Abstract
Children in pediatric long-term care facilities (pLTCF) represent a highly vulnerable population and infectious outbreaks occur frequently, resulting in significant morbidity, mortality, and resource use. The purpose of this quasi-experimental trial using time series analysis was to assess the impact of a 4-year theoretically based behavioral intervention on infection prevention practices and clinical outcomes in three pLTCF (288 beds) in New York metropolitan area including 720 residents, ages 1 day to 26 years with mean lengths of stay: 7.9–33.6 months. The 5-pronged behavioral intervention included explicit leadership commitment, active staff participation, work flow assessments, training staff in the World Health Organization “‘five moments of hand hygiene (HH),” and electronic monitoring and feedback of HH frequency. Major outcomes were HH frequency, rates of infections, number of hospitalizations associated with infections, and outbreaks. Mean infection rates/1000 patient days ranged from 4.1–10.4 pre-intervention and 2.9–10.0 post-intervention. Mean hospitalizations/1000 patient days ranged from 2.3–9.7 before and 6.4–9.8 after intervention. Number of outbreaks/1000 patient days per study site ranged from 9–24 pre- and 9–18 post-intervention (total = 95); number of cases/outbreak ranged from 97–324 (total cases pre-intervention = 591 and post-intervention = 401). Post-intervention, statistically significant increases in HH trends occurred in one of three sites, reductions in infections in two sites, fewer hospitalizations in all sites, and significant but varied changes in the numbers of outbreaks and cases/outbreak. Modest but inconsistent improvements occurred in clinically relevant outcomes. Sustainable improvements in infection prevention in pLTCF will require culture change; increased staff involvement; explicit administrative support; and meaningful, timely behavioral feedback.
Keywords: hand hygiene, healthcare-associated infections, long-term care, pediatrics
Introduction
While relatively small in number, children with complex, chronic medical conditions have a disproportionate impact on the US health care system and consume extensive health care resources and Medicaid dollars; the ongoing long-term care of these children and youth accounts for 40% of all medical expenditures for children overall,1 and this unique population is growing in complexity and number. Over a 5-year period, hospitalization rates of children with diagnoses of more than one complex chronic condition increased 100%, from 83 per 100,000 in 1991–1993 to 166 per 100,000 in 2003–2005.2 Many of these children receive care in their homes, but others live in approximately 100 US pediatric post-acute long-term care facilities (pLTCF: http://pediatriccomplexcare.org/) or in skilled nursing facilities intended for adults due to lack of pediatric facilities in some regions of the country.
Children in pLTCF represent a highly vulnerable population; outbreaks occur frequently, resulting in significant morbidity, mortality, and resource use.3 Further, changes to health care delivery for children have led to increased acuity and device use requiring increased levels of care and transfer to acute care facilities. Many policies and practices developed for infection prevention among the elderly in LTCF or for children in acute care facilities may not be applicable for pLTCF,4 but infection prevention policies and practices in pLTCF have not previously been described and we found no published research designed to prevent infections in pLTCF.5
The effectiveness of HH in reducing infections has been consistently demonstrated in a variety of health care and public settings, and both the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) have published guidelines for hand hygiene (HH) in health care facilities.6–9 Nevertheless, HH practices by health care professionals have remained suboptimal despite a number of interventions.10–12 Authors of a recent Cochrane review concluded that the quality of interventions to improve HH practices “remains disappointing” and there is “urgent need to undertake methodologically robust research to explore the effectiveness of soundly designed and implemented interventions.”13 In preliminary work, we found that the frequency of HH among pLTCF was low and that many indications for HH were missed.14 Some efforts to improve HH have been attempted in nursing homes for older adults with varying levels of success.15,16 One recent clinical trial demonstrated improved HH in children’s day care centers in the Netherlands following an educational intervention,17 but none have used a behavioral framework. Additionally, to our knowledge there have been no previous efforts to improve HH in pLTCF.
