMCs, PBMCs combined with Granulocytes, and Granulocytes exposed to TBT for 24 h, 48 h, and 6 days.
| Percent Viable Cells | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 24 h exposure |
[TBT] nM | NK | monocyte- depleted PBMCs |
PBMCs | PBMCs with Granulocytes |
Granulocytes |
| 0 | 95±3 | 97±2 | 98±1 | 97±2 | 98±1 | |
| 2.5 | 99±1 | 96±1 | 98±2 | 96±4 | 95±2 | |
| 5 | 98±1 | 97±2 | 95±2 | 96±1 | 97±1 | |
| 10 | 97±0 | 97±2 | 93±3 | 94±3 | 95±2 | |
| 25 | 98±1 | 95±4 | 95±3 | 97±2 | 95±1* | |
| 50 | 96±2 | 94±5 | 86±5* | 95±3 | 94±1* | |
| 100 | 96±3 | 97±2 | 80±4* | 98±0 | 96±2 | |
| 200 | 89±2* | 89±1* | 61±3* | 91±8 | 91±5 | |
| 48h exposure | ||||||
| 0 | 93±3 | 95±3 | 95±2 | 97±2 | 98±3 | |
| 2.5 | 96±2 | 96±2 | 95±4 | 97±3 | 96±4 | |
| 5 | 96±4 | 97±3 | 97±3 | 92±5 | 95±4 | |
| 10 | 96±1 | 97±2 | 92±3 | 97±4 | 97±3 | |
| 25 | 95±1 | 96±3 | 93±1 | 95±5 | 95±3 | |
| 50 | 94±4 | 93±7 | 89±7 | 95±3 | 96±4 | |
| 100 | 94±5 | 90±10 | 76±7* | 92±5 | 91±5 | |
| 200 | 72±18 | 79±5* | 49±5* | 82±13 | 79±7* | |
| 6 day exposure | ||||||
| 0 | 68±15 | 80±8 | 89±6 | 75±8 | 92±3 | |
| 2.5 | 75±3 | 87±7 | 89±8 | 76±13 | 89±7 | |
| 5 | 75±6 | 87±8 | 83±14 | 76±2 | 91±2 | |
| 10 | 70±15 | 83±8 | 85±5 | 82±6 | 91±4 | |
| 25 | 68±5 | 85±12 | 88±7 | 84±8 | 93±3 | |
| 50 | 64±9 | 76±16 | 69±10 | 73±14 | 92±4 | |
| 100 | 47±10 | 67±15 | 48±11* | 59±12 | 78±13 | |
| 200 | 34±6* | 44±20* | 50±9* | 48±20 | 62±2* | |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in viability compared to control cells (cells treated with vehicle alone), p<0.05
Viability of NK cells, MD-PBMCs, PBMCs, PBMCs+ Granulocytes, and Granulocytes exposed to DBT
Table 2 shows the effects of DBT exposures (0.05–5 µM) on the viability of the various immune cell preparations. NK cells exposed to DBT for 48 h showed significant reductions in viability (around 35%) at 2.5 and 5 µM. No statistically significant change in viability as compared to control cells was seen after 6 days of exposure. MD-PBMCs and PBMCs both showed a significant decrease in viability with exposure to 5 µM DBT for 24 h. The viability of MD-PBMCs at 48 h was significantly reduced by exposure to 1 (15%), 2.5 (40%), and 5 µM (41%) DBT. After 6 days of exposure 0.5 µM DBT also caused significant reductions in viability of MD-PBMCs. The viability of PBMCs+granulocytes and granulocytes alone was not significantly affected by exposure to DBT.
Table 2.
Percent viability of NK cells, Monocyte-depleted PBMCs, PBMCs, PBMCs combined with Granulocytes, and Granulocytes exposed to DBT for 24 h, 48 h, and 6 days.
| Percent Viable Cells | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 24 h exposure |
[DBT] µM | NK | monocyte- depleted PBMCs |
PBMCs | PBMCs with Granulocytes |
Granulocytes |
| 0 | 92±6 | 94±3 | 98±1 | 98±1 | 97±2 | |
| 0.05 | 97±3 | 98±2 | 95±4 | 99±2 | 97±2 | |
| 0.1 | 98±2 | 98±1 | 97±3 | 98±2 | 96±4 | |
| 0.25 | 98±1 | 97±1 | 97±1 | 99±1 | 96±3 | |
| 0.5 | 95±4 | 91±8 | 97±4 | 98±1 | 93±8 | |
| 1 | 92±5 | 92±4 | 90±10 | 94±7 | 95±4 | |
| 2.5 | 88±1 | 74±15* | 80±17 | 88±10 | 93±5 | |
| 5 | 80±7 | 80±9* | 77±8* | 93±7 | 93±5 | |
| 48 h exposure | ||||||
| 0 | 97±1 | 94±2 | 98±2 | 98±2 | 95±3 | |
| 0.05 | 97±0 | 96±2 | 95±4 | 96±4 | 94±6 | |
| 0.1 | 96±1 | 97±1* | 97±4 | 96±3 | 93±7 | |
| 0.25 | 93±0* | 96±2 | 97±2 | 95±5 | 93±6 | |
| 0.5 | 90±1* | 94±3 | 96±3 | 95±5 | 89±9 | |
| 1 | 79±12 | 80±11* | 86±3* | 92±7 | 92±3 | |
| 2.5 | 61±6* | 56±20* | 74±20 | 81±15 | 91±6 | |
| 5 | 64±8* | 55±10* | 85±21 | 85±13 | 87±10 | |
| 6 day exposure | ||||||
| 0 | 70±15 | 87±8 | 88±14 | 76±19 | 64±33 | |
| 0.05 | 75±8 | 89±8 | 84±15 | 71±24 | 62±40 | |
| 0.1 | 78±7 | 86±7 | 81±21 | 77±21 | 64±36 | |
| 0.25 | 74±9 | 81±13 | 78±14 | 69±26 | 62±37 | |
| 0.5 | 68±9 | 67±13* | 74±25 | 61±27 | 50±22 | |
| 1 | 69±3 | 52±11* | 70±25 | 60±23 | 53±31 | |
| 2.5 | 58±7 | 47±9* | 69±23 | 47±30 | 54±28 | |
| 5 | 49±19 | 52±8* | 71±22 | 56±22 | 47±23 | |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in viability compared to control cells (cells treated with vehicle alone), p<0.05
Effects of TBT Exposure on Secretion of IL-6 by NK cells
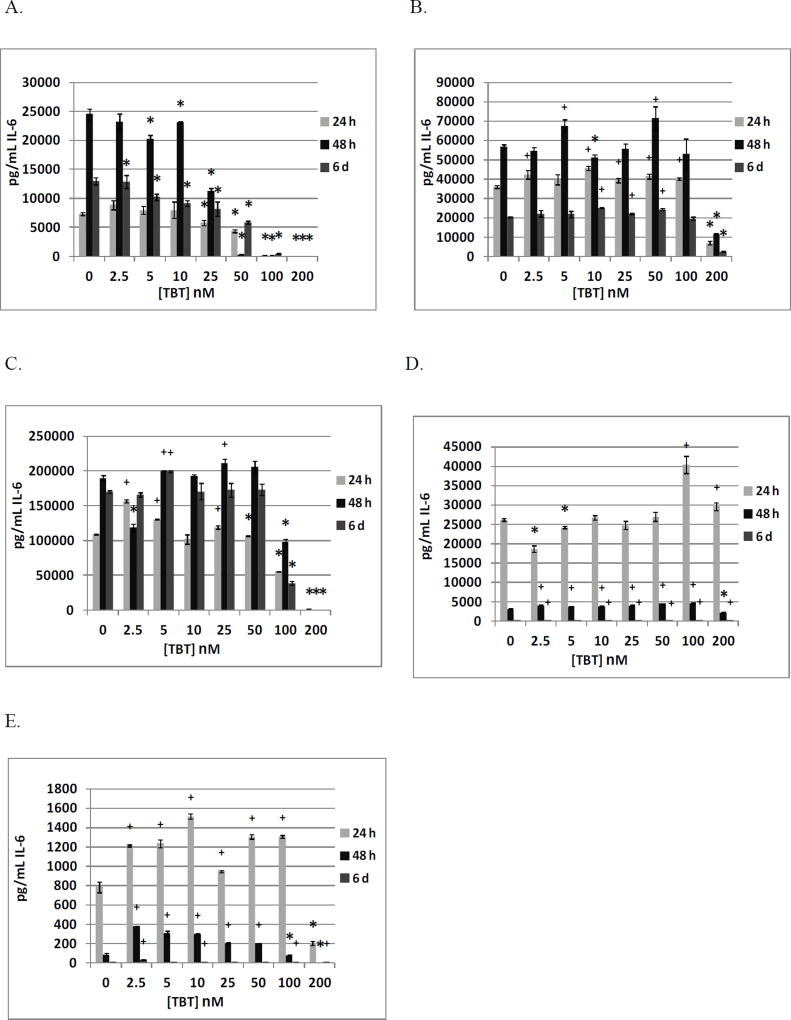
Table 3 shows the effects of TBT (0, 2.5, 5, 10, 25, 50, 100, and 200 nM) exposure on IL-6 secretion from NK cells. Cells from 4 different donors were exposed to TBT for 24 h, 48 h, and 6 days (KB=Key Biologic buffy coat). All donors showed significant decreases in IL-6 secretion when exposed to 100 and 200 nM TBT at 24 h, 48 h, and 6 days. Figure 1A shows the decreases from a representative experiment (KB169).
