Abstract
Objectives
To prospectively examine the independent contribution of major depressive disorder (MDD), generalized anxiety disorder (GAD), and selective serotonin reuptake inhibitors (SSRIs) use to changes in bone metabolism in older adolescents and emerging adults.
Methods
Medically-healthy 15 to 20 year-olds who were unmedicated or within one month of starting an SSRI were prospectively followed. Psychiatric functioning and medication treatment were assessed monthly. Every four months, trabecular and cortical volumetric bone mineral density (vBMD) at the radius and markers of bone metabolism were evaluated. Every eight months, total body less head areal bone mineral content and lumbar spine (LS) areal BMD were determined. Linear mixed effects regression analysis examined associations between bone measures on the one hand and MDD, GAD, and SSRI indices on the other.
Results
Two hundred and sixty four participants were followed for 1.51±0.76 years. After adjusting for age, sex, vitamin D concentration, physical activity, lean mass or grip strength, and time in the study, MDD severity was associated with increasing LS aBMD. Similarly, SSRI use was associated with increasing LS aBMD and bone formation in female participants. In contrast, SSRI use was associated with decreasing LS aBMD in males. After accounting for depression, GAD was independently, albeit weakly, associated with increased bone mineralization.
Conclusions
In older adolescents and emerging adults, MDD and GAD are associated with increasing bone mass, particularly in the lumbar spine and in females, while SSRIs are associated with increasing bone mass in females but decreasing bone mass in males.
Keywords: adolescents, emerging adults, bone mineral density, bone mass, bone markers, depression, major depressive disorder, anxiety, selective serotonin reuptake inhibitors
Introduction
Major depressive disorder (MDD) is characterized by depressed mood, anhedonia, neuro-vegetative symptoms (e.g., insomnia, anorexia, fatigue), and cognitive symptoms (e.g., concentration difficulty).(1) MDD is associated with low bone mass,(2,3) increasing one’s lifetime risk for osteoporosis and fractures, as well as medical expenditures.(4,5) The effects of MDD on bone mass has been attributed to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, other hormonal abnormalities, autonomic nervous system dysfunction, and subclinical inflammation.(6) In addition, lifestyle factors prevalent in MDD, such as poor dietary intake, physical inactivity, and smoking also affect bone mineralization.(5) Notably, the association of MDD with bone mass holds across the age spectrum; however, other factors moderate it. For instance, bone mass may be more strongly impacted by MDD in women than in men, and in clinical populations compared to epidemiologic samples.(2,3) Additionally, psychotropic medications, particularly selective serotonin reuptake inhibitors (SSRIs), may accelerate bone loss and increase fracture risk.(7–10)
Elucidating the independent effect of MDD and SSRIs on bone metabolism requires a prospective design. Given that bone mass rapidly accrues during adolescence and that peak bone mass achieved by early adulthood is a major determinant of future osteoporosis risk, we conducted a two-year prospective study examining the skeletal effects of SSRIs in older adolescents.(11,12) We hypothesized that, after accounting for depression severity, SSRI use would be associated with reduced bone mineral content as operationalized via total body less head (TBLH) areal bone mineral content (aBMC) and trabecular volumetric bone mineral density (vBMD) at the ultradistal radius. We further hypothesized that SSRI use would be associated with reduced bone formation, as measured by serological markers of bone metabolism. Given the high comorbidity between MDD and generalized anxiety disorder (GAD), we further investigated the association between GAD and bone mineralization.
Methods
Participants
Between 09/2010 and 12/2014, 15 to 20 year-old participants were recruited into this longitudinal observational cohort study, from outpatient and inpatient clinical settings as well as by advertisement and word of mouth.(11,12) Enrollment was restricted to individuals not taking psychotropics or those who were within one month of starting an SSRI. Treatment with psychotropics, other than SSRIs, during the two years prior to study entry led to exclusion, with the exception of use of benzodiazepines, trazodone, α2-agonists, or psychostimulants. The presence of an eating disorder, substance dependence, pregnancy, significant medical or surgical history, the chronic use of medications potentially affecting bone metabolism, or plans to move out of state within a year also led to exclusion.
Procedures
The local Institutional Review Board approved the study and informed consent and assent were obtained. After completing the baseline visit, the participants returned for a follow-up every four months, for up to two years. Between in-person visits, they were contacted by phone monthly.
During all contacts, participants were queried about their medical history and medication use. Adherence was based on self-report and pharmacy records. At every in-person visit, the Inventory of Depressive Symptomatology (IDS),(13) the Beck Depression Inventory (BDI-II),(14) the Beck Anxiety Inventory (BAI),(15) and the modified version of the Physical Activity Questionnaire for Adolescents (PAC-A)(16) were administered. In addition, height and weight were measured following standard procedures.(11)
Clinical diagnoses, based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR),(17) incorporated information from the medical records, the self- and researcher-completed symptom rating scales, a structured diagnostic interview completed at study entry, and an unstructured interview by a child psychiatrist.(11,12) Meeting criteria for a major depressive episode (MDE) and GAD, following the DSM-IV-TR, was determined for each week during the study period, using the Longitudinal Interval Follow-up Evaluation for Adolescents (A-LIFE).(11,12)
At study entry and every eight months, a whole-body and a lumbar spine (LS) dual energy x-ray absorptiometry (DXA) scan were obtained using a Hologic QDR DELPHI-4500A unit or a Hologic Discovery A unit (Hologic, Inc, Bedford, MA). The two DXA units were cross-calibrated.(11) vBMD at the nondominant radius (4% and 20% sites) was measured, at study entry and every four months, with peripheral quantitative computed tomography (pQCT), using a Stratec XCT-2000 scanner (Stratec, Inc., Pforzheim, Germany). Image analysis was performed using the manufacturer’s software package, version 6.0, as previously described.(11) pQCT scans compromised by movement were rejected. Quality control and calibration of the equipment were performed daily. To estimate musculoskeletal fitness, grip strength was measured using a Jamar Plus hand dynamometer (model number: 12-0604; Patterson Medical, Bolingbrook, IL, USA) following the manufacturer-recommended protocol. Two measurements for each hand, alternating sides, were obtained and the one of highest magnitude on the side that was scanned was used in the analyses.
