Abstract
In aged mice, conventional naive B cells decrease and a new population of age-associated B cells (ABC)3 develops. When aged unprimed mice are infected with influenza virus, there is a reduced generation of helper CD4 T cell subsets and germinal center B cells, leading to limited production of IgG Ab and less generation of conventional long-lived plasma cells, compared to young. However, we find an enhanced non-follicular (GL7−) ABC response that is helper T cell-independent, but requires high viral dose and pathogen recognition pathways. The infection-induced ABC (iABC) include IAV-specific Ab-secreting cells, some of which relocate to the bone marrow and lung, and persist for >4 wk., suggesting they may provide significant protection. We also speculate there is a shift with increased age to dependence on TLR-mediated pathogen-recognition in both B and CD4 T cell responses.
Keywords: aging, immune response, B cell subsets, T-independent response, pathogen recognition, antibody production, antiviral response, influenza virus
Graphical Abstract
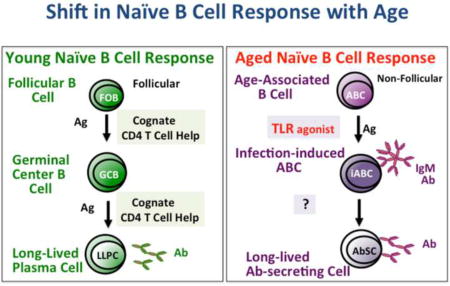
As animals age the follicular B cell response (FOB) (pictured on left), responsible for most persistent antibody (Ab) production from long-lived plasma cells (LLPC), is reduced due to diminished CD4 help that is required for the generation of the germinal center B cells (GCB) response and to intrinsic defects in the B cells. We speculate that in the aged, a new response occurs in addition to the residual the FOB response. In response to infection, age-associated B cells (ABC), triggered by the antigen (Ag) and toll like receptor (TLR) signaling develop into infection-induced effectors (iABC) and some of these effectors develop into long-lived antibody-secreting cells (LL-AbSC).
Introduction
As animals age, there are dramatic shifts in their immune response. These include a striking decrease in their Ab responses to vaccines and a heightened susceptibility to infections [1,2]. We and others have shown there is a dramatic decrease in the number and function of naive CD4 T cells with age [3,4] that leads to a reduced generation of helper T cell effectors [2,5]. As a consequence, there is also a reduced generation of germinal center B cell (GCB) responses, and generation of fewer isotype-switched Ab-secreting plasma cells (PC), long-lived PC (LLPC) [2,5–7] as well as reduced generation of memory B cells all of which are critical for effective immunity. The generation of memory CD4 T cells from aged naive cells is also severely compromised [8,9]. Thus many vaccines are ineffective in the elderly, leaving them highly vulnerable to newly emergent pathogens, such as SARS and to rapidly evolving viruses, such as influenza [2].
It has been previously established that the genesis of new B cells in the bone marrow wanes with age and there are fewer conventional naive B cells, including a marked reduction in follicular B (FOB) that make up the majority in the young (about 80% in spleen) [1]. With age, the non-follicular marginal zone B cells (MZB), which make an early IgM response, remain a small fraction of total (<10% in spleen) [10] and there are few B1 B cells in adult lymphoid organs [11].
Michael Cancro and collaborators were the first to point out the increase with age of another phenotypically distinct (CD23−/CD21/35−), resting population that they called "age-associated B cells" (ABC) [12,13]. Meanwhile, Philippa Marrack and her colleagues used the same name, ABC, to describe an activated/memory population that expresses CD11b or CD11c, requires TLR7 for generation and expresses high levels of the transcription factor T-bet and is associated with autoimmunity [14,15]. The relationship of different subpopulations of ABC, and their exact origin and function are not yet clearly understood.
Here we summarize studies in which we followed the response of a naive-like phenotype population from unimmunized aged mice, like that described originally by Cancro as ABC to influenza virus infection and, as will be discussed, we show that these ABC are the direct precursors of activated effector and Ab-secreting B cells we call iABC. We believe that it is critical to subdivide ABC into naive-like (ABC) and antigen (Ag)-induced (iABC) subsets to study their origin and function, and to compare them to presumed endogenously activated cells some of which have autoimmune potential. Furthermore, we suggest that the unique properties of ABC and iABC especially the requirements for iABC induction, argue they have a specialized role in immunity to pathogens in the aged, where FOB responses are compromised. We focus on the ability of ABC to respond independently of helper CD4 T cells and on their dependence on TLR signals and propose these are part of a wider shift to greater reliance on pathogen-associated danger signals with age.
Identification of a Novel B cell Response Following IAV Infection
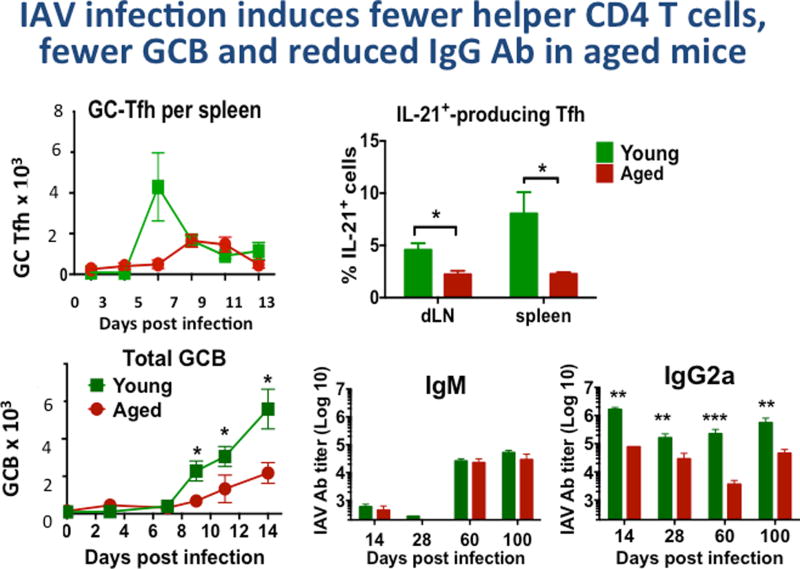
We chose a model of infection with influenza A virus (IAV) to probe age-associated changes in immunity since we knew live influenza A infection (IAV) in young mice, generates high levels of specific Ab, both short term and persisting, which provide strong protection against re-infection with the same strain of IAV [16]. In aged mice there is a reduced but substantial response to IAV infection (Figure 1) [2,4,16,17]. We compared the size and kinetics of CD4 T and B cell response to the live PR8 virus in young vs. aged mice. We found that in the aged, the generation helper T cells (Tfh, GC-Tfh, Th-producing IL-21) was reduced several-fold, as was generation of germinal center B cells (GCB) and both short and long-term isotype-switched antibody (Ab) to IAV (Figure 1). We show GC-Tfh and IL-21-producing CD4 effectors, Tfh were increased earlier and to higher levels in the young (not shown). In contrast, the production of IgM Ab was little affected even several months later (Figure 1 and Zhang. W, unpublished). This suggested that helper T cell dependent B cell response is selectively impaired.
