Abstract
Neuroimaging evidence suggests that executive functions (EF) depend on brain regions that are not closely tied to specific cognitive demands but rather to a wide range of behaviors. A multiple-demand (MD) system has been proposed, consisting of regions showing conjoint activation across multiple demands. Additionally, a number of studies defining networks specific to certain cognitive tasks suggest that the MD system may be composed of a number of sub-networks each subserving specific roles within the system. We here provide a robust definition of an extended MDN (eMDN) based on task-dependent and task-independent functional connectivity analyses seeded from regions previously shown to be convergently recruited across neuroimaging studies probing working memory, attention and inhibition, i.e., the proposed key components of EF. Additionally, we investigated potential subnetworks within the eMDN based on their connectional and functional similarities. We propose an eMDN network consisting of a core whose integrity should be crucial to performance of most operations that are considered higher cognitive or EF. This then recruits additional areas depending on specific demands.
Keywords: Executive functioning, functional connectivity, hierarchical clustering, higher cognitive functions, meta-analytical connectivity modeling
Introduction
Executive functioning is central to coordinated, goal-directed behavior and thought to play a major role in a wide range of different psychiatric and neurological diseases (Zelazo & Müller, 2002). However, despite its significance and the consequent effort directed towards investigating it, the true nature of executive abilities remains rather elusive. One of the main reasons for this is that executive functioning is probably not a single process but should be rather regarded as a “macro-construct” that includes different aspects of mental functioning (Zelazo et al., 1997). Moreover, the lack of a clear formal definition of executive functioning is also due to the nature of the aspects that constitute it, the relationship among these and their contribution to the overall concept (Lezak, 1982). Mirroring this lack of formal definition of executive functioning, several brain regions and networks have been implicated as the brain’s underpinning of executive functioning. In this perspective, the network aspect is particularly important, as there is a growing consensus that higher cognitive, including “executive”, functions depend on distributed networks rather than any particular region in isolation (Corbetta & Shulman, 2002). In addition, it has been shown that “executive networks” seem to sustain a wide range of cognitive functions (Federenko et al., 2013; Cabeza & Nyberg, 2000), prompting the term multiple-demand network (MD; Duncan & Owen, 2000; Duncan, 2010). Unfortunately, different perspectives, operationalizations and traditions have resulted in a rather diverse co-existence of labels for brain networks associated with executive control. One is the aforementioned multiple-demand network as defined by convergent activation across multiple cognitive tasks in fMRI (Duncan, 2010). A very similar example is the cognitive control network, which has been described as a network that includes a set of cortical regions that are consistently co-active during cognitive control tasks (Cole & Schneider 2007). Other comparable networks include the fronto-parietal control system (Vincent et al., 2008), the superordinate cognitive control network (Niendam et al., 2012), the task-positive network (Fox et al., 2005), and the extrinsic mode network (Hugdahl et al., 2015). In addition, there seems to be some convergence with concepts such as the salience network (Seeley et al., 2007), the ventral attention network (VAN) (Vossel et al., 2014; Japee et al., 2015) and the dorsal attention network (DAN) (Corbetta et al., 2008; Corbetta & Schulman, 2002), besides other functional networks such as the working memory network (Rottschy et al., 2012), the vigilant attention network (Langner & Eickhoff, 2013), and the inhibitory control network (Cieslik et al., 2015).
Inspecting these various networks, it quickly becomes evident, that virtually all of them indicate the posterior-medial frontal cortex [pre-supplementary motor area and adjacent middle cingulate cortex (pre-SMA/MCC)], the bilateral anterior insula (aINS), intraparietal sulcus (IPS), and posterior inferior frontal sulcus (IFS) as regions contributing to executive processing. Interestingly, these regions were suggested as part of a multiple-demand network (MDN) by Duncan (2010) and emerged from a recent integration (Müller et al., 2015) of three large-scale neuroimaging meta-analyses on working memory (Rottschy et al., 2012), vigilant attention (Langner & Eickhoff, 2013), and inhibitory control (Cieslik et al., 2015).
In turn, there are also several brain regions not included in these rather conservative definitions of regions of the MDN but can nevertheless be found in several of the aforementioned networks. These include, e.g., the basal ganglia and thalamus, the more anterior IFS / dorsolateral prefrontal cortex, or the dorsal premotor cortex. In addition, the MDN as suggested by Duncan (2010) or Müller et al. (2015) are based on the (most robust) convergence of activation data and do not directly consider the perspective of a distributed neural network. In light of these two observations, it seems likely that the previously established regions of the MDN entertains close interactions with several other regions that may be considered as an extended MDN (eMDN) complementing the original regions.
Mapping and characterizing this broader MDN is the core aim of this study. In more detail, this entails the computation of robust connectivity maps for each original MDN region by combining task-free and task-based functional connectivity analyses. The eMDN is then identified by convergence across multiple of these robust connectivity maps for the seed regions. Next, we functionally characterize the ensuing eMDN regions by an objective analysis of experimental paradigms that evoke activation of these regions. Finally, we investigate potential cliques of regions within the extended MDN based on similarities in connectional and functional profiles.
Methods
Seed definition
The seed regions for this work were based on the meta-analytically defined multiple-demand network of Müller et al. (2015), which was defined by performing a conjunction across three large-scale neuroimaging meta-analyses on working memory (Rottschy et al., 2012, covering e.g., n-back, Sternberg or delayed match-to sample tasks), vigilant attention (Langner & Eickhoff, 2013, covering e.g., stimulus detection or simple reaction tasks), and inhibitory control (Cieslik et al., 2015, covering, e.g., Stroop, Simon or Flanker tasks). The regions present in the resulting conjunction consist of the bilateral anterior insula, bilateral inferior frontal junction/gyrus, right middle frontal gyrus, right intraparietal sulcus and the posterior medial frontal cortex extending from the midcingulate cortex to the (pre-) supplementary motor area.
