Abstract
Infectious diseases are the result of molecular cross-talks between hosts and their pathogens. These cross-talks are in part mediated by Host-Pathogen Protein-Protein Interactions (HP-PPI). HP-PPI play crucial roles in infections, as they may tilt the balance either in favor of the pathogens’ spread or their clearance. The identification of host proteins targeted by viral or bacterial pathogenic proteins necessary for the infection can provide insights into their underlying molecular mechanisms of pathogenicity, and potentially even single out pharmacological intervention targets. Here, we review the available methods to study HP-PPI, with a focus on recent mass spectrometry based methods to decipher bacterial – human infectious diseases and examine their relevance in uncovering host cell rewiring by pathogens.
Introduction to Host-Pathogen Protein-Protein Interactions
Infectious diseases reflect the evolutionary balance between a host and its pathogen. In order to ensure their survival and propagation, pathogens have developed numerous intricate tools to subvert their hosts’ defense mechanisms. Understanding how pathogens actively rewire host cell defenses is of particular interest in infectious disease research. Ultimately by identifying host-directed targets for pharmacological intervention, this field of research may contribute to eradicate the public health burden caused by these agents.
The molecular mechanisms underlying pathogenic rewiring of host cells are widely varied. However, as protein complexes and their interaction networks into which they are organized comprise the primary functional modules of the cell [1], we can predict that the disruption of these host networks are likely to be a key strategy for manipulation by pathogens. Re-wiring of the host’s proteome, also known as pathogenic hijacking, generally includes intervention at multiple stages of signaling pathways and cellular functions to ensure the robustness of the virulent intervention [2]. This hijacking by protein-protein interactions may be carried out by evolutionarily derived partial molecular mimicry [3], which consists of virulent proteins having evolved similar structures or motifs to the host proteins to mediate such HP-PPI. It has further been proposed that the phenotypic impact of a pathogen is directly proportional to its ability to rewire the host interactome, and that the impacts of individual virulent proteins are linked to their number of interactions with host proteins [4]. Thus, mapping the host-pathogen protein interactome may provide valuable insights into the biological functions of virulence factor proteins, highlight interactions critical to the pathogens’ progression and spread, and improve our overall understanding on the molecular basis of pathogenicity.
In this review, we aim to summarize the methods available to characterize HP-PPI, consider their utility by providing biological insights, and present some outlook into the how the field may develop going forward. Even though we are primarily concerned with the possibilities of characterizing HP-PPI from the perspective of bacterial pathogens, a survey of the literature indicates that significantly more work has been done for viruses in this area [5]. As such, an examination of lessons learned from studies of interactions between viruses and hosts should also be instructive.
It is well established that due to their minimal genomes and by being obligate parasites, viruses rely on HP-PPI as a mean to carry out the pleiotropic functions of their proteins by hijacking various host protein modules to either avoid their clearance or enable their spread. For example, by mapping the Influenza A – human PPI network, viral proteins were reported to be highly inter-connected thus forming functional modules, and to interact with a greater number of host proteins compared to the average degree of connectivity in the human interactome [6]. The HP-PPI map further enabled the identification of multiple molecular mechanisms employed by the virus to manipulate its host, including how Influenza proteins intervene in the WNT/ß-catenin pathways as a mean to modulate the host’s interferon production [6].
Unfortunately, the characterization of bacterial HP-PPI has lagged behind. The reason for this disparity most likely reflects differences in feasibility. That is, testing all proteins produced by a viral genome for interactions with a host proteome requires significantly less effort than that for bacterial genomes due to their increased genomic complexity. Nonetheless there is increasing amount of evidence that bacteria also rewire host cellular pathways via HP-PPI [2]. Pathogenic bacteria can interact with their host’s proteome by three main mechanisms. First, bacterial membrane proteins are an obvious interaction point, as they are located at the physical interface between both organisms. Secondly, bacteria might secrete effector proteins (also known as virulence factors) into the host cell where they can interact with the host proteome. Secreted effector proteins are of particular interest as they are frequently required for full virulence [7]. Additionally, some bacterial pathogens such as certain Shigella dysenteriae or Escherichia Coli strains express Shiga toxins generally during their lytic cycle [8] or release these toxins through Outer Membrane Vesicles during their growth phase [9], leading to the inhibition of protein synthesis or activation of the apoptotic pathways of their host cells. As the number of bacterial host-pathogen interaction studies increases, they demonstrate that while bacteria generally do not rely on host cell machinery for the purpose of replication as directly as viruses do, they do seem to disrupt the immune response [10] and interact preferentially with the hosts’ cytoskeleton as a mean of motility, invasion of the host tissues [11] and escape of phagocytic cells [12]. For instance, Mycobacterium tuberculosis (Mtb), an intracellular parasite, is known to modulate the host’s immune response and prevent its bacterial clearance by suppression of autophagy. Recent work has shown that a secreted Mtb factor, PE_PGRS47, locates in the host’s cytosol and inhibits the Major Histocompatibility Complex II mediated antigen presentation, thereby partially suppressing the autophagy of the Mtb containing macrophages in chronic stages of infections [13]. By mapping such host interactors, HP-PPI studies could hint us towards the molecular mechanisms behind certain virulence factors like this PE-PGRS47. In this review, we will describe the available methodologies to achieve such goals and discuss their impact on mechanistic understanding or host cell rewiring.
Protein-Protein Interactions detection methods
Yeast2Hybrid
Historically first, the Yeast Two Hybrid (Y2H) method has been extensively used to detect direct physical interactions between two ectopically expressed tagged proteins in yeasts [14]. Although this method generates direct binary interaction datasets at high throughput, the need for exhaustive screens hampers its feasibility, and its technical challenges such as the non-physiological expression system provokes high rates of false negatives [15]. Nonetheless, many studies in the field of infectious diseases have successfully employed Y2H screens to investigate (near) genome-wide virus-host interactions [16–23], to compare homologous viral proteins from various strains [24,25], or to systematically map bacterial effector proteins – host interactions [26–31] (see supplementary table 1). In the context of Mycobacterium tuberculosis infections, a Y2H screen along with functional validations, enabled the discovery of a molecular mechanism by which an effector protein, named EsxH, targets the Endosomal Sorting Complexes Required for Transport (ESCRT) necessary for endosomal membrane trafficking, thereby impairing the phagosomal maturation and fusion with the lysosomes [28].