Figure 1. IAV infection of Aged Mice induces fewer helper CD4 T cells, fewer GCB cell and reduced IgG Ab.
Young (2–3 mo.) and aged (20 mo.) B6 female mice, were infected with A/PR8/34 influenza (EID 500, corresponding to 0.2LD50 for young B6 mice) introduced by the intranasal route as in previous studies [29]. We determined generation of helper T cell subsets and germinal center B cells (GCB). GC-Tfh (CXCR5hi, Bcl6hi, GL-7+ CD4+ T cells by FACS) were enumerated in groups of mice harvested at 0, 3, 5, 7, 9, 11 and 13 days post infection. Potential helper CD4 T cells producing IL-21 were determined by intracellular cytokine staining [7] and are expressed as the % of total CD4 T cells.
We determined total GCB by FACS staining of CD19+ cells with labeled Ab to CXCR5, Bcl6 and GL-7. In a similar more extended kinetics experiment, serum was collected from young and aged mice at, 28, 60, 100 dpi and IgM, IgG1, IgG2a, IgG2b and IgG3 specific for PR8 were determined by isotype-specific ELISA as previously [16]. Significant differences between values for young and aged groups are indicated by * is ≤0.05, **≤0.01, ***≤0.005. TFH were also reduced in age, as were plasma cells in bone marrow and spleen at 40 and 100 days (not shown). Total IgG, IgG1 and IgG2b were also reduced significantly in the aged compared to young at each time point (not shown).
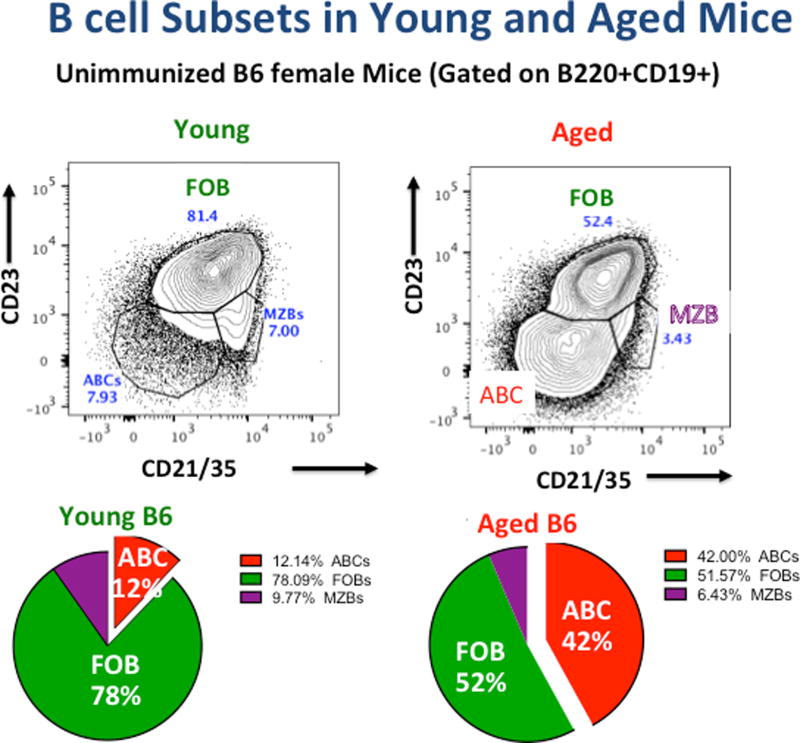
When we looked more carefully at the B cell subsets that developed in aged mice, we noted an enhanced population of CD23−/CD21−/35− B cells in the unimmunized aged mice, which we identified as the same ABC described by Cancro based on their similar distribution and phenotype [12]. The percentage of ABC increased progressively with age and by 18–20 months they represented a major population in female B6 mice (Figure 2) with slightly smaller population in male mice as previously reported [12]. After immunization of aged mice with live IAV, we noticed an unexpected age-associated population that increased following infection. In addition to expected GCB-phenotype Fashi/GL7+ B cells, there was a substantial population of Fashi/GL7− B cells, a phenotype rare in young immunized mice and pictured in Figure 3. We called these infection-induced ABC (iABC), because of their phenotype after IAV infection (see Figure 4) and we postulated they came from the ABC, as we show in Figure 5. The infection-induced generation of this putative Fas+/GL7− effector B cell population, increased progressively with age, was greater in females than males, and in B6 compared to BALB/c mice. They appeared in spleen, blood, lung and even bone marrow, but were low in draining lymph nodes (dLN) (Zhang. W et al, unpublished). The phenotype and characteristics of our IAV infection-generated iABC subset corresponded to the more activated ABC seen by Cancro after in vitro activation of freshly isolated ABC from aged normal mice [11] and by Marrack in autoimmune prone mice [12,13]. Our iABC were more numerous than the ABC population in the autoimmune-prone mice [13,14], and iABC in aged mice were equivalent in number to IAV-induced GCB cells in those mice (Figure 3, bottom). This led us to propose that IAV infection efficiently drove naive IAV-specific ABC in unimmunized aged mice to differentiate into antibody-secreting iABC, just as FOB are driven to become GCB, and that this ABC response might provide a substantial fraction of the IAV-specific antibody seen in response of aged mice. We set out to test this hypothesis.