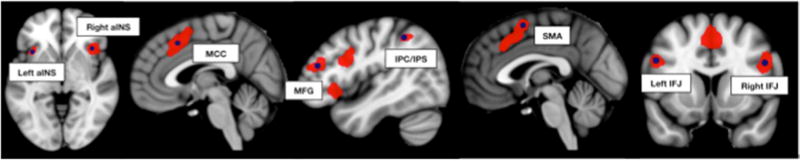
To ensure that the functional connectivity analyses of all seed regions were based on the same number of voxels, in spite of unequal cluster sizes, we represented each seed by a 5 mm sphere around its center of gravity. The only exception to this approach was the posterior medial frontal cortex cluster whose center of gravity was located between MCC and pre-SMA. Given the presence of distinct peaks within both MCC and pre-SMA, both of these were retained as seed coordinates. Thus, eight seed coordinates (Figure 1; Table 1) were used for whole-brain resting-state and meta-analytic connectivity modeling analyses that were intersected to define the robust, state-independent connectivity map for each seed.
Figure 1.
Seed regions (shown in dark blue) derived from the meta-analytically defined multiple-demand network by Müller et al. 2015 (shown in red).
Table 1.
Seed coordinates derived from the meta-analytically defined multiple-demand network by Müller et al. (2015)
| x | y | z | |
|---|---|---|---|
| Right aINS | 38 | 22 | −2 |
| Left aINS | −36 | 18 | −2 |
| Right IFJ/IFG | 48 | 10 | 28 |
| Left IFJ/IFG | −48 | 10 | 30 |
| Right MFG | 44 | 38 | 20 |
| Right IPC/IPS | 44 | −44 | 46 |
| MCC | 4 | 20 | 44 |
| pre-SMA | −2 | 6 | 58 |
Resting-state functional connectivity
Seed-based RS analysis was used to investigate the task-independent functional connectivity of each original MDN region. Resting-state fMRI images of 192 healthy volunteers were obtained from the Enhanced Nathan Kline Institute – Rockland Sample (Nooner et al., 2012). The local ethics committee of the Heinrich-Heine University in Düsseldorf approved re-analysis of the data. During RS acquisition, subjects were instructed to look at a fixation cross, not think about anything in particular and not to fall asleep. Images were acquired on a Siemens TimTrio 3T scanner using BOLD contrast [gradient-echo EPI pulse sequence, TR = 1.4 s, TE = 30 ms, flip angle = 65°, voxel size = 2.0 mm × 2.0 mm × 2.0 mm, 64 slices]. Physiological and movement artifacts were removed from the RS data by using FIX (FMRIB’s ICA-based Xnoiseifier, version 1.061 as implemented in FSL 5.0.9; Salimi-Khorshidi et al. 2014; Griffanti et al. 2014), which decomposes the data into independent components (ICs) and identifies noise components using a large number of distinct spatial and temporal features via pattern classification. Unique variance related to the identified artefactual ICs is then regressed from the data together with 24 movement parameters (including derivatives and 2nd order effects as previously described and evaluated; cf. Satterthwaite et al., 2013). Data were further preprocessed using SPM8 (Wellcome Trust Centre for Neuroimaging, London) and in-house Matlab scripts. The first four scans were excluded prior to further analyses, the remaining EPI images corrected for head movement using a two-pass (alignment to the initial volume followed by alignment to the mean after the first pass) affine registration. The mean EPI image for each subject was then spatially normalized to the ICBM-152 reference space using the “unified segmentation” approach (Ashburner & Friston, 2005). The resulting deformation was applied to the individual EPI volumes, which were subsequently smoothed with a 5-mm FWHM Gaussian kernel to improve the signal-to-noise ratio and to compensate for residual anatomic variations. The time-course of each seed was extracted per subject by computing the first eigenvariate of the time-series of all voxel within 5 mm of the seed coordinate. To reduce spurious correlations, variance explained by the mean white matter and cerebral spinal fluid signal were removed from the time series, which was subsequently band-pass filtered preserving frequencies between 0.01 and 0.08 Hz. The processed time-course of each seed was then correlated with the (identically processed) time-series of all other gray-matter voxels in the brain using linear (Pearson) correlation. The resulting correlation coefficients were transformed into Fisher’s z-scores, which were entered in a second-level ANOVA for group analysis including age and gender as covariates of no interest. The data was then subjected to non-parametric permutation based inference and thresholded at p < 0.05 corrected for multiple comparisons on the cluster level.
Meta-analytical connectivity modeling (MACM)
Meta-analytical connectivity modeling (MACM) was used to characterize the whole-brain connectivity of each seed region during the execution of experimental tasks through the identification of significant co-activations with the seed across many individual experiments (Eickhoff et al., 2009; Laird et al., 2013). It thus benefits from the fact that a large number of such studies are now available in a highly standardized format through the BrainMap database (Fox et al., 2014; Laird et al., 2011). First, all experiments that feature at least one focus of activation in a particular seed region were identified in BrainMap. Next, the retrieved experiments were subjected to a quantitative meta-analysis using the revised activation likelihood estimation (ALE) algorithm (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012). This algorithm treats the activation foci reported in the experiments as spatial probability distributions rather than single points, and aims at identifying brain areas that show convergence of activation across experiments. Importantly, convergence was assessed across all the activation foci reported in these experiments. Consequently, any significant convergence outside the seed indicates consistent co-activation and hence functional connectivity. Statistical significance was assessed at p < 0.05 after correction for multiple comparisons (Eickhoff et al., 2016).