Theoretical underpinnings
Reported variations in approaches and results to improve HH may be explained in part by the fact that they have not been based on behavioral theoretical underpinnings. Two recent literature reviews of interventions aimed to improve clinician practices concluded that no single approach (e.g., academic detailing, feedback, education, reminders) was clearly superior.18,19 Hysong and colleagues20 conducted a qualitative study to explore the characteristics of feedback that were associated with high levels of adherence to clinical practice guidelines. When compared to facilities with low adherence to guidelines, the characteristics of feedback depicted in Figure 1 (timely, individualized, nonpunitive, customized), were associated with high levels of adherence. The authors coined the term “actionable feedback” to describe this model.
Figure 1.
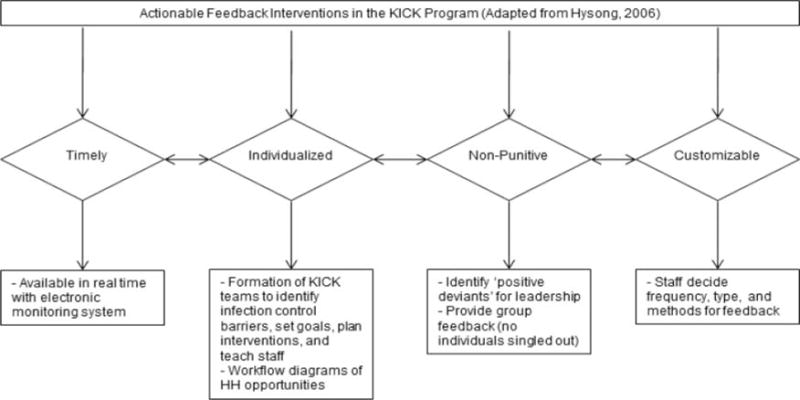
Actionable feedback model with intervention components included.
A multimodal approach to improve HH conducted in an acute care hospital was associated with a significant and sustained increase in HH. Small teams from quality assurance, infection prevention, and clinical staff for each unit were formed to identify barriers, set goals for compliance, and make detailed diagrams of workflow for common tasks corresponding to the WHO “5 Moments for HH” (before and after patient care, before an aseptic procedure, after contact with blood or body fluids, and after touching the patient environment).21 Their approach fit well with the theoretical constructs of the actionable feedback model, and is consistent with recent research emphasizing the importance of a safety culture to reduce health care–associated infections.22,23 In previous work, we successfully applied these components of the actionable feedback model to develop an antimicrobial stewardship program,24 which led us to apply this model to the pediatric long-term care setting. Hence, the aim of this project was to measure the impact of a theoretically based infection prevention behavioral intervention on HH frequency among staff and visitors, rates of infection, and number of acute care hospitalizations and outbreaks among patients in pLTCF.
Methods
Design
In this 4-year (7/1/12–6/30/16) quasi-experimental project (5R01HS021470), data were collected from three pLTCF at baseline and following the intervention based on the theoretical underpinnings described. The study was approved by the appropriate institutional review boards with a waiver of documentation of consent for parents and staff.
Sample and setting
The three participating pLTCF with on-site schools, described in Table 1, are located in the NYC metropolitan area. All children who resided at the facilities when the baseline phase commenced or were admitted during the study period were enrolled.
Table 1.
Characteristics of Study Facilities and Children.
| Site 1 | Site 2 | Site 3 | |
|---|---|---|---|
| Beds | 54 | 137 | 97 |
| Resident rooms | 14 | 63 | 52 |
| Classrooms | 5 | 14 | 4 |
| Total children enrolled | 93 | 205 | 422 |
| Mean (range) age at enrollment | 6.29 years (1 day-19 years) | 7.36 years (20d-20 years) | 4.16 years (1d-26 years) |
| n (%) males | 50 (54) | 99 (48) | 212 (50) |
| Race | |||
| n (%) caucasian | 49 (53) | 28 (14) | 93 (22) |
| n (%) black | 14 (15) | 69 (34) | 138 (33) |
| n (%) Asian | 8 (9) | 27 (13) | 67 (16) |
| n (%) other or unspecified* | 22 (24) | 81 (40) | 124 (29) |
| Length of stay for enrolled children | 33.6 months (7 d-10 years) | 15.7 months (1 day-20 years) | 7.9 months (1 day-21 years) |
| n (%) with feeding tubes (nasogastric, jejunostomy, gastrostomy, or gastrostomy-jejunostomy) | 83 (89) | 168 (82) | 287 (68) |
| n (%) with central venous catheter | 5 (5) | 1 (0.7) | 41 (10) |
| n (% with tracheotomy | 35 (38) | 90 (44) | 93 (22) |
| n (%) with ventilator | 9 (10) | 19 (9) | 20 (5) |
| Staff | |||
| Physicians/nurse practitioners | 1 | 4 | 10 |
| Registered nurses | 45 | 141 | 100 |
| Nursing assistants | 65 | 18 | 100 |
| Therapists (e.g., recreational, respiratory, art, rehabilitation) | 29 | 78 | 44 |
| Total | 140 | 241 | 254 |
Intervention
The Keep It Clean for Kids (KICK) Project included five components.