Table 3.
Effects of 24 h, 48 h, 6 day exposures to TBT on IL-6 secretion from highly purified human NKs.
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | KB166 | KB167 | KB168 | KB169 |
| 0 | 4685±174 | 1618±31 | 12391±260 | 7374±250 |
| 2.5 | 5236±302 | 1375±109* | 8175±78* | 8893±785 |
| 5 | 4930±229 | 1141±31* | 8138±263* | 8004±688 |
| 10 | 4764±4 | 990±16* | 7777±277* | 8008±1380 |
| 25 | 4700±148 | 646±32* | 6996±206* | 5795±402* |
| 50 | 4405±124 | 419±24* | 5052±97* | 4393±278* |
| 100 | 2935±89* | ND* | 135±42* | 170±63* |
| 200 | 84±241* | ND* | ND* | ND* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | KB166 | KB167 | KB168 | KB169 |
| 0 | 4424±92 | 2139±61 | 16394±501 | 24580±941 |
| 2.5 | 4616±982 | 3934±204+ | 13359±88* | 23221±1332 |
| 5 | 3991±464 | 3635±611+ | 12967±466* | 20260±589* |
| 10 | 3745±260* | 1092±136 | 12166±341 | 23077±201* |
| 25 | 3527±190* | 1199±128* | 9540±156* | 11293±437* |
| 50 | 3682±329* | 1438±108* | 6342±149* | 345±34* |
| 100 | 2432±63* | 45±64* | 1201±56* | ND* |
| 200 | 29±10* | 357±171* | 96±44* | ND* |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | KB166 | KB167 | KB168 | KB169 |
| 0 | 4030±200 | 1635±35 | 15482±575 | 13027±592 |
| 2.5 | 8341±396+ | 2194±140+ | 1710±132* | 12893±1138* |
| 5 | 7310±138+ | 3036±177+ | 14510±2402 | 10243±509* |
| 10 | 8299±179+ | 1541±201 | 19291±920+ | 9154±516* |
| 25 | 4780±17+ | 2160±65+ | 8625±231* | 8232±1225* |
| 50 | 3791±63 | 672±82* | 1920±99* | 5871±269* |
| 100 | 3291±76* | ND* | 1710±33* | 477±33* |
| 200 | ND* | ND* | 15463±297 | ND* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05 ND = not detectable.
Figure 1.
Effects of 24 h, 48 h and 6 day exposures to TBT on IL-6 secretion from highly purified human NK cells, monocyte-depleted PBMCs, PBMCs, PBMCs plus granulocytes, and granulocytes in individual donors. A) NK cells exposed to 0–200 nM TBT (donor KB169). B) Monocyte-depleted PBMCs exposed to 0–200 nM TBT (donor F156). C) PBMCs exposed to 0–200 nM TBT (donor F261). D) PBMCs plus granulocytes exposed to 0–200 nM TBT (donor F261). E) Granulocytes exposed to 0–200 nM TBT (donor F261). * Indicates a significant decrease in secretion and + indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone).
Effects of TBT Exposure on Secretion of IL-6 by MD-PBMCs
Table 4 summarizes the effects of exposing MD-PBMCs from 4 individual donors to TBT on IL-6 secretion (F=filter obtained from the Red Cross). Significant decreases in IL-6 secretion were seen in cells from all donors at 200 nM TBT. Additionally, cells from 3 of 4 donors showed an increase in secretion after exposure for 24 h, 48 h, or 6 days at one or more concentration of TBT. The maximum increase of IL-6 in cells from one donor was 1.34 fold (F137 at 2.5 nM after 48 h). Figure 1B shows the effects of TBT exposures for an individual donor (F156).
Table 4.
Effects of 24 h, 48 h, 6 day exposures to TBT on IL-6 secretion from monocyte-depleted PBMCs.
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F137 | F142 | F145 | F156 |
| 0 | 1495±113 | 64607±3102 | 8647±309 | 36151±711 |
| 2.5 | 1171±57* | 39411±9167* | 6675±42* | 42362±2282+ |
| 5 | 1105±72* | 38274±8450* | 6763±474* | 39758±2410 |
| 10 | 1072±49* | 47239±4826* | 6814±164* | 45813±1123+ |
| 25 | 1011±70* | 55389±2496* | 6689±508* | 39288±1090+ |
| 50 | 809±57* | 45285±2781* | 5680±313* | 41484±1238+ |
| 100 | 758±86* | 47883±1614* | 4897±157* | 40276±777+ |
| 200 | 241±168* | 2285±72* | ND* | 6958±796* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F137 | F142 | F145 | F156 |
| 0 | 7059±349 | 94610±2681 | 11822±509 | 56627±1086 |
| 2.5 | 9471±661+ | 96509±397 | 7847±87* | 54410±1896 |
| 5 | 6646±447 | 82913±2441* | 6827±205* | 67550±3147+ |
| 10 | 7533±1649 | 80267±3758* | 7673±615* | 51100±1749* |
| 25 | 6844±506 | 77903±424* | 7021±201* | 55759±2608 |
| 50 | 6386±239 | 72954±2077* | 7026±46* | 71502±6127+ |
| 100 | 3567±436* | 68994±1794* | 7200±313* | 53157±7680 |
| 200 | ND* | 3337±455* | ND* | 11518±501* |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F137 | F142 | F145 | F156 |
| 0 | 223±6 | 46608±1494 | 9920±489 | 20362±315 |
| 2.5 | 165±17* | 57987±1806+ | 8187±347* | 22137±1585 |
| 5 | 116±23* | 59399±7568 | 7781±305* | 21855±1738 |
| 10 | 102±8* | 57118±493+ | 6417±445* | 25086±288+ |
| 25 | 106±17* | 57137±272+ | 7035±200* | 22047±361+ |
| 50 | 84±3* | 55719±981+ | 6472±421* | 24310±479+ |
| 100 | 32±11* | 49993±753+ | 4787±248* | 19560±792 |
| 200 | ND* | 6379±131* | ND* | 2515±240* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05 ND = not detectable.
Effects of TBT Exposure on Secretion of IL-6 by PBMCs
The effects of exposures to TBT on secretion of IL-6 from PBMCs from 4 donors are shown in Table 5a. All donors showed significant decreases in IL-6 secretion after 24 h and 6 days of exposure to 200 nM TBT. Cells from each of the donors showed increased secretion of IL-6 at one or more TBT exposure after 24 and 48 h. The range of maximum increases after 24 h was 1.1 fold (F252, 2.5 nM), 1.4 fold (F257, 50 nM), 1.4 fold (F261, 2.5 nM), and 2.4 fold (F264, 2.5 nM). The effects of TBT exposures for a representative experiment are shown in Figure 1C (F261).
Table 5.