Figure 2. Naive B cell Subsets in Young and Aged Mice.
Spleens of unimmunized young and aged B6 mice were analyzed by FACS as in our recent study [7] and CD19+/ B220+ cells were analyzed for expression of CD23 and CD21/35. FOB express high CD23 and CD21/35; MZB are CD23−/ CD21/35+ and ABC express neither. A representative young and aged analysis is shown. Similar patterns have been seen in all analyses (>10) in female B6 mice. Also shown are pie charts derived from the distribution of subsets in a recent group of 5 young and aged female B6 mice.
Figure 3. B cell subsets in young and aged IAV-infected mice.
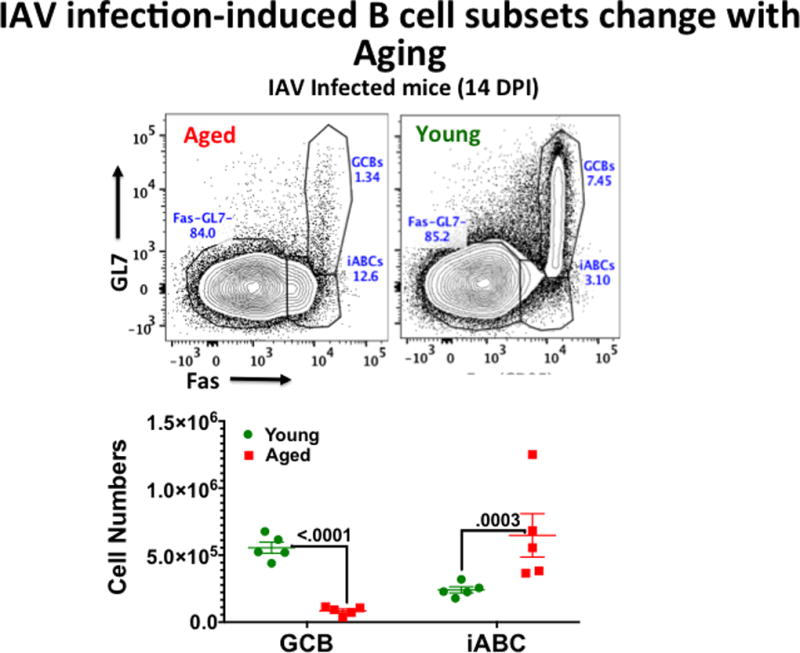
Young and Aged B6 were immunized as in Figure 1. At 14dpi, CD19+/B220 positive gated B cells were analyzed for expression of Fas and GL7. GL7hi, FAS+ cells are GCB and GL7−, Fas+ cells are influenza-induced ABC (iABC). Numbers of each in groups of 4 aged and young mice in one experiment are shown.
Figure 4. Phenotype of naive ABC and IAV-induced effector iABC.
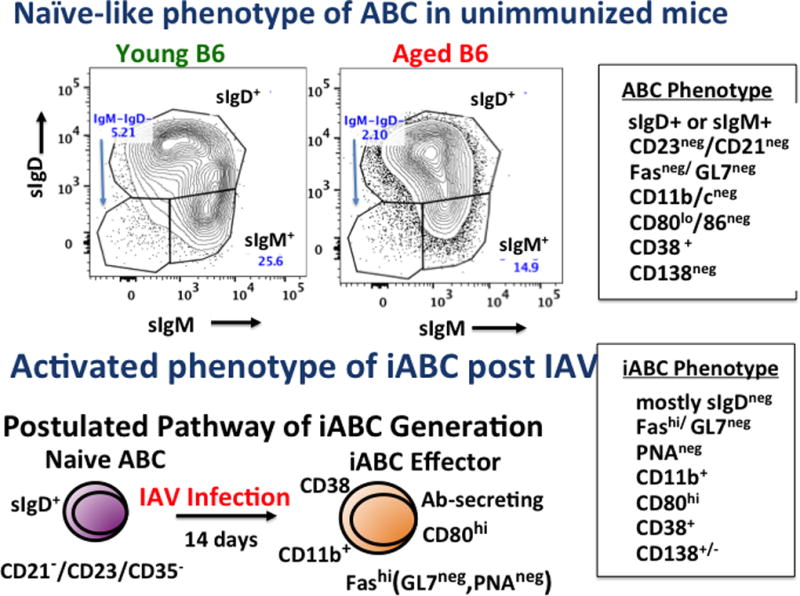
The boxes on the right show the phenotype of ABC (CD19+/B220+gated CD21−/CD23/35−) from unimmunized mice (above) and iABC (CD19+/B220+gated Fas+/GL7−), both from aged mice. This phenotype is representative of those seen in B6 and BALB/c female and male mice. The top FACS plots indicate the high fraction of ABC in naive cells are sIgD+. The bottom is the model we propose for the generation of iABC effector cells from naive ABC in unimmunized mice.
Figure 5. ABC precursors become iABC Ab-Secreting Effectors.
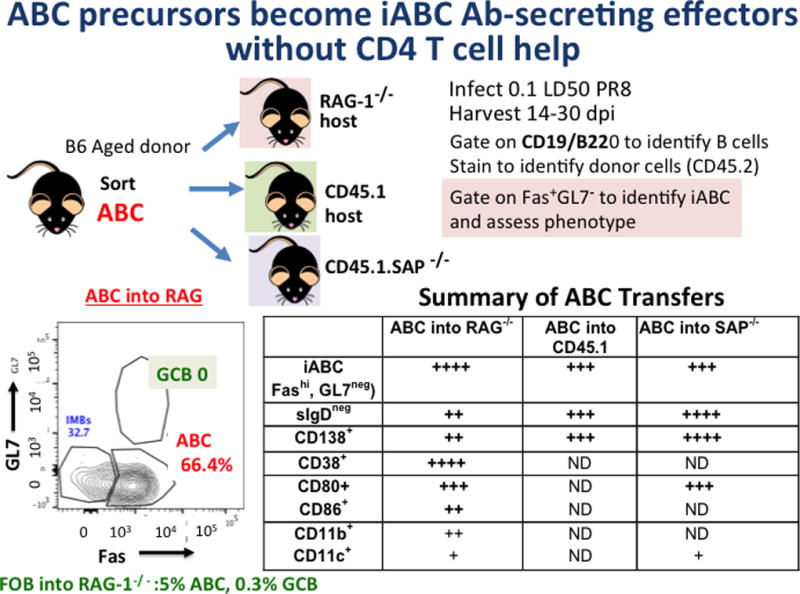
In a series of separate experiments FACS-sorted ABC (CD19+/B220+gated CD21−/CD23/35−) were isolated from aged B6 females (CD45.2) and between 2 and 4 × 106 ABC were transferred into congenic hosts, including RAG-1−/−, B6.CD45.1, B6.SAP/−, and CD45.1. The hosts were infected with 0.4LD50 PR8 IAV. Weight loss was monitored to ensure infection. After 14–30 dpi spleen and sometimes bone marrow and lung were harvested and FACS analysis was used to identify donor B cells (CD45.2+ CD19+, B220+) and determine their phenotype. Shown lower left is staining to identify iABC and GCB. Not all stains were done in each transfer as indicated by ND.