At every in-person visit, a fasting (in 98.88% of the time) blood sample was obtained to measure plasma 25-OH-vitamin D (Abbott, Wiesbaden, German). The median time of the blood draw was 9:38 AM (Q1: 8:45AM – Q3: 11:30AM). In addition, serum concentration of intact parathyroid hormone (iPTH), bone-specific alkaline phosphatase (BAP), osteocalcin, and C-terminal telopeptide (CTX-1), was determined using a multiplex platform (Immunodiagnostic Systems, United Kingdom, Supplemental Table S1).
Statistical Analysis
Body mass index (BMI) was computed as weight/height2 (kg/m2) and lean body mass index (LBMI) as lean mass/height2 (kg/m2). Age- and sex-specific Z-scores were then derived.(18,19) Age-sex-height-race-specific Z-scores for TBLH aBMC and LS aBMD were also generated, based on the Bone Mineral Density in Childhood Study.(20)
In order to capture the change in the BDI-II and BAI, the mean score over the interim visits, up to and including the score at the visit when a DXA scan was obtained, was used as the predictor in the relevant models.
Differences between participants taking SSRIs at study entry vs. not on SSRIs were evaluated using Student’s t test for continuous variables and the chi square test for categorical variables.
The association between MDD, GAD, and SSRIs, on the one hand, and skeletal measures, on the other, was examined by fitting linear mixed effects regression models.(21) Different indices of MDD and GAD were used, including DSM-IV-TR-based diagnoses, number of weeks meeting diagnostic criteria, and scale scores.(11) SSRI treatment was characterized in terms of duration of use and dose, accounting for adherence. Given that SSRI adherence was missing for only between 1.7 and 2.6% of observations, the EM algorithm was used to impute missing values.(22) All models included adjustment for age (years) at study entry, sex, level of physical activity, LBMI Z-score or grip strength, and vitamin D concentration at study entry (Supplemental Figure). Measures of body size [i.e., height Z-score or forearm length (cm)] were also included as covariates, in the relevant analyses. Participant-specific random intercepts and slopes were modeled assuming an unstructured covariance matrix. Duration of study participation was the time metric in the analysis. Maximum likelihood (ML) methods were used for estimation, which yields unbiased estimates under the assumption that the missing data mechanism is ignorable.(23) The covariates of interest were analyzed as time-dependent covariates and decomposed into a between-subject and a within-subject component.(24) Importantly, the former represents a cross-sectional effect while the latter represents an individual slope effect (i.e., change over time). Cohen’s f2 effect sizes were calculated to assess the relative strength of MDD, GAD, and SSRI measures in the linear mixed-effects regression models (with f2≥ 0.02, f2≥ 0.15, and f2≥ 0.35 representing small, medium, and large effect sizes, respectively).(25,26) Sample size estimation using nQuery Advisor 7.0 based on a two group-two repeated measures model, showed that 120 participants per group (on SSRIs vs. not) would provide ≥ 80% power to detect a significant (p<0.0125) SSRI by time interaction effect at year 1 of the study and ≥ 98% power at year 2, assuming a 30% drop out rate during year 1 and a medium effect size.
All hypothesis tests were two-tailed and analyses utilized procedures from SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC).
Results
Participants
Of the 279 participants enrolled in the study, 264 contributed to this analysis after exclusions for psychosis and bipolar disorder, the timing of SSRI use, having a genetic condition or substance use disorder, and missing data. As would be expected, participants in the SSRI group were more likely to have MDD and GAD and to score higher on all measures related to these disorders, when compared to the no-SSRI group (Table 1). They were also less likely to complete the 2-year study (61% vs. 80%, p=0.0007 and Supplemental Table S2). Participants in the SSRI group also had significantly lower baseline bone strength index and cortical thickness and higher circulating iPTH (Supplemental Table S3).
Table 1.