Identification of the extended multiple-demand network
The key aim of our study was the definition of an extended multiple-demand system by identifying the brain regions that are strongly connected to multiple of the original MDN regions as defined by Müller et al (2015). We identified areas that show robust task-independent as well as task-dependent functional connectivity with multiple of the seeds (Amft et al., 2014) using the workflow outlined in figure 2. First we generated the task-independent (RS) and task-dependent (MACM) whole-brain functional connectivity map for each seed. Then a conjunction analysis was performed across the RS and MACM functional connectivity maps for each seed using the minimum statistic(Nichols et al., 2005). This resulted in eight consensus functional connectivity maps, showing the areas consistently interacting with each seed across different brain states (cf. Clos et al., 2014, Hardwick et al., 2015). The extended multiple-demand network was then delineated by identifying all regions that were significantly connected with multiple seeds, i.e., regions in which the consensus connectivity maps of at least half of the seeds overlapped. In order to exclude smaller regions of putatively spurious overlap an additional extent-threshold of 10 continuous voxels was applied.
Figure 2.
Workflow used for the delineation of the extended multiple demand network entailing the computation of task-free (RS-FC) and task-based (MACM) connectivity maps for each seed region which were then converged to identify the eMDN.
Functional Characterization of extended MDN regions
The resulting regions (represented by their peak coordinates) of the extended multiple-demand network were then functionally characterized based on the metadata from the BrainMap database (Laird et al., 2009, Laird et al., 2011, & Fox et al., 2002), using both forward and reverse inference, as performed in previous studies (Müller et al., 2013, Rottschy et al., 2013). The key idea behind this approach is to identify all experiments that activate a particular region of interest and then analyze the experimental meta-data describing the experimental settings that were employed in these. This allows statistical inference on the type of tasks that evoke activation in a particular region.
In BrainMap, tasks are coded along two dimensions. Behavioral domains (BD) describe the cognitive processes probed by an experiment, while paradigm classes (PC) label the respective task used. In the forward inference approach, the functional profile was determined by identifying taxonomic labels for which the probability of finding activation in the respective region/set of regions was significantly higher than the overall (a priori) chance across the entire database. That is, we tested whether the conditional probability of activation given a particular label [P(Activation|Task)] was higher than the baseline probability of activating the region(s) in question per se [P(Activation)]. Significance was established using a binomial test [p < 0.05, corrected for multiple comparisons using false discovery rate (FDR)]. In the reverse inference approach, the functional profile was determined by identifying the most likely behavioral domains, given activation in a particular region/set of regions. This likelihood P(Task|Activation) can be derived from P(Activation|Task) as well as P(Task) and P(Activation) using Bayes’ rule. Significance (at p < 0.05, corrected for multiple comparisons using FDR) was then assessed by means of a chi-squared test.
Clustering of extended MDN regions
The last objective was to elucidate the relationships between eMDN regions and to identify potential cliques among them, i.e., sub-networks within the eMDN. To this end, we performed hierarchical cluster analysis of the eMDN regions based on their: (1) resting-state functional connectivity patterns, (2) whole-brain co-activation maps, and (3) functional profiles yielded by the BrainMap based decoding. Resting-state functional connectivity between all regions of the identified eMDN was computed using the FSLNets toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). Partial temporal correlations between all regions’ time series data were computed to estimate pairwise functional connectivity (Marrelec et al. 2006). For each pairwise connection, Fisher’s Z–transformed functional connectivity values were submitted to one-sample t-tests. The resulting t values, reflecting connection strength as well as consistency across the sample, were z-transformed (into units of the standard normal distribution).This connectivity matrix was then fed into the WARD clustering.
Of note, all features entered the analyses without any thresholding for significance, which is a distinction from the analyses described above but necessary in order to preserve the full pattern of the respective connectional and functional profiles. The concept behind hierarchical clustering is to group the initial elements (regions) in a stepwise fashion such that elements within a cluster have features that are as homogeneous as possible while different clusters are maximally distinct from each other. This was achieved through an agglomerative approach in which clusters initially formed by individual regions that are subsequently merged according to their similarity using standardized Euclidean distances and Ward’s incremental sum of squares method (Eickhoff, 2011; Timm, 2002). This hierarchical approach then revealed cliques of eMDN regions at different level of granularity based on resting-state connectivity, MACM co-activation and functional activation patterns.
Contrast analyses of functional differences
Finally, to examine the specificity of the functional profiles of the three different cliques we performed contrast analyses which were constrained to the experiments in BrainMap that activated the regions belonging to each of the 3 clusters. This was done using the “Behavioural Domain” meta-categories in the BrainMap database and forward inference to compare the activation probabilities between each pair of cliques given a particular behavioural domain, compared with the a priori probability of any focus to lie in either of the two compared cliques. This was done by means of a binomial test (p < 0.05, FDR-corrected for multiple comparisons).
Results
The extended multiple-demand network
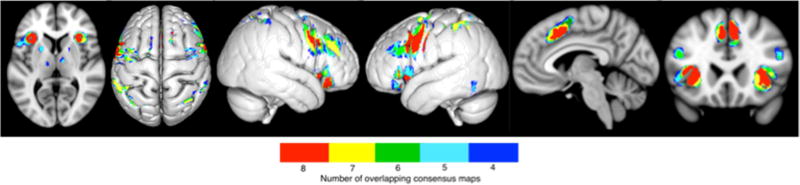
The extended multiple-demand network was defined in a stepwise fashion, first identifying regions that were robustly connected with all of the seed regions, i.e., found in all eight consensus connectivity maps. This yielded six clusters located in the bilateral inferior frontal junction (IFJ) extending into the inferior frontal gyrus, the bilateral anterior insula (aINS), and the bilateral pre-SMA (Figure 3 in red). When reducing the required overlap to seven consensus connectivity maps, four additional clusters emerged. These were located in the bilateral intraparietal sulcus (IPS), right middle frontal gyrus (MFG) extending into the inferior frontal sulcus (IFS), as well as in the left dorsal pre-motor cortex (dPMC) (Figure 3 in yellow). At a threshold of six overlapping consensus connectivity maps an additional cluster in the left MFG/IFS was observed (Figure 3 in green). The network formed from five overlapping connectivity maps additionally included the right dorsal premotor cortex, the left putamen, the left thalamus and the left ITG (Figure 3 in cyan). Finally, when reducing the required overlap to four, i.e., half of the computed consensus connectivity maps, clusters in the right putamen and in the right thalamus were additionally observed (Figure 3 in blue). The peak coordinates of all the resulting regions can be found in Table 2.