Component 1: Explicit leadership commitment
Multiple meetings with medical and nursing administrators in each facility were conducted prior to and throughout the study. A member of the project research team was present at each site at least weekly, formal and informal meetings with administration and staff were ongoing, and leaders were actively involved in planning interventions and timing.
Component 2: Active participation of the staff
KICK teams were formed at each site to create “buy-in,” individualize the feedback, and develop tailored interventions. Team composition and size were determined by the staff and varied by site; members included clinicians, educational staff, family members, or administrators. Initially, each KICK team had a structured set of three meetings with the research team in which site staff (a) determined barriers to HH and infection prevention success and set their own goals and timelines; (b) discussed relevance of the WHO HH guidelines for their setting25; and (c) identified preferences for providing feedback of data obtained from electronic monitoring of HH frequency, e.g., how often to provide feedback, which data would be meaningful, in what format, and how often, and (d) planned facility-specific staff communication and training strategies. Following these structured meetings, teams appointed a staff leader and were encouraged to continue to meet and develop motivational and informational projects and materials. Each study site was provided with $1500–2000 per year to develop such training materials.
Component 3: Conducting work flow assessments
KICK teams identified work flow patterns for common, repetitive patient care tasks (e.g., diaper change, tracheostomy care) and developed and distributed visual diagrams to identify HH opportunities.26,27
Component 4: Training staff in the WHO “5 Moments for HH”
The KICK teams led staff training efforts for the “5 Moments for HH”25 using materials available in WHO toolkits. Staff also had hands-on experience performing HH observations using a standardized instrument (http://www.who.int/gpsc/5may/tools/en/).
Component 5: Electronic HH monitoring and feedback
A wireless communication technology that automatically captured HH events was mounted into all soap and hand sanitizer dispensers in each study facility including the schools (DebMed® GMS™ Group Monitoring System, http://debmed.com/usa/debmed-gms/). At the outset of the project, we validated that electronically generated data matched data obtained by direct observation. The system provided several standard graphical report options to display HH frequency for specified locations (e.g., individual dispenser, room, unit, or entire facility) during specified time frames (e.g., day, week, month, or year). The reports could also be customized to include goals or benchmarks set by the sites. Data were available for download in spreadsheet format, allowing each site to tailor what data were presented in conjunction with the dispenser usage reports, e.g., creating a figure to show HH before and after an outbreak. This feedback strategy allowed for both individualization to a specific unit or dispenser as well as customizability regarding frequency and format of reports, essential components of the actionable feedback.28 We met with infection prevention staff and administration at each site to discuss how they would like to provide feedback to staff, including who would receive the feedback (e.g., all staff or selected staff) as well as the frequency and format (email, signs, personal communication, etc.) of the feedback.
Families received communications and some educational materials from the research team and we attended health fairs and other family-related events to discuss the project and provide information regarding HH and infection prevention. We also interviewed a purposive sample family members as well as staff to assess their knowledge, attitudes, and reported practices regarding infection prevention.29,30 However, visitors to the children were quite rare; a large proportion of children had no visitors, a few had visitors several times/week and there were one or two family caretakers across the three sites who were present almost daily. Further, most children were unable to perform their own hand hygiene. Hence, although all HH dispensers in the facilities (in public spaces as well as in patient rooms) were included in the project, the vast majority of HH was from the staff.