Baseline Demographic and Clinical Characteristics of the Participants as a Group and Split Based on SSRI Use at Study Entry [Mean±SD, unless noted otherwise]
| Variable | Total Sample N=264 |
No-SSRI Group N=137 |
SSRI Group N=127 |
p-value |
|---|---|---|---|---|
|
| ||||
| Age, years | 18.9±1.6 | 19.0±1.5 | 18.8±1.7 | >0.40 |
|
| ||||
| Female Sex, n (%) | 159 (60) | 76 (55) | 83 (65) | >0.10 |
|
| ||||
| Time since Menarche, years | 6.3±2.3 | 6.5±2.3 | 6.1±2.2 | >0.20 |
|
| ||||
| White Race, n (%) | 233 (88) | 119 (87) | 114 (90) | >0.30 |
|
| ||||
| Hispanic, n (%) | 22 (8) | 11 (8) | 11 (9) | >0.80 |
|
| ||||
| BMI Z-score | 0.43±0.93 | 0.40±1.00 | 0.46±0.90 | >0.50 |
|
| ||||
| LBMI Z-score | −0.52±0.93 | −0.53±0.94 | −0.52±0.91 | >0.90 |
|
| ||||
| Physical Activity Score* | 2.0±0.7 | 2.0±0.7 | 2.0±0.8 | >0.30 |
|
| ||||
| Estimated Daily Caloric Intake, kcal | 1762±941 | 1715±816 | 1814±1064 | >0.40 |
|
| ||||
| Cigarette Use, n (%) | 41 (16) | 16 (12) | 25 (20) | <0.08 |
|
| ||||
| Alcohol Use, n (%) | 183 (69) | 95 (69) | 88 (69) | >0.90 |
|
| ||||
| Psychiatric Characteristics | ||||
|
| ||||
| MDD at Study Entry, n (%) | <0.0001 | |||
| - Symptomatic | 131 (50) | 29 (21) | 102 (80) | |
| - In Full Remission | 42 (16) | 27 (20) | 15 (12) | |
| - Never | 91 (34) | 81 (59) | 10 (8) | |
| MDD at Study End, n (%) | <0.0001 | |||
| - Symptomatic | 74 (28) | 20 (15) | 54 (43) | |
| - In Full Remission | 113 (43) | 47 (34) | 66 (52) | |
| - Never | 77 (29) | 70 (51) | 7 (6) | |
|
| ||||
| Percent Time Meeting Full MDE Criteria¶ | 23.9±29.9 | 11.2±23.5 | 37.6±29.9 | <0.0001 |
|
| ||||
| IDS Score | 13.9±10.8 | 7.9±8.1 | 20.3±9.5 | <0.0001 |
|
| ||||
| BDI Score | 11.1±10.5 | 5.7±6.5 | 16.9±10.8 | <0.0001 |
|
| ||||
| GAD at Study Entry, n (%) | 73 (28) | 23 (17) | 50 (39) | <0.0001 |
|
| ||||
| Percent Time Meeting GAD Criteria¶ | 39.5±37.7 | 25.0±33.5 | 55.1±35.9 | <0.0001 |
|
| ||||
| BAI Score | 8.3±8.6 | 4.4±5.0 | 12.6±9.6 | <0.0001 |
|
| ||||
| SSRI Use at Study End, n (%) | 65 (25) | 2 (1) | 63 (50) | <0.0001 |
|
| ||||
| Duration of SSRI Use, years | 0.44±0.67 | 0.02±0.18 | 0.90±0.70 | <0.0001 |
MDD/MDE: major depressive disorder/episode, IDS: inventory of depressive symptomatology, BDI/BAI: Beck depression and Beck anxiety inventory, BMI: Body Mass Index, BMI Z-score: age-sex-specific BMI Z-score, FMI Z-score: age-sex-specific fat mass index Z-score, GAD: generalized anxiety disorder, LBMI Z-score: age-sex-specific lean body mass index Z-score, SSRI: selective serotonin reuptake inhibitor.
Physical Activity Score 1=low, 5=high.
Percent time meeting full MDE or GAD criteria capture the percentage of weeks where the participant met DSM-IV-TR criteria for a major depressive episode or generalized anxiety disorder, based on the Longitudinal Interview Follow-up Evaluation for Adolescents (A-LIFE).
Bolded results are statistically significant (p<0.05) and italicized and bolded results are marginally significant (p<0.10).
Primary Skeletal Outcomes
On average, TBLH aBMC significantly increased by 23.9±80.8 g/cm2 over the course of the study (p<0.0001) while trabecular vBMD at the ultradistal radius significantly decreased by 2.7±10.8 mg/cm3 (p=0.0002). After adjusting for age, sex, level of physical activity, LBMI Z-score, and vitamin D concentration at study entry, the number of weeks in MDE was associated with lower cross-sectional age-sex-race-height-specific TBLH aBMC Z-score but not with its change over time (within subject effect) (Table 2, Figure 1). In contrast, after adjusting for the relevant covariates, the number of weeks in MDE was not significantly associated with trabecular vBMD (Table 2). Substituting the BDI-II or IDS score for the number of weeks in MDE yielded comparable, albeit weaker, findings.
Table 2.