Affinity Purification – and Immuno Purification – Mass Spectrometry
In the past two decades, improvements of mass spectrometry (MS) based proteomics in combination with Affinity Purification (AP) methods have enabled the systematic detection of PPI in near physiological environments [32] (see Figure 1). Most commonly, it consists of fusing an affinity epitope tag to a bait protein, followed by a single or double biochemical affinity- or immuno- purification (IP) steps in native lysis conditions. The purified bait, along with the non-covalently bound interacting proteins or macromolecular protein complexes (preys), are then identified and quantified via standard bottom up proteomics. To filter out non-specific interactions, this strategy relies on quantitative comparisons with control purifications.
Figure 1.
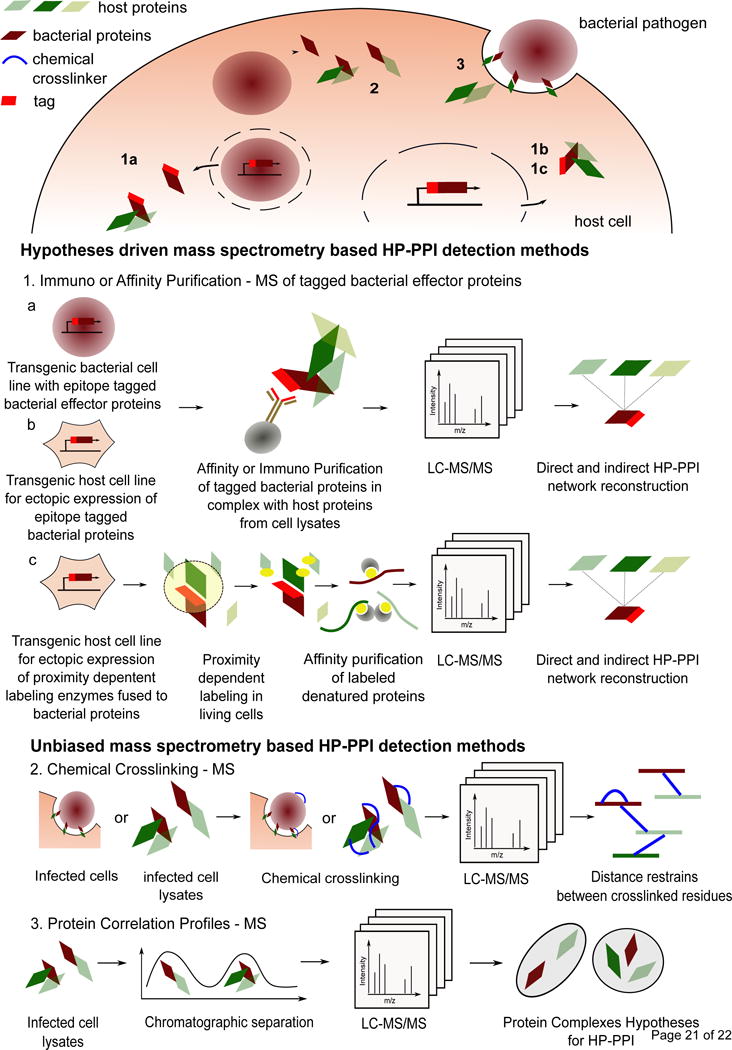
Mass spectrometry based methods for Host-Pathogen Protein-Protein Interactions detections in the context of bacterial infections. AP/IP-MS from epitope tagged bacterial effector proteins (1a) post infections of their host cells enables the identification of physiological HP-PPI. Along with other hypotheses driven methods such as ectopic expressions of tagged bacterial proteins in the host environment (1b and 1c), they can lead to near comprehensive identifications of HP-PPI. However, because they rely on the prior tagging of the proteins of interest, they are limited by the number of proteins that can be cloned and expressed in the relevant cellular systems. Chemical Crosslinking – MS (2) and Protein Correlation Profiles – MS (3) methods, although less sensitive, do not require prior knowledge and tagging of bacterial proteins and thus allow de novo discovery of physiological and endogenous HP-PPI directly from infected cells (2 and 3).
In the field of infectious diseases, AP-MS is commonly applied to systematically map the interactome of individual virulent proteins ectopically expressed in the host’s environment [24,33–44], to monitor single virulent proteins [45] or upon infection [46] (see supplementary table 1). A related strategy uses immobilized recombinant bacterial effectors on beads [47] combined with AP-MS from their incubation with human plasma.
Although expressing single virulent genes in host environments is informative, it is believed that during the course of infections, the host-pathogen interactomes undergo infection stage-dependent dynamic changes [48], influenced by the hosts’ responses and by the other co-expressed virulent proteins. Thus, some groups have generated replication-competent, epitope tagged viruses which enabled the spatio-temporal monitoring of empirical and quantitative changes upon viral infectious of host cells, including for Alphavirus Sindbis [48] and most recently HIV infected human cells [49]. Based on similar principles, Mousnier A et al. and subsequently So EC et al. respectively developed and applied a double purification based method coupled to mass spectrometry to enable the identification of HP-PPI of bacterial effector proteins in host cells upon infections of Legionella pneumophila. This study, amongst others findings, described how three effector proteins may target up to 25 Rab GTPases individually during the course of infections [50,51] (see supplementary table 1).
Proximity Dependent Labeling – Mass Spectrometry
BioID has recently emerged as a new possibility to detect transient and weaker PPI [52] complementary to AP-MS [53]. This method relies on the fusion of a mutated promiscuous biotin ligase BirA* to the bait protein. During an incubation with high biotin concentrations, neighboring proteins to the fused BirA*-bait protein undergo proximity dependent biotinylation reactions. Biotin-conjugated proteins, potential direct or indirect interactors of the bait, can then be affinity purified using streptavidin-coated affinity matrices and quantified by mass spectrometry (see Figure 1). Because the identification of interactions does not depend on the native purification conditions, weak, transient and insoluble interactions such as for membrane proteins can be readily identified [52]. BioID has been applied as a mean to obtain comprehensive interactome information of selected bacterial proteins [54] belonging to the human pathogen, Chlamydia psittaci (see supplementary table 1). A variation of this proximity labelling strategy, called APEX, enables much faster reaction times (~30 seconds), and opens up the possibility of time-resolved proximity measurements [55].