ABC In Unimmunized Mice Have a “Naive-like” B cell Phenotype
It is important to clarify the terminology of age-associated B cell subsets. In some recent studies Marrack’s group [12,14,15] has used the term ABC to describe the B cells that are defined by expression of high levels of T-bet as well as CD11c/CD11b expression[14] both of which imply they are activated or perhaps memory cells. In contrast the original ABC described by Cancro, were identified by their lack of CD21 and CD23 [13] and had a naive-like phenotype. We provide evidence below that indicates that indeed the bulk of the initial ABC in the unimmunized mice have a “naive-like” phenotype and after IAV infection they are the precursors of the more differentiated and/or activated iABC cells. We speculate that the T-bethi B cells seen in the extensive studies of Marrack and their colleagues are activated or memory cells. To indicate these subsets are distinct, we use ABC to connote the original “naive-like” phenotype cells in unimmunized mice and the term iABC to refer to those effector B cells generated by infection with IAV, but please bear in mind this is not the convention followed by others.
We first asked if ABC from unimmunized aged mice were naive B cells. We found that most splenic CD19+/B220+ ABC (defined by non-expression of CD23 and CD21/CD35) express a phenotype consistent with naive B cells in both young and aged unimmunized mice (Figure 4, box, upper right) (Zhang W and Kugler-Umana, unpublished). They are small, non-dividing cells, and most express sIgD either alone or with IgM, which is consistent with naive B cells. They do not express Fas, which is high on effector B cells and autoimmune B cells. Furthermore, ABC lack GL7 expression and do not bind PNA, molecules associated with follicular location, further distinguishing them from GCB. ABC from conventional unimmunized mice express low levels of costimulatory receptors CD80 and CD86 needed for Ag-presentation, and of adhesion receptors CD11b or CD11c involved in migration. Importantly, they do not express the canonical marker of Ab-secreting cells CD138 (Figure 4 box, upper right). This is consistent with the phenotype described by Cancro’s group [13] and contrasts with that of the cells called ABC in autoimmune-prone mice by Marrack’s group, that we interpret as having a more activated/memory phenotype, and that are defined by their expression of CD11c and to some extent CD11b and sometimes T-bet and CD86 [12,14,15]. Such activation marker-expressing cells were rare in our unimmunized ABC population.
Next, we have characterized in detail the phenotype of the CD19+/B220+ Fashi/GL7− IAV-induced population of iABC, depicted in Figure 3, which is not present in unimmunized mice. The box on the bottom right of Figure 4, indicates the corresponding phenotype. The iABC, mostly analyzed at 13–14 days post infection (dpi) with sublethal doses of IAV, differ from GCB (not shown) in their lack of GL7 and PNA, indicating they are non-follicular, but they otherwise have a differentiated effector B cell phenotype. Most have lost sIgD, and many express CD11b and CD80. A smaller fraction expresses CD11c and CD86, consistent with an effector phenotype. Most GCB in young mice CD38, but both iABC and GCB in aged mice are mixed for CD38 expression. Importantly, a substantial fraction that increases with time after infection up to 20 dpi or so, are CD138 positive. As expected for effectors responding to infection, iABC are found in the lung, which is the site of infection and replication of IAV, but also in the bone marrow (BM) and peripheral blood (our unpublished results).
Thus, we suspected that naive-like ABC respond to infection with IAV by becoming iABC effectors that include Ab-secreting cells (AbSC), as depicted on the bottom of Figure 4, and we set out to rigorously test this.
ABC are precursors of IAV-infection induced iABC
We carried out several transfer experiments to test our hypothesis that most of the effector iABC develop from naive ABC following IAV infection. We have summarized key studies in Figure 5. We transferred FACS-sorted ABC (CD19+, B220+CD23−/CD21/35−) from unimmunized aged (18–20 months) B6 mice into young RAG-1 deficient, intact CD45.1 disparate hosts, or recently into hosts deficient in SLAM-associated protein (SAP), which negates the ability of CD4 T cells to become TFH. In each case hosts were immediately immunized with IAV, and we followed the development of donor B cell (CD19+/B220+) into effectors and analyzed their phenotype to determine if they were iABC. In all cases, the donor ABC transfer generated a substantial population of effector B cells in host spleens (>105) with lower numbers in lung and bone marrow. These expressed the phenotype of iABC, including high expression of Fas with low GL7, the signature for the iABC. Most of the donor cell effectors had lost expression of sIgD and had become CD38+. A substantial subpopulation of donor effectors expressed CD138, indicating they were Ab-secreting cells. In RAG-1−/− mice, lacking T, B and NKT cells, where their derivation from ABC is most definitive, most of the donor effectors expressed upregulated CD80 and CD11b and some expressed modest increases in CD11c and CD86, indicating there was considerable heterogeneity of among the effector B cells. In these RAG hosts, the ABC precursors did not give rise to any GCB. The key phenotypic markers of effector B cells, loss of sIgD, expression of Fas and transition to CD138, Ab-secreting cells were seen in all the hosts. Since the CD45.1 hosts are wildtype except for the allelic difference in CD45, and the SAP−/− hosts also have a normal distribution of lymphocytes, we conclude the response is not dependent hosts being lymphopenic and that conversion to iABC is not substantially altered by the absence of T cells, at least over the 1st two wk of response. These results strongly support the model that Ag-specific cohorts of naive ABC become iABC after exposure to IAV virus. It is striking that the iABC population derived from IAV infection is substantial (Figure 2).