Parameter Estimates (Standard Errors) for MDD-, GAD-, and SSRI-Related Variables from Linear Mixed Effects Regression Analysis Models for Relevant Skeletal Outcomes
| Weeks in MDD | Weeks in GAD | SSRI Use | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Within | Between | Within | Between | Within | Between | |
|
| ||||||
| TBLH aBMC Z-score | 0.0004 (0.0008) | −0.0144 (0.0057) | 0.0012 (0.0006) | −0.0044 (0.0044) | 0.0042 (0.0360) | 0.3151 (0.3346) |
| Males | 0.0020 (0.0017) | −0.0365 (0.0135) | 0.0015 (0.0010) | −0.0060 (0.0100) | −0.0833 (0.0532) | 0.9245 (0.6745) |
| Females | 0.0001 (0.0009) | −0.0095 (0.0061) | 0.0011 (0.0007) | −0.0039 (0.0048) | 0.0551 (0.0475) | 0.0843 (0.3748) |
|
| ||||||
| LS aBMD Z-score | 0.0035 (0.0010) | −0.0137 (0.0073) | 0.0018 (0.0007) | −0.0055 (0.0055) | 0.0288 (0.0478) | 0.0578 (0.4223) |
| Males | 0.0047 (0.0025) | −0.0131 (0.0154) | 0.0003 (0.0016) | −0.0017 (0.0107) | −0.2380 (0.0819) | 1.0694 (0.7435) |
| Females | 0.0030 (0.0011) | −0.0143 (0.0084) | 0.0021 (0.0009) | −0.0074 (0.0066) | 0.1632 (0.0577) | −0.2720 (0.5159) |
|
| ||||||
| Trabecular vBMD | −0.0363 (0.0301) | −0.1920 (0.3277) | −0.0089 (0.0223) | 0.2113 (0.2524) | 0.6960 (1.7072) | 2.7123 (20.2226) |
| Males | 0.0046 (0.0757) | 0.4728 (0.7559) | 0.0263 (0.0480) | 0.9238 (0.5370) | 0.9350 (3.4149) | 11.8618 (43.4000) |
| Females | −0.0382 (0.0308) | −0.3461 (0.3566) | −0.0125 (0.0240) | 0.0251 (0.2809) | 0.8015 (1.8936) | 0.7662 (22.5163) |
|
| ||||||
| Cortical vBMD | 0.0115 (0.0356) | −0.1783 (0.1713) | −0.0245 (0.0265) | −0.2014 (0.1293) | 2.1156 (2.0622) | −31.2229 (10.1531) |
| Males | −0.0552 (0.0777) | −0.1684 (0.4487) | −0.0677 (0.0500) | 0.0383 (0.3158) | 4.0194 (3.7524) | −47.7074 (24.2898) |
| Females | 0.0573 (0.0374) | −0.1871 (0.1670) | 0.0094 (0.0290) | −0.2417 (0.1285) | 2.9009 (2.2694) | −28.1480 (10.1579) |
|
| ||||||
| Osteocalcin/CTX-1 Ratio | −0.1006 (0.0925) | −0.0124 (0.1494) | 0.0936 (0.0673) | −0.0307 (0.1131) | 11.2839 (5.4034) | 2.3695 (8.9355) |
| Males | −0.4508 (0.1976) | 0.0678 (0.3596) | −0.0877 (0.1293) | −0.1212 (0.2435) | 2.8653 (10.2700) | 13.6745 (18.2788) |
| Females | −0.0696 (0.1087) | −0.0273 (0.1592) | 0.1183 (0.0822) | −0.0292 (0.1244) | 11.9645 (6.5339) | 0.0488 (10.0649) |
|
| ||||||
| BAP/CTX-1 Ratio | 0.0067 (0.0670) | −0.0684 (0.1331) | 0.0765 (0.0489) | −0.0815 (0.1013) | 11.1269 (3.8731) | −8.2515 (8.0445) |
| Males | −0.0352 (0.1660) | 0.5791 (0.3149) | 0.0523 (0.1042) | 0.2068 (0.2189) | 8.5635 (8.3274) | 6.0490 (16.9248) |
| Females | −0.0145 (0.0761) | −0.2173 (0.1372) | 0.0519 (0.0579) | −0.2260 (0.1067) | 11.1028 (4.4853) | −14.0120 (8.7258) |
TBLH aBMC Z-score: Total body less head areal bone mineral content age-sex-race-height-specific Z-score. LS aBMD Z-score: Lumbar spine areal bone mineral density age-sex-race-height-specific Z-score. Trabecular vBMD: Trabecular volumetric bone mineral density measured at the 4% non-dominant radius. Cortical vBMD was measured at the 20% non-dominant radius. CTX-1: C-terminal telopeptide. BAP: Bone-specific alkaline phosphatase. The base model included the following “standard covariates”: baseline age, sex (for overall sample), lean body mass index Z-score (for DXA-based measures) or forearm length (for pQCT-based measures) or age-sex-specific height and body mass index Z-scores (for bone markers), physical activity (for DXA-based measures) or grip strength (for pQCT-based measures), vitamin D concentration, and time in the study. All models included the standard covariates in addition to the predictor specified in each model. Current Depression Status included: 0=Never depressed, 1=Full Remission, 2=Remitting, 3=Partial Remission, 4=Relapse, 5= Major Depressive Episode (MDE). Weeks in MDE and weeks in GAD reflect the number of weeks where the participant met DSM-IV-TR criteria for a MDE or generalized anxiety disorder, based on the Longitudinal Interview Follow-up Evaluation for Adolescents (A-LIFE). Selective Serotonin Reuptake Inhibitor (SSRI) use reflects aggregate use between visits, in years. SSRI dose reflects the cumulative dose of SSRI taken by participants. Both SSRI use and dose were adjusted for adherence as captured by self-report and pharmacy records. BAI: Beck Anxiety Inventory. BDI-II: Beck Depression Inventory. IDS: Inventory of Depressive Symptomatology. Bolded results are statistically significant (p<0.05) and italicized and bolded results are marginally significant (p<0.10).
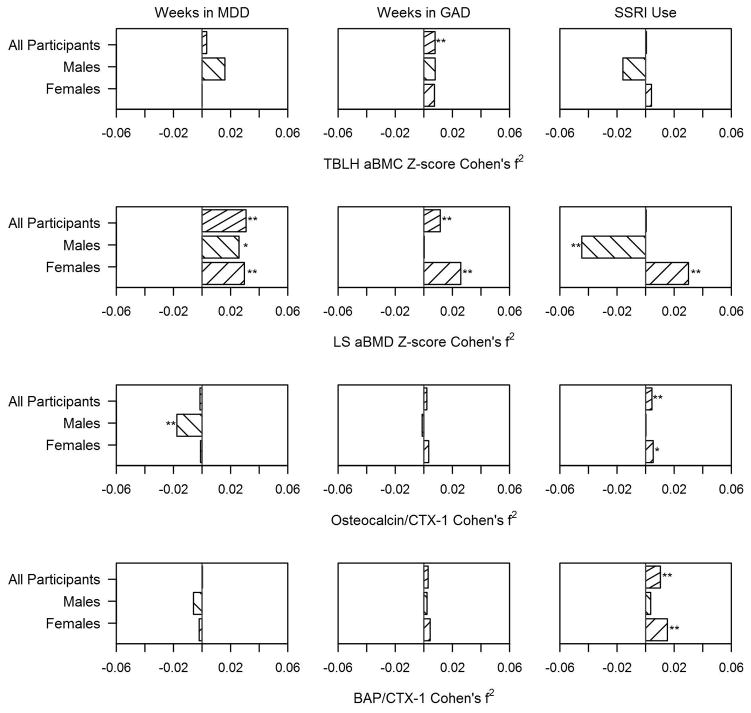
Figure 1.