Chemical Crosslinking – Mass Spectrometry
Chemical Crosslinking coupled to Mass Spectrometry (XL-MS) consists of chemically crosslinking proximal reactive side chains of exposed specific amino acids from native proteins in monomeric states or in protein complexes, followed by an MS based, bottom up approach to identify the crosslinked peptides and infer their proteins. XL-MS thus yields fixed distance restraints between bound residues, suggesting direct physical intra-protein or inter-protein interactions between crosslinked peptides belonging to the same or distinct proteins respectively [56] (see Figure 1). Chemical crosslinking reactions can be performed on purified protein samples [57] using GFP epitope tags [58], on cell lysates [59] or on living cells such as on the pathogen Pseudomonas aeruginosa [60]. Although having gained popularity in recent years to study the topology of protein networks, decipher the architecture of macromolecular complexes, and provide insights into domain-resolution protein interactions, XL-MS has not yet been widely applied to study HP interactions due to its challenging utilization. One exception is the unbiased study of live human epithelial H292 cells infected with A. baumannii which led to the identification of 46 HP-PPI [61] (see supplementary table 1).
Protein Microarray Based Technologies
Membrane proteins play pivotal roles in infections by mediating host-pathogen recognition, docking, adhesion, invasion and secretion. Regrettably, their lack of solubility and their necessity of remaining in lipid-rich environment highly impairs their interactome mapping via conventional methods such as AP-MS. To overcome these challenges, Glick Y et al. introduced a screening method for HP interactions, adequate for transmembrane proteins [62] named the human Membrane Protein Array (MPA). Similarly, several studies have developed and applied Protein Micro Array technologies [63] including Nucleic Acid Programmable [64] or AVEXIS (AVidity-based Extracellular Interaction Screen) [65,66] to study soluble and transmembrane HP interactions (see supplementary table 1).
Discussion
There are a variety of methods available for detecting PPI, where each of them may be applied to answer different questions and come with their own advantages or disadvantages (see Table 1). Many methods have been successfully applied to the HP-PPI field and lead to the discovery of important biological insights. For instance, although human host interactors of viruses and bacteria range across all biological functions, common or pathogen specific themes can be observed within pathogenic groups by meta-analysis of HP-PPI studies. First, viral proteins and to a lesser extent secreted bacterial effector proteins [30], are both more likely to interact with host hub proteins (highly connected proteins in the host network) [16,17,21] and bottleneck proteins (central proteins to many signaling pathways) [10,31,67] for an increased efficiency in altering host cellular processes. Secondly, by performing gene ontology enrichment analysis on the host targets, viral pathogens seems to unavoidably disturb cellular processes as they rely on the transcriptional machinery, whereas bacteria tend to mesh with the immune response to prevent their clearance [10]. Thirdly, the manipulation of the host ubiquitin pathways by viruses [68] and bacterial effector proteins [69] is a recurrent finding. By controlling protein degradation and cell signaling, ubiquitination is a critical regulator of various cellular processes such as inflammatory responses, vesicular trafficking and cell cycle, altogether making it an ideal target to hijack for bacterial and viral pathogenicity. Indeed, there is increasing amounts of evidence that numerous human bacterial pathogens hijack and modulate the host ubiquitin processes utilizing molecular mimicry to impair the hosts’ defense systems, including the ubiquitin-dependent autophagy, the NF-κB and the inflammatory signaling pathways [70,71].
Table 1.
An overview of the main advantages and disadvantages for the commonly used HP-PPI detection methods. Although Y2H and Protein microarray based technologies are high throughput and could theoretically test any gene combinations, they are based on non-physiological experimental conditions and may identify only binary PPI. Techniques such as AP-MS and proximity-dependent labeling coupled to MS, on the other hand, generate physiologically relevant PPI with information about the Post Translational Information (PTM) states of the identified prey proteins and can detect entire protein complexes. Similarly, but in an unbiased manner as it may detect proteome-wide PPI without the need of prior genetic engineering of the pathogens or host cells, PCP-MS and XL-MS on infected cells may detect de novo HP-PPI in physiologically relevant conditions. Furthermore, mass spectrometry based methods may be coupled to quantitative proteomics to monitor in a time course-compatible manner, qualitative and quantitative changes of proteins complexes between biological conditions. Unfortunately, the sensitivity of proteome-wide HP-PPI like PCP- and XL-MS remain their largest drawback and would probably never gain the same sensitivity as for more targeted methods like AP-MS to study specific protein complexes. Lastly, although XL-MS may be applied on purified protein complexes and provide valuable information their structural arrangements and topologies, it requires high amounts of purified protein complexes and the data analysis remains challenging.
| Yeast 2 Hybrid (Y2H) | ||
|---|---|---|
| advantages | high throughput ● existing human and pathogen ORFeome collections ● universality – any cDNA from any protein is testable | |
| disadvantages | need for exhaustive screens ● non-physiological experimental conditions ● detects only binary interactions ● no PTM information | |
| Affinity Purification – Mass Spectrometry (AP-MS) | ||
| advantages | high throughput ● sensitive ● detects entire protein complexes ● PTM sensitive ● when using antibodies against the bait of interest, can be applied from infected tissues directly | |
| disadvantages | need for transgenic cell lines ● needs additional experimental data to distinguish direct from indirect interactors ● the identification of PPI depends on the biochemical extraction conditions | |
| Chemical Crosslinking – Mass Spectrometry (XL-MS) on: | ||
| purified protein complexes | infected cells or infected cell lysates | |
| advantages | provides information on interacting protein domains ● residue to residue resolution | |
| information on the topology and structural arrangement of protein complexes | whole-proteome ● adequate for soluble and membrane proteins ● can be applied on infected tissues directly | |
| disadvantages | for predefined and purified protein complexes only ● need for large amounts of purified protein complexes ● complex data acquisition and analysis | low sensitivity/resolution ● complex data acquisition and analysis |
| Proximity dependent labelling strategies – Mass Spectrometry | ||
| BioID | Ascorbate Peroxidase-based Proximity Tagging (APEX) | |
| advantages | sensitive ● appropriate for weak and transient interactions ● adequate for resolving the spatial organizations of the tagged proteins ● identification of PPI does not depend on the biochemical extraction conditions | |
| suitable for soluble and transmembrane proteins | fast reaction times, amenable for time course experiments for temporal resolutions | |
| disadvantages | long reaction times, not suitable for time course experiments | so far applicable to membrane proteins only |
| hard to distinguish direct from indirect/proximal interactors | ||
| Protein microarray based technologies | ||
| Nucleic Acid Programmable Protein Array (NAPPA) | Human Membrane Protein Array (hMPA) | |
| advantages | high throughput ● universality – any cDNA from any protein is testable ● no need for protein purification compared to classical protein microarrays ● gene size does not seem to affect its final intensity | high throughput ● physiological for membrane proteins ● recognition against the entire pathogen ● naturally occurring PTM on the surface of tested pathogen |
| disadvantages | non-physiological ● only binary interactions are detected ● no PTM information | for membrane proteins only ● no PTM on the expressed protein |
| Protein Correlation Profiling (PCP) | ||
| advantages | whole proteome studies ● unbiased ● stoichiometric and quantitative information readily available | |
| disadvantages | dynamic range of protein abundances between host and pathogen might be too important ● low sensitivity ● hard to detect kiss and run interactions | |
Numerous approaches could be employed to further improve the quality and completeness of HP-PPI networks. These include combining orthogonal PPI detection methods [53,72,73], considering strain specific variation in dependence on the host cellular modules [74], to acknowledge the genetic diversity of both hosts and pathogens [75] and to beware of host cell-type dependent HP-PPI [33]. The use of more physiological systems for studying these interactions is also a proximal goal, such as adopting more disease relevant cells or transgenic animal models for the ectopic expression of tagged pathogenic proteins. Likewise, employing infection systems where the virulent proteins are tagged within the pathogen could provide dynamic and more physiological maps of the HP-PPI.
