Abstract
Objective
To demonstrate how maladaptive emotion regulation (ER) lead to diabetes distress (DD), with subsequent effects on management and metabolic outcomes among adults with type 1 diabetes.
Methods
Data are based on pre-intervention assessment for a random controlled trial to reduce DD. Patients were recruited in California, Oregon, Arizona and Ontario, Canada. After screening and consent, patients completed an online assessment and released their most recent laboratory HbA1C. Structural equation modeling was used to define an ER measurement model and test for significant pathways.
Results
Three ER mechanisms combined into a single construct: emotion processing, non-judgment of emotions, non-reactivity to emotions. Models indicated a significant pathway from ER and cognitions to DD to disease management to metabolic control.
Conclusions
As hypothesized, the three ER mechanisms formed a single, coherent ER construct. Patients with poor ER reported high DD; and high DD was linked to poor diabetes management and poor metabolic control.
Practice implications
Identifying both the level of DD and the ER mechanisms that lead to high DD should be explored in clinical settings. Helping T1Ds to become more aware, less judgmental and less reactive behaviorally to what they feel about diabetes and its management may reduce DD.
Keywords: diabetes, emotion management, diabetes distress
1. Introduction
Diabetes distress (DD) refers to the ongoing worries, fears and burdens associated with managing a chronic disease like diabetes over time [1]. It is significantly associated with poor disease management and poor glycemic outcomes, it tends to be chronic as opposed to episodic, and its point-prevalence among both type 1 (T1D) and type 2 adults is approximately 40% [2–5]. DD, therefore, remains a significant clinical concern.
Despite traditional approaches through education and behavior change [6], relatively little is known about how best to intervene clinically to address DD, in part because there is little understanding of the underlying emotional mechanisms that lead to the development and chronicity of DD. For example, why are some patients able to modulate the emotional effects of ongoing disease-related burdens, fears of complications and worries about health care from affecting their quality of life and disease management, whereas others with similar life circumstances and similar disease management demands find themselves emotionally distressed and burdened [7]? Knowing more about what leads to these differences can assist in the development of new, novel and informed interventions.
This report addresses this question in two ways. First, we identify potential mechanisms or emotion management from the literature and determine empirically if they can be combined into an underlying latent construct or unitary emotion regulation (ER) composite. Second, we test a framework that outlines the pathways through which this ER construct is linked to DD, and how DD relates to diabetes self-management and to glycemic outcomes. These analyses are important for two major reasons. First, describing the relationship between ER and DD will enable clinicians to identify patients at risk for DD over time. Second, identifying these mechanisms and their pathways of operation will permit the development of targeted interventions that address ER directly to prevent or reduce DD among adults with diabetes.
1.1 Emotion Regulation And Diabetes Distress
Research indicates that people do not passively experience their emotions. Instead, they actively try to modulate or regulate them [8]. For example, an adaptive response to a diagnosis of a diabetes complication might be to mobilize physical and cognitive resources. A maladaptive response, however, might be to become paralyzed, ruminative, or impulsive [9]. “Emotion regulation” is a term used to capture the range of both conscious (top-down) and automatic (bottom-up) mechanisms that people use to manage the emotional reactions they experience in response to threats, burdens, and fears. There are a host of definitions of ER tied to different theoretical approaches [10]; however, we define ER as efforts made implicitly or explicitly to influence the experience, expression and effects of emotions [11].
A long and complex research literature has evolved that describes the primary adaptive and maladaptive aspects of ER [10]. An overview of this literature suggests that maladaptive ER can lead to chronic negative affect, as in DD. This negative affect narrows cognitions, reduces creative thinking, and promotes negative self-judgments [9]. Thus, an escalating cycle occurs over time to up-regulate negative affect and down-regulate positive affect.
ER is considered distinct from personality and use of specific ER mechanisms within individuals tends to be stable over time [10, 12, 13]. Furthermore, ER interacts directly with cognitive processes, such that the mood that results as a function of ER influences subsequent perceptions and judgments, and affects selective attention and cognitive flexibility. These “mood congruent biases” also affect information processing, problem solving, and the interpretation and meaning of newly received information [7]. Thus, cognitive processes and ER mechanisms are jointly linked with DD. Interestingly, the ER-mood relationship is directional: ER affects mood, but not vice versa [14].
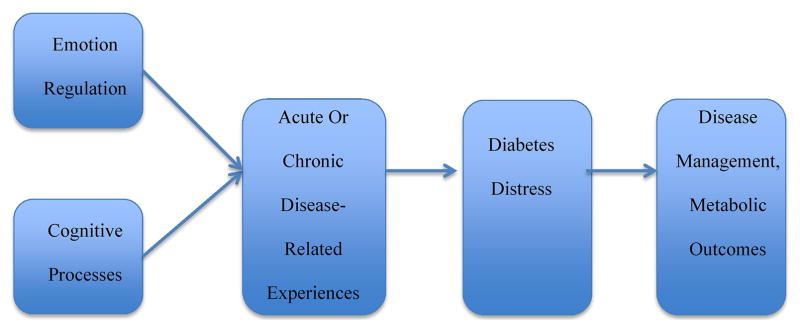
The effects of ER on health and well-being have been extensively documented [10, 14, 15]. In diabetes, maladaptive ER has been linked to less frequent blood glucose monitoring, more hypoglycemic episodes, elevated HbA1C, and more problematic self-care behaviors [13, 16]. The implicit top-down or bottom-up regulatory choices one makes over time, combined with cognitive processes, directly affects the experience of emotional burden (DD), which, in turn, has a direct impact on disease management and glycemic outcomes [16]. We outline this framework graphically in Figure 1.