Cohen’s f2 effect size comparing the “within-subject” association, from the linear mixed effects regression model, of weeks meeting criteria for major depressive disorder (MDD) and for generalized anxiety disorder (GAD), and use of selective serotonin reuptake inhibitors (SSRI) with the skeletal outcomes in the entire samples and in males and female participants. Effect sizes for inverse associations have been assigned a negative value. Top panel: TBLH aBMC (Total body less head areal bone mineral content). Second panel: LS aBMD (Lumbar spine areal bone mineral density). Third panel: Ratio of osteocalcin to CTX-1 (C-terminal telopeptide). Bottom panel: Ratio of bone-specific alkaline phosphatase (BAP) to CTX-1. One asterisk denotes marginally significant results (p < 0.10) and two denote significant results (p < 0.05).
In addition, neither duration of treatment with SSRIs nor the cumulative dose were significantly associated with either primary outcomes but number of weeks in GAD were associated with increased TBLH aBMC Z-score over time (Table 2, Figure 1). This remained the case even after including weeks meeting DSM-IV-TR criteria for MDE and GAD concurrently in the overall model (p<0.04).
Secondary Skeletal Outcomes
Similar analyses were conducted for secondary skeletal outcomes, without adjustment for the number of statistical tests conducted (Tables 3 and S4). Cross-sectionally, in the group overall, there was only a statistical trend (p<0.10) for weeks meeting criteria for MDE (and for BDI score) to be associated with lower LS aBMD Z-score (Table 3). Longitudinally, however, the number of weeks meeting MDE criteria was significantly associated with an increase in LS aBMD Z-score, but a decline in polar section modulus (Tables 3 and S4, Figure 1). The number of weeks meeting GAD criteria showed similar association with polar section modulus (Supplemental Table S4). However, when weeks meeting criteria for MDE and GAD were concurrently included in the model for polar section modulus, only the association with the former remained significant (within-subject effect of weeks in MDE β= −0.0926, SE= 0.0387, p=0.0168 vs. weeks in GAD β= −0.0130, SE= 0.0287, p=0.6522).
Table 3.
Summary of the Findings for the Longitudinal Associations (Within-Subject Effect) Between Indices of Depression, SSRI use, and GAD on the One Hand and Skeletal Outcomes on the Other
| MDD Indices | SSRI Indices | GAD Indices | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| TBLH aBMC Z-score | ~ | ~ | ~ | ~ | ~ | ~ |
| LS aBMD Z-score | ↑ | ↑ | ↓ | ↑ | ~ | ↑ |
| Trabecular vBMD | ~ | ~ | ~ | ~ | ~ | ~ |
| Polar Section Modulus | ~ | ↓ | ~ | ↑ | ~ | ↓ |
| Bone Formation Relative to Bone Resorption | ↓ | ~ | ~ | ↑ | ~ | ~ |
MDD: Major depressive disorder, SSRI: Selective serotonin reuptake inhibitors, GAD: Generalized anxiety disorder, TBLH aBMC Z-score: Total body less head areal bone mineral content age-sex-race-height-specific Z-score. LS aBMD Z-score: Lumbar spine areal bone mineral density age-sex-race-height-specific Z-score. vBMD: Volumetric bone mineral density.
Denotes the absence of any significant longitudinal association. ↑: Denotes the presence of a significant positive association between the predictors of interest and the slope of the outcome. ↓: Denotes the presence of a significant inverse association between the predictors of interest and the slope of the outcome.
In the overall sample, SSRI use was positively associated with longitudinal change in bone strength index and, cross-sectionally, with polar section modulus (Supplemental Table S4) and with periosteal and endosteal circumferences (data not shown).
There was no association between weeks meeting criteria for MDE and bone markers in the overall sample (Tables 2 and S4). In contrast, SSRI use was positively associated with increasing osteocalcin to CTX-1 and bone-specific alkaline phosphatase to CTX-1 ratios over time (Table 2).
Sex Effect
Next, we examined the differential associations in males and females. While, cross-sectionally, weeks meeting criteria for MDE was inversely related to DXA-based measures in both sexes, the magnitude of the associations appeared stronger in males (Table 2, Figure 1). Of particular interest, SSRI use was associated with significantly decreasing LS aBMD Z-score over time in males but significantly increasing LS aBMD Z-score in females (Table 2). When the models predicting LS aBMD Z-score in females were concurrently adjusted for weeks meeting criteria for MDE and for SSRI use, along with the other covariates, both remained significant (weeks in MDE β= 0.003±0.001, p=0.0235 and SSRI use β= 0.145±0.058, p=0.0128). The number of weeks meeting MDE criteria was associated with a decrease in periosteal and endosteal circumferences primarily in males (data not shown), but also somewhat in females, leading to a significant inverse association with change over time in polar section modulus, primarily in females (Supplemental Table S4). SSRI use was cross-sectionally associated with lower cortical vBMD but larger polar section modulus, primarily in females (Tables 2 and S4). It was also associated with increasing bone strength index, primarily in females (Supplemental Table S4).