| a. Effects of 24 h, 48 h, 6 day exposures to TBT on IL-6 secretion from PBMCs. | ||||
|---|---|---|---|---|
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 107735±4735 | 123182±4413 | 108921±458 | 46005±308 |
| 2.5 | 119503±1019+ | 157545±2460+ | 156436±1916+ | 109857±1151+ |
| 5 | 106508±3257 | 160273±656+ | 130376±688+ | 90252±1561+ |
| 10 | 89450±4474* | 158333±1001+ | 102073±6556 | 80770±2100+ |
| 25 | 1365±220* | 150697±105+ | 119042±2900+ | 86894±964+ |
| 50 | 100201±8444 | 172636±10351+ | 106618±315* | 83240±4790+ |
| 100 | 12540±1176* | 120030±1211 | 55527±793* | 90844±901+ |
| 200 | 1386±477* | ND* | ND* | ND* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 86054±3721 | 159936±402 | 189796±3587 | 141±370 |
| 2.5 | 112033±8360+ | 177617±1486+ | 118288±5352* | 104303±210+ |
| 5 | 112825±3112+ | 199994±2634+ | 200393±321+ | 98768±3031+ |
| 10 | 114158±1589+ | 198545±1328+ | 192884±1895 | 94040±1152+ |
| 25 | 79846±6771 | 219299±7449+ | 211481±5805+ | 99091±1283+ |
| 50 | 10596±1016+ | 233965±1424+ | 205656±8477 | 159333±1266+ |
| 100 | ND* | 155183±1086+ | 98358±3466* | 147333±1748+ |
| 200 | ND* | 2371±822* | ND* | 52990±573+ |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 143040±5912 | 164368±443 | 170226±2050 | 66006±1860 |
| 2.5 | 124754±5201* | 182069±2296+ | 165820±2790 | 77252±1936+ |
| 5 | 131230±4148* | 149609±939* | 199386±1616+ | 81020±2811+ |
| 10 | 150126±4018 | 197011±2704+ | 170226±11833 | 107049±1158+ |
| 25 | 156564±12441 | 212598±522+ | 172835±9901 | 97831±611+ |
| 50 | 150583±1126 | 217287±1795+ | 173241±8388 | 131513±1254+ |
| 100 | 94735±5760* | 154345±1202* | 38864±2611* | 228035±1313+ |
| 200 | 2011±695* | 7816±80* | ND* | 11223±100* |
| b. Effects of 24 h, 48 h, 6 day exposures to TBT on IL-6 secretion from PBMCs plus Granulocytes. | ||||
|---|---|---|---|---|
|
| ||||
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 24852±1323 | 37539±1932 | 26205±352 | 3124±3 |
| 2.5 | 30787±1395+ | 44261±1248+ | 18660±906* | 3107±16 |
| 5 | 38369±748+ | 46178±1355+ | 24221±203* | 3317±22+ |
| 10 | 37506±157+ | 55233±1673+ | 26693±593 | 3155±17 |
| 25 | 47101±460+ | 59678±966+ | 24774±982 | 3191±36 |
| 50 | 48068±1024+ | 66622±3007+ | 26985±1150 | 3514±69+ |
| 100 | 68199±958+ | 77344±2115+ | 40481±2245+ | 4951±47+ |
| 200 | 20591±432* | 22733±300* | 29652±1043+ | 4079±18+ |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 8475±122 | 17469±302 | 3058±96 | 1085±18 |
| 2.5 | 9798±245+ | 23335±325+ | 3991±109+ | 1112±28 |
| 5 | 13232±1047+ | 24021±1180+ | 3800±15+ | 1183±30+ |
| 10 | 10586±367+ | 35240±198+ | 3823±81+ | 1216±7+ |
| 25 | 17818±316+ | 23488±540+ | 3980±122+ | 1385±45+ |
| 50 | 17101±364+ | 42078±994+ | 4429±48+ | 1642±40+ |
| 100 | 28636±786+ | 52935±293+ | 4577±166+ | 3264±32+ |
| 200 | 8182±121* | 15926±198* | 2188±62* | 2387±5+ |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 280±34 | 1063±21 | 13±0 | 86±3 |
| 2.5 | 219±4 | 1080±187 | 16±1+ | 345±23+ |
| 5 | 375±14+ | 1159±7+ | 16±2 | 94±12 |
| 10 | 278±51 | 1782±18+ | 26±0+ | 64±5* |
| 25 | 531±4+ | 2380±7+ | 30±0+ | 117±12+ |
| 50 | 924±27+ | 3718±15+ | 35±2+ | 650±3+ |
| 100 | 3318±51+ | 7225±28+ | 97±2+ | 1581±31+ |
| 200 | 355±37 | 429±37* | 65±2+ | 347±16+ |
| c. Effects of 24 h, 48 h, 6 day exposures to TBT on IL-6 secretion from Granulocytes. | ||||
|---|---|---|---|---|
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 122±2 | 137±11 | 785±53 | 137±13 |
| 2.5 | 882± 41+ | 507±3+ | 1214±11+ | 460±9+ |
| 5 | 1044±13+ | 300±5+ | 1238±42+ | 326±25+ |
| 10 | 963±30+ | 313±20+ | 1515±25+ | 309±23+ |
| 25 | 315±20+ | 219±34+ | 948±10+ | 209±2+ |
| 50 | 299±35+ | 161±1 | 1304±21+ | 516±19+ |
| 100 | 229±23+ | 162±6+ | 1306±17+ | 339±16+ |
| 200 | ND* | ND* | 206±22* | ND* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 6±6 | 110±15 | 87±14 | 1292±25 |
| 2.5 | 733±20+ | 949±12+ | 382±0+ | 1329±38 |
| 5 | 1635±15+ | 885±35+ | 305±27+ | 1428±42+ |
| 10 | 1380±15+ | 1260±12+ | 297±13+ | 1474±9+ |
| 25 | 365±6+ | 1110±51+ | 201±8+ | 1710±62+ |
| 50 | 394±19+ | 635±25+ | 203±2+ | 2068±55+ |
| 100 | 274±5+ | 163±13+ | 80±8* | 4328±45+ |
| 200 | ND* | ND* | ND* | 3106±6+ |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [TBT] nM | F252 | F257 | F261 | F264 |
| 0 | 9±0 | 5±1 | 6±1 | 4±1 |
| 2.5 | 25±1+ | 48±1+ | 29±1+ | 14±+ |
| 5 | 22±1+ | 53±1+ | 9±2 | 7±1+ |
| 10 | 12±1+ | 18±1+ | 9±1+ | 9±2+ |
| 25 | 10±0+ | 8±0+ | 8±1 | 8±1+ |
| 50 | 10±1 | 9±1+ | 7±2 | 8±1+ |
| 100 | 9±1 | 8±1+ | 10±1+ | 6±1 |
| 200 | 8±0* | 4±0 | 8±1+ | 4±1 |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05 ND = not detectable.
Effects of TBT Exposure on Secretion of IL-6 by PBMCs + Granulocytes
Effects of exposing PBMCs+granulocytes (prepared from the same 4 donors as PBMCs) to TBT on IL-6 secretion are shown in Table 5b. Cells from 3 of the 4 donors showed a significant decrease in IL-6 secretion at 200 nM TBT after either 24 h or 48 h of exposure, with 3 of the 4 donors showing a significant decrease at this concentration after 48 h. As with PBMCs alone, the combination of PBMCs+granulocytes also showed TBT-induced increases in IL-6 secretion in cells from all donors at each length of exposure. However, TBT-induced increases were of the greatest magnitude after 6 days (all donors) (11.8 fold for F252, 6.8 fold for F257, 7.5 fold for F261, 18.4 fold for F264). The increases are shown from an individual donor in Figure 1D (F261).
Effects of TBT Exposure on Secretion of IL-6 by Granulocytes
The effects of exposures to TBT on secretion of IL-6 were examined in granulocytes prepared from the same 4 donors as were PBMCs (results shown in Table 5c). All donors showed significant decreases of IL-6 secretion at 200 nM TBT after 24 h length of exposure. Significant increases in IL-6 secretion were seen at TBT levels ranging from 2.5–100 nM after 24 h and TBT levels ranging from 5–50 nM after 48 h for all donors. The fold increases seen after 48 h exposures to 5 nM TBT were 273 (F252), 8 (F257), 3.5 (F261), and 1.1 (F264). Minimal IL-6 secretion was noted after 6 days of exposure. However, 2.5 nM TBT induced substantial increases in the granulocytes from each of the donors after 6 days of exposure (2.8 fold for F252, 9.6 fold for F257, 4.8 fold for F261, and 3.5 fold for F264). Figure 1E shows the effects of TBT exposures from an individual donor (F261).
Effects of DBT Exposure on Secretion of IL-6 by NK cells
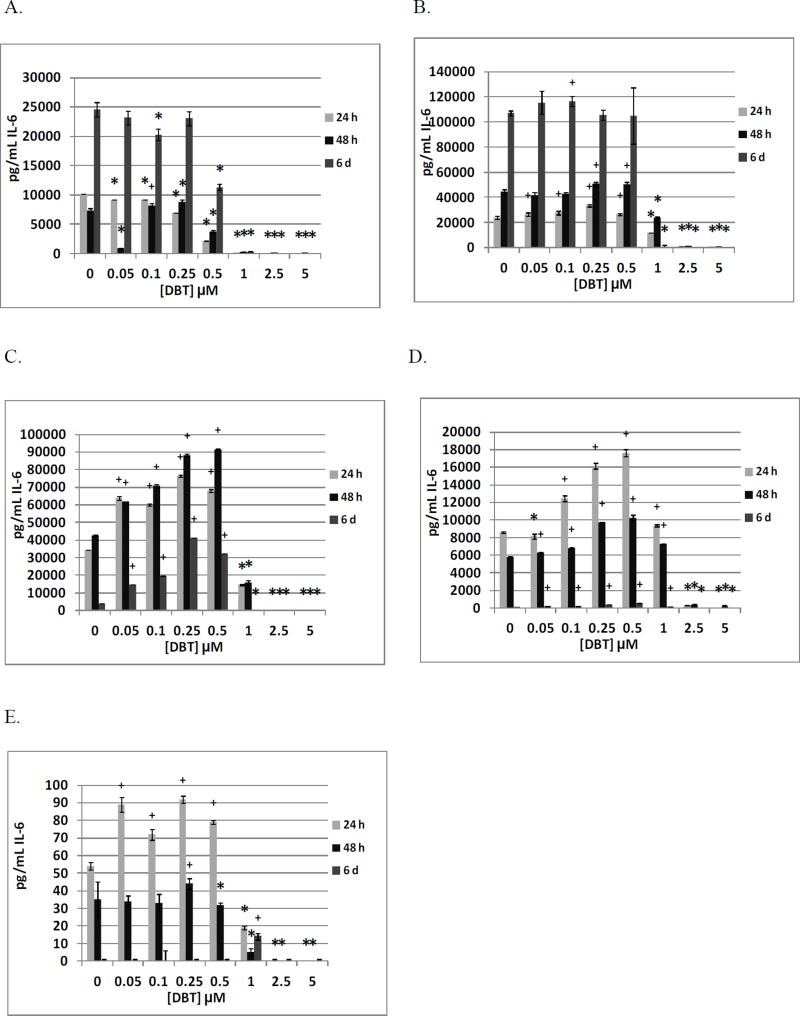
The effects of DBT (0, 0.05, 0.1, 0.25, 0.05, 1, 2.5 and 5 µM) exposures on IL-6 secretion from NK cells are shown in Table 6. Cells from 3 different donors were exposed to DBT for the same lengths of exposure as were used in the TBT studies. All donors showed significant decreases in IL-6 secretion when exposed to 0.5, 1, 2.5, and 5 µM DBT at all lengths of exposure. Additionally, all donors showed increased IL-6 secretion with exposures to one or more concentration of DBT in the range of 0.05 to 0.25 µM DBT after 24 h and/or 48 h exposures. The increases were rather modest ranging from 1.2–1.5 fold. Figure 2A shows the effects of DBT exposures at each length of exposure for a representative experiment (KB169).