Figure 1.
An emotion regulation – diabetes distress framework.
1.2 Which ER Processes Are Most Related To DD?
A number of studies have documented specific ER mechanisms that are linked to the emergence of negative affect (high DD) and poor disease management behavior. These include: lack of self-compassion (not treating the self with understanding and delivering harsh self-criticism), lack of mindfulness (not accepting events as they are and then over- or underreacting), viewing events as a reflection of self-worth, lack of self-empathy, unawareness, lack of clarity and low tolerance of emotion, unwillingness to engage with emotion (impulsive reactions), rumination or suppression of emotion, and a narrow repertoire of responses to those circumstances that caused the emotion [14, 17–20]. In contrast, primary adaptive ER mechanisms can be summarized as: awareness, mindfulness, clarity, acceptance, and understanding of emotion, self-compassion and self-empathy, and a readiness and ability to act planfully. These mechanisms have become the basis for a number of measurement instruments used in clinical research [21] and they have been used as primary targets for interventions to enhance adaptive ER responses [22].
1.3 Overview
DD results in large part as a function of an inability to self-regulate one’s ongoing emotional response to the burdens, strains and specific fears associated with diabetes management over time. Maladaptive ER mechanisms include increased levels of rumination, negative self-evaluation, impulsivity, and avoidance that increase DD, which, in turn, affect disease management and metabolic outcomes.
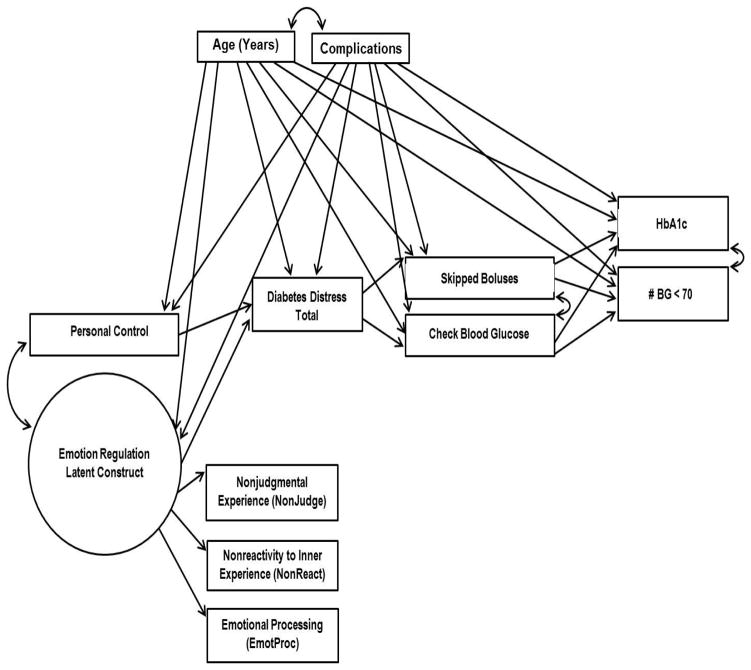
The hypothesized linkages between ER and DD, and the subsequent effects of this relationship on disease management and level of HbA1C, have not as yet been tested empirically in diabetes. Documentation of these linkages is critical because it enables the development of informed interventions to reduce or prevent DD by targeting those maladaptive ER mechanisms that lead to DD. In Figure 2 we expand upon the framework presented in Figure 1 by adding diabetes-specific variables that enable a comprehensive test of the potential processes involved. In preparation for testing the framework, we first describe the construction of an empirical or latent ER measurement construct that captures some of the critical ER mechanisms that are hypothesized to be significantly related to DD. We then test the complete framework using structural equation modeling (SEM) in a sample of adults with type 1 diabetes (T1D). We hypothesize a single generic ER measurement construct composed of three ER mechanisms derived from the review presented above (nonjudgment of emotions, nonreactivity to emotions, emotional processing). As a test of the framework, we then hypothesize significant pathways of association between ER and personal control with DD, and significant pathways between DD, disease management and HbA1C, as illustrated in Figure 2.
Figure 2.
Pathways of interest in the theoretical model.