Importantly, these transfers also indicate that the generation of initial iABC (mostly the responses were assessed at 14 dpi) is independent of CD4 T cell help since it occurs in both RAG-1−/− and of follicular T cell help since it occurs in SAP−/− hosts. Further experiments in intact aged B6 hosts depleted of T cells with anti-Thy1.2, support the same conclusion, as injection of IAV led to no impairment of the generation of iABC, as defined here, but a major reduction in GCB (our unpublished results). We have yet to determine whether the generation of long-lived cells has any T-dependency, since we could not follow the responses in RAG-1−/− hosts much past 21 dpi, presumably because their lack of T and NKT cell-mediated immunity led to reduced protection. Studies to resolve this are in progress.
For comparison, we also transferred FOB from the unimmunized mice to the help-deficient mice (RAG-1−/− and SAP−/−) hosts. The FOB did not give rise to significant effectors, as expected because that depends on GC-TFH, which are absent in both these hosts [18,19]. In transfers to intact CD45.1 mice, the FOB gave rise to a population of GCB that was equivalent in size to the iABC population (Figure 5). They did not yield iABC. These experiments are critical because they provide strong evidence that in unimmunized mice, without any signs of autoimmunity, the ABC are the major precursors of iABC that develop due to IAV infection. Since these are short-term experiments they do not rule out other pathways to effector ABC that could require a longer timeframe nor do they rule out the presence of some memory ABC due to previous antigen encounters. The fact that iABC develop in intact CD45 disparate, as well as SAP−/− hosts, indicates they can respond in environments that do not favor abnormal homeostatic proliferation. The T-independence of iABC generation contrasts with what is known for FOB development into GCB, which is strictly CD4 helper dependent even following viral infection, as indicated by its loss in SAP−/− mice as reported previously [16,19].
Role of TLR stimulation in the Generation of Ab-secreting ABC
There is mounting evidence that generation of effector iABC in our IAV infection model depends on stimulation by pathogen-associated molecular patterns (PAMP). We see more efficient generation of iABC when we use live IAV compared to inactivated IAV, and more iABC when we use doses of virus that are higher than the low doses that optimally generate GCB (Kugler-Umana, unpublished). This has poised a technical challenge in the RAG-1−/− deficient hosts because many of the hosts succumb as early as 14–21 dpi to the dose necessary for strong iABC. Thus, we have not looked at late times in the RAG-1−/− hosts. We plan to re-address the issue of virus dose in intact hosts.
We also find that naive ABC express upregulated levels of multiple TLR indicating their readiness to respond to TLR-signaling (Zhang, unpublished). We have examined the generation of iABC in aged WT vs. TLR-7−/− BALB/c mice. We found that few iABC develop in the TLR7 deficient mice (Kugler-Umana, unpublished. These observations suggest that the response to IAV is dependent on both IAV Ag and IAV induction of PRR pathways. This is consistent with the requirements for TLR triggering to generate activated ABC reported by Cancro [13] and Marrack groups [14]. It is not clear whether the TLR agonists act directly on the ABC or through an indirect mechanism or both. In Cancro’s in vitro study TLR agonist stimulation was required for in vitro proliferation of ABC from unimmunized mice [13] and more recent studies by Rubtsov and Marrack that indicate the generation of ABC is dependent on TLR agonists in their autoimmune models [14,20].
What is the relationship between ABC reported in the different models?
Over the last several years Marrack and Cancro have reported that high expression of T-bet is a signature of CD11chi ABC in autoimmune prone mice [14,20] and have delineated a pathway in which TLR triggering of B cells and their response to IFNγ leads to T-bet expression that in turn induces the high levels of CD11c and CD11b and CD80/86 [12]. They have also presented evidence that suggests T-bet is essential for development B cells that are effective in antiviral responses [15] and autoimmunity [14] as well as required for generation of ABC [20]. However, we note that T-bet expression is also required for differentiation of conventional B cell effectors, especially those generated by interaction with Th1 cells and termed Be1 by Lund [21], that secrete IFNγ and play a key role in IgG2a protective Ab production in chronic infections [21,22] and T-bet also plays roles in other immune cells of different types including Th1 CD4 effectors [23]. Thus T-bet seems to be a common feature of multiple effector B cell subsets, not just activated ABC.
To explore the role of T-bethi expression and our iABC that develop as a result of influenza infection [15], we compared the expression of two key transcription factors T-bet and Bcl6 in IAV-induced iABC vs. GCB and to the naive precursors of each (ABC and FOB from unimmunized mice). In our preliminary experiment the initial ABC and FOB in both young and aged B6 mice expressed the same low level of T-bet, consistent with a naive phenotype. After infection, neither GCB of aged or young mice significantly upregulated T-bet, but a fraction of the iABC expressed elevated T-bet. GCB, as expected strongly upregulated Bcl6 a canonical marker of GCB derived from FOB, while iABC did not upregulate Bcl6 (Kugler-Umana et al., unpublished). It will be very interesting to further determine if the iABC or GCB upregulate other key B cell-differentiation associated transcription factors (TF) and if TF upregulation correlates with highest expression in CD80 and CD11b and with Ab-secreting (CD138+) fraction of infection-induced iABC. We conclude T-bet is likely to drive effector programs in multiple B and T cell subsets, including some iABC.