Table 6.
Effects of 24 h, 48 h, 6 day exposures to DBT on IL-6 secretion from highly purified human NKs.
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | ||
|---|---|---|---|
|
| |||
| [DBT] µM | KB130 | KB155 | KB169 |
| 0 | 8077±166 | 3220±118 | 10146±309 |
| 0.05 | 9055±322+ | 3867±286+ | 9124±267* |
| 0.1 | 7864±480 | 3973±210+ | 9234±196* |
| 0.25 | 4963±596* | 3811±216+ | 6985±209* |
| 0.5 | ND* | 1569±139* | 2155±103* |
| 1 | ND* | 63±0* | 66±5* |
| 2.5 | ND* | ND* | ND* |
| 5 | ND* | ND* | ND* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | ||
|---|---|---|---|
|
| |||
| [DBT] µM | KB130 | KB155 | KB169 |
| 0 | 8422±204 | 10064±680 | 7338±72 |
| 0.05 | 12286±1696+ | 11037±510 | 871±518* |
| 0.1 | 9106±218+ | 10011±245 | 8203±377+ |
| 0.25 | 6710±136* | 8517±759* | 8768±9* |
| 0.5 | 1467±83* | 2908±100* | 3754±206* |
| 1 | 719±97* | 544±71* | 318±24* |
| 2.5 | 629±87* | ND* | 36±14* |
| 5 | 764±68* | ND* | 28±8* |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | ||
|---|---|---|---|
|
| |||
| [DBT] µM | KB130 | KB155 | KB169 |
| 0 | 8834±530 | 13743±209 | 24580±941 |
| 0.05 | 8161±1807 | 11926±877 | 23221±1332 |
| 0.1 | 7559±908 | 12697±904 | 20260±589* |
| 0.25 | 5030±30* | 10789±538* | 23077±201 |
| 0.5 | 416±119* | 2697±199* | 11293±437* |
| 1 | ND* | ND* | 345±34* |
| 2.5 | ND* | ND* | ND* |
| 5 | ND* | ND* | ND* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05 ND = not detectable.
Figure 2.
Effects of 24 h, 48 h and 6 day exposures to DBT on IL-6 secretion from highly purified human NK cells, monocyte-depleted PBMCs, PBMCs, PBMCs plus granulocytes, and granulocytes in individual donors. A) NK cells exposed to 0–5 µM DBT (donor KB169). B) Monocyte-depleted PBMCs exposed to 0–5 µM DBT (donor F152). C) PBMCs exposed to 0–5 µM DBT (donor F285). D) PBMCs plus granulocytes exposed to 0–5 µM DBT (donor F285). E) Granulocytes exposed to 0–5 µM DBT (donor F285). * Indicates a significant decrease in secretion and + indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone).
Effects of DBT Exposure on Secretion of IL-6 by MD-PBMCs
Table 7 shows the effects of exposing MD-PBMCs to DBT on IL-6 secretion. Cells from 5 different donors were exposed to DBT. All donors showed significant decreases at 2.5 and 5 µM DBT at all lengths of exposure. Additionally, all donors showed DBT-induced increases in secretion at one or more length of exposure with concentrations of DBT in the range of 0.5–0.25 µM. The effects of DBT exposures for a representative experiment are shown in Figure 2B (F152).
Table 7.
Effects of 24 h, 48 h, 6 day exposures to DBT on IL-6 secretion from monocyte-depleted PBMCs.
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
|
| |||||
| [DBT] µM | F142 | F145 | F152 | F163 | F166 |
| 0 | 6583±1729 | 10955±346 | 23788±920 | 58347±2724 | 113749±2245 |
| 0.05 | 62944±2481 | 11337±911 | 26616±1374+ | 59569±654+ | 128203±6793+ |
| 0.1 | 63811±3934 | 9296±298* | 27626±1290+ | 84036±4023+ | 113633±4815 |
| 0.25 | 69633±481* | 9150±149* | 33545±833+ | 111013±8251+ | 14444±581* |
| 0.5 | 40967±4574* | 3369±173* | 26525±779+ | 111013±8251+ | 11971±1006* |
| 1 | ND* | ND* | 11758±219* | 114524±839+ | 8609±1011* |
| 2.5 | ND* | ND* | 727±52* | ND* | ND* |
| 5 | ND* | ND* | 30±52* | ND* | ND* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
|
| |||||
| [DBT] µM | F142 | F145 | F152 | F163 | F166 |
| 0 | 112226±7067 | 11665±282 | 44353±1914 | 122785±7126 | 190821±7442 |
| 0.05 | 66314±3381* | 13018±1151 | 41839±2357 | 76845±4077* | 242779±16720+ |
| 0.1 | 74717±3591* | 11422±433 | 42564±1418 | 92633±1151* | 244363±6224+ |
| 0.25 | 78792±8955* | 11089±115* | 50704±1374+ | 84330±2257* | 247029±3178+ |
| 0.5 | 65384±1586* | 2412±298* | 50295±1771+ | 90148±4587* | 178404±13542 |
| 1 | 6591±218* | ND* | 23605±1095* | 630±1261* | 39904±2032* |
| 2.5 | ND* | ND* | 1570±54* | ND* | 9988±661* |
| 5 | ND* | ND* | 1020±54* | ND* | 8863±781* |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
|
| |||||
| [DBT] µM | F142 | F145 | F152 | F163 | F166 |
| 0 | 90029±2082 | 8847±73 | 107282±1965 | 26669±1165 | 119022±6314 |
| 0.05 | 105705±27762 | 87938±4504+ | 115333±9391 | 29565±251+ | 136489±6076* |
| 0.1 | 107114±17400 | 58278±3297+ | 116487±4223+ | 26367±801 | 129711±3272 |
| 0.25 | 91495±3511 | 40981±1125+ | 105590±4165 | 22966±2170 | 12467±677* |
| 0.5 | 81686±16334 | 3841±128* | 104897±22387 | 492±54* | 130867±10975 |
| 1 | 8790±380* | 4156±145* | ND* | 25857±602 | 11222±252* |
| 2.5 | ND* | 4047±378* | ND* | 3242±289* | ND* |
| 5 | 238±436* | 411±238* | ND* | 497±117* | ND* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05 ND = not detectable.
Effects of DBT Exposure on Secretion of IL-6 by PBMCs
Table 8a summarizes the effects of exposing PBMCs from 4 individual donors to DBT on IL-6 secretion. Significant decreases were seen at the 5 µM concentration in cells from each of the donors at all lengths of exposure. Cells from the vast majority of donors also showed very significant decreases with exposures to 1 and 2.5 µM DBT. Significant increases were seen at lower concentrations of DBT (0.05 to 0.5 µM) in cells from all donors after 48 h and 6 day exposures. Cells from 3 of the 4 donors also showed increases after 24 h of exposure. Figure 2C shows the effects of DBT exposures from an individual donor (F285).