2. Methods
2.1 Patients
Using patient registries and contacts with local online groups, we recruited T1D patients from community and academic settings in California (San Francisco Bay Area, Los Angeles, Sacramento, San Diego), Tucson, Arizona, Portland, Oregon, and Toronto, Canada. Inclusion criteria were: patient ≥19 years of age, diagnosis of T1D for at least 12 months, ability to read, write and speak English, mean item score of ≥ 2 on the T1-Diabetes Distress Scale (T1-DDS) indicating at least moderate DD, a recently recorded HbA1c ≥ 7.5%, no severe complications (end-stage renal disease), absence of psychosis and dementia, and availability of a computer with Internet access for online assessment.
2.2 Procedure
The data collected for this report were based on baseline, pre-intervention assessment for a study called T1-REDEEM (Reducing Distress and Enhancing Effective Management). This was a randomized clinical trial to test the relative effectiveness of two interventions to reduce DD among distressed T1Ds with elevated HbA1C.
Human subjects approval was received at each site from the appropriate university or community review board. Recruitment involved a combination of opt-in and opt-out procedures, based on the requirements of the site institutional review board. For sites that approved an opt-out procedure, the research team mailed letters to each patient on the registry informing them of the study and telling them that a project representative would contact them by phone within two weeks unless they opted out of the call by returning an enclosed post card or calling an 800 number. For sites requiring an opt-in procedure, letters were mailed to patients on the registry describing the project. Those interested were encouraged to call our 800 number. During contact with patients identified by both recruitment procedures, the project was explained, informed consent was obtained, and screening commenced, including administration of the T1-DDS and permission to obtain their latest clinic recorded HbA1C. If a timely HbA1C was not available (within three months), a pre-paid lab slip was mailed to the participant for collection at a local facility. All recruited and consented patients were then sent an email with a unique personal code to access the HIPAA-protected online survey. Upon completion of the survey and a recorded HbA1C, patients received a $25 gift card for their time. Data were collected in 2015–2016 and analyzed in 2017.
2.3 Measures
Patient age in years and number of complications from a list of 14 [23] were recorded.
Three scales were selected as potential contributors to the ER construct, based on the review of ER mechanisms above. The Non-Judging of Inner Experience Scale (NonJudge) is an 8-item subscale (alpha = .95) from the Five Facet Mindfullness Scale [21]. Items are reversed-scored on a 5-point scale from “never or rarely true” to “very often or always true,” and include, “I tell my self that I shouldn’t be feeling the way I am feeling” and “I think some of my emotions are bad or inappropriate and I shouldn’t be feeling them.” The Nonreactivity to Inner Experience Scale (NonReact) is a 7-item subscale (alpha = .89) from the Five Facet Mindfullness Scale [21]. Items include, “I perceive my feelings and emotions without having to react to them,” and “When I have distressing thoughts or images I can just notice them and then let them go.” The Emotional Processing Scale (EmotProc) is a 4-item subscale (alpha = .87) from the Emotional Approach and Coping Scale [24]. Items are scored on a 4-point scale from “I haven’t been doing this at all” to “I’ve been doing this a lot,” and include, “I take time to figure out what I am feeling,” and “I realize that my feelings are valid and important.” These three scales reflect acceptance of emotion without self-criticalness; non-impulsive, planned reactions to emotions; and engagement with and understanding emotions.
Because ER and cognitions interact to affect DD, we included the Personal Control subscale from the Revised Illness Perception Questionnaire [25] as a generic surrogate to assess disease-related cognitions. It is a 6-item (5 response options from “strongly disagree” to “strongly agree” (alpha = .90), with items such as, “I have the power to influence my disease,” and “The course of my illness depends on me.” Two items are reverse scored.
T1-DDS is a 28-item scale (alpha = .91) [26] that assesses overall level of DD. There are six response options from “not a problem” to “a very serious problem.” Items include, “Feeling discouraged when I see high blood glucose numbers that I cannot explain” and “Worried that I will develop serious long-term complications no matter how hard I try.”
Self-management behavior was assessed by self-reports of two common management tasks: missed insulin boluses and frequency of blood glucose checks per day over the past week. Participants were asked: “How many times did you typically miss or skip a bolus that you probably should have taken during a typical day over the past week?” and “How many times did you typically check your blood sugar during a typical day over the past week?” Responses were from 0 to 10 or more.
Laboratory-assessed HbA1c was obtained from clinic records for tests within 3 months of survey completion or, if unavailable, through a lab slip provided by the project. Number of hypoglycemic episodes (defined as blood glucose < 70 mg/dl) in the past 7 days were self-reported.
Although not included in Figure 2, we also included a measure of depression symptoms. The Patient Health Questionnaire 8 (PHQ8)[27] is an 8-item scale with each item linked to a depression symptom defined by the DSM-V as part of Major Depressive Disorder. The suicide item was omitted, which does not affect the scale’s reliability or validity.
2.4 Data Analysis
SEM was used to examine hypothesized relationships between the ER latent factor, personal control, DD, diabetes management and glycemic control variables (Figure 2). Models were estimated using Mplus software (version 6.1). Mplus uses an Expectation Maximization algorithm that allows for the handling of missing data, enabling the inclusion of all participants’ data in the analyses. Analyses were specified to estimate regression parameters, covariances, means, and variances.