The systematic study of bacterial-host interactions brings additional challenges. The first is to identify all secreted proteins upon infection, where in silico predictions and experimental findings don’t always corroborate [76]. To help the identification of virulence factors from membrane-contained intracellular bacterial pathogens, one could consider purifying intact pathogen-containing compartments or vacuoles, and characterizing their proteome by mass-spectrometry to find new virulence factors that associate with the host membranes [77]. Secondly, due to their increased genomic complexity compared to viruses, the generation of transgenic cell lines to ectopically express each putative secreted protein would be highly time-consuming. Thirdly, bacterial systems generally lack adequate genetic tools preventing endogenous tagging of their secreted proteins. Thusly, we hypothesize that more global approaches for bacterial – host PPI detection may be useful. In the last years, numerous groups have been working towards developing methods which do not require genetically engineered cells to systematically identify in an unbiased manner endogenous protein complexes in physiological samples by correlating protein profiles (PCP-MS) across various biochemical separations or chromatographic techniques [78,79]. Not only does this mass-spectrometry based approach yields lists of putative protein complexes, but it also reports stoichiometric and quantitative information for the identified components. Unfortunately, despite tremendous improvements in the field, the sensitivity remains the limiting factor. It is especially problematic in infectious diseases, where the dynamic ranges in terms of protein abundances from pathogenic organisms are generally several orders of magnitude lower than those of the host proteome [80].
In any case, regardless of which methods were employed, it is imperative to validate and functionally characterize the discovered HP-PPI to understand how they impact the course of infections. To do so, interaction studies can be coupled to endogenous host interaction maps [81,82] or to functional genomic screens to measure the fitness cost upon disruptions of either pathogenic or host molecular components [21], as was done for the HCV interactome [33]. By measuring sets of phenotypes such as pathogenic replication or host cell death, functional studies have the benefice of being able to simultaneously identify the positive, negative or neutral impact on the infection of targeted bacterial [83] or host factors [74,84]. Altogether, interaction studies, biochemical characterizations and functional screens may not only identify host – pathogen interactions, but also inform us on their phenotypic impacts and their molecular mechanisms for bacterial or viral pathogenicity.
Supplementary Material
Highlights.
Bacterial pathogens, like viruses, rewire host proteomes to enable their replication and spread
The elucidation of physical protein-protein interactions between hosts and pathogens can lead to biological insights into the mechanisms of pathogenic rewiring of host systems
The field of protein-protein interactions is moving from employing binary interaction methods towards more physiological, unbiased and quantitative mass-spectrometry based approaches and is gaining applications into studies focusing on infectious diseases
We hypothesize that host-pathogen protein-protein interaction studies focusing on bacterial pathogens will transition to more physiologically relevant and endogenous experimental systems
Coupling host-pathogen protein-protein interaction studies to functional assays can help measuring the phenotypic impacts of given interspecies interactions
Acknowledgments
We apologize to the research teams whose work could not be cited due to space limitations. We thank Audrey van Drogen and Emanuela Milani for critical reading of the manuscript. CN and BCC were supported by a Swiss National Science Foundation Ambizione grant (PZ00P3_161435). ABE was supported by the SystemsX.ch project TbX and the National Institutes of Health project Omics4TB Disease Progression (U19 AI106761)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- 1.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology [Internet] Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 2.Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens [Internet] Nature. 2007;449:827–834. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- 3.Elde NC, Malik HS. The evolutionary conundrum of pathogen mimicry [Internet] Nat Rev Microbiol. 2009;7:787–797. doi: 10.1038/nrmicro2222. [DOI] [PubMed] [Google Scholar]
- 4 **.Crua Asensio N, Muñoz Giner E, de Groot NS, Torrent Burgas M, Lotteau V. Centrality in the host–pathogen interactome is associated with pathogen fitness during infection [Internet] Nat Commun. 2017;8:14092. doi: 10.1038/ncomms14092. The examination of bacteria-host interactomes suggested that the phenotypic impact and pathogenic fitness of given bacterial proteins are related to their ability to interact with hub host proteins and their overall number of interactions with their host. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lum KK, Cristea IM. Proteomic approaches to uncovering virus-host protein interactions during the progression of viral infection [Internet] Expert Rev Proteomics. 2016;13:325–40. doi: 10.1586/14789450.2016.1147353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapira SD, Gat-Viks I, Shum BOV, Dricot A, de Grace MM, Wu L, Gupta PB, Hao T, Silver SJ, Root DE, et al. A Physical and Regulatory Map of Host-Influenza Interactions Reveals Pathways in H1N1 Infection [Internet] Cell. 2009;139:1255–1267. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green ER, Mecsas J. Bacterial Secretion Systems: An Overview. [Internet] Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesh VL. Induction of apoptosis by Shiga toxins. [Internet] Future Microbiol. 2010;5:431–53. doi: 10.2217/fmb.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunsmann L, Rüter C, Bauwens A, Greune L, Glüder M, Kemper B, Fruth A, Wai SN, He X, Lloubes R, et al. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain [Internet] Sci Rep. 2015;5:13252. doi: 10.1038/srep13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durmuş Tekir S, Cakir T, Ulgen KÖ. Infection Strategies of Bacterial and Viral Pathogens through Pathogen-Human Protein-Protein Interactions. [Internet] Front Microbiol. 2012;3:46. doi: 10.3389/fmicb.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colonne PM, Winchell CG, Voth DE. Hijacking Host Cell Highways: Manipulation of the Host Actin Cytoskeleton by Obligate Intracellular Bacterial Pathogens. [Internet] Front Cell Infect Microbiol. 2016;6:107. doi: 10.3389/fcimb.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostowy S, Shenoy AR. The cytoskeleton in cell-autonomous immunity: structural determinants of host defence [Internet] Nat Rev Immunol. 2015;15:559–573. doi: 10.1038/nri3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saini NK, Baena A, Ng TW, Venkataswamy MM, Kennedy SC, Kunnath-Velayudhan S, Carreño LJ, Xu J, Chan J, Larsen MH, et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47 [Internet] Nat Microbiol. 2016;1:16133. doi: 10.1038/nmicrobiol.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields S, Song O. A novel genetic system to detect protein protein interactions [Internet] Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 15.Mehta V, Trinkle-Mulcahy L, Mehta V, Trinkle-Mulcahy L. Recent advances in large-scale protein interactome mapping [Internet] F1000Research. 2016;5:782. doi: 10.12688/f1000research.7629.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calderwood MA, Venkatesan K, Xing L, Chase MR, Vazquez A, Holthaus AM, Ewence AE, Li N, Hirozane-Kishikawa T, Hill DE, et al. Epstein-Barr virus and virus human protein interaction maps. [Internet] Proc Natl Acad Sci U S A. 2007;104:7606–11. doi: 10.1073/pnas.0702332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Chassey B, Navratil V, Tafforeau L, Hiet MS, Aublin-Gex A, Agaugué S, Meiffren G, Pradezynski F, Faria BF, Chantier T, et al. Hepatitis C virus infection protein network. [Internet] Mol Syst Biol. 2008;4:230. doi: 10.1038/msb.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapira SD, Gat-Viks I, Shum BOV, Dricot A, de Grace MM, Wu L, Gupta PB, Hao T, Silver SJ, Root DE, et al. A Physical and Regulatory Map of Host-Influenza Interactions Reveals Pathways in H1N1 Infection [Internet] Cell. 2009;139:1255–1267. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Salwinski L, Zhang C, Chu D, Sampankanpanich C, Reyes NA, Vangeloff A, Xing F, Li X, Wu T-T, et al. An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus [Internet] PLoS Pathog. 2011;7:e1002297. doi: 10.1371/journal.ppat.1002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulbahce N, Yan H, Dricot A, Padi M, Byrdsong D, Franchi R, Lee D-S, Rozenblatt-Rosen O, Mar JC, Calderwood MA, et al. Viral Perturbations of Host Networks Reflect Disease Etiology [Internet] PLoS Comput Biol. 2012;8:e1002531. doi: 10.1371/journal.pcbi.1002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khadka S, Vangeloff AD, Zhang C, Siddavatam P, Heaton NS, Wang L, Sengupta R, Sahasrabudhe S, Randall G, Gribskov M, et al. A physical interaction network of dengue virus and human proteins. [Internet] Mol Cell Proteomics. 2011;10:M111.012187. doi: 10.1074/mcp.M111.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mairiang D, Zhang H, Sodja A, Murali T, Suriyaphol P, Malasit P, Limjindaporn T, Finley RL. Identification of New Protein Interactions between Dengue Fever Virus and Its Hosts, Human and Mosquito [Internet] PLoS One. 2013;8:e53535. doi: 10.1371/journal.pone.0053535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolan PT, Zhang C, Khadka S, Arumugaswami V, Vangeloff AD, Heaton NS, Sahasrabudhe S, Randall G, Sun R, LaCount DJ, et al. Identification and comparative analysis of hepatitis C virus–host cell protein interactions [Internet] Mol Biosyst. 2013;9:3199. doi: 10.1039/c3mb70343f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozenblatt-Rosen O, Deo RC, Padi M, Adelmant G, Calderwood MA, Rolland T, Grace M, Dricot A, Askenazi M, Tavares M, et al. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. [Internet] Nature. 2012;487:491–5. doi: 10.1038/nature11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller M, Jacob Y, Jones L, Weiss A, Brino L, Chantier T, Lotteau V, Favre M, Demeret C. Large Scale Genotype Comparison of Human Papillomavirus E2-Host Interaction Networks Provides New Insights for E2 Molecular Functions [Internet] PLoS Pathog. 2012;8:e1002761. doi: 10.1371/journal.ppat.1002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blasche S, Arens S, Ceol A, Siszler G, Schmidt MA, Häuser R, Schwarz F, Wuchty S, Aloy P, Uetz P, et al. The EHEC-host interactome reveals novel targets for the translocated intimin receptor. [Internet] Sci Rep. 2014;4:7531. doi: 10.1038/srep07531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27 *.Wallqvist A, Memišević V, Zavaljevski N, Pieper R, Rajagopala SV, Kwon K, Yu C, Hoover TA, Reifman J. Using host-pathogen protein interactions to identify and characterize Francisella tularensis virulence factors. [Internet] BMC Genomics. 2015;16:1106. doi: 10.1186/s12864-015-2351-1. By screening for Francisella tularensis putative virulence factors that interacted with their host using a Y2H approach, Wallqvist A. et al coupled with functional validations, could show that a subset of them were as predicted, involved in virulence and lethality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M, Köster S, Penberthy K, Kubota Y, Dricot A, et al. Mycobacterium tuberculosis Type VII Secreted Effector EsxH Targets Host ESCRT to Impair Trafficking [Internet] PLoS Pathog. 2013;9:e1003734. doi: 10.1371/journal.ppat.1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Memisević V, Zavaljevski N, Pieper R, Rajagopala SV, Kwon K, Townsend K, Yu C, Yu X, DeShazer D, Reifman J, et al. Novel Burkholderia mallei virulence factors linked to specific host-pathogen protein interactions [Internet] Mol Cell Proteomics. 