Overall, the ABC we find in unimmunized aged mice have a phenotype and function that mirrors those reported originally by Cancro and colleagues [14]. The fact that most are sIgD+ and do not express activation markers, suggests they a large fraction of ABC are resting cells that are either naive or have limited differentiation from naive cells. On the other hand, the iABC we generate following IAV infection, share multiple key features with the autoimmune-generated ABC of the Marrack group [14,20], to ABC generated by other infections [20] and to ABC generated by in vitro stimulation with TLR and anti-BcR [13,24]. The similarities include non-follicular distribution of the subset, dependence on TLR stimulation, and expression of upregulated CD11b or CD11c and of CD80 and /or CD86. We have used high Fas expression as a signature to define the iABC induced by IAV. Production of a unique cytokine profile including TNF described by Riley and colleagues [9] is another possible common feature of effector or memory ABC, and our iABC make upregulated RNA for TNF and for IL-10. We plan further studies to correlate cytokine production and other phenotypes of iABC. Based on these similarities, we favor the hypothesis that naive-like ABC in unimmunized mice give rise to distinct but related subsets of activated effector ABC, most likely depending on the precise Ag and pathogen recognition pathways that induce them. We present a possible model in Figure 6. We further speculate that some ABC-phenotype B cells in unimmunized mice may represent memory cells generated by previous encounter with exogenous or endogenous Ag, a hypothesis we will explore further in transfer models to determine signatures of a “memory-phenotype” ABC.
Figure 6. Hypothesis: A Spectrum of Age-Associated B Cells.
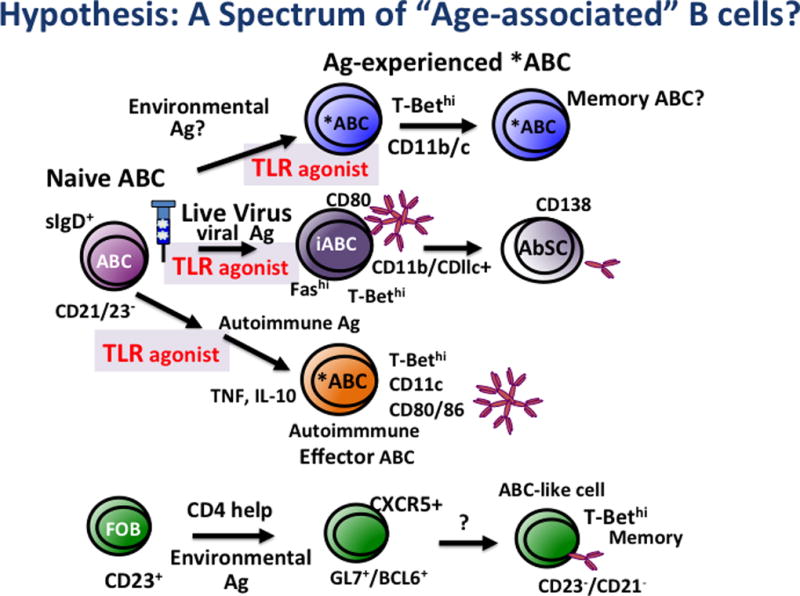
Represents the possible origin of various naive ABC and Ag-induced iABC.
Cancro reported that a small subset of young CD23+-enriched FOB cells transferred to adoptive hosts underwent multiple divisions over months, dependent on Class II and CD154 expression [12,25]. A fraction of the resulting population was highly-divided, expressed T-bet at enhanced levels and was also CD21−/CD23−. They suggested, based on these results that these T-bethi cells were ABC and concluded that ABC in unimmunized mice are derived from FOB [13,25] through "environmental antigen exposure" and "T-dependent mechanisms" and that ABC thus represent a memory B cell population. Although the population was small, they argued that over time it would accumulate. However, the low frequency of this population calls into question the conclusion that this is the major pathway for ABC generation. We suggest that in unimmunized aged mice, the CD23−/CD21/35− population may be a mix of predominantly naive sIgD+ cells (naive ABC) and a smaller subset of resting or activated “innate memory”-like B cells with a memory phenotype. We suggest care needs to be taken to better define the naive vs memory ABC and that they should be considered separately in studies of their potential function and of pathways of ABC development.
In recently published studies, Gearhart and Cancro addressed the question of the lineage of ABC by analyzing BcR diversity and the frequency of mutations, among subsets of B cells in unimmunized aged mice [25]. They found that all three putatively “naive” B cell subsets they analyzed, ABC (CD21−/CD23−) FOB and MZB, expressed similar, diverse VH heavy chain and VK light chains. They did find a several-fold increase of mutations in these chains in sorted ABC compared to FOB and MZB, and upregulation of genes associated with activation/memory in ABC (CD23−, CD21/35−) from aged mice and some in IAV-primed mice long after infection. They again argued from this that ABC are memory cells derived from innate Ag-stimulated division of FOB. However, comparison of mutation rates in a known population of memory B cell had a considerably greater mutation rate than they found in ABC. We would argue the presence of a population of memory ABC in addition to more naive-like ABC could explain the increase in mutation rate and reduction of ABC in aged CD154 deficient mice. Obviously further analysis of the precursors and pathways of subsets of effector ABC generated in the different models is needed to further elucidate their pathways of development and functional abilities, and further identify distinguishing properties [20,26].
Does the Response of ABC Provide Protective Immunity in the Aged?
Our results indicate ABC respond to viral Ag, expand and differentiate into effector Ab-secreting iABC by a pathway that is suited to respond to infection. In unimmunized mice, IAV infection provides high, sustained Ag due to viral replication to high titers in the lung before clearance at about day 10. Virus also provides equally sustained viral RNA to interact with viral-sensing TLR3, 7 and 9, and other PRR not yet evaluated. The ABC described in unimmunized aged mice by Cancro [13,24] and studied for their functional potential by our group, accumulate progressively with age such that they become almost as numerous as FOB in female B6 mice and represent a major subset in BALB/c mice (Figure 1). Moreover, IAV-specific iABC are a major component of the B cell effector response in aged mice (Figure 3). Studies in several infections are compatible with a role for T-bethi ABC in protection against pathogenic viruses. Hence it seems likely iABC play a protective role after infection and we may well see synergy between memory CD4 T cells and the primed iABC in protection (27,28).