A preliminary, saturated measurement model was first specified to evaluate the tenability of the theoretical ER latent factor, which was hypothesized to be comprised of three observed variables: NonJudge, NonReact, and EmotProc. A second model was specified to relate the ER latent factor to DD. Then a full model was specified to provide a more comprehensive picture of the hypothesized relations between ER, cognitions, DD, and the other variables in the model. The final model included age and diabetes complications as covariates, and covaried the two glycemic control variables, the two diabetes management variables, DD, and the ER latent construct with the observed personal control variable. Specific indirect effects were estimated and tested for all combinations of paths leading from ER and personal control, to the glycemic control variables.
3. Results
Across all sites, 347 patients were eligible after baseline assessment, and of these, 301 (86%) participated. There were no significant differences between those patients who were eligible and agreed to participate and those eligible who declined on gender, ethnicity, education, and insulin use. However, those who participated reported significantly higher T1-DDS scores (mean = 2.9, SD = 0.6) than those who did not (mean = 2.7, SD = 0.9); t(528) = 3.20, p = .001. Across all sites, average age was 45.05 (15.0) years, education was 15.4 (3.6) years, percent female was 69.1%, mean DD score was 2.9 (.6), and mean HbA1C was 8.8 (1.1). As expected, significant differences in diabetes status and demographics occurred across sites to assure a diverse sample (Table 1).
Table 1.
Description of the sample (Mean [SD] or % by location (N = 301)
| Variable | Site | p* Value | ||||||
|---|---|---|---|---|---|---|---|---|
| San Francisco | Sacramento | Los Angeles | San Diego | Portland | Tucson | Canada | ||
| n=100 | n=26 | n=16 | n=50 | n=58 | n=25 | n=26 | ||
| Age (yr) | 42.6 (15.6) | 44.9 (14.7) | 46.4 (10.3) | 50.4 (14.5) | 40.8 (14.7) | 42.8 (16.8) | 43.7 (13.1) | 0.06 |
| Diagnosis age(yr) | 19.4 (13.4) | 18.4 (14.3) | 27.5 (15.8) | 22.6 (15.7) | 19.1 (13.9) | 21.0 (14.5) | 19.1 (11.1) | 0.32 |
| Education (yr) | 16.4 (3.0) | 16.0 (3.3) | 15.8 (4.9) | 15.2 (2.8) | 14.8 (4.4) | 14.4 (3.0) | 14.1 (4.5) | 0.02 |
| No. complications | 2.6 (2.3) | 2.5 (2.1) | 2.9 (3.1) | 3.2 (3.0) | 2.6 (2.4) | 3.1 (2.9) | 2.5 (2.1) | 0.84 |
| Female (%) | 73% | 54% | 94% | 64% | 74% | 64% | 58% | 0.08 |
| Ethnicity (%) | 0.1 | |||||||
| Asian | 4% | 8% | 6% | 2% | 0% | 0% | 4% | |
| Afric. American | 1% | 0% | 0% | 2% | 5% | 0% | 0% | |
| Latino | 9% | 11% | 6% | 12% | 2% | 24% | 0% | |
| Native American | 0% | 4% | 0% | 0% | 0% | 0% | 0% | |
| Pacific Islander | 0% | 0% | 0% | 2% | 2% | 0% | 0% | |
| White | 80% | 77% | 69% | 78% | 88% | 72% | 85% | |
| Multiple/Other | 6% | 0% | 19% | 4% | 3% | 4% | 11% | |
| Insulin pump(%) | 61% | 58% | 69% | 58% | 74% | 64% | 89% | 0.09 |
| NonJudge | 3.6 (1.1) | 3.6 (1.0) | 3.9 (1.0) | 3.6 (1.0) | 3.5 (1.0) | 3.1 (0.9) | 3.5 (1.0) | 0.26 |
| NonReact | 3.2 (0.8) | 3.1 (0.8) | 3.1 (0.7) | 3.3 (0.8) | 3.1 (0.7) | 3.3 (0.8) | 3.1 (0.8) | 0.81 |
| Personal control | 24.7 (3.6) | 24.4 (3.4) | 25.4 (3.2) | 25.0 (3.2) | 24.9 (3.3) | 24.6 (4.4) | 25.3 (2.4) | 0.95 |
| EmotProc | 2.5 (0.8) | -- | 2.6 (0.8) | 2.4 (0.8) | 2.4 (0.8) | 2.7 (0.8) | 2.2 (0.6) | 0.24 |
| Diabetes distress | 2.9 (0.6) | 2.9 (0.6) | 3.2 (0.6) | 2.8 (0.7) | 2.9 (0.6) | 3.0 (0.6) | 2.8 (0.6) | 0.22 |
| Glucose monitoring | 5.5 (2.5) | 4.5 (2.2) | 5.1 (2.6) | 4.9 (2.8) | 3.7 (2.2) | 4.6 (2.5) | 4.6 (2.6) | 0.003 |
| Missed boluses | 1.3 (1.6) | 1.2 (1.6) | 1.5 (1.9) | 1.3 (1.5) | 1.6 (1.8) | 1.8 (2.4) | 1.1 (1.6) | 0.63 |
| HbA1c % | 8.6 (0.9) | 8.9 (1.3) | 8.5 (0.8) | 8.8 (1.0) | 9.0 (1.2) | 9.3 (1.6) | 9.0 (1.2) | 0.02 |
| No. hypo episodes | 3.0 (2.2) | 2.4 (2.3) | 2.4 (1.7) | 2.3 (1.7) | 2.3 (2.2) | 2.4 (1.7) | 2.5 (1.2) | 0.36 |
Chi-square or one-way analysis of variance, as appropriate.