Similarly, sex differences emerged in the associations with bone markers. The number of weeks meeting criteria for MDE was associated with a decrease in osteocalcin to CTX-1 ratio and, cross-sectionally, positively associated with the ratio of BAP to CTX-1 in males only (Table 2, Figure 1). The BDI-II and IDS scores yielded comparable findings (data not shown). In contrast, SSRI use was associated with increased ratio of bone formation to resorption primarily in females.
Indices of GAD were associated with an increase in LS aBMD Z-score but a decrease in polar section modulus only in females (Tables 2 and S4, Figure 1). Of note, when weeks meeting criteria for MDE and GAD were concurrently included in the model for LS aBMD Z-score in females, only the association with the former remained significant (within-subject effect of weeks in MDE β= 0.0022, SE= 0.0013, p=0.0900 vs. weeks in GAD β= 0.0013, SE= 0.0010, p=0.1709).
Discussion
To our knowledge, this is the first longitudinal study in older adolescents and emerging adults specifically designed to examine the independent effects of MDD, GAD, and SSRI treatment on bone metabolism. A complex picture emerged of cross-sectional and longitudinal associations that varied depending on sex and skeletal site or skeletal outcome (Table 3).
Whether MDD ought to be considered an independent risk factor for osteoporosis has been debated for more than a decade.(6) This question is confounded by the association of depression with a number of unfavorable lifestyle and health-related factors (e.g., antidepressant use, smoking, physical inactivity, poor diet, etc.) that, themselves, may increase the risk for osteoporosis.(4,5) Thus, ideally, these confounding factors, along with other established risk factors, should be considered when examining the cross-sectional and longitudinal association between MDD and bone mass. Yet, only a minority of studies are prospectively designed to specifically address this question, which requires a thorough assessment of MDD severity, documentation of pharmacological treatment, and an accounting for confounding factors.
Notwithstanding these shortcomings, a few studies have examined the association between depression and bone mass in youth. For instance, in one study in adolescent girls, depressive symptoms were found to be associated with lower total body aBMC.(27,28) In contrast, in another smaller study, only male adolescents with MDD were found to have lower aBMD at the hip and femoral neck.(29) Finally, in a prospective study from Portugal, no cross-sectional or longitudinal associations between depressive symptoms and aBMD at the radius were identified either in adolescent boys or girls.(30) This contrasts with findings from a meta-analysis showing significant association between depressive symptoms and prospective bone loss at the hip and the lumbar spine, in adults.(31) In the current analysis, the cross-sectional associations between TBLH aBMC and MDD indices appear to be primarily driven by male participants, while the opposite is true for LS aBMD. In addition, only females showed an inverse association between MDD indices and polar section modulus at the radius. Overall, these findings suggest that sex may moderate which skeletal sites are affected by depression. We speculate that MDD may be associated with relatively lower bone mass at sites rich in trabecular bone (e.g., LS) or in non-weight-bearing bone in females but relatively lower weight-bearing cortical bone mass in males.(32) Of course, this hypothesis requires testing by measuring bone mass in the lower extremities.
Surprisingly, depression burden (as captured by the number of weeks meeting MDE criteria) was associated with increased bone mass at the lumber spine over time, both in male and female participants. Given that the models adjusted for vitamin D concentration and physical activity, it is unclear what other factors may account for this finding. It is likely not driven by anxiety because, when the model adjusted for both weeks in MDE and GAD concurrently, only the former remained significant. This finding carries two possible implications: First, the fact that, cross-sectionally but not longitudinally, MDD indices were associated with lower bone mass suggests that this finding may reflect genetic pleiotropy, whereby a gene variant is associated with seemingly distinct traits, that may or may not be causally linked.(33,34) Second, the positive association between MDD burden and increases in bone mass over time implies that more research is needed before MDD is established as a risk factor for bone loss.(6) Whether the association is moderated by age is important to examine given that most positive prospective studies have been conducted in the elderly.(31)
Bone cells express the serotonin transporter and several serotonin receptors.(10) In fact, SSRI-treated mice, as well as mice lacking the serotonin transporter gene, have been found to exhibit abnormal bone mass accrual.(10) Of particular interest is that fluoxetine has been recently shown to affect bone metabolism, similarly in adult male and female mice, via two distinct pathways: a peripheral anti-resorptive immediate effect and a central, delayed one, that stimulates sympathetic signaling, promoting bone resorption.(8) Over time, the latter effect overtakes the anti-resorptive one, resulting in net bone loss.(8) In humans, however, SSRIs appear to reduce cardiac sympathetic control in 18 to 65 year-olds.(35) Thus, it is unclear to what extent the recent pre-clinical findings apply to humans and to what extent they vary by age or sex, given that our participants were older adolescents and that SSRI use was associated with increased bone mass at the lumbar spine in our female participants but decreased bone mass in males. Similarly, although the effects were not significant for TBLH aBMC, perhaps due to the limited duration of the study, the estimates for males and females were again of opposite sign (Table 2). Of note, while the preclinical study used fluoxetine,(8) our participants received primarily one of four SSRIs (citalopram/escitalopram, fluoxetine, and sertraline). However, unlike the differential SSRI associations with adiposity we have reported elsewhere,(12) we found no significant differences in skeletal effects between individual SSRIs (data not shown).