Consistent with the KICK theme, the study had two phases: Revving Up and Taking Off. The Revving Up period was the planning phase during which the HH monitoring system was activated to provide baseline data, but no feedback to staff was provided. In addition, staff KICK teams were formed to plan tailored interventions. During the Taking Off period, the interventions were “rolled out” and implemented over time. Initiation of phases was staggered across the three study sites over a 3-month period. Because behavior change has proven to be exceedingly difficult, we hypothesized that even with an intense and multifaceted intervention such as those planned with the KICK project, changes would be incremental and occur over a prolonged period of time. Hence, research staff visited each setting on a regular basis, generally at least weekly. During these visits, research staff talked with clinical personnel and administrators to trouble shoot, reinforce the activities of the KICK teams and infection prevention staff, support KICK teams, and provide educational materials.
Assessment of outcomes
Each of the study facilities employed a fulltime infection control professional responsible for surveillance and reporting. A fulltime surveillance officer hired for the study collaborated with site staff to make weekly site visits and collect data on all primary and secondary outcomes, using definitions and methods described next.
HH frequency was obtained from the electronic monitoring system.
Rates of infections and hospitalizations
Ongoing medical record reviews to identify clinician-diagnosed infections were conducted by the research surveillance officer. Data collected included clinician diagnosis (e.g., pneumonia), signs and symptoms of infection, diagnostic testing, and associated hospital admissions.31
Number of outbreaks
We retrospectively collected outbreaks and the number of children affected by each outbreak reported, as mandated, from the NY State Department of Health Nosocomial Outbreak Reporting Application (NORA). For this study, outbreaks of acute respiratory infections were defined as ≥ 2 cases of the same laboratory-confirmed pathogen or ≥ 2 cases on the same unit with the same symptoms.32
Statistical analysis
All analyses were carried out individually by site. For each outcome we calculated overall means before and after the intervention. However, since means do not accurately reflect trends over time, interrupted time series analyses were conducted.
Impact on HH frequency
We examined mean HH episodes/day by staff and visitors for each week using an interrupted time series analysis with segmented regression to compare changes in HH trends, modeled by slopes and levels before and after implementation of the intervention. Because only a proportion of the children in the facility attended school each day and HH in the schools was relatively stable over time, we analyzed the school HH frequency data separately and did not include those data in the hypothesis testing. Since the outcome variable was a count of HH events/day for each week, we used a negative binomial model in form of
where T1 is the number of weeks from the start of the observation. INT is a dummy variable for pre- or post-intervention, T2 is weeks since the intervention. The testing and interpretation of the model are: β0: the baseline intercept; β1: the slope prior to the intervention; β2: the change of level immediately after the intervention as we wanted to test an immediate effect due to the novelty of the intervention; and β3: the change of slope from pre to post intervention.
Impact on infections and hospitalizations
Similarly, an interrupted time series design with logistic segmented regression was used to compare differences in levels and slopes of infection rates and hospitalization (transfers to acute care) associated with infection between pre- and post-intervention periods by site. We examined monthly rates, defined as number of infections or hospitalizations per 1000 patient days.
Impact on number and size of outbreaks
We counted number of reported outbreaks and number of infections associated with each outbreak per 1000 patient days during the pre and post intervention periods and assessed trends using the hurdle negative binomial model33 to simultaneously compare the probability of an outbreak and, when an outbreak occurred, the number of infections (a zero-truncated count) associated with that outbreak.
Results
In total, 720 residents were enrolled with mean ages of 4.1, 7.1, and 6.3 years and average lengths of stay of 7.9–33.6 months in the three sites. The majority had feeding tubes, about one-third had tracheostomies, and smaller proportions were on ventilators and/or had central venous lines (Table 1). Overall means for HH frequency, infections and hospitalizations are summarized in Table 2, and Table 3 summarizes changes for HH, infections and hospitalizations in level immediately after implementation of the intervention and in the slope/trend in the time series analysis.
Table 2.