3.1 Hypothesis 1: Forming a Latent ER Construct
The three ER measures loaded significantly on a single latent factor, making it a viable and reliable indicator. Standardized factor loadings were b = .57 (p < .001) for NonJudge, b = .23 (p = .009) for EmotProc, and b = .79 (p < .001) for NonReact.
A subsequent model showed that the ER factor was significantly negatively associated with DD (b = −.40, p < .001). Fitting procedures for this model were: χ2(2) = 3.44, p = .18, Comparative Fit Index (CFI) = .99, Tucker-Lewis Index (TLI) = .96, and Root Mean Square Error of Approximation (RMSEA) = .05. Generally acceptable SEM fit index values are a nonsignificant chi-square, CFI and TLI equal to or greater than .95, and .06 or less for the RMSEA.
3.2 Hypothesis 2: Testing the Model
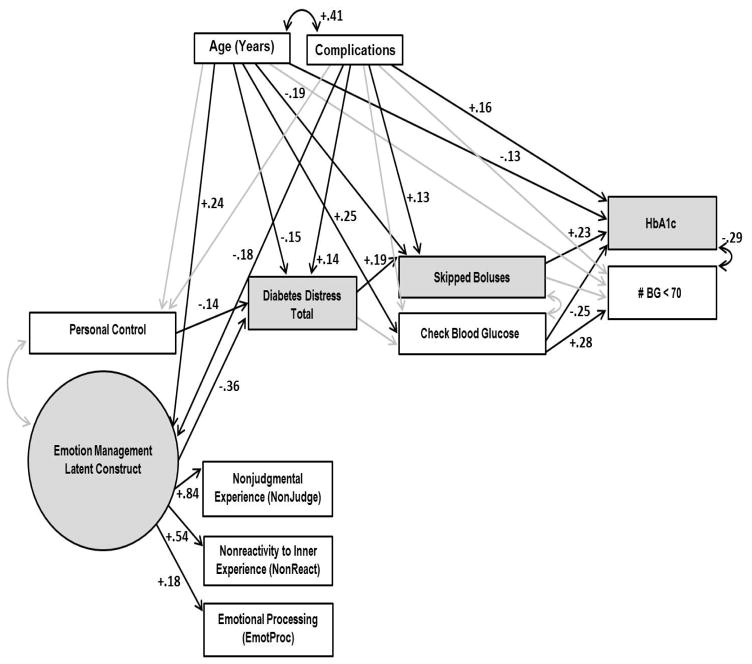
Figure 3 illustrates the complete model. Model fitting procedures indicated a good fit to the data: χ2(26) = 20.77, p = .75, CFI = 1.00, TLI = 1.04, and RMSEA = .000. Standardized regression coefficients are presented in Table 2.
Figure 3.
The full model with correlation and standardized regression coefficients. Shading indicates the significant indirect effect.
Table 2.