GAD was significantly associated with several skeletal outcomes. However, all became non-significant when the models concurrently accounted for depression severity. The only exception was the longitudinal increase in TBLH aBMC Z-score which remained positively associated with the number of weeks meeting GAD criteria, even after adjusting for the number of weeks meeting MDE criteria. In the one prior study that examined the association between anxiety symptoms and bone mass in adolescent females, a similarly positive association was reported, in participants who smoked cigarettes.(27) It is unclear, however, if this finding would have persisted, after adjusting for depression severity. Examination of the independent effect of anxiety disorders on bone mineralization is needed given their high prevalence, their potential impact on sympathetic nervous system signaling, and their high comorbidity with depressive disorders. Of note, population-based studies have largely failed to show an association between depression and bone mass.(2,3) We propose that this may be due, at least in part, to the fact that the depression severity scales used in epidemiologic studies are not suited for distinguishing between depression and anxiety disorders. Not only are these disorders often comorbid, they may even affect bone metabolism in opposing ways, as suggested by our findings.
This study’s unique design has generated novel and somewhat unexpected findings that should be considered in light of its limitations. First, the study was powered to examine the effect of SSRIs on TBLH aBMC, trabecular vBMD, and bone formation. However, two of the primary hypotheses were not supported by the data. Instead, given the presence of significant differences for several outcomes, the findings were interpreted separately for male and female participants. To that effect, examining the actual estimates was needed, given that the lack of statistical significance for some of the outcomes in males may reflect lower statistical power rather than genuine sex differences. Second, multiple secondary analyses were conducted, potentially leading to spurious findings (i.e., type I errors). However, importantly, the various outcomes are correlated; thus, individual analyses should not be treated as independent tests, which led us to highlight the overall emerging picture as opposed to focusing on each test separately. Moreover, as we have argued elsewhere,(11) some apparent inconsistencies across MDD indices may actually reflect the accuracy with which the burden of disease is captured by each index as opposed to true inconsistency. Of course, given practical and ethical challenges, this long-term study was not randomized. Therefore, it is unknown what other confounders may underlie the findings. It is also unclear whether a more extended follow up would have altered the findings, given that 1.5 years of follow-up may not have been enough to observe a substantial and lasting effect of psychopathology or its treatment on bone mineralization. In addition, this study was not powered to examine sex differences. While it did highlight the most dramatic differences between male and female participants, it was likely underpowered to detect smaller differences. Similarly, although the normative data from the Bone Mineral Density in Childhood Study account for race,(20) a larger sample could have also allowed more detailed stratification of the analyses by race and ethnicity. Furthermore, in designing the study, we sought to balance the need for repeated measurements with safety concerns, given that both pQCT and DXA involve radiation, albeit minimal. It would have been useful to measure bone mass at the hip and tibia to examine other weight-bearing sites rich in trabecular and cortical bone. Finally, we sought to account for important confounders, including physical activity. Current methods to collect such information in real time may have improved validity.
Conclusion
In sum, in this prospective study of older adolescents and emerging adults, designed specifically to examine the association between depression, SSRI use and bone mass, a complex picture emerged, whereby depression was associated with low bone mass cross-sectionally but with greater, site-specific, bone mass accrual over time. In contrast, SSRI use was independently associated with a decrease in bone mass accrual in male participants and an increase in females, again in a site-specific manner. The reason for the sex differences we observed warrants further investigation. Future research should also explore mechanisms, as well as interventions to attenuate these treatment effects in males.
Supplementary Material
Flowchart detailing the variables addressed in the analyses. DSM: Diagnostic and Statistical Manual of Mental Disorders, A-LIFE: Longitudinal Interview Follow-up Evaluation for Adolescents, BDI: Beck Depression Inventory, IDS: Inventory for Depressive Symptomatology, BAI: Beck Anxiety Inventory, TBLH aBMC: Total body less head areal bone mineral content, vBMD: volumetric bone mineral density, LS aBMD: lumbar spine areal bone mineral density, CTX-1: C-terminal telopeptide.
Acknowledgments
This work was funded by the National Institute of Mental Health (R01MH090072) and the National Center for Research Resources (2UL1TR000442-06). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The authors would like to thank the participants and their families, as well as the research team.
Footnotes
Supplemental data have been included with the submission.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflict of interest to report.
Clinical Trial Registration: clinicaltrials.gov NCT02147184
Contributors’ Statement
Chadi A. Calarge: Dr. Calarge conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted.
James A. Mills: Mr. Mills conducted the statistical analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Babette S. Zemel, Kathleen F. Janz, Trudy L. Burns, Janet A. Schlechte, and William H. Coryell: These co-authors contributed to data analysis and interpretation, reviewed and revised the manuscript, and approved the final manuscript as submitted. Drs. Coryell and Schlechte also contributed to study design and data collection.