Mean Changes by Study Site in Daily Hand Hygiene/Bed and Mean Infections and Hospitalizations/1000 Patient Days Before and After Initiation of the Intervention.
| Pre-Intervention: Revving Up | Intervention: Taking Off | |
|---|---|---|
| Site 1 | ||
| Study period | Sept ’12 – Mar ’13 | Apr ’13 – Dec ’15 |
| Mean daily hand hygiene frequency per bed (+/− standard deviation) | 19.52 (2.00) | 21.17 (1.84) |
| Mean infections/1000 patient days (+/− standard deviation) | 10.39 (3.72) | 10.03 (6.02) |
| Hospitalizations: mean/1000 patient days (standard deviation) | 2.26 (3.98) | 9.83 (6.58) |
| Site 2 | ||
| Dates | Oct ’12 – Apr ’13 | May ’13 – Dec ’15 |
| Mean daily hand hygiene frequency per bed (+/− standard deviation) | 17.01 (1.44) | 21.02 (3.15) |
| Mean infections/1000 patient days (+/− standard deviation) | 4.47 (3.05) | 5.51 (2.00) |
| Hospitalizations: mean/1000 patient days (standard deviation) | 6.81 (6.39) | 7.94 (4.62) |
| Site 3 | ||
| Dates | Nov ’12 – May ’13 | Jun ’13 – Dec ’15 |
| Mean daily hand hygiene frequency per bed (+/− standard deviation) | 29.46 (1.09) | 28.17 (1.48) |
| Mean infections/1000 patient days (+/− standard deviation) | 4.09 (1.92) | 2.89 (1.33) |
| Hospitalizations: mean/1000 patient days (standard deviation) | 9.70 (6.00) | 6.40 (3.91) |
Table 3.
Trends in Weekly Hand Hygiene Frequency/Day/Bed, Monthly Infections and Hospitalizations/1000 Patient Days in 3 Pediatric Long-Term Care Facilities.
| Site | Site | Change in Level (β2)
|
Change in Slope (β3)
|
||
|---|---|---|---|---|---|
| Estimate (SE) | P value | Estimate (SE) | P value | ||
| Hand hygiene frequency | 1 | 0.0161 (0.029) | 0.59 | −0.0031 (0.0008) | 0.0003 |
| 2 | −0.076 (0.028) | 0.0065 | −0.0006 (0.0014) | 0.68 | |
| 3 | −0.124 (0.024) | < 0.0001 | −0.0045 (0.0012) | < 0.0001 | |
| Infection | 1 | 0.069 (0.23) | 0.78 | 0.10 (0.05) | 0.029 |
| 2 | −1.048 (0.190) | < 0.0001 | −0.215 (0.04) | < 0.0001 | |
| 3 | −0.875 (0.189) | < 0.0001 | −0.197 (0.04) | < 0.0001 | |
| Hospitalizations | 1 | −1.135 (0.389) | 0.0035 | −0.964 (0.21) | < 0.0001 |
| 2 | −0.815 (0.148) | < 0.0001 | −0.318 (0.04) | < 0.0001 | |
| 3 | −0.411 (0.124) | 0.0009 | −.188 (0.26) | < 0.0001 | |
Changes in HH frequency
Mean HH frequency was higher in the post-intervention periods for Sites 1 and 2 and was slightly lower in Site 3 (Table 2). As depicted in Table 3 and Figure 2, each site demonstrated a different pattern of HH over time. At Site 1, there was no change in HH level immediately following the intervention (p = 0.59) and the slope decreased slightly during the post-intervention follow-up period (p = 0.0003). At Site 2, the HH level decreased immediately following the intervention (p = 0.0065) and the slope was unchanged during the post-intervention follow-up period (p = 0.68) At Site 3 the level decreased immediately following the intervention (p < 0.0001) and the slope decreased during the post-intervention follow-up period There were no significant changes in HH trends in the schools.
Figure 2.
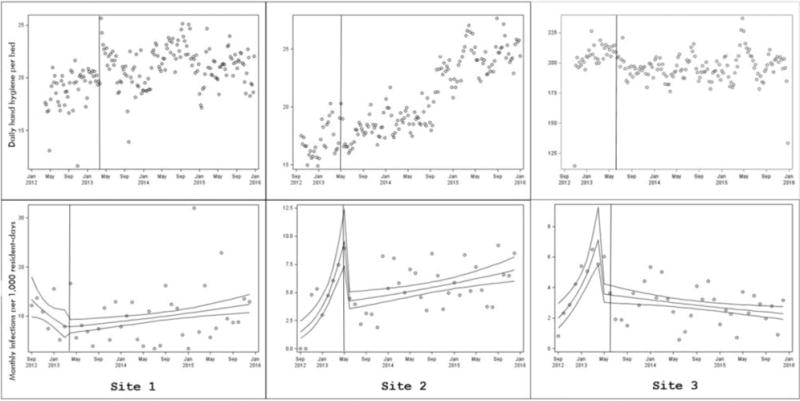
Top: Markers depict total daily HHHH events per bed, excluding school units. Vertical line is date of onset of intervention. Generalized linear mixed models with negative binomial distribution were used to assess post-intervention changes in HHHH events accounting for clustering by unit. Bottom: Markers depict total monthly infections per 1000 resident-days. Slopes represent estimated means (95% confidence intervals) modeled using logistic segmented regression. Note. Graph axes differ by site.