Figure 3.
Results showing the different resulting extended networks depending on the number of overlapping consensus maps. Eight overlapping consensus maps yielded clusters located in the bilateral IFJ/IFG, bilateral aINS, and bilateral pre-SMA (in red). Seven overlapping maps resulted in the addition of the bilateral IPS, right MFG/IFS and left dPMC (in yellow). Six overlapping maps resulted in the addition of the left MFG/IFS (in green). Five overlapping maps additionally included the right dPMC, left putamen, left thalamus and left ITG (in cyan). Four overlapping maps resulted in the addition of the right putamen and right thalamus (in blue).
Table 2.
Peak coordinates of resulting regions of the extended multiple demand network
| Number of overlapping consensus maps |
Region | Coordinates of main peak | ||
|---|---|---|---|---|
| x | y | z | ||
| 8 | Left IFJ | −46 | 6 | 30 |
| Right IFJ | 50 | 12 | 28 | |
| Left aINS | −32 | 20 | 2 | |
| Right aINS | 36 | 22 | 0 | |
| Left SMA/pre-SMA | −4 | 14 | 44 | |
| Right SMA/pre-SMA | 6 | 18 | 46 | |
| 7 | Left IPS | −32 | −52 | 46 |
| Right IPS | 32 | −58 | 48 | |
| Right MFG/IFS | 44 | 36 | 20 | |
| Left dPMC | −28 | −4 | 52 | |
| 6 | Left MFG/IFS | −44 | 32 | 22 |
| 5 | Right dPMC | 32 | 0 | 52 |
| Left Putamen | −20 | 6 | 4 | |
| Right Thalamus | 10 | −12 | 8 | |
| Left ITG | −46 | −60 | −10 | |
| 4 | Right Putamen | 22 | 6 | 4 |
| Left Thalamus | −10 | −16 | 6 | |
Functional Characterization
The functional profiles of each of the eMDN regions were characterized based on the behavioral domain and paradigm class meta-data from the BrainMap database. This revealed that the left IFJ was associated with language and working memory, where as the right IFJ was associated with spatial cognition and attention. Both IPS clusters were associated with working memory while the right IPS was found to be associated to functions related to spatial cognition, reasoning, perception, attention, action control and language. Both aINS clusters showed associations with language. The right aINS showed additional associations with action preparation, cognition and sensation. The bilateral pre-SMA were associated with language and working memory. Moreover, the left pre-SMA was associated with speech exection while the right pre-SMA was associated with working memory and attention. The subcortical clusters, namely the bilateral putamen and the bilateral thalamus, were all found to be associated with sensation and action. The cluster in the right MFG/IFS was linked to cognition and working memory, that in the left MFG/IFS to reasoning and working memory. Both dPMC clusters were related to action, perception, working memory, action control. In addition, the left dPMC cluster was linked to attention, the right to spatial cognition. Finally, the cluster in the left ITG was linked to emotion, perception, language, and memory.
Clustering of extended MDN regions
The hierarchical clustering revealed several cliques within the eMDN that were present across the different features, i.e., resting-state connectivity, MACM co-activation and function but also feature-specific patterns (Figure 4).
Figure 4.
Clustering of the extended MDN regions based on resting state connectivity, whole brain co-activation and behavioural domains and paradigm classes. The different colours represent the grouping that resulted from the different clustering analyses.
Across all analyses, the bilateral thalamus and putamen were consistently grouped together. In turn, the bilateral aINS and bilateral pre-SMA showed high similarity in resting-state connectivity and co-activation profiles but were slightly more divergent in terms of their functional profile. In the latter, the bilateral pre-SMA formed a cluster with the left IPS, which was then joined by the bilateral MFG/IFS and later by the bilateral aINS. In clustering based on MACM co-activation, the bilateral MFG/IFS also showed a close connection to the aINS and pre-SMA cluster. Conversely, in RS clustering the bilateral MFG/IFS showed a closer association with the bilateral IFJ. Additionally, the bilateral IFJ, the bilateral IPS and the left ITG showed high similarity in the co-activation and functional clustering. However, when the feature being assessed was RS connectivity the bilateral IPS was first closely grouped with the ITG, which was then joined by clusters consisting of the bilateral IFJ and the bilateral MFG/IFS and another cluster consisting of the bilateral dPMC. On the other hand, the bilateral dPMC formed a rather separate cluster in the clustering based on function and co-activation as highlighted in purple in Figure 4.
The hierarchical clustering results thus revealed three main cliques within the eMDN, namely a subcortical cluster, i.e., bilateral putamen and thalamus, a cluster consisting of the pre-SMA, aINS and the MFG/IFS and another cluster consisting of the IFJ, IPS, dPMC and left ITG, with the latter being less consistent across features.