References
- 1.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Association; 2013. Fourth ed. [Google Scholar]
- 2.Schweiger JU, Schweiger U, Huppe M, Kahl KG, Greggersen W, Fassbinder E. Bone density and depressive disorder: a meta-analysis. Brain Behav. 2016 Aug;6(8):e00489. doi: 10.1002/brb3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yirmiya R, Bab I. Major depression is a risk factor for low bone mineral density: a meta-analysis. Biological psychiatry. Meta-Analysis Research Support, Non-U S Gov’t. 2009 Sep 1;66(5):423–32. doi: 10.1016/j.biopsych.2009.03.016. Epub 2009/05/19. [DOI] [PubMed] [Google Scholar]
- 4.Osteoporosis prevention, diagnosis, and therapy. Jama. 2001 Feb 14;285(6):785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 5.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016 Apr;27(4):1281–386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cizza G, Primma S, Csako G. Depression as a risk factor for osteoporosis. Trends in endocrinology and metabolism: TEM. Research Support, N.I.H., Intramural Review. 2009 Oct;20(8):367–73. doi: 10.1016/j.tem.2009.05.003. Epub 2009/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haney EM, Warden SJ, Bliziotes MM. Effects of selective serotonin reuptake inhibitors on bone health in adults: time for recommendations about screening, prevention and management? Bone. Research Support, N.I.H., Extramural Review. 2010 Jan;46(1):13–7. doi: 10.1016/j.bone.2009.07.083. Epub 2009/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortuno MJ, Robinson ST, Subramanyam P, Paone R, Huang YY, Guo XE, et al. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med. 2016 Oct;22(10):1170–9. doi: 10.1038/nm.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawson KS, Dixon D, Civitelli R, Peterson TR, Mulsant BH, Reynolds CF, 3rd, et al. Bone Turnover with Venlafaxine Treatment in Older Adults with Depression. J Am Geriatr Soc. 2017 May 26; doi: 10.1111/jgs.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warden SJ, Robling AG, Haney EM, Turner CH, Bliziotes MM. The emerging role of serotonin (5-hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5) Bone. Research Support, N.I.H., Extramural Review. 2010 Jan;46(1):4–12. doi: 10.1016/j.bone.2009.06.029. Epub 2009/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calarge CA, Butcher BD, Burns TL, Coryell WH, Schlechte JA, Zemel BS. Major depressive disorder and bone mass in adolescents and young adults. J Bone Miner Res. 2014 Oct;29(10):2230–7. doi: 10.1002/jbmr.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calarge CA, Mills JA, Janz KF, Burns TL, Coryell WH, Zemel BS. Body Composition in Adolescents during Treatment with Selective Serotonin Reuptake Inhibitors. Pediatrics. doi: 10.1542/peds.2016-3943. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996 May;26(3):477–86. doi: 10.1017/s0033291700035558. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. New York, NY: Harper & Row; 1967. [Google Scholar]
- 15.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988 Dec;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 16.Janz KF, Lutuchy EM, Wenthe P, Levy SM. Measuring activity in children and adolescents using self-report: PAQ-C and PAQ-A. Med Sci Sports Exerc. 2008 Apr;40(4):767–72. doi: 10.1249/MSS.0b013e3181620ed1. [DOI] [PubMed] [Google Scholar]
- 17.Association AP. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 18.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics Comparative Study. 2002 Jan;109(1):45–60. doi: 10.1542/peds.109.1.45. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 19.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. Comparative Study Research Support, N.I.H., Extramural. 2013 Jul;98(1):49–56. doi: 10.3945/ajcn.112.053611. Epub 2013/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. The Journal of clinical endocrinology and metabolism. Multicenter Study Research Support, N.I.H., Extramural. 2011 Oct;96(10):3160–9. doi: 10.1210/jc.2011-1111. Epub 2011/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer; 2000. [Google Scholar]
- 22.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–76. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 23.Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: Wiley; 2002. [Google Scholar]
- 24.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2011. [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1998. [Google Scholar]
- 26.Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A Practical Guide to Calculating Cohen’s f(2), a Measure of Local Effect Size, from PROC MIXED. Front Psychol. 2012;3:111. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorn LD, Pabst S, Sontag LM, Kalkwarf HJ, Hillman JB, Susman EJ. Bone mass, depressive, and anxiety symptoms in adolescent girls: variation by smoking and alcohol use. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. Research Support, N.I.H., Extramural. 2011 Nov;49(5):498–504. doi: 10.1016/j.jadohealth.2011.03.008. Epub 2011/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorn LD, Susman EJ, Pabst S, Huang B, Kalkwarf H, Grimes S. Association of depressive symptoms and anxiety with bone mass and density in ever-smoking and never-smoking adolescent girls. Archives of pediatrics & adolescent medicine. Comparative Study Research Support, N.I.H., Extramural. 2008 Dec;162(12):1181–8. doi: 10.1001/archpedi.162.12.1181. Epub 2008/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazeli PK, Mendes N, Russell M, Herzog DB, Klibanski A, Misra M. Bone density characteristics and major depressive disorder in adolescents. Psychosom Med. 2013 Feb;75(2):117–23. doi: 10.1097/PSY.0b013e3182821e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lourenco S, Lucas R, da Silva DF, Ramos E, Barros H. Depressive symptoms are not associated with forearm bone accrual during adolescence. Arch Osteoporos. 2014;9:173. doi: 10.1007/s11657-014-0173-4. [DOI] [PubMed] [Google Scholar]
- 31.Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG. Depression, fracture risk, and bone loss: a meta-analysis of cohort studies. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA Meta-Analysis Review. 2010 Oct;21(10):1627–35. doi: 10.1007/s00198-010-1181-x. Epub 2010/03/06. [DOI] [PubMed] [Google Scholar]
- 32.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008 Feb;23(2):205–14. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016 Jul;48(7):709–17. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013 Jul;14(7):483–95. doi: 10.1038/nrg3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Licht CM, Penninx BW, de Geus EJ. Effects of antidepressants, but not psychopathology, on cardiac sympathetic control: a longitudinal study. Neuropsychopharmacology. 2012 Oct;37(11):2487–95. doi: 10.1038/npp.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart detailing the variables addressed in the analyses. DSM: Diagnostic and Statistical Manual of Mental Disorders, A-LIFE: Longitudinal Interview Follow-up Evaluation for Adolescents, BDI: Beck Depression Inventory, IDS: Inventory for Depressive Symptomatology, BAI: Beck Anxiety Inventory, TBLH aBMC: Total body less head areal bone mineral content, vBMD: volumetric bone mineral density, LS aBMD: lumbar spine areal bone mineral density, CTX-1: C-terminal telopeptide.