We examined the level of IAV specific antibody produced exclusively by iABC after transfer of ABC to RAG-1−/− hosts, which have no B cells. Figure 7 indicates the levels of IAV-specific IgM and IgG recovered in serum up to 25 days post transfer and infection. There was a substantial level of IAV-specific IgM produced by the ABC population responding to IAV infection. Unfortunately, the RAG-1−/− hosts without CD4 T cells, succumbed to the infection, so we were unable to evaluate later time points, so we have not yet established that iABC and the Ab they produce are protective. We are currently using alternate hosts in the transfer models to evaluate Ab produced by the CD138+ Ab-secreting cell which we find develops later in bone marrow and spleen and which also may include more IgG–switched cells. We are also using revised transfer models that contain CD4 T cells, so as to provide the CD4 response that can synergy with Ab to combat IAV to address possible protective roles of ABC-produced Ab [27,28].
Figure 7. iABC make IAV-specific IgM Ab in RAG-1−/− Hosts.
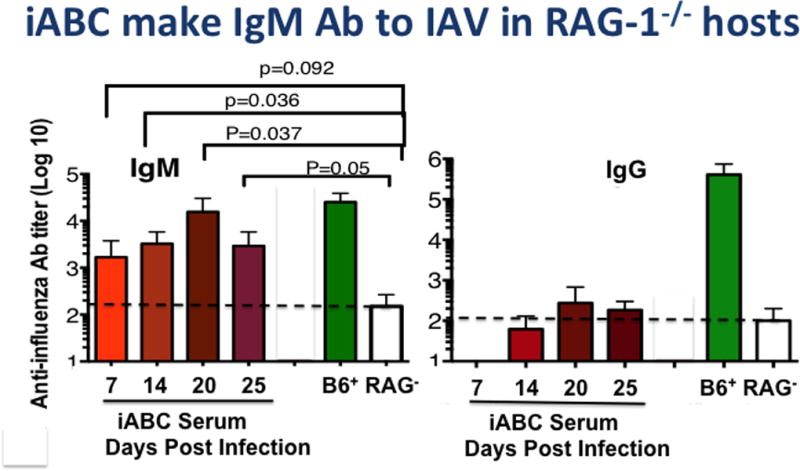
Sorted ABC from aged B6 female mice (106) were transferred to Rag-1−/− hosts, which were then infected with PR8 as in Figure 5. At 7, 14, 20, and 25 dpi sera were collected. ELISA assays of the IgM and IgG anti-PR8 titers in these sera (red), in pooled control serum from young infected B6 mice (>21 dpi, green) and from unimmunized RAG-1−/− (clear) are shown.
An Age-Associated Shift to Greater Dependence on Viral-Sensing Pathways
To us, one of the most intriguing observations is that the development of a substantial ABC population that requires recognition of pathogen via viral sensing pathways, indicates a shift in the requirements for inducing aged, as compared to young, naive B cell responses. This is seen in both ABC and FOB responses (Figure 8). First, in young mice, the response is dominated by FOB cells, which are highly dependent on CD4 T cell help, but not as dependent on PRR stimulation. With advanced age, the number of FOB are diminished and, as in the young, and their response is dependent on CD4 T cell help. Our recent studies indicate that the generation of aged CD4 help is more dependent on PRR stimulation, in that the APC that initiate the CD4 their response need to be highly activated. Since the CD4 helper response is more PRR dependent, GCB generation is indirectly more dependent on pathogen recognition [7]. Second ABC, as described here, become a major component of the primary B cell response and they are highly dependent on direct PRR stimulation. This raises the possibility that with age there is a programmed shift in response that facilitates those responses associated with activation of PAMP and reduces those to inert antigens. We postulate this may indicate a programmed shift selected by evolution to restrain responses to non-pathogens, responses that are less crucial to survival and perhaps cause un-needed inflammation and immunopathology, while retaining ability to respond to dangerous pathogens.
Figure 8. Response of Aged Unimmunized B Cells to IAV infection: Shift to TLR Dependence.
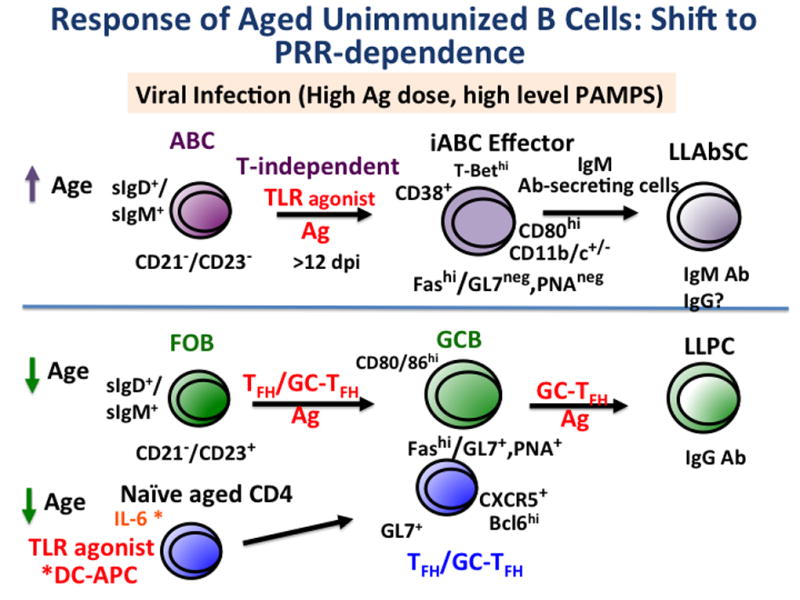
We depict the major pathways used by aged B cell subsets in a response to low dose sub-lethal IAV. As noted in Figures 2 and 3, two naive-phenotype cells predominate in unimmunized mice, ABC and FOB. The ABC response on top half of figure, increases with age. It requires Ag and also TLR agonists for substantial response, but they do not require CD4 T cell help to become effector iABC and Ab-secreting cells as shown in Figure 5. In contrast, the FOB response decreases with age, and remains helper T cell dependent, while generation of T helper cells is reduced (Figure 2). Our recently published studies indicate the FOB response to inactivated influenza vaccine, can be markedly enhanced when the aged naive CD4 receive their cognate signals by TLR-agonist treated DC (*DC-APC) [7]. Thus both the ABC and FOB responses in the aged are much more dependent on pathogen-associated danger signals, which in the case of IAV.
Summary.