Impact on infection rates
Mean infection rates/1000 patient days ranged from 4.1–10.4 prior to intervention and 2.9–10.0 following implementation of the intervention. As depicted in Figure 2, Site 1 had been experiencing a decrease in infection rate prior to the intervention whereas Sites 2 and 3 had been experiencing increases. At Site 1 there was no change in infection level immediately following the intervention (p = 0.79); the pre- and post-intervention slopes were significantly different (p = 0.0001) and changed from negative to positive. At Site 2 there was a significant drop in infections immediately following the intervention (p < 0.0001); the slope was significantly decreased compared to the pre-intervention period (p < 0.0001), although it remained positive. At Site 3, there was a significant drop in infections immediately following the intervention (p < 0.0001); the pre- and post-intervention slopes were significantly different (p < 0.0001) and changed from positive to negative.
Impact on hospitalizations
Monthly mean hospitalizations/1000 patient days ranged from 2.3–9.7 before and 6.4–9.8 after intervention. The change in slope for hospitalizations decreased significantly for all three sites.
Impact on number and size of outbreaks
Mean observation time for outbreaks was 1217 days pre- and 975 days post-intervention. Number of outbreaks per study site ranged from 9–24 pre- and 9–18 post-intervention (total = 95); number of cases/outbreak ranged from 97–324 (total cases pre-intervention = 591 and post-intervention = 401, Table 4). Again, sites differed in results. At post-intervention when compared with baseline, Site 1 had more outbreaks (p = 0.08) and similar cases during each outbreak (p < 0.5); at Site 2 the number of outbreaks was similar (p = 0.32), but there were fewer cases per outbreak (p < 0.001); and there were fewer outbreaks (p = 0.05) and more cases during an outbreak (p < 0.001) at Site 3. One or more respiratory viruses were identified as the primary cause of 90.5% (86/95) of outbreaks and 90.4% (897/992) of the individual infections.
Table 4.
Mean Outbreaks and Associated Cases/100 Patient Days/Site Before and After KICK Intervention.
| Site | Pre-Intervention
|
Post-Intervention
|
||||||
|---|---|---|---|---|---|---|---|---|
| #Observation Days | #Outbreaks | #Cases | Mean Cases/100 Patient Days | #Observation Days | #Outbreaks | #Cases | Mean Cases/100 Patient Days | |
| 1 | 1250 | 9 | 129 | 10.32 | 944 | 18 | 150 | 15.89 |
| 2 | 1216 | 17 | 324 | 26.64 | 976 | 18 | 154 | 15.78 |
| 3 | 1186 | 24 | 138 | 11.64 | 1005 | 9 | 97 | 9.65 |
Pre-post intervention comparisons:
Site 1: More outbreaks (p=0.08), more infections per outbreak (p=0.50)
Site 2: More outbreaks (p=0.32), fewer infections per outbreak (p<0.001)
Site 3: Fewer outbreaks (p=0.05), more infections per outbreak (p<0.001)
Discussion
In this intervention, there were statistically significant but small reductions in infections in two sites, reductions in hospitalizations in all sites, and varied changes in the numbers of outbreaks and the number of cases associated with each outbreak. All of the study sites experienced modest HH increases prior to the implementation of the intervention, possibly due to the installation of the monitoring system and/or our team’s initial communication with the facilities during the pre-intervention phases. At Sites 1 and 3, this upward trend was not sustained during the intervention period. Site 1 had no significant change in HH level immediately after the intervention, and the slope changed significantly from positive prior to the intervention to negative following the intervention. Site 3 had a drop in HH level immediately after the intervention, and this downward trend continued, resulting in a significant decrease in slope. At Site 2, there was a drop immediately following the intervention, although after that brief decrease, the HH frequency rose again and continued to steadily improve at the same rate it had prior to the intervention, resulting in no change in slope.