Table 8.
| a. Effects of 24 h, 48 h, 6 day exposures to DBT on IL-6 secretion from PBMCs. | ||||
|---|---|---|---|---|
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 34255±142 | 14998±986 | 32382±357 | 95826±2410 |
| 0.05 | 63720±1078+ | 61442±2081+ | 92678±862+ | 112870±348+ |
| 0.1 | 60002±523+ | 65886±1367+ | 53085±3654+ | 118145±724+ |
| 0.25 | 76453±765+ | 69788±4877+ | 63289±641+ | 119362±362+ |
| 0.5 | 68152±745+ | 53195±3027+ | 61937±861+ | 61681±3093* |
| 1 | 14593±438* | 16405±1398 | 13622±735* | 17275±703* |
| 2.5 | ND* | ND* | ND* | ND* |
| 5 | ND* | ND* | ND* | ND* |
| 48 h | Interleukin 6 secreted in ng/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 42735±177 | 53716±1836 | 30850±33 | 97222±962 |
| 0.05 | 61846±122+ | 76212±1266+ | 45764±344+ | 148278±631+ |
| 0.1 | 70898±912+ | 87289±884+ | 43992±772+ | 157389±1549+ |
| 0.25 | 88325±798+ | 108349±711+ | 63154±343+ | 124000±2333+ |
| 0.5 | 91577±266+ | 91289±1299+ | 52811±348+ | 83444±1678* |
| 1 | 15717±1110* | 49716±872* | 27078±788* | 23944±1798* |
| 2.5 | ND* | ND* | 50±216* | ND* |
| 5 | ND* | ND* | ND* | ND* |
| 6 day | Interleukin 6 secreted in ng/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 3922±9 | 11020±543 | 35576±77 | 115832±599 |
| 0.05 | 14426±4+ | 18824±471+ | 50986±239+ | 152672±2344+ |
| 0.1 | 19597±8+ | 24039±245+ | 58209±77+ | 168474±3388+ |
| 0.25 | 41418±6+ | 37294±1387+ | 46682±674+ | 139042±905+ |
| 0.5 | 32052±1+ | 42980±1682+ | 30716±1216* | 89264±1864* |
| 1 | ND* | 15294±204+ | 7323±155* | 21314±226* |
| 2.5 | ND* | ND* | 116±51* | ND* |
| 5 | ND* | ND* | ND* | ND* |
| b. Effects of 24 h, 48 h, 6 day exposures to DBT on IL-6 secretion from PBMCs plus Granulocytes. | ||||
|---|---|---|---|---|
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 8587±89 | 8593±60 | 12411±381 | 15883±675 |
| 0.05 | 8138±302* | 10178±136+ | 15173±84+ | 18437±737+ |
| 0.1 | 12487±328+ | 9162±324 | 14586±300+ | 18727±658+ |
| 0.25 | 16162±363+ | 11058±195+ | 14788±307+ | 20621±407+ |
| 0.5 | 17618±430+ | 10477±130+ | 15558±28+ | 21298±388+ |
| 1 | 9364±102+ | 7475±34* | 7244±146* | 17080±404 |
| 2.5 | 265±25* | 520±17* | 0±18* | 2804±60* |
| 5 | ND* | ND* | ND* | 267±92* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 5797±38 | 6346±13 | 11944±111 | 11795±238 |
| 0.05 | 6270±58+ | 9284±64+ | 15322±317+ | 13838±531+ |
| 0.1 | 6792±88+ | 7465±315+ | 14925±466+ | 15217±419+ |
| 0.25 | 9783±17+ | 11645±78+ | 17219±17+ | 19809±226+ |
| 0.5 | 10257±307+ | 11280±107+ | 15552±110+ | 20011±851+ |
| 1 | 7253±34+ | 9277±30+ | 8777±93* | 16104±2294 |
| 2.5 | 394±11* | 95±16* | ND* | 1150±264* |
| 5 | 215±45* | ND* | ND* | ND* |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 21±10 | 30±10 | 1843±18 | 49±4 |
| 0.05 | 161±4+ | 498±12+ | 1845±49 | 348±244 |
| 0.1 | 159±13+ | 531±9+ | 1991±36 | 656±166+ |
| 0.25 | 359±15+ | 260±6+ | 2699±16+ | 456±37+ |
| 0.5 | 550±15+ | 316±18+ | 2638±22+ | 321±26+ |
| 1 | 99±10+ | 108±5+ | 1020±35* | 107±8+ |
| 2.5 | ND* | ND* | ND* | 37±5* |
| 5 | ND* | ND* | ND* | 26±54 |
| c. Effects of 24 h, 48 h, 6 day exposures to DBT on IL-6 secretion from Granulocytes. | ||||
|---|---|---|---|---|
| 24 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 54±2 | 46±2 | 16±1 | 148±0 |
| 0.05 | 89±4+ | 71±10+ | 16±4 | 197±8+ |
| 0.1 | 72±3+ | 65±8+ | 10±1* | 254±9+ |
| 0.25 | 92±2+ | 93±25 | 17±3 | 22±1* |
| 0.5 | 79±1+ | 65±2+ | 1±2* | 252±3+ |
| 1 | 19±1* | 15±1* | 0±1* | 162±4+ |
| 2.5 | ND* | ND* | ND* | 17±0* |
| 5 | ND* | ND* | ND* | 8±0* |
| 48 h | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | 35±10 | 58±2 | 36±1 | 149±4 |
| 0.05 | 34±3 | 57±9 | 34±0* | 143±1 |
| 0.1 | 33±5 | 59±4 | 31±2* | 136±2* |
| 0.25 | 44±3+ | 78±6+ | 45±3+ | 221±8+ |
| 0.5 | 32±1* | 70±7 | 30±3* | 231±3+ |
| 1 | 5±2* | 20±0* | 6±1* | 131±9* |
| 2.5 | ND* | ND* | ND* | ND* |
| 5 | ND* | ND* | ND* | ND* |
| 6 day | Interleukin 6 secreted in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
|
| ||||
| [DBT] µM | F285 | F290 | F294 | F299 |
| 0 | ND | 6±0 | ND | 4±0 |
| 0.05 | ND | 22±1+ | ND | 9±1+ |
| 0.1 | ND | 23±4+ | ND | 5±1 |
| 0.25 | ND | 18±4+ | ND | 6±1 |
| 0.5 | ND | 10±3 | ND | 4±0 |
| 1 | 14±2+ | 7±3 | ND | 7±1 |
| 2.5 | ND | 4±2 | ND | 7±1+ |
| 5 | ND | ND* | ND | 6±1+ |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05 ND = not detectable.
Effects of DBT Exposure on Secretion of IL-6 by PBMCs + Granulocytes
Effects of exposing PBMCs+granulocytes (prepared from the same 4 donors as PBMCs) to DBT on IL-6 secretion are shown in Table 8b. After 24 and 48 h of exposure, all donors showed significant decreases in IL-6 secretion at 2.5 and 5 µM DBT. All lengths of exposure displayed significant increases at 0.25 µM DBT for all donors. Figure 2D shows the effects of TBT exposures for an individual donor (F285).
Effects of DBT Exposure on Secretion of IL-6 by Granulocytes
The effects of exposures to DBT on secretion of IL-6 were examined in granulocytes prepared from the same 4 donors as were PBMCs (results shown in Table 8c). Cells from all donors showed significant decreases in IL-6 secretion at 2.5 and 5 µM DBT after 24 h and 48 h exposures. However, significant increases were seen at DBT concentrations of 0.05, 0.1, and 0.5 µM for 3 of the 4 donors after 24 h of exposure. Cells from the fourth donor (F294) showed a small but significant increase in IL-6 secretion after 48 h. Cells from 3 of the 4 donors continued to show DBT-stimulated increases in IL-6 secretion after 6 days while the secretion from control cells was nonexistent or very low. Cells from the fourth donor (F294) showed no IL-6 secretion under any condition after 6 days. Figure 2E shows the effects of TBT exposures for an individual donor (F285).
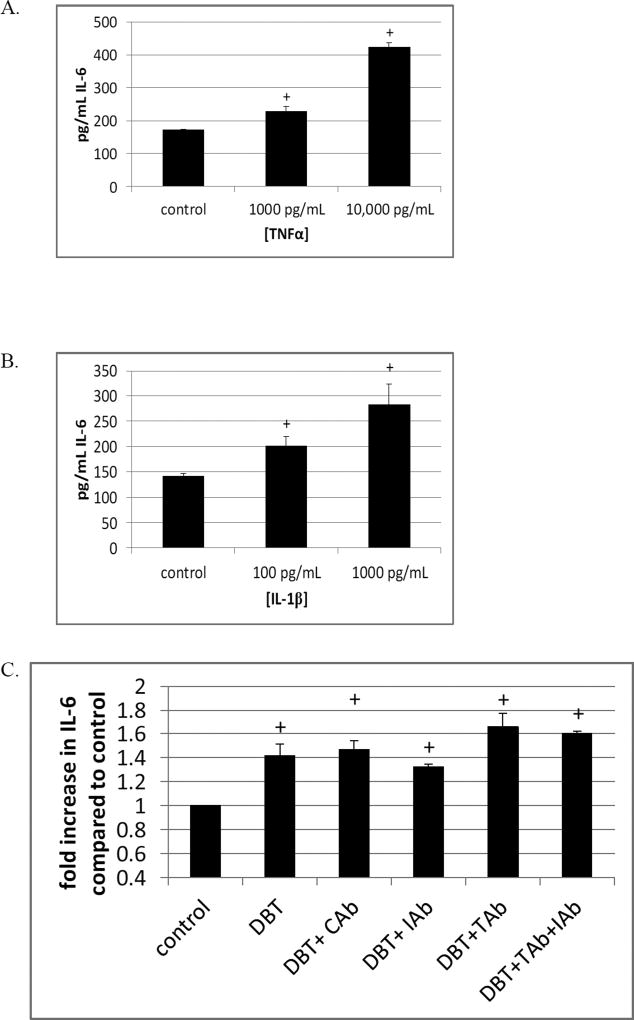
Ability of TNFα and IL-1β to stimulate IL-6 secretion
The effects of TNFα to elicit secretion of IL-6 from MD-PBMCs were examined. Figure 3A shows that incubation of MD-PBMCs for 24 h with 10,000 pg/mL of TNFα increased the secretion of IL-6 by 2.5 fold. When the amount of TNFα was 1000 pg/mL the fold increase in IL-6 secretion was 1.3 fold. When MD-PBMCs were treated with 1000 pg/mL of IL-1β for 24 h there was a 2 fold increase in IL-6 secretion. A 24 h exposure of the cells to 100 pg/ mL IL-1β caused a 1.4 fold increase (Figure 3B). Antibodies to TNFα (TAb), IL-1β (IAb) and an isotype control (CAb) were added to PBMCs prior to exposure to DBT in order to see if DBT-induced secretion of either of these cytokines was responsible for a portion of the increase seen with BT exposure. As seen in Figure 3C, there was no decrease in DBT-induced elevation of IL-6 when either TNFα or isotype control antibodies were present. There was a very slight decrease in DBT-induced IL-6 secretion when IL-1β antibody was present. However, when both TNF and IL-1β antibodies were present there was no decrease.