Contrast analyses of functional differences
The results of the contrast analyses of the functional profiles of the three cliques further highlighted some functional differences between the sub-networks. Namely, the results indicated that the subcortical cluster showed the strongest associations with functions that are related to perception and action execution when compared to the other two clusters. Additionally, the cluster consisting of the bilateral IFJ, IPS, dPMC and left ITG was strongly associated with functions such as action execution, spatial cognition, action observation and motor learning when compared to the cluster consisting of the bilateral pre-SMA, aINS and the MFG/IFS. Inversely, the latter cluster was found to be more strongly associated with functions such as interoception, speech execution, social cognition and emotion when compared to the cluster consisting of the bilateral IFJ, IPS, dPMC and left ITG. The results of these analyses are shown in Figure 5.
Figure 5.
Functional differences between each pair of sub-networks (cluster consisting of the pre-SMA, aINS and the MFG/IFS is shown in green; cluster consisting of the IFJ, IPS, dPMC and left ITG is shown in red; sub-cortical cluster i.e., bilateral putamen and thalamus is shown in yellow). “Behavioral Domain” meta-categories in the BrainMap database were used to perform forward inference to determine the above-chance differences in activating either set of regions given a particular behavioural domain. The baserate denotes the general probability of BrainMap activation of the given seeds.
Discussion
The goal of the current study was to establish a robust definition of the extended multiple-demand network (eMDN) comprising regions that are either part of the previously meta-analytically defined MDN (Müller et al., 2015) or closely connected to multiple of these regions. To achieve this we first performed task-dependent and task-independent functional connectivity analyses seeded from the original MDN regions and performed a per-seed conjunction analysis resulting in a consensus connectivity map of each seed region. Subsequently, eMDN regions were defined by identifying those locations where at least half of these consensus connectivity map overlapped. The delineated eMDN regions where then functionally characterized by the paradigms that evoke activation in these regions. Finally, we employed hierarchical clustering based on similarities in task-dependent and task-independent functional connectivity as well as functional profiles to identify cliques of regions within this eMDN.
The extended multiple-demand network
In total, we identified 17 regions in which the consensus functional connectivity maps of more than half of the seed-regions overlapped and which we hence consider part of the eMDN. All these regions resonate well with regions previously implicated as part of the multiple-demand system by Duncan (2010, 2013) based on various task-activation studies and results from non-human primates. That is, by taking a complementary approach starting from a network that was robustly defined over hundreds of neuroimaging findings and mapping regions consistently connected to these across mental states (cf. Amft et al., 2014), we corroborated previous views of the multiple-demand system. We would argue that our work reconciles previous accounts of an MDN (Müller et al., 2015; Duncan, 2010) together with the reported involvement of several additional areas in “executive” networks. In particular, we note that a full overlap of all consensus connectivity maps was only found in regions that were part of the original network resulting from a conjunction across three large-scale meta-analyses dealing of activation data for working memory (Rottschy et al. 2012), attention (Langner and Eickhoff 2013) and inhibitory control (Cieslik et al. 2015). In turn, however, two regions implicated as MDN regions from the activation data (right MFG and IPS) did not show a full overlap of all consensus connectivity maps and were thus not part of the connectivity core. From this, we would argue that only the bilateral inferior frontal junction (IFJ) extending into the inferior frontal gyrus, the bilateral anterior insula (aINS), and the bilateral pre-SMA extending into the anterior midcingulate cortex (aMCC) should be considered the core eMDN based on consistent activation in executive control tasks and strong interconnectivity. Such core networks have been suggested to play a key role in enabling high levels of functional diversity and functional synchronization between brain regions (van den Heuvel & Sporns, 2013). Most evidently, this core eMDN is made up of regions that make up the saliency network, namely the bilateral pre-SMA and bilateral aINS (Seeley et al., 2007). This network has been previously shown to play an important role in executive processing and cognitive control by initiating and maintaining cognitive sets, coordination behavioural responses and guiding behavior in general (Menon, 2010) and is thus of no surprise that it forms part of our core eMDN. Interestingly the bilateral pre-SMA and the bilateral aINS are here joined by the bilateral IFJ. The inclusion of the IFJ in the eMDN core might be related to the role that it plays in task switching and cognitive control in general (Brass et al., 2005).
Our core eMDN is then complemented by a range of other regions that are likewise robustly connected and involved in various aspects of executive processes as detailed below, forming the extended multi-demand network.
Moreover, the regions that have been found to be part of the eMDN all seem to converge with other networks that have been found in previous studies to represent regions involved in executive functioning. Such networks include the cognitive control network (Cole & Schneider 2007), the fronto-parietal control system (Vincent et al., 2008), the superordinate cognitive control network (Niendam et al., 2012), and the task-positive network (Fox et al., 2005) among others. This convergence, together with the fact that the eMDN is defined by looking at functional connectivity across different brain states might suggest a possible integration of such networks.
Hierarchical Clustering
The hierarchical clustering revealed several cliques within the eMDN that were largely consistent across features, i.e., resting-state connectivity, MACM co-activation and function. Most notably, the subcortical structures, i.e., bilateral putamen and thalamus always clustered together. Furthermore, pre-SMA, aINS and MFG/IFS showed close associations, as did the IFJ, IPS, dPMC and left ITG, though the latter was less consistent across features (Figure 6). These results noticeably disrupt the aforementioned core eMDN.