Regression effects and correlations in the final model
| Variable | b or r (p) |
|---|---|
| Regression Effects | |
| Emotion Regulation → Diabetes Distress | −.36 (<.001) |
| Personal Control → Diabetes Distress | −.14 (.01) |
| Diabetes Distress → Missed Insulin Boluses | .19 (.001) |
| Diabetes Distress → Blood Glucose Monitoring | −.06 (.33) |
| Missed Insulin Boluses → HbA1c | .23 (<.001) |
| Blood Glucose Monitoring → HbA1c | −.25 (<.001) |
| Missed Insulin Boluses → Hypoglycemic Episodes | .05 (.35) |
| Blood Glucose Monitoring → Hypoglycemic Episodes | .28 (<.001) |
| Age → Emotion Regulation | .24 (.001) |
| Age → Personal Control | −.02 (.72) |
| Age → Diabetes Distress | −.15 (.02) |
| Age → Missed Insulin Boluses | −.19 (.002) |
| Age → Blood Glucose Monitoring | .25 (<.001) |
| Age → HbA1c | −.13 (.03) |
| Age → Hypoglycemic Episodes | .06 (.36) |
| Complications → Emotion Regulation | −.18 (.01) |
| Complications → Personal Control | −.10 (.10) |
| Complications → Diabetes Distress | .14 (.02) |
| Complications → Missed Insulin Boluses | .13 (.03) |
| Complications → Blood Glucose Monitoring | −.02 (.77) |
| Complications → HbA1c | .16 (.01) |
| Complications → Hypoglycemic Episodes | .05 (.40) |
| Correlations | |
| Emotion Regulation with Personal Control | .09 (.23) |
| Missed Insulin Boluses with Blood Glucose Monitoring | −.12 (.21) |
| HbA1c with Hypoglycemic Episodes | −.29 (<.001) |
| Age with Complications | .41 (<.001) |
The pathway between ER and DD was significant (b = −.36; p < .001), as was the pathway between personal control and DD (b = −.14, p = .01). Higher levels of both ER and personal control were associated with lower DD. In turn, lower DD was significantly associated with fewer missed insulin boluses (b = .19, p = .001), which was linked with lower HbA1c (b = .23, p < .001). While DD was not directly linked with blood glucose monitoring, greater frequency of monitoring was associated with both lower HbA1c (b = −.25, p < .001) and fewer hypoglycemic episodes (b = .28, p < .001). HbA1c and number of hypoglycemic episodes were significantly negatively correlated (r = −.25, p < .001). Age and diabetes complications were significantly correlated with most variables in the model, and with each other (r = .41, p < .001). Younger age and more complications were significantly associated with lower ER, higher DD, and poorer management and glycemic control. As hypothesized, only one specific indirect pathway from ER to HbA1c was significant—the path from ER to DD to missed boluses to HbA1c (b = −.02, p = .02).
The final full model explained 70% of the variance in NonJudge, 29% in NonReact, 21% in DD, 18% in HbA1c, 9% in missed insulin boluses, 9% in hypoglycemic episodes, 7% in blood glucose monitoring, and 3% in EmotProc. EmotProc loaded significantly on the latent factor, but the variance explained by this variable in the model was nonsignificant.
3.3 Additional Analyses: The Impact of Depression Symptoms
Given the reported interactions between DD and depression symptoms [28], we also had the opportunity to investigate the impact of depression symptoms in the tested models. We ran the same SEM in two ways: first by substituting PHQ8 for T1-DDS, and second by including both PHQ8 and T1-DDS in the same model.
The model substituting PHQ8 for T1-DDS demonstrated a good fit to the data: (χ2(26) = 23.35, p = .61, CFI = 1.00, TLI = 1.02, and RMSEA = .000). There was a significant pathway between ER and depression symptoms (b = −.64, p < .001), and between depression symptoms and missed boluses (b = .12, p = .03). In contrast to the DD model, however, the pathway between personal control and depression symptoms was nonsignificant; and age and number of complications were unrelated to depression symptoms. Also, there was no significant pathway from both ER and personal control through DD to the diabetes management variables. Thus, although ER was linked to depression symptoms, there was no significant pathway to management and metabolic outcomes.
The second supplemental analysis included both depression symptoms and DD in the same model. It too yielded a good fit to the data: χ2(30) = 26.52, p = .65, CFI = 1.00, TLI = 1.02, and RMSEA = .000. Although DD and depression symptoms were significantly related to each other (r = .43), and ER was significantly related to both DD (b = −.36, p < .001) and to depression symptoms (b = −.63, p < .001), personal control was significantly associated only with DD (b = −.14, p = .01). Most importantly, only DD was significantly related to missed boluses (b = .17, p = .01), not depressive symptoms. There was one significant indirect effect, from ER to DD to missed insulin boluses to HbA1c (b = −.02, p = .03); depressive symptoms was not significantly included in these relationships. Thus, only DD carried the pathway from ER to skipped boluses to glycemic outcomes, not depression symptoms.
4. Discussion and conclusion
4.1 Discussion
In a sample of distressed T1Ds with elevated HbA1C, we identified three emotion regulation mechanisms that coalesce into a single, coherent ER factor linked with DD: NonReact, NonJudge and EmotProc. As hypothesized, patients with higher DD tend to make critical self-judgments about their emotions, react to them impulsively or ruminatively and without a plan, and are rarely consciously aware or mindful of their emotional experiences related to diabetes.
Following Hypothesis 2, our findings conform with the general ER literature [16] to suggest that there is a significant pathway from both ER and cognitive processes to DD, followed by subsequent significant linkages to diabetes management and glycemic outcomes. This significant pathway documents the important role that ER plays as a precursor to the known linkages from DD, management and glycemic control. Patient age and number of complications affect these processes, with greater significance for younger patients and those with more diabetes-related complications.
A number of existing diabetes-related interventions have been used to address DD; unfortunately, they do so indirectly [6, 29–31]. That is, although many highlight issues concerning the emotional side of diabetes, they do not necessarily target specific ER mechanisms for intervention. For example, the Stanford Chronic Disease Management Program [32] addresses the feelings, beliefs and expectations that are tied to diabetes self-efficacy, but the program does not directly target modifications in the use of ER mechanisms to help reduce DD. From our review, only general mindfulness-based approaches target at least some of these mechanisms directly [33]. What is needed now are efforts to identify other ER mechanisms that may be relevant, followed by the development of targeted intervention programs to help patients improve the adaptive use of ER to alleviate or drastically reduce DD.