2013;12:3036–51. doi: 10.1074/mcp.M113.029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer MD, Neff C, Dufford M, Rivera CG, Shattuck D, Bassaganya-Riera J, Murali TM, Sobral BW. The Human-Bacterial Pathogen Protein Interaction Networks of Bacillus anthracis, Francisella tularensis, and Yersinia pestis [Internet] PLoS One. 2010;5:e12089. doi: 10.1371/journal.pone.0012089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang H, Ke Y, Wang J, Tan Y, Myeni SK, Li D, Shi Q, Yan Y, Chen H, Guo Z, et al. Insight into bacterial virulence mechanisms against host immune response via the Yersinia pestis-human protein-protein interaction network. [Internet] Infect Immun. 2011;79:4413–24. doi: 10.1128/IAI.05622-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gingras A-C, Gstaiger M, Raught B, Aebersold R. Analysis of protein complexes using mass spectrometry [Internet] Nat Rev Mol Cell Biol. 2007;8:645–654. doi: 10.1038/nrm2208. [DOI] [PubMed] [Google Scholar]
- 33 *.Ramage HR, Kumar GR, Verschueren E, Johnson JR, Von Dollen J, Johnson T, Newton B, Shah P, Horner J, Krogan NJ, et al. A Combined Proteomics/Genomics Approach Links Hepatitis C Virus Infection with Nonsense-Mediated mRNA Decay [Internet] Mol Cell. 2015;57:329–340. doi: 10.1016/j.molcel.2014.12.028. To generate a list of high confidence host targets interacting with the Hepatitis C Virus, Ramage H. et al. employed a two-step approach: they first established a HP-PPI map by ectopically expressing all HCV proteins individually in host cells amenable for large-scale AP-MS, and then performed a functional genomic screen in which they knocked down host targets identified in the AP-MS screen to assess their impact on viral replications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34 **.Mirrashidi KM, Elwell CA, Verschueren E, Johnson JR, Frando A, Von Dollen J, Rosenberg O, Gulbahce N, Jang G, Johnson T, et al. Global Mapping of the Inc-Human Interactome Reveals that Retromer Restricts Chlamydia Infection [Internet] Cell Host Microbe. 2015;18:109–121. doi: 10.1016/j.chom.2015.06.004. A pioneering study that systematically mapped the HP-PPI using an AP-MS approach of nearly all secreted Chlamydia Inc-family bacterial effector proteins with their human host, leading to amongst other findings, how host cells may restrict Chlamydia infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35 **.Jäger S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, et al. Global landscape of HIV–human protein complexes [Internet] Nature. 2011;481:365. doi: 10.1038/nature10719. The first systematic AP-MS screen of all HIV proteins against the host proteome in two cell lines. This study provided significant insight into the cellular systems targeted by HIV to enable survival and replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe T, Kawakami E, Shoemaker JE, Lopes TJS, Matsuoka Y, Tomita Y, Kozuka-Hata H, Gorai T, Kuwahara T, Takeda E, et al. Influenza Virus-Host Interactome Screen as a Platform for Antiviral Drug Development [Internet] Cell Host Microbe. 2014;16:795–805. doi: 10.1016/j.chom.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis ZH, Verschueren E, Jang GM, Kleffman K, Johnson JR, Park J, Von Dollen J, Maher MC, Johnson T, Newton W, et al. Global Mapping of Herpesvirus-Host Protein Complexes Reveals a Transcription Strategy for Late Genes [Internet] Mol Cell. 2015;57:349–360. doi: 10.1016/j.molcel.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichlmair A, Kandasamy K, Alvisi G, Mulhern O, Sacco R, Habjan M, Binder M, Stefanovic A, Eberle C-A, Goncalves A, et al. Viral immune modulators perturb the human molecular network by common and unique strategies [Internet] Nature. 2012;487:486–490. doi: 10.1038/nature11289. [DOI] [PubMed] [Google Scholar]
- 39.Germain M-A, Chatel-Chaix L, Gagné B, Bonneil É, Thibault P, Pradezynski F, de Chassey B, Meyniel-Schicklin L, Lotteau V, Baril M, et al. Elucidating novel hepatitis C virus-host interactions using combined mass spectrometry and functional genomics approaches. [Internet] Mol Cell Proteomics. 2014;13:184–203. doi: 10.1074/mcp.M113.030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White EA, Kramer RE, Tan MJA, Hayes SD, Harper JW, Howley PM. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. [Internet] J Virol. 2012;86:13174–86. doi: 10.1128/JVI.02172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White EA, Sowa ME, Tan MJA, Jeudy S, Hayes SD, Santha S, Münger K, Harper JW, Howley PM. Systematic identification of interactions between host cell proteins and E7 oncoproteins from diverse human papillomaviruses. [Internet] Proc Natl Acad Sci U S A. 2012;109:E260–7. doi: 10.1073/pnas.1116776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auweter SD, Bhavsar AP, de Hoog CL, Li Y, Chan YA, van der Heijden J, Lowden MJ, Coombes BK, Rogers LD, Stoynov N, et al. Quantitative mass spectrometry catalogues Salmonella pathogenicity island-2 effectors and identifies their cognate host binding partners. [Internet] J Biol Chem. 2011;286:24023–35. doi: 10.1074/jbc.M111.224600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kane JR, Stanley DJ, Hultquist JF, Johnson JR, Mietrach N, Binning JM, Jónsson SR, Barelier S, Newton BW, Johnson TL, et al. Lineage-Specific Viral Hijacking of Non-canonical E3 Ubiquitin Ligase Cofactors in the Evolution of Vif Anti-APOBEC3 Activity [Internet] Cell Rep. 2015;11:1236–1250. doi: 10.1016/j.celrep.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colpitts TM, Cox J, Nguyen A, Feitosa F, Krishnan MN, Fikrig E. Use of a tandem affinity purification assay to detect interactions between West Nile and dengue viral proteins and proteins of the mosquito vector [Internet] Virology. 2011;417:179–187. doi: 10.1016/j.virol.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamayoshi S, Noda T, Ebihara H, Goto H, Morikawa Y, Lukashevich IS, Neumann G, Feldmann H, Kawaoka Y. Ebola Virus Matrix Protein VP40 Uses the COPII Transport System for Its Intracellular Transport [Internet] Cell Host Microbe. 