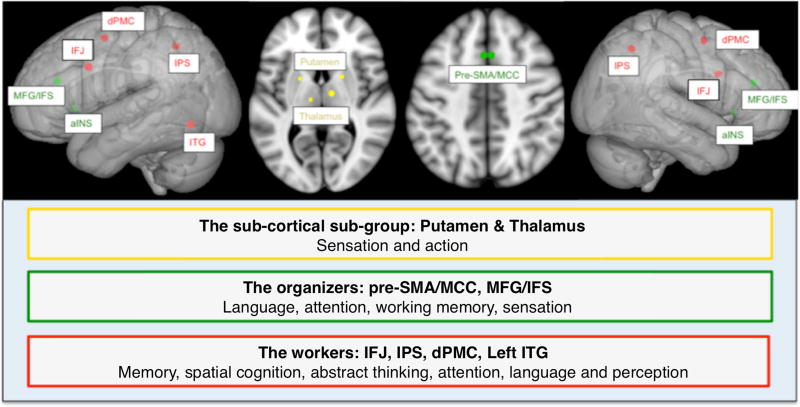
Figure 6.
Summary figure illustrating results obtained from hierarchical clustering and functional decoding analyses revealing three main cliques.
The subcortical sub-group: Putamen and Thalamus
The four subcortical nodes of the eMDN, i.e., regions in the bilateral putamen and the mediodorsal nucleus of the thalamus, formed the most consistent clique within the delineated executive network as they were clustered together based on resting-state connectivity, task co-activation and functional recruitment patterns. As evidenced by the functional decoding and contrast analyses via the BrainMap database, all of these regions are associated to sensorimotor processes, such as action execution and inhibition, as well as the perception of music, pain and visual stimuli. These results are thus well in line with the current literature describing the thalamus as a station, which relays nearly all incoming information from the outside world to the cortex (McCormick & Bal, 1994; Behrens et al., 2003). Furthermore, both the putamen and the thalamus have been frequently linked to pain perception (Starr et al., 2011; Kogler et al., 2015) and pain-related motor responses (Jones et al., 1991; Coghill et al., 1994). Additionally, consistent with our findings implicating this subgroup to action-related functions, both the putamen (Arsalidou et al., 2013). and the thalamus (Sommer, 2003; Guillery & Sherman, 2002) have been previously linked to motor functions and movement regulation.
Importantly in the context of the eMDN, several studies have already implicated both the thalamus and the putamen to hold key roles in executive functioning as part of a system that links different regions, including the (pre-) frontal cortex, via subcortical loop including the struatum and thalamus (Alexander et al., 1986; Alvarez & Emory, 2006). This view has been supported by tracer studies in experimental animals revealing dense connections between prefrontal and subcortical structures (Alexander et al., 1990; Joel & Weiner, 1994; Middleton & Strick, 1997; Tanaka, 1976; Markowitsch et al., 1985). Likewise patients with basal ganglia pathology, e.g., those suffering from Huntington’s and Parkinson’s disease, have well documented deficits in executive functioning (Elliott, 2003), highlighting the critical role of cortico-striatal-thalamic circuits for executive processes. In summary, the presence of a subcortical clique within the eMDN is well supported by several streams of previous literature. Its interaction with (pre-) frontal and parietal cortices is classically conceptualized as parallel segregated processing loops, each connected to a different area of the cortex (Alexander et al., 1986). One is the motor loop that connects premotor and sensory areas to the primary motor cortex via the putamen and the ventral lateral thalamus (Alexander, 1986; Lehericy et al., 2006). In contrast, a cognitive loop connects (pre-)frontal areas, the caudate nucleus and the mediodorsal thalamus (Alexander, 1986; Tanaka, 1976). At first glance, these studies seem to contradict our findings which highlighted the role of the putamen and the mediodorsal thalamus in executive functioning. However more recent anatomical and physiological evidence has extended the classical view of the cortico-striatal loops and suggests substantial interaction between these loops (Houk, 2001; Seger, 2006). Consequently, cortico-striatal-thalamic circuits are now described as a spiral through which information cascading from one loop to the next rather than individual segregated loops (Haber & Knuston, 2010).
In conclusion, the presence of a distinct clique of subcortical nodes within the eMDN reflects the integration of cortico-striatal-thalamic loops into the executive system. We would argue that within a framework of interacting subcortical processing loops, these regions should play a key role in processing information from the outside environment and interacting with it.
Pre-SMA/MCC, aINS and MFG/IFS
Bilateral pre-SMA/MCC, aINS and MFG/IFS formed another clique that was associated with a broad functional profile including many higher cognitive processes such as language, working memory, sensation, action preparation, and attention. In contrast to the subcortical group this set showed some heterogeneity based on the assessed feature, which may be best described as a tight clustering of aINS and pre-SMA/MCC based on functional connectivity whereas the MFG/IFS shows similarities to these particularly in task-based features, i.e. function and co-activation profiles.
The broad cognitive profile of this group is consistent with previous findings from a wide range of brain imaging studies associating these regions, especially the aINS and the pre-SMA/MCC (often referred to as dACC, see Müller et al., 2015), to numerous, cognitive and affective processes (for review see: Menon & Uddin, 2010). Moreover and in line with our clustering results these two regions are well recognized as being closely related to each other, forming together what has been termed the salience network (Menon & Uddin, 2010). This network has been discussed in the initiation and maintenance of cognitive sets (Dosenbach et al., 2006), the coordination of behavioral responses (Medford & Critchley, 2010) and more general the guidance of behavior by identifying the most relevant among several intra- and extra-personal stimuli (Seeley et al., 2007). All of these processes are essential aspects of executive functioning and cognitive control. Hence, our observation that the “saliency regions” aINS and pre-SMA/MCC form the core of the MDN is well in line with the rich literature on this network. We would thus focus on two aspects that may be of particular relevance to the current findings. First, it has been argued that the “saliency network” bridges sensory, emotional and cognitive information (Craig, 2009; Gogolla et al., 2014; Menon & Uddin, 2010) through switching between the executive and the default mode network (Sridharan et al., 2008). Therefore, the aINS and pre-SMA/MCC may not only represent core regions of the eMDN but moreover initiate and orchestrate the engagement of other regions in the eMDN upon commencement of cognitive tasks. Second, it has been shown, that these regions form a convergent morphological substrate of mental illness as only the aINS and pre-SMA/MCC showed consistent atrophy in a large scale meta-analysis over structural neuroimaging studies in a wide range of psychiatric disorders (Goodkind et al., 2015). The core role of these regions within the executive eMDN as demonstrated in the current study thus resonates well with the widespread impairments in executive functioning across various mental disorders (Elliott, 2003).