We also find that, although depression symptoms and DD are significantly correlated, they act differently with the other variables in the models. Unlike the significant ER to DD to HbA1C pathway, the similar pathway from ER to depression symptoms to HbA1C is not significant; and the significant impact of ER on depression symptoms is eliminated when DD is added to the model. Similar findings have been reported elsewhere [34, 35]. Thus, the emotional distress reflected by depression symptoms may primarily be due to the burdens and concerns that result from diabetes and its management, as reflected by DD, and not necessarily by psychopathology.
Several study limitations should be acknowledged. First, the data submitted to the models are cross-sectional. Thus, causation cannot be implied. Further exploration with longitudinal data is needed. Second, the sample only included adults with T1D who had both high DD and elevated HbA1C. It is unclear how the models would operate when patients with a fuller range of DD and HbA1C are included; however, a more complete range would better explicate the potential application of ER across the entire range of diabetes experience. Last, we only explored the impact of three potential ER mechanisms. The impact of other ER mechanisms should be explored.
5. Conclusion
Although the relationship between DD and diabetes-related outcomes has been well-documented in both cross-sectional and longitudinal studies, the emotional processes and mechanisms that serve as precursors to DD have remained largely unexplored. Our results indicate that the ways in which patients with T1D regulate their diabetes-related emotions have a direct impact on their level of DD, with implications for subsequent self-management and metabolic outcomes. These findings suggest the importance of at least three ER mechanisms that can serve as targets for intervention and prevention to reduce DD and enhance diabetes management and quality of life.
6. Practice implications
These findings suggest new approaches to intervention and DD risk assessment. Although it is critical to document a patient’s level of DD in clinical care, it may be equally important to identify the emotional mechanisms that underlie the expression and experience of a patient’s DD. Are patients even aware of their underlying diabetes-related emotions? Do they react to them impulsively and without reflection? Do they devalue their importance or become self-blaming? Examining these mechanisms is a critical next step in clinical inquiry because doing so creates a point of entry into the very processes that lead to DD. Because the literature suggests that ER tends to be stable over time, evaluation of these mechanisms also enables the identification of individuals at risk for DD before the stresses and burdens of care increase.
HIGHLIGHTS.
We showed that 3 emotion regulation mechanisms formed a single factor.
Emotion regulation was directly related to level of diabetes distress (DD).
Pathways from emotion regulation to DD affected management and glycemic outcomes.
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health RO1DK094863, R18DK108039.
We gratefully acknowledge the assistance of the following site collaborators: Andrew Almanns MD, Marina Basina MD, Charles Choe MD, Sara Kim MD, Ann Peters MD, Karen Weihs MD, and Patricia Wu MD. We also acknowledge the contributions of our Project Associates: Meredith Craven, Britnee Ochabski and Hannah Martin.
Footnotes
Authorship: All authors have contributed to the research and article preparation. All authors approve of the submitted version. Lawrence Fisher and Danielle Hessler designed and wrote the initial draft, William Polonsky undertook the initial editing and final review, Lisa Stryker designed and undertook the data analyses, and Susan Guzman, Ian Bloomer and Umesh Masharani added sections to the text and completed final editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes a call for greater precision and clarity. Diabetic Med. 2014;31:764–772. doi: 10.1111/dme.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsjii S, Hayashino Y, Ishii H. Diabetes distress, but not depessive symptoms, is associated with glycaemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 1) Diabetic Med. 2012;29:1451–1455. doi: 10.1111/j.1464-5491.2012.03647.x. [DOI] [PubMed] [Google Scholar]
- 3.Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabtes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med. 2012;35:299–304. doi: 10.1007/s10865-011-9359-z. [DOI] [PubMed] [Google Scholar]
- 4.Fisher L, Hessler DH, Polonsky WH, Masharani U, Peters AL, Blumer I, Stryker DB. The prevalence of depression in type 1 diabetes and the problem of over-diagnosis. Diabetic Med. 2015 doi: 10.1111/dme.12973. [DOI] [PubMed] [Google Scholar]
- 5.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress among adults with type 2 diabetes. Diabetic Med. 2008;25:1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturt J, Dennick K, Hessler DH, Hunter BM, Oliver J, Fisher L. Effective interventions for redusing diabetes distress: systematic review and meta-analysis. International Diabetic Nursing. 2016;12:40–55. [Google Scholar]
- 7.Joorman J, Stanton CH. Examining emotion regulation in depression: a review and future directions. Behav Res Ther. 2016;86:35–49. doi: 10.1016/j.brat.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. Guilford Press; New York: 2007. pp. 3–24. [Google Scholar]