Improving suboptimal HH behaviors has been shown to be feasible, but exceedingly difficult, requiring intensive, long-term, multifaceted interventions.21,34–39 Knowing that, we developed this theoretically based intensive intervention implemented over a period of several years in three pLTCF, a relatively untapped research setting. Despite extensive efforts, overall results of the intervention were inconsistent; given the time and effort expended over several years, the costs were high with no evidence of sustainability. Since the majority of infections in these study facilities were respiratory it is possible that social distancing and other interventions would have a stronger impact on curtailing transmission, but such interventions may be difficult given the high levels of mobility and interaction among of the children and staff in pLTCF.
Challenges to change
There are likely three major reasons for the minimal impact of our intervention. First, children in these facilities stay for long periods of time, often their entire lives. Some of the children are mobile, all have frequent and close interactions (including hugging, kissing) with staff as well as other children, and essentially all of the children intermingle in school and group programming which is ongoing throughout the day. This setting is vastly different than a hospital where patients are often bed-bound or have limited activity outside their rooms. Hence, the ability to “contain” microorganisms in pLTCF is limited, and the potential for reducing the number of outbreaks is also limited. A promising result in our study was that when an outbreak did occur, there were significantly fewer cases in two of the three study sites following the intervention, suggesting that control measures or other ongoing efforts may have been more effectively implemented.
Second, while the electronic HH monitoring system could generate graphs presented in multiple ways, despite multiple meetings and seminars with staff and administrators to teach them how to access and use the data, there were challenges in providing staff with feedback. Each site decided how to disseminate the HH data. At some sites, the graphs were available only to certain individuals such as the infection control officer or administrator and other sites displayed results on monitors and in areas frequented by staff, but when we queried staff members, many were either unaware or only vaguely recalled seeing the data, and others could not interpret the meaning of the graphs or relate them to their daily work. Some staff reported that the numbers were meaningless because they did not trust the accuracy of the monitoring system. In fact, lack of accuracy of electronically collected HH data and the potential punitive use of these data have been cited in other studies as major concerns of staff.28,40 Others have shown that when feedback was provided only to managers, HH rates were not improved, but when staff received direct feedback, performance improved significantly.41
The Joint Commission mandates monitoring of HH practices and has set a high standard for adherence, but has not specified monitoring methods. Direct observation is still considered the “gold standard” despite data confirming that observation is costly, has poor validity and reliability, and overestimates actual practice.42–45 A meta-analysis of 19 studies concluded that effective feedback should be frequent and accompanied by specific suggestions for improvement.46 In this study, data were made available primarily to a small number of individuals, and frontline staff were often uninformed or unaware of what actions could/should be taken in response to the results. Electronic HH monitoring has a number of advantages over the current observational standard, including increased accuracy, minimizing the “Hawthorne Effect,” and saving time.43,47 Clearly however, as electronic monitoring of HH becomes increasingly adopted it will necessitate considerable orientation and buy in from administrators and staff before it can effectively influence behavior rather than used solely for the purpose of surveillance.
The third possible reason for minimal impact of the intervention related to the perceived needs and priorities of staff members. Despite evidence to the contrary,14,29 individual staff members reported to us that their own infection prevention behaviors were fine, and that others were the problem. Further, in qualitative interviews we conducted, staff members expressed doubt about the efficacy of alcohol-based sanitizers and stated that they preferred to continue with what they had done for years—use soap and water handwashing.30 The direct care providers reported that they treated the children like their family members and many staff members prioritized this role over that of being a health care provider. Family members we interviewed often shared staff views, reporting, for example, that they “always” washed their hands, as did staff, and expressed the opinion that infections were inevitable in their children.