The MFG in turn has not been discussed in the context of the saliency network. This fits with our observation that while being part of the same clique, albeit not consistently, this region is neither part of the MDN core based on convergent activation in executive tasks nor by virtue of consistent interaction with all seeds. This position as a relative outsider in the clique matches the fact that the MFG has previously been discussed as part of a ventral attention network (Corbetta & Shulman, 2002). Interestingly, however, this network has been often associated with functions that overlap with those attributed to the salience network, namely identifying and responding to behaviorally relevant stimuli (Vossel et al., 2014; Japee et al., 2015). As moreover the aINS is likewise often considered part of the ventral attention network, we would argue that the integration of the MFG/IFS into the clique formed by the aINS and pre-SMA/MCC may reflect often-neglected convergence between the concept of the salience and ventral attention network, respectively. In this context, it is interesting to note, that in contrast to the salience network, the ventral attention network has been reported to be strongly right-lateralized. This bodes well with our observation in the clustering on functional properties revealing close similarities between the right MFG/IFS and the salience regions while the left MFG/IFS joins this group at a later stage.
We therefore conclude that the bilateral pre-SMA/MCC, bilateral aINS and bilateral MFG/IFS form the core of the multi demand system that is not only relevant for integrating different information on the internal and external environment but also plays a key role in engaging the eMDN by regulating activity of other networks. These regions thus can be thought of as the managers of the eMDN, explaining their involvement in virtually all cognitive tasks.
IFJ, IPS, dPMC and left ITG
The remaining regions of the eMDN, i.e., the bilateral IFJ, IPS, dPMC and the left ITG were less consistently organized into any distinct cliques than the previously discussed sets. We would see this as an indication that these regions form, in contrast to the subcortical nodes related to sensorimotor processes and the core aINS, pre-SMA/MCC, MFG/IFS cluster, more flexible, most likely task- and brain-state dependent associations. In other words, whereas the regions above represent the coordinating core of the eMDN and are hence consistently (co-) recruited by cognitive tasks, the regions discussed in this section are integrated more flexibly into the system.
In the task-based based clustering we found a relatively close association between the IFJ and IPS. This mirrors previous findings that have linked these regions to higher-level processes (Neubert et al., 2014) such as the processing of infrequent stimuli (Verbruggen et al., 2010), preparatory cognitive control (Chikazoe et al., 2009), spatial orientating and re-orientating (Corbetta et al., 1998; Thiel et al., 2004), information updating (Vossel et al., 2011) and feedback processing (Hirose et al., 2009). Convergently, our functional decoding also associated this sub-group with functions such as spatial cognition, reasoning, and working memory.
Interestingly, the clustering analysis based on RS-FC linked the IPS with the dPMC. While at odds with the task-based results discussed previously, this association is also well supported by literature proposing that the IPS and dPMC are part of a dorsal attention network related to the top-down control of visual attention and motor preparation (Corbetta et al., 2008; Corbetta & Schulman, 2002; Genon et al., 2016). Within this dorsal attention network, the IPS and the dPMC are thought to be involved in shifting maintaining spatial attention to peripheral locations (Hopfinger et al., 2000; Kelley et al., 2008). In our view, the fact that two incongruent findings of the current study, linking the IPS with either the IFJ or the dPMC are both well supported by the current literature serves to highlight the proposed flexible recruitment of different components within this clique during the performance of cognitive tasks depending presumably on the exact demands. Our results and findings of previous studies therefore indicate that the nodes within this rather loose group fulfill specific roles needed for the implementation of higher cognitive functions while under the coordination and recruitment by previously discussed core regions and can thus be compared to the workers of a system.
Conclusion
In this study we provide a robust definition of an extended multiple-demand network (eMDN) based on task-dependent and task-independent functional connectivity analysis seeded from regions previously shown to be convergently recruited across neuroimaging studies probing working memory, attention and inhibition, i.e., the proposed key components of executive functioning. The eMDN was differentiated into three cliques, including a subcortical group mainly related to sensorimotor processing, a core of potential organizers (bilateral pre-SMA/MCC, aINS, MFG/IFS), and a more heterogeneous set of workers dynamically recruited based on task demands.
The proposed structure of the eMDN as the most likely neurobiological substrate for executive processes also holds important implications for the understanding of the psychological structure of executive functions. In particular, we would propose a core system whose integrity should be crucial to performance of most operations that may be considered higher cognitive or executive functions. This explains the inter-correlation of performance in different executive function tests and is consistent with the general factor of intelligence (g) (Jensen, 1998). The core network then dynamically recruits additional areas of the eMDN depending on the demands of the individual tasks, explaining divergences between the performance in different tasks probing the executive system and the presence of isolated clinical impairment. In this regard, further studies should be carried out in order to corroborate these findings and link the different regions and sub-networks to executive performance in health and disease.