- 9.Quoidbach J, Mikolajcak M, Gross JJ. Positive interventions: an emotion regulation perspective. Psychol Bull. 2015;141:655–693. doi: 10.1037/a0038648. [DOI] [PubMed] [Google Scholar]
- 10.Nigg JT. On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk taking and inhibition for developmental psychopathology. J Child Psychol Psychiat. 2016;58:361–383. doi: 10.1111/jcpp.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jazzaieri H, Morrison AS, Goldin PR, Gross JJ. The role of emotion and emotion regulation in social anxiety disorder. Curr Psychiat Reports. 2015;17:531–540. doi: 10.1007/s11920-014-0531-3. [DOI] [PubMed] [Google Scholar]
- 12.Stanton K, Rozek DC, Stasick-O’Brian SM, Ellickson-Larew S, Watson D. Atransdiagnostic approach to examining the incremental predictive power of emotion regulation and basic personality dimensions. J Abnormal Psychol. 2016;125:960–975. doi: 10.1037/abn0000208. [DOI] [PubMed] [Google Scholar]
- 13.Guendelman S, Medeiros S, Rampes H. Mindfulness and emotion regulation: insights from neurobiological, psychological and clinical studies. Frontiers Psychol. 2017;8:1–23. doi: 10.3389/fpsyg.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diedrich A, Burger J, Kirchner M, Berking M. Adaptive emotion regulation mediates the relationship between self-compassion and depression in individuals with unipolar depression. Psychol Psychother: Theory, Res Pract. 2016;89:1–17. doi: 10.1111/papt.12107. [DOI] [PubMed] [Google Scholar]
- 15.Blair C, Diamond A. Biological processes in prevention and intervention: the promotion of self-regulation as a means oif preventing failure. Devel Psychopathol. 2008;20:899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lansing AH, Berg CA. Adolescent self-regulation as a foundation for chronic illness management. J Ped Psychol. 2014;39:1091–1096. doi: 10.1093/jpepsy/jsu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure and initial validation of the Difficulties in Emotion Regulation Scale. J Psychopathol Behav Assess. 2004;26:41–54. [Google Scholar]
- 18.Berking M, Poppe C, Luhmann M, Wupperman P, Jaggi V, Seifritz E. Is the association between various emotion regulation skills and mental health mediated by the ability to modify emotions? Results from two cross-sectional studies. Journal of Behav Ther Exper Psychiat. 2012;43:931–937. doi: 10.1016/j.jbtep.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Neff KD, Kirpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Personality. 2007;41:139–154. [Google Scholar]
- 20.Baer RA, Smith GT, Lykins E, Button D, Kreitemeyer J, Sauer S, Walsh E, Duggan D, Williams JM. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15:329–342. doi: 10.1177/1073191107313003. [DOI] [PubMed] [Google Scholar]
- 21.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 22.Maes S, Karoly P. Self-regulation and intervention in physical health and Illness: a review. Appl Psychol: An Internat Rev. 2005;54:267–299. [Google Scholar]
- 23.Fisher L, Hessler DH, Glasgow RE, Arean PA, Masharani U, Naranjo D, Stryker LA. REDEEM: a practical trial to reduce diabetes distress. Diabetes Care. 2014;36:2551–2558. doi: 10.2337/dc12-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanton AL, Kirk SB, Cameron CL, Danoff-Burg S. Coping through emotional approach: scale construction and validation. J Person Soc Psychol. 2000;78:1150–1169. doi: 10.1037//0022-3514.78.6.1150. [DOI] [PubMed] [Google Scholar]
- 25.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. [Google Scholar]
- 26.Fisher L, Polonsky WH, Hessler DH, Masharani U, Blumer I, Peters AL, Stryker LA, Bowyer V. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Compl. 2015;29:572–577. doi: 10.1016/j.jdiacomp.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ 8: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez JS, Fisher L, Polonsky WJ. Depression in diabetes: Have we been missing something important? Diabetes Care. 2011;34:236–239. doi: 10.2337/dc10-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang TS, Funnell MM. Peer leader training manual. International Diabetes Federation; 2011. [Google Scholar]
- 30.Miller WR, Rollnick S. Helping people change. Guilford Press; New Yor, NY: 2013. Motivational interviewing. [Google Scholar]
- 31.Fisher L, Hessler DH, Naranjo D, Polonsky WH. AASAP: A program to increase recruitment and retention in clinical trials. Patient Educ Counsel. 2011;86:372–7. doi: 10.1016/j.pec.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow JH, Wright CC, Sheasby JE, Turner AP, Hainsworth JM. Self-management approaches for people with chronic conditions: a review. Patient Educ Counsel. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cogntive therapy and mindfullness-based stress reduction improve mental health and well-being? A systematic reivew. of mediation studies. Clin Psychol Rev. 2015;37:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress and not clinical depression or depressive affect is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher L, Skaff MM, Mullan JT, Arean P, Mohr DC, Masharani U, Glasgow R, Laurencin G. Clinical depression vs. distress among patients with type 2 diabetes: Not just a question of semantics. Diabetes Care. 2007;30:542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]