The major limitation of the current study was that the intervention was based on a theory that feedback results in behavior change, but the feedback staff received was inconsistent across sites and often did not even reach frontline providers of care. Hence, it was not possible to fully evaluate the potential impact of the actionable feedback model. As with any field trial, many factors likely varied across study sites and temporal changes occurred throughout the pre- and post-intervention periods which may have had an impact on our outcomes of interest. For example, although we used the NYS Department of Health NORA system to identify outbreaks, there was no standard definition regarding how many children constituted an outbreak and sites used their own discretion in reporting outbreaks. Further, personnel and/or environmental changes over time likely affected reporting as well as clinical practices. Because planning for this study occurred over a long period, it is possible that some changes in HH frequency occurred during the baseline period. Finally, we were unable to establish a denominator for the HH monitoring system and provide staff feedback regarding compliance percentages, which has been done successfully in acute care settings.48–50
Conclusions
These findings highlight challenges for identifying effective infection prevention strategies in post-acute pediatric settings and potentially in other skilled nursing facilities and home care. Although the patient populations and environmental settings in such health care settings pose a “perfect storm” for outbreaks and cross-transmission, deeply ingrained attitudes and practices are unlikely to change without a culture shift involving front-line staff and administration. It is likely that our varied results were reflective of pre-existing and long-standing differences in the culture of each site and staff readiness for change.
In acute care settings we and others found incremental but sustained increases in HH only when there was explicit, overt support from top level administrators and frontline staff members who planned and “owned” the practice change and served as role models.51,52 Since participants did not consistently receive feedback and given the theory on which the treatment was based, this may explain the lack of more significant improvements. Sustainable improvements and next steps for infection prevention in chronic care and home-like settings will likely require ongoing long-term efforts to inculcate infection prevention into the institutional culture, increasing staff involvement and mindfulness, assuring explicit administrative support, and providing meaningful and timely feedback on behavior.53
Acknowledgments
We express our gratitude to the administration and staff of the pediatric long-term care facilities who participated in this project and to other trainees and colleagues who also contributed (B. Loyland, A. Hessels, S. Wilmont, M. Burgermaster). The Electronic Hand Hygiene Compliance Monitoring System used in the study was provided by DebMed, Charlotte, NC.
Funding
This study was funded by the Agency for Healthcare Research and Quality (5R01HS021470). The Electronic Hand Hygiene Compliance Monitoring System used in the study was provided by DebMed, Charlotte, NC.
References
- 1.Maternal and Child Health Bureau of the Department of Health and Human Services. National Survey of Children with Special Health Care Needs. 2011 http://www.cdc.gov/nchs/slaits/cshcn.htm#09-10.
- 2.Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010 Oct;126:638–46. doi: 10.1542/peds.2009-1658. [DOI] [PubMed] [Google Scholar]
- 3.Murray MT, Heitkemper E, Jackson O, et al. Direct costs of acute respiratory infections in a pediatric long-term care facility. Influenza and Other Respir Viruses. 2016 Jan;10:34–36. doi: 10.1111/irv.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JA. Infection control in pediatric extended care facilities. Infect Control Hosp Epidemiol. 2006 Jun;27:598–603. doi: 10.1086/504937. [DOI] [PubMed] [Google Scholar]
- 5.Castle NG, Wagner LM, Ferguson-Rome JC, Men A, Handler SM. Nursing home deficiency citations for infection control. Am J Infect Control. 2011 May;39:263–9. doi: 10.1016/j.ajic.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Aiello AE, Larson EL. What is the evidence for a causal link between hygiene and infections? Lancet Infect Dis. 2002;2:103–10. doi: 10.1016/s1473-3099(02)00184-6. [DOI] [PubMed] [Google Scholar]
- 7.Boyce JM, Farr BM, Jarvis WR, et al. Guideline for hand hygiene in the healthcare setting. Am J Infect Control. 2002;30:S1–S46. doi: 10.1067/mic.2002.130391. [DOI] [PubMed] [Google Scholar]
- 8.Pittet D, Allegranzi B, Boyce J, World Health Organization World Alliance for Patient Safety First Global Patient Safety Challenge Core Group of E The World Health Organization Guidelines on Hand Hygiene in Health Care and their consensus recommendations. Infect Control Hosp Epidemiol. 2009 Jul;30:611–22. doi: 10.1086/600379. [DOI] [PubMed] [Google Scholar]
- 9