Acknowledgments
Funding
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/4-1, LA 3071/3-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modelling for the Human Brain” and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 7202070 (HBP SGA1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Amft M, Bzdok D, Laird A, Fox P, Eickhoff S. Definition and characterization of the extended default mode network. Brain Struct Funct. 2014 doi: 10.1007/s00429-013-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum. Brain Mapp. 2013;34:3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Behrens T, Johansen-Berg H, Woolrich M, Smith S, Wheeler-Kingshott C, Boulby P, Barker G, Sillery E, Sheehan K, Ciccarelli O. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn. Sci. (Regul. Ed.) 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 2009;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Mueller VI, Eickhoff CR, Langner R, Eickhoff SB. Three key regions for supervisory attentional control: Evidence from neuroimaging meta-analyses. Neuroscience & Biobehavioral Reviews. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Rottschy C, Laird AR, Fox PT, Eickhoff SB. Comparison of structural covariance with functional connectivity approaches exemplified by an investigation of the left anterior insula. Neuroimage. 2014;99:269–280. doi: 10.1016/j.neuroimage.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J. Neurosci. 1994;14:4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? the anterior insula and human awareness. Nature reviews neuroscience. 2009;10 doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. (Regul. Ed.) 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate based activation likelihood estimation meta analysis of neuroimaging data: A random effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, Bzdok D, Eickhoff CR. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. Executive functions and their disorders. Br. Med. Bull. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Mapping context and content: the BrainMap model. Nature Reviews Neuroscience. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL, Laird AR, Eickhoff SB. Meta-Analysis in Human Neuroimaging: Computational Modeling of Large-Scale Databases. Annu. Rev. Neurosci. 2014;37:409–434. doi: 10.1146/annurev-neuro-062012-170320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Li H, Fan L, Muller VI, Cieslik EC, Hoffstaedter F, Reid AT, Langner R, Grefkes C, Fox PT, Moebus S, Caspers S, Amunts K, Jiang T, Eickhoff SB. The Right Dorsal Premotor Mosaic: Organization, Functions, and Connectivity. Cereb. Cortex. 2016 doi: 10.1093/cercor/bhw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS. Identification of a common neurobiological substrate for mental illness. JAMA psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Lesage E, Eickhoff CR, Clos M, Fox P, Eickhoff SB. Multimodal connectivity of motor learning-related dorsal premotor cortex. Neuroimage. 2015;123:114–128. doi: 10.1016/j.neuroimage.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S, Chikazoe J, Jimura K, Yamashita K, Miyashita Y, Konishi S. Sub-centimeter scale functional organization in human inferior frontal gyrus. Neuroimage. 2009;47:442–450. doi: 10.1016/j.neuroimage.2009.04.094. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Houk JC. Neurophysiology of frontal-subcortical loops. Frontal-subcortical circuits in psychiatry and neurology. 2001:92–113. [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, Specht K. On the existence of a generalized non-specific task-dependent network. Frontiers in human neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Frontiers in systems neuroscience. 2015;9:23. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AR. The g factor: The science of mental ability 1998 [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc. Biol. Sci. 1991;244:39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cereb. Cortex. 2008;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Müller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B. Psychosocial versus physiological stress—Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Frontiers in neuroinformatics. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, Fox PT. Networks of task co-activations. Neuroimage. 2013;80:505–514. doi: 10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res. 2011 doi: 10.1186/1756-0500-4-349. Notes 4, 349-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 2013;139:870. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Bardinet E, Tremblay L, Van de Moortele PF, Pochon JB, Dormont D, Kim DS, Yelnik J, Ugurbil K. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb. Cortex. 2006;16:149–161. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- Lezak MD. The problem of assessing executive functions. International journal of Psychology. 1982;17:281–297. [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B. Cortical and subcortical afferent connections of the primate's temporal pole: a study of rhesus monkeys, squirrel monkeys, and marmosets. J. Comp. Neurol. 1985;242:425–458. doi: 10.1002/cne.902420310. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Krainik A, Duffau H, Pélégrini-Issac M, Lehéricy S, Doyon J, Benali H. Partial correlation for functional brain interactivity investigation in functional MRI. Neuroimage. 2006;32:228–237. doi: 10.1016/j.neuroimage.2005.12.057. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr. Opin. Neurobiol. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and function. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar output channels. Int. Rev. Neurobiol. 1997;41:61–82. doi: 10.1016/s0074-7742(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Eickhoff SB. Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Structure and function. 2015;220:2401–2414. doi: 10.1007/s00429-014-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert F, Mars RB, Thomas AG, Sallet J, Rushworth MF. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron. 2014;81:700–713. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe S, Tobe R, Mennes M, Benedict M, Moreno A, Panek L, Brown S, Zavitz S, Li Q. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Frontiers in neuroscience. 2012;6:152. doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz J, Zilles K, Laird A, Fox P, Eickhoff S. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Structure and function. 2013;218:1551–1567. doi: 10.1007/s00429-012-0476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA. The basal ganglia in human learning. Neuroscientist. 2006;12:285–290. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- Sommer MA. The role of the thalamus in motor control. Curr. Opin. Neurobiol. 2003;13:663–670. doi: 10.1016/j.conb.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka D. Thalamic projections of the dorsomedial prefrontal cortex in the rhesus monkey (Macaca mulatta) Brain Res. 1976;110:21–38. doi: 10.1016/0006-8993(76)90206-7. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. Neuroimage. 2004;21:318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Timm N. Applied multivariate analysis. 2002 [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within experiment and within group effects in activation likelihood estimation meta analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel Martijn P, Sporns O. Network hubs in the human brain. Trends Cogn. Sci. (Regul. Ed.) 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Weidner R, Fink GR. Dynamic coding of events within the inferior frontal gyrus in a probabilistic selective attention task. J. Cogn. Neurosci. 2011;23:414–424. doi: 10.1162/jocn.2010.21441. [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Carter A, Reznick JS, Frye D. Early development of executive function: A problem-solving framework. Review of general psychology. 1997;1:198. [Google Scholar]
- Zelazo PD, Müller U. Executive function in typical and atypical development 2002 [Google Scholar]