2008;3:168–177. doi: 10.1016/j.chom.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malik-Soni N, Frappier L. Proteomic profiling of EBNA1-host protein interactions in latent and lytic Epstein-Barr virus infections. [Internet] J Virol. 2012;86:6999–7002. doi: 10.1128/JVI.00194-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47 *.Posner MG, Upadhyay A, Abubaker AA, Fortunato TM, Vara D, Canobbio I, Bagby S, Pula G. Extracellular Fibrinogen-binding Protein (Efb) from Staphylococcus aureus Inhibits the Formation of Platelet-Leukocyte Complexes. [Internet] J Biol Chem. 2016;291:2764–76. doi: 10.1074/jbc.M115.678359. By incubating the Staphylococcus aureus Extracellular Fibrinogen-binding protein Efb with human platelet lysates and then using an AP-MS approach, Posner M. et al. could identify physiologically relevant HP-PPI and gained insights into how the pathogen inhibits platelet activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cristea IM, Carroll J-WN, Rout MP, Rice CM, Chait BT, MacDonald MR. Tracking and elucidating alphavirus-host protein interactions. [Internet] J Biol Chem. 2006;281:30269–78. doi: 10.1074/jbc.M603980200. [DOI] [PubMed] [Google Scholar]
- 49 **.Luo Y, Jacobs EY, Greco TM, Mohammed KD, Tong T, Keegan S, Binley JM, Cristea IM, Fenyö D, Rout MP, et al. HIV–host interactome revealed directly from infected cells [Internet] Nat Microbiol. 2016;1:16068. doi: 10.1038/nmicrobiol.2016.68. By generating two replication and infection competent HIV strains with 2 tagged viral proteins, Luo Y. et al. could monitor empirical and quantitative, time course-dependent changes in the host-pathogen interactomes upon HIV-Human lymphocytes infections, altogether providing physiological HP-PPI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mousnier A, Schroeder GN, Stoneham CA, So EC, Garnett JA, Yu L, Matthews SJ, Choudhary JS, Hartland EL, Frankel G. A New Method To Determine In Vivo Interactomes Reveals Binding of the Legionella pneumophila Effector PieE to Multiple Rab GTPases [Internet] MBio. 2014;5:e01148-14–e01148-14. doi: 10.1128/mBio.01148-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.So EC, Schroeder GN, Carson D, Mattheis C, Mousnier A, Broncel M, Tate EW, Frankel G. The Rab-binding Profiles of Bacterial Virulence Factors during Infection. [Internet] J Biol Chem. 2016;291:5832–43. doi: 10.1074/jbc.M115.700930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells [Internet] J Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambert J-P, Tucholska M, Go C, Knight JDR, Gingras A-C. Proximity biotinylation and affinity purification are complementary approaches for the interactome mapping of chromatin-associated protein complexes [Internet] J Proteomics. 2015;118:81–94. doi: 10.1016/j.jprot.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54 *.Mojica SA, Hovis KM, Frieman MB, Tran B, Hsia R, Ravel J, Jenkins-Houk C, Wilson KL, Bavoil PM. SINC, a type III secreted protein of Chlamydia psittaci, targets the inner nuclear membrane of infected cells and uninfected neighbors. [Internet] Mol Biol Cell. 2015;26:1918–34. doi: 10.1091/mbc.E14-11-1530. By employing the BioID method, a Chlamydia psittaci secreted protein named SINC was found to interact with inner nuclear membrane proteins. This study hinting towards how SINC modulates nuclear structure, signaling, chromatin organization and gene silencing of its host. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lobingier BT, Hüttenhain R, Eichel K, Miller KB, Ting AY, von Zastrow M, Krogan NJ. An Approach to Spatiotemporally Resolve Protein Interaction Networks in Living Cells [Internet] Cell. 2017;169:350–360.e12. doi: 10.1016/j.cell.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leitner A, Faini M, Stengel F, Aebersold R. Crosslinking and Mass Spectrometry: An Integrated Technology to Understand the Structure and Function of Molecular Machines [Internet] Trends Biochem Sci. 2016;41:20–32. doi: 10.1016/j.tibs.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Makowski MM, Willems E, Jansen PWTC, Vermeulen M. Cross-linking immunoprecipitation-MS (xIP-MS): Topological Analysis of Chromatin-associated Protein Complexes Using Single Affinity Purification [Internet] Mol Cell Proteomics. 2016;15:854–865. doi: 10.1074/mcp.M115.053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Pellarin R, Fridy PC, Fernandez-Martinez J, Thompson MK, Li Y, Wang QJ, Sali A, Rout MP, Chait BT. A strategy for dissecting the architectures of native macromolecular assemblies [Internet] Nat Methods. 2015;12:1135–1138. doi: 10.1038/nmeth.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rinner O, Seebacher J, Walzthoeni T, Mueller L, Beck M, Schmidt A, Mueller M, Aebersold R. Identification of cross-linked peptides from large sequence databases [Internet] Nat Methods. 2008;5:315–8. doi: 10.1038/nmeth.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navare AT, Chavez JD, Zheng C, Weisbrod CR, Eng JK, Siehnel R, Singh PK, Manoil C, Bruce JE. Probing the Protein Interaction Network of Pseudomonas aeruginosa Cells by Chemical Cross-Linking Mass Spectrometry [Internet] Structure. 2015;23:762–773. doi: 10.1016/j.str.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61 **.Schweppe DK, Harding C, Chavez JD, Wu X, Ramage E, Singh PK, Manoil C, Bruce JE. Host-Microbe Protein Interactions during Bacterial Infection [Internet] Chem Biol. 2015;22:1521–1530. doi: 10.1016/j.chembiol.2015.09.015. This unbiased and untargeted chemical crosslinking of live human cells infected with A. baumannii lead to the de novo and predicted identifications of 46 interspecies PPIs, including with the known virulence factor OmpA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62 **.Glick Y, Ben-Ari Y, Drayman N, Pellach M, Neveu G, Boonyaratanakornkit J, Avrahami D, Einav S, Oppenheim A, Gerber D. Pathogen receptor discovery with a microfluidic human membrane protein array. [Internet] Proc Natl Acad Sci U S A. 2016;113:4344–9. doi: 10.1073/pnas.1518698113. Glick et al. developed a protein micro-array based device coupled with a microfluidics platform to detect whole virus-host membrane protein interactions, and applied it to discover new cellular receptors of the Simian Virus 40 and the Hepatitis delta virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63 *.Scietti L, Sampieri K, Pinzuti I, Bartolini E, Benucci B, Liguori A, Haag AF, Lo Surdo P, Pansegrau W, Nardi-Dei V, et al. Exploring host-pathogen interactions through genome wide protein microarray analysis [Internet] Sci Rep. 2016;6:27996. doi: 10.1038/srep27996. By using a protein-microarray based technology, Scietti L. et al. detected previously uncharacterized host interactors for two bacterial pathogens including an endothelial receptor and the human complement component. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu X, Decker KB, Barker K, Neunuebel MR, Saul J, Graves M, Westcott N, Hang H, LaBaer J, Qiu J, et al. Host–Pathogen Interaction Profiling Using Self-Assembling Human Protein Arrays [Internet] J Proteome Res. 2015;14:1920–1936. doi: 10.1021/pr5013015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bushell KM, Sollner C, Schuster-Boeckler B, Bateman A, Wright GJ. Large-scale screening for novel low-affinity extracellular protein interactions [Internet] Genome Res. 2008;18:622–630. doi: 10.1101/gr.7187808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartholdson SJ, Crosnier C, Bustamante LY, Rayner JC, Wright GJ. Identifying novel Plasmodium falciparum erythrocyte invasion receptors using systematic extracellular protein interaction screens [Internet] Cell Microbiol. 2013;15:1304–1312. doi: 10.1111/cmi.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dyer MD, Murali TM, Sobral BW, Wille A, Buhlmann P. The Landscape of Human Proteins Interacting with Viruses and Other Pathogens [Internet] PLoS Pathog. 2008;4:e32. doi: 10.1371/journal.ppat.0040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Randow F, Lehner PJ. Viral avoidance and exploitation of the ubiquitin system [Internet] Nat Cell Biol. 2009;11:527–534. doi: 10.1038/ncb0509-527. [DOI] [PubMed] [Google Scholar]
- 69.Zhou Y, Zhu Y. Diversity of bacterial manipulation of the host ubiquitin pathways [Internet] Cell Microbiol. 2015;17:26–34. doi: 10.1111/cmi.12384. [DOI] [PubMed] [Google Scholar]
- 70.Hicks SW, Galán JE. Exploitation of eukaryotic subcellular targeting mechanisms by bacterial effectors [Internet] Nat Rev Microbiol. 2013;11:316–326. doi: 10.1038/nrmicro3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashida H, Kim M, Sasakawa C. Exploitation of the host ubiquitin system by human bacterial pathogens [Internet] Nat Rev Microbiol. 2014;12:399–413. doi: 10.1038/nrmicro3259. [DOI] [PubMed] [Google Scholar]
- 72.Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O’Connor L, Li M, et al. Large-scale mapping of human protein–protein interactions by mass spectrometry [Internet] Mol Syst Biol. 2007;3:89. doi: 10.1038/msb4100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Futschik ME, Chaurasia G, Herzel H. Comparison of human protein protein interaction maps [Internet] Bioinformatics. 2007;23:605–611. doi: 10.1093/bioinformatics/btl683. [DOI] [PubMed] [Google Scholar]
- 74.Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, Rao KVS, Peters PJ. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. [Internet] Cell. 2010;140:731–43. doi: 10.1016/j.cell.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Shah PS, Wojcechowskyj JA, Eckhardt M, Krogan NJ. Comparative mapping of host-pathogen protein-protein interactions. [Internet] Curr Opin Microbiol. 2015;27:62–8. doi: 10.1016/j.mib.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang G, Xia Y, Song X, Ai L. Common Non-classically Secreted Bacterial Proteins with Experimental Evidence [Internet] Curr Microbiol. 2016;72:102–111. doi: 10.1007/s00284-015-0915-6. [DOI] [PubMed] [Google Scholar]
- 77.Herweg J-A, Hansmeier N, Otto A, Geffken AC, Subbarayal P, Prusty BK, Becher D, Hensel M, Schaible UE, Rudel T, et al. Purification and proteomics of pathogen-modified vacuoles and membranes. [Internet] Front Cell Infect Microbiol. 2015;5:48. doi: 10.3389/fcimb.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Havugimana PC, Hart GT, Nepusz TS, Yang H, Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, et al. A Census of Human Soluble Protein Complexes [Internet] Cell. 2012;150:1068–1081. doi: 10.1016/j.cell.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kristensen AR, Gsponer J, Foster LJ. A high-throughput approach for measuring temporal changes in the interactome [Internet] Nat Methods. 2012;9:907–909. doi: 10.1038/nmeth.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt F, Völker U. Proteome analysis of host-pathogen interactions: Investigation of pathogen responses to the host cell environment [Internet] Proteomics. 2011;11:3203–3211. doi: 10.1002/pmic.201100158. [DOI] [PubMed] [Google Scholar]
- 81.König R, Zhou Y, Elleder D, Diamond TL, Bonamy GMC, Irelan JT, Chiang C, Tu BP, De Jesus PD, Lilley CE, et al. Global Analysis of Host-Pathogen Interactions that Regulate Early-Stage HIV-1 Replication [Internet] Cell. 2008;135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uetz P, Dong Y-A, Zeretzke C, Atzler C, Baiker A, Berger B, Rajagopala SV, Roupelieva M, Rose D, Fossum E, et al. Herpesviral Protein Networks and Their Interaction with the Human Proteome [Internet] Science (80–.) 2006;311:239–242. doi: 10.1126/science.1116804. [DOI] [PubMed] [Google Scholar]
- 83.Beaulieu AM, Rath P, Imhof M, Siddall ME, Roberts J, Schnappinger D, Nathan CF. Genome-Wide Screen for Mycobacterium tuberculosis Genes That Regulate Host Immunity [Internet] PLoS One. 2010;5:e15120. doi: 10.1371/journal.pone.0015120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park RJ, Wang T, Koundakjian D, Hultquist JF, Lamothe-Molina P, Monel B, Schumann K, Yu H, Krupzcak KM, Garcia-Beltran W, et al. A genome-wide CRISPR screen identifies a restricted set of HIV host dependency factors [Internet] Nat Genet. 2016;49:193–203. doi: 10.1038/ng.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