Figure 3.
Effects of TNFα and IL-1β on secretion of IL-6. A) Cells exposed to 10,000 and 1000 pg/ mL TNFα for 24 h. B) Cells exposed to 1000 and 100 pg/ mL IL-1β for 24 h. C) TNFα antibody (TAb), IL-1β antibody (IAb), and an isotype control antibody (CAb) were added to cells 1 h prior to exposure to 0.25 µM DBT for 24 h. Data are from representative experiment and results were repeated in cells from additional donors. + Indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone).
Discussion
BTs, TBT and DBT, have been used in a variety of industrial applications such as antifouling paints (TBT) (Gipperth, 2009) and stabilizer of plastics (DBT) (Forsyth et al., 1992; Forsyth and Jay, 1997; Sadiki et al., 1996). Levels of BTs have been found as high as 261 nM TBT (Kannan et al., 1995; Whalen et al., 1999) and 0.3 µM DBT in human blood (Kannan et al., 1999; Whalen et al., 1999). Both BTs decrease the ability of human NK cells to destroy tumor cells (Dudimah et al., 2007a; Dudimah et al., 2007b; Thomas et al., 2004; Whalen et al., 2002) and alter the secretion of TNFα, IFNγ, and IL-1β from immune cells (Brown and Whalen, 2015; Brown et al., 2017; Hurt et al., 2013; Lawrence et al., 2015). IL-6 is necessary for normal immune functions (Naitoh et al., 1988; Spangelo et al., 1989); however, elevation of this cytokine can lead to inflammatory diseases and carcinomas (Gabay, 2006; Hong et al., 2007). We investigated whether BTs alter the secretion of IL-6 from immune cells in an ex vivo human model. Increasingly complex cell preparations were used (NK cells, MD-PBMCs, PBMCs, PBMC+granulocytes, and granulocytes) to investigate whether certain cell types or combinations of cell types were more susceptible to alterations of IL-6 secretion by either TBT or DBT.
TBT induced both increases and decreases in IL-6 secretion from human immune cells. The highest concentration of TBT (200 nM) decreased IL-6 secretion from all cell preparations at one or more length of incubation. These decreases were seen in NK cells and monocyte-depleted (MD)-PBMCs (predominantly T and NK lymphocytes) from all donors after a 24 h exposure to TBT. However, in PBMCs, PBMCs+granulocytes, and granulocytes alone the length of exposure at which a decrease was seen with exposure to 200 nM TBT varied among cells from different donors. This indicated that the presence of monocytes and granulocytes changed the effectiveness of 200 nM TBT in inhibiting IL-6 secretion. Increases in IL-6 secretion seen with exposures to TBT were consistently seen in MD-PBMCs and PBMCs. However, while TBT induced increases in 3 of 4 donors in NK cells, the length of exposure and concentration of TBT at which the increases occurred varied from donor to donor.
When monocytes and granulocytes were present the TBT-induced decreases in IL-6 became less consistent but TBT-induced increases were seen in all donors at all lengths of exposure. While PMBCs and granulocytes prepared from every donor showed TBT-induced increases in IL-6 secretion, it was the granulocytes that showed the greatest sensitivity to TBT. The baseline secretion of IL-6 is much lower in granulocytes than in PBMCs. For example, PBMCs from donor F257 had a baseline secretion of 123,182 pg/mL at 24 h and a maximal TBT-induced increase in IL-6 secretion of 1.4 fold (50 nM). Granulocytes from this same donor had a baseline secretion of 137 pg/mL with an increase of 3.7 fold in IL-6 secretion induced by 2.5 nM TBT. This same pattern was seen across donors. Thus, results of this study indicate that higher concentrations of TBT are most effective at decreasing IL-6 secretion in cell preparations that do not contain monocytes or granulocytes. Conversely, concentrations of TBT of 100 nM and below are most effective at elevating IL-6 secretion when monocytes and granulocytes are present. Granulocytes are the most sensitive cell type to TBT-induced elevation of IL-6 secretion. As neutrophils are the most abundant white blood cell, the ability of TBT to induce very large increases in their normally low secretion of the important pro-inflammatory cytokine, IL-6, could lead to very significant disruption of the immune response leading to unwarranted inflammation.
DBT also altered IL-6 secretion from each of the immune cell preparations that were examined. The two highest concentrations of DBT (5 and 2.5 µM) induced a nearly complete blockage of IL-6 secretion from NK cells, MD-PBMCs, PBMCs, PBMCs+granulocytes, and granulocytes. These DBT concentrations caused some decrease in viability, but the decrease in IL-6 secretion was far greater than any decreases seen in viability at any of the time points or concentrations. Lower concentrations of DBT (ranging from 0.05 to 0.5 µM) induced elevation of IL-6 secretion in NK cells, MD-PBMCs, PBMCs, PBMCs+granulocytes and granulocytes at one or more length of incubation. As was seen with TBT, the length of exposure and the concentration of DBT at which the increases in IL-6 occurred in NK cells varied from donor to donor. Past studies have shown that DBT (at lower concentrations) is able to increase both IL-1β and TNFα secretion from human immune cell preparations that contain T-lymphocytes and monocytes (Hurt et al., 2013; Brown et al., 2017). Again, similar to TBT, the increases induced by DBT exposures occurred most consistently when monocytes were present. As mentioned above, granulocyte secretion of IL-6 was more sensitive to TBT-induced elevation than were other cell types. This was not the case for DBT. For instance, PBMCs from donor F294 showed an increase in IL-6 secretion of 2 fold when exposed to 0.25 µM DBT for 24 h. PBMCs+granulocytes from this same donor showed only a 1.2 fold increase under the same conditions and granulocytes alone showed no increase. Thus, unlike the results seen with TBT, DBT appears to be most effective at increasing IL-6 elevation from monocytes rather than granulocytes.
It is known that other cytokines such as IL-1β and TNFα are able to stimulate IL-6 production in a variety of cells including skeletal muscle cells (Luo et al., 2003; McGee et al., 1995; Ostrowski et al., 1998b; Ostrowski et al., 1998a; Pedersen et al., 2001; Tilg et al., 1997; Tosato and Jones, 1990). Monocyte production of IL-6 is stimulated by IL-1β (Tosato and Jones, 1990). Thus, we investigated whether BT-stimulated T lymphocyte (or monocyte) production of one of these cytokines may be responsible for the increase in IL-6. Previously, we have shown that TBT and DBT are able to induce elevations in MD-PBMC production of both TNFα and IL-1β (Hurt et al., 2013; Lawrence et al., 2015; Brown and Whalen 2015). Thus, either TNFα and/or IL-1β secreted by monocytes or T lymphocytes in response to either TBT or DBT could explain, at least in part, the BT-induced increases in IL-6 secretion from immune cells seen in this study. Here we showed that when cells were treated with either TNFα or IL-1β (“addition experiments”) there was a significant increase in IL-6 production by these cells, IL-1β was more effective than TNFα in its ability to stimulate this increase. However, there was no major loss of BT-induced increases in IL-6 when antibodies to IL-1β or TNFα were added prior to exposure to DBT. Thus, while the “addition experiments” suggest that IL-1β and/ or TNFα might be in part responsible for the increases in IL-6 secretion stimulated by BTs, the results of experiments using antibodies to diminish levels of DBT-induced IL-1β or TNFα did not corroborate those results. However, there are complications in the antibody experiments that could obscure the interpretation of the results. For instance, the incubation conditions allow for the cells to become closely packed (due to settling in the tube) and this might limit access of the antibody to areas where high concentrations of secreted IL-1β and TNFα would occur. Thus, the competition between these cytokines’ receptors on the cell surface and the antibody for DBT-generated IL-1β and TNFα might greatly favor the receptors. Additionally, it has been shown that the receptors for TNF generally have significantly higher affinities for the cytokine than do many antibodies (Lang et al., 2016; Song et al., 2008).