While there is still much to learn about their origin, their function and how they are best induced, studies of the naive ABC response to influenza infection indicate a unique T helper independent pathway that results in robust generation of Ab-secreting cells. The studies point to the conclusion that aged naive B cell responses, require both Ag recognition and stimulation of pathogen recognition pathways, favoring naive B cell responses to dangerous pathogens and reducing responses to more inert antigens.
Highlights.
Aged naive CD4 T cells make fewer helpers producing IL-21 and TFH and GC-TFH
Naive B cell response in aged results in fewer GCB and IgG Ab-secreting cell
With age a new age-associated B cell population (ABC) generates Ab-secreting cells
The ABC response is T-independent but depends on TLR stimulation and Ag
In the aged both B and CD4 T cell responses require higher levels of PAMPS
Acknowledgments
We thank Richard W. Dutton for discussion and for help editing of the manuscript.
Funding: This work has been funded by the National Institutes of Health [R37AG025805 to S. Swain, P01AG021600 to L. Haynes and IMSD grant 1R25GM113686 to University of Massachusetts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: ABC: Age-associated B cells; Ab: antibody, AbSC: Ab-secreting cells, Ag: antigen, APC: antigen-presenting cells, dpi: days post infection, FOB: follicular B cells GC: germinal center, iABC: infection-induced ABC, IAV: influenza A virus; Ig: immunoglobulin, PAMP: pathogen-associated molecular pattern; PRR: pathogen recognition receptor, PC: plasma cells, LLPC: long-lived PC, TLR: toll-like receptor,
References
- 1.Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: Composition and function. Biogerontology. 2010;11:125–137. doi: 10.1007/s10522-009-9256-9. [DOI] [PubMed] [Google Scholar]
- 2.McElhaney JE, Kuchel GA, Zhou X, Swain SL, Haynes L. T-cell immunity to influenza in older adults: A pathophysiological framework for development of more effective vaccines. Front. Immunol. 2016;7:41. doi: 10.3389/fimmu.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol. Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 4.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8447–52. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Brahmakshatriya V, Swain SL. CD4 T cell defects in the aged: Causes, consequences and strategies to circumvent. Exp. Gerontol. 2014;54:67–70. doi: 10.1016/j.exger.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton SM, Maue AC, Swain SL, Haynes L. Bone marrow precursor cells from aged mice generate CD4 T cells that function well in primary and memory responses. J. Immunol. 2008;181:4825–4831. doi: 10.4049/jimmunol.181.7.4825. 181/7/4825 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmakshatriya V, Kuang Y, Devarajan P, Xia J, Zhang W, Vong AM, Swain SL. IL-6 Production by TLR-Activated APC Broadly Enhances Aged Cognate CD4 Helper and B Cell Antibody Responses In Vivo. J. Immunol. 2017;198 doi: 10.4049/jimmunol.1601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci. Rep. 2016;6:25051. doi: 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholz JL, Diaz A, Riley RL, Cancro MP, Frasca D. A comparative review of aging and B cell function in mice and humans. Curr. Opin. Immunol. 2013;25:504–510. doi: 10.1016/j.coi.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol. 2011;186:3441–3451. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothaeusler K, Baumgarth N. B-cell fate decisions following influenza virus infection. Eur. J. Immunol. 2010;40:366–377. doi: 10.1002/eji.200939798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, Bengsch B, Linderman SL, Stelekati E, Spolski R, Wherry EJ, Hunter C, Hensley SE, Leonard WJ, Cancro MP. Cutting Edge: IL-4, IL-21, and IFN- Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J. Immunol. 2016;197:1023–1028. doi: 10.4049/jimmunol.1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013;110:E3216–24. doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamperschroer C, Dibble JP, Meents DL, Schwartzberg PL, Swain SL. SAP Is Required for Th Cell Function and for Immunity to Influenza. J. Immunol. 2006;177 doi: 10.4049/jimmunol.177.8.5317. [DOI] [PubMed] [Google Scholar]
- 17.Rojek JM, Perez M, Kunz S. Cellular entry of lymphocytic choriomeningitis virus. J. Virol. 2008;82:1505–17. doi: 10.1128/JVI.01331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamperschroer C, Roberts DM, Zhang Y, Weng NP, Swain SL. SAP enables T cells to help B cells by a mechanism distinct from Th cell programming or CD40 ligand regulation. J Immunol. 2008;181:3994–4003. doi: 10.4049/jimmunol.181.6.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–7. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 20.Rubtsova K, Rubtsov AV, Cancro MP, Marrack P. Age-Associated B Cells: A T-bet- Dependent Effector with Roles in Protective and Pathogenic Immunity. J. Immunol. 2015;195:1933–1937. doi: 10.4049/jimmunol.1501209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. Regulation of IFN-gamma production by B effector 1 cells: essential roles for T-bet and the IFN-gamma receptor. J. Immunol. 2005;174:6781–6790. doi: 10.4049/jimmunol.174.11.6781. 174/11/6781 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Barnett BE, Staupe RP, Odorizzi PM, Palko O, Tomov VT, Mahan AE, Gunn B, Chen D, Paley MA, Alter G, Reiner SL, Lauer GM, Teijaro JR, Wherry EJ. Cutting Edge: B Cell-Intrinsic T-bet Expression Is Required To Control Chronic Viral Infection. J. Immunol. 2016;197 doi: 10.4049/jimmunol.1500368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann C, Bonilla WV, Fröhlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, Löhning M. T-bet– and STAT4–dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc. Natl. Acad. Sci. 2015;112:201418549. doi: 10.1073/pnas.1418549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naradikian MS, Hao Y, Cancro MP. Age-associated B cells: Key mediators of both protective and autoreactive humoral responses. Immunol. Rev. 2016;269:118–129. doi: 10.1111/imr.12380. [DOI] [PubMed] [Google Scholar]
- 25.Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, Gearhart PJ. Age-Associated B Cells Express a Diverse Repertoire of VH and VK Genes with Somatic Hypermutation. J. Immunol. 2017:1601106. doi: 10.4049/jimmunol.1601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–11. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4 + T cells protect against influenza through multiple synergizing mechanisms. J. Clin. Invest. 2012;122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. 177/5/2888 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Román E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]