In addition to their effect on cytokine secretion shown here and in previous studies (Hurt et al., 2013; Brown and Whalen, 2015; Lawrence et al., 2015; Brown et al., 2017), both TBT and DBT have been shown to induce apoptosis of thymocytes in vitro (Gennari et al., 1997) and degeneration of the thymus (maturation site of T-cells) in rats (Snoeij et al., 1988). Comparison of TBT and DBT effects on IL-6 secretion in NK cells from the same donor at the same concentrations (50 and 100 nM) showed that TBT inhibited IL-6 secretion from NK cells by 97.7% at 100 nM whereas DBT inhibited secretion by 9%. When the relative effectiveness of TBT and DBT were compared in MD-PBMCs from the same donor, we found that TBT inhibited IL-6 secretion at 50 or 100 nM while DBT either had no effect or stimulated IL-6 secretion at these same concentrations. The greater effectiveness of TBT over DBT has been seen previously when comparing the effects of TBT and DBT on IL-1β secretion (Brown et al., 2017). Taken together, these comparisons indicate that DBT may be generally less effective at inducing an increase in both IL-6 and IL-1β secretion than is TBT. This may be due to the fact that DBT is less hydrophobic than TBT due to the loss of the third butyl group (Laughlin et al., 1986; Harper, 2005) and is less able to enter the cell to bind to intracellular targets (such as MAPKs (Brown et al., 2017) that appear to be involved in the compound-induced increases in IL-1β secretion which appear to be at least in part responsible for inducing increases in IL-6 as discussed above. This study also suggests that DBT has a lower affinity for those intracellular components that are responsible for causing decreased IL-6 secretion and thus is somewhat less effective at inducing that effect.
A previous study demonstrated that DBT alters IL-1β secretion from increasingly reconstituted preparations of human immune cells (Brown et al., 2017). As was seen with IL-6 secretion in the current study, higher concentrations of DBT (5 and 2.5 µM) decreased the secretion of IL-1β, while lower concentrations (0.1 and 0.05 µM) increased the secretion of IL-1β. However, in contrast to the effects of DBT on IL-6 secretion, the secretion of IL-1β was increased when NK cells were exposed to lower concentrations of DBT for at each length of incubation. As mentioned above, there was no consistent increase in secretion of IL-6 by NK cells in response to DBT exposures. Additionally, the greater sensitivity of granulocytes to TBT-induced increases in IL-6 secretion that was seen in the current study did not occur when looking at granulocyte secretion of IL-1β (Brown and Whalen, 2015).
These results suggest that TBT and DBT-induced alterations of IL-6 secretion as well as other pro-inflammatory cytokines from immune cells may potentially affect immune function and cancer invasiveness. Monobutyltin (MBT), which can be a breakdown product of both TBT and DBT, was not examined in this study as it showed very limited effects on immune cell function in previous studies (Whalen et al., 1999) and no effects at levels measured in human blood (Kannan et al., 1999). The levels of TBT and DBT found in human blood samples are in the range of TBT and DBT concentrations that elevated IL-6 levels. The fact that both of these compounds are able to alter IL-6 secretion suggests that at higher concentrations they may decrease immune competency. Lower concentrations of either TBT or DBT may lead to increased levels of IL-6 which could produce chronic inflammation. Increased inflammation in the absence of a microorganism is referred to as “sterile inflammation” (Chen and Nunez, 2010). Compound such as TBT and DBT may have the capacity to induce sterile inflammation by their ability to increase the production of pro-inflammatory cytokines such as IL-6 and other pro-inflammatory cytokines (Hurt et al., 2013; Brown and Whalen, 2015; Lawrence et al., 2015; Brown et al., 2017). The results of this study, as well as previous studies, indicate that these two compounds may be more of a problem in terms of causing chronic inflammation rather than loss of immune competence in exposed individuals due to the fact that a large percentage of the individuals whose blood levels were measured had levels in the range where effects on IL-6 secretion were seen ex vivo (Whalen et al., 1999; Kannan et al., 1999). Interestingly, a rise in Rheumatoid arthritis (Myasoedova et al., 2010) (one of the several diseases where Il-6 is elevated) has been noted over the period of 1955–2007 as the use of compounds such as TBT and DBT with their concomitant contamination of the human system has been increasing.
In summary, both TBT and DBT are able to alter the secretion of IL-6 in a range of human immune cell preparations. Both compounds decrease the secretion of IL-6 at the highest concentration (TBT=200 nM, DBT= 5 and 2.5 µM)) from NK cells, MD-PBMCs, PBMCs, PBMCs+granulocytes, and granulocytes alone. TBT (50–2.5 nM) and DBT (0.5–0.05 µM) are also able to increase the secretion of IL-6 at lower exposure levels and this is effect is most consistent when either T lymphocytes or monocytes are present. The cell type that is most sensitive to TBT-induced increases in IL-6 are granulocytes. Cell preparations containing T lymphocytes and/or monocytes were the most sensitive to DBT-induced elevation of IL-6 secretion.
Acknowledgments
Grants U54CA163066 and 4T34GM007663 from the National Institutes of Health
References
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Advances in Immunology. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. Environment International. 2008;34:292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, Müllberg J, Tostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in Crohn disease and experimental colitis in vivo. Nature Medicine. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- Brown S, Whalen M. Tributyltin alters secretion of interleukin 1 beta from human immune cells. Journal of Applied Toxicology. 2015;35:895–908. doi: 10.1002/jat.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Tehrani S, Whalen Dibutyltin-induced alterations of interleukin 1 beta secretion from human immune cells. Journal of Applied Toxicology. 2017;37:181–191. doi: 10.1002/jat.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature Reviews/Immunology. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. Journal of Cellular Biochemistry. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Gibson C, Whalen MM. Effect of dibutyltin on ATP levels in human natural killer cells. Environmental Toxicology. 2007a;22:117–123. doi: 10.1002/tox.20252. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effect of tributyltin (TBT) on ATP levels in human natural killer (NK) cells: Relationship to TBT-induced decreases in NK function. Journal of Applied Toxicology. 2007b;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Epstein RL, Phillippo ET, Harr R, Koscinski W, Vosco G. Organotin residue determination in poultry and turkey sample survey in the United States. J. Agric Food Chem. 1991;39:917–921. [Google Scholar]
- Fent K. Ecotoxicology of organotin compounds. CRC Critical Review of Toxicology. 1996;26:1–117. [PubMed] [Google Scholar]
- Forsyth DS, Weber D, Cldroux C. Determination of butyltin, cyclohexyltin and phenyltin compounds in beers and wines. Food Additives and Contaminants. 1992;9:161–169. doi: 10.1080/02652039209374058. [DOI] [PubMed] [Google Scholar]
- Forsyth DS, Jay B. Organotin leachates in drinking water from chlorinated polyvinyl chloride (CPVC) pipe. Applied Organometallic Chemistry. 1997;11:551–558. [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Research and Therapy. 2006;8:S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signaling in inflammation. Nature Rev. Drug Disc. 2009;8:480–481. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, McGourty J, Brasher KK, King JA, Gillis S, Mosley B, Ziegler SF, Cosman D. The IL-6 signal transducer, gp130: An Oncostatin M receptor and affinity converter for the LIF Receptor. Science. 1992;255:1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- Gennari A, Potters M, Seinen W, Pieters R. Organotin-Induced Apoptosis as Observed in Vitro Is Not Relevant for Induction of Thymus Atrophy at Antiproliferative Doses. Toxicology and Applied Pharmacology. 1997;147:259–266. doi: 10.1006/taap.1997.8265. [DOI] [PubMed] [Google Scholar]
- Gipperth L. The legal design of the international and European Union ban on tributyltin antifouling paint: Direct and indirect effects. Journal of Environmental Management. 2009;90:S86–S95. doi: 10.1016/j.jenvman.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Hall LJ, Clare S, Dougan G. NK Cells Influence Both Innate and Adaptive Immune Responses after Mucosal Immunization with Antigen and Mucosal Adjuvant. J. Immunol. 2010;84:4327–4337. doi: 10.4049/jimmunol.0903357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. Toxicological profile for tin and tin compounds. United States Agency for Toxic Substances and Disease Registry; US EPA: 2005. [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochemical Journal. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Matsuda T, Nakajima K. Signal transduction through gp130 that is shared among the receptors for the interleukin 6 related cytokine subfamily. Stem Cells. 1994;12:262–277. doi: 10.1002/stem.5530120303. [DOI] [PubMed] [Google Scholar]
- Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- Hurst SM, Wilkinson ST, McLouglin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- Hurt K, Hurd-Brown T, Whalen M. Tributyltin and dibutyltin alter secretion of tumor necrosis factor alpha from human natural killer cells and a mixture of T cells and natural killer cells. Journal of Applied Toxicology. 2013;33:503–510. doi: 10.1002/jat.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, Birren SJ, Yasukawa K, Kishimoto T, Anderson DJ, Stahl N, Yancopoulos GD. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R. Occurrence of butyltin residues in certain foodstuffs. Bulletin of Environmental Contamination and Toxicology. 1995;55:510–516. doi: 10.1007/BF00196029. [DOI] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. Occurrence of butyltin compounds in human blood. Environmental Science and Technology. 1999;33:1776–1779. [Google Scholar]
- Kimbrough RD. Toxicity and health effects of selected organotins compounds: A Review. Environmental Health Perspectives. 1976;14:51–56. doi: 10.1289/ehp.761451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood Journal. 1989;74:1–10. [PubMed] [Google Scholar]
- Kuijpers TW, Tool ATJ, van der Schoot CE, Ginsel LA, Onderwater JJM, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 2013;78:1105–1111. [PubMed] [Google Scholar]
- Lang I, Fullsack S, Wyzgol A, Fick A, Trebing J, Carmona Arana JA, Schafer V, Weisenberger D, Wajant H. Binding studies of TNF receptor superfamily (TNFRSF) receptors on intact cells. J. Biol. Chem. 2016;291:5022–5037. doi: 10.1074/jbc.M115.683946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RB, Guard HE, Coleman WM., III Tributyltin in seawater: Speciation and octanol-water coefficient. Environ. Sci. Technol. 1986;20:201–204. doi: 10.1021/es00144a016. [DOI] [PubMed] [Google Scholar]
- Lawrence S, Reid J, Whalen MM. Secretion of interferon gamma from human immune cells is altered by exposure to tributyltin and dibutyltin. Environmental Toxicology. 2015;30:559–571. doi: 10.1002/tox.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, He S. Induction of IL-6 release from human T cells by PAR-1 and PAR-2 agonists. Immunology and Cell Biology. 2006;84:461–466. doi: 10.1111/j.1440-1711.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Modrell B, Aruffo A, Marken JS, Taga T, Yasukawa K, Murakami M, Kishimoto T, Shoyab M. Interleukin-6 signal transducer gp130 mediates oncostatin M signaling. The Journal of Biological Chemistry. 1992;267:16763–16766. [PubMed] [Google Scholar]
- Loganathan BG, Kannan K, Sajwan KS, Owen DA. Butyltin compounds in freshwater ecosystems. In: Lipnick RL, Hermens J, Jones K, Muir D, editors. Persistent, Bioaccumulative and Toxic Chemicals I: Fate and Exposure. American Chemical Society; Washington, DC: 2001. pp. 134–149. ACS Monograph Series vol. 772. [Google Scholar]
- Loganathan BG. Persistent organic chemicals in the Pacific Basin countries: An overview. In: Loganathan BG, Khim JS, Kodavanti PR, Masunaga S, editors. Persistent Organic Chemicals in the Environment: Status and trends in the Pacific Basin Countries I. American Chemical Society and Oxford University Press; 2016. pp. 1–15. ACS Symposium Series Vol. 1243. [Google Scholar]
- Luo G, Hershko DD, Robb BW, Wray CJ, Hasselgren PO. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-κB. American Journal of Physiology-Regulatory, Integrative, and Comparative. 2003;284:1249–1254. doi: 10.1152/ajpregu.00490.2002. [DOI] [PubMed] [Google Scholar]
- Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee DW, Bamberg T, Vitkus SJD, McGhee JR. A synergistic relationship between TNF-α, IL-1β, and TGF-β1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology. 1995;86:6–11. [PMC free article] [PubMed] [Google Scholar]
- Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE. Filter Buffy Coats (FBC): A source of peripheral blood leukocytes recovered from leukocyte depletion filters. Journal of Immunological Methods. 2005;307:150–166. doi: 10.1016/j.jim.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, Imura H. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochemical and Biophysical Research Communications. 1988;155:1459–1463. doi: 10.1016/s0006-291x(88)81305-6. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Hori S, Nakazawa H. Determination of dibutyltin and dioctyltin compounds in PVC food containers, wrappage and clothes by reversed phase HPLC with column switching. Eisei Kagaku. 1990;36:155–220. [Google Scholar]
- Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Research. 2005;65:10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation in plasma cytokines in humans in response to treadmill running. Journal of Physiology. 1998a;513:889–894. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that IL-6 is produced in skeletal muscle during intense long-term muscle activity. Journal of Physiology. (London) 1998b;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Schjerling P. Muscle derived interleukin-6: possible biological effects. Journal of Physiology. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, Rosenthal A, Taga T, Paoni NK, Wood Wi. Cardiotrophin-1: Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. The Journal of Biological Chemistry. 1995;270:10915–10922. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo R-F, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB Journal. 2004;18:370–372. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- Roper WL. Toxicological profile for tin. US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, USA 1992 [Google Scholar]
- Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. International Journal of Biological Science. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiki AI, Williams DT, Carrier R, Thomas B. Pilot study on the contamination of drinking water by organotin compounds from PVC materials. Chemosphere. 1996;32:2389–2398. doi: 10.1016/0045-6535(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Sadiki AI, Williams DT. A Study on Organotin Levels in Canadian Drinking Water Distributed Through PVC Pipes. Chemosphere. 1999;38:1541–1548. doi: 10.1016/s0045-6535(98)00374-9. [DOI] [PubMed] [Google Scholar]
- Scheller J, Grotzinger Rose-John S. Updating interleukin-6 classic and trans-signaling. Signal Transduction. 2006;6:240–259. [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Snoeij NJ, Penninks AH, Seinen W. Dibutyltin and tributyltin compounds induce thymus atrophy in rats due to selective action on thymic lymphoblasts. International Journal of Immunopharmacology. 1988;10:891–899. doi: 10.1016/0192-0561(88)90014-8. [DOI] [PubMed] [Google Scholar]
- Song M-Y, Park S-K, Kim C-S, Yoo TH, Kim B, Kim M-S, Kim Y-S, Kwag WJ, Lee B-K, Baek K. Characterization of a novel anti-human TNF-α murine monoclonal antibody with high binding affinity and neutralizing activity. Exp. Mol. Med. 2008;40:35–42. doi: 10.3858/emm.2008.40.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangelo BL, Judd AM, Isakson PC, MacLeod RM. Interleukin-6 stimulates anterior pituitary hormone release in vitro. Endocrinology. 1989;125:575–577. doi: 10.1210/endo-125-1-575. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tanabe S, Takeuchi I, Miyazaki N. Distribution and specific bioaccumulation of butyltin compounds in a marine ecosystem. Archives of Environmental Contamination and Toxicology. 1999;37:50–61. doi: 10.1007/s002449900489. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Prudente M, Mizuno T, Hasegawa J, Iwata H, Miyazaki N. Butyltin contamination in marine mammals from north Pacific and Asian coastal waters. Environmental Science and Technology. 1998;32:193–198. [Google Scholar]
- Thomas LD, Shah H, Green SA, Bankhurst AD, Whalen MM. Tributyltin exposure causes decreased granzyme B and perforin levels in human natural killer cells. Toxicology. 2004;200:221–233. doi: 10.1016/j.tox.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunology Today. 1997;18:428–432. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- Tosato G, Jones KD. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood Journal. 1990;75:1305–1310. [PubMed] [Google Scholar]
- Wester PW, Krajnc EI, van Leeuwen FXR, Loeber JG, van der Heijden CA, Vaessen HA, Helleman PW. Chronic toxicity and carcinogenicity of bis(tri-n-butyltin)oxide (TBTO) in the rat. Food and Chemical Toxicology. 1990;28:179–196. doi: 10.1016/0278-6915(90)90006-9. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of Environmentally Relevant Concentrations of Butyltins on Human Natural Killer Cells in Vitro. Environmental Research. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Ghazi S, Loganathan BG, Hatcher F. Expression of CD16, CD18 and CD56 in Tributyltin-exposed human natural killer cells. Chemico-Biological Interactions. 2002;139:159–176. doi: 10.1016/s0009-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Yamada S, Fuji Y, Mikami E, Kawamura N, Hayakawa J. Small-scale survey of organotin compounds in household commodities. Journal of AOAC International. 1993;76:436–441. [Google Scholar]
- Yin T, Taga T, Tsang MLS, Yasukawa K, Kishimoto T, Yang YC. Involvement of IL-6 signal transducer gp130 in IL-11-mediated signal transduction. The Journal of Immunology. 1993;151:2555–2561. [PubMed] [Google Scholar]
- Zimmermann M, Arruda-Silva F, Bianchetto-Aguilera F, Finotti G, Calzetti F, Scapini P, Lunardi C, Cassatella MA, Tamassia N. IFNα enhances the production of IL-6 by human neutrophils activated via TLR8. Sci. Rep. 2016;6:19674. doi: 10.1038/srep19674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Bianchetto-Aguilera F, Castellucci M, Rossato M, Costa S, Lunardi C, Ostuni R, Girolomoni G, Natoli G, Bazzoni F, Tamassia N, Cassatella MA. Chromatin remodelling and autocrine TNFa are required for optimal interleukin-6 expression in activated human neutrophils. Nat. Commun. 2015;6:6061. doi: 10.1038/ncomms7061. [DOI] [PubMed] [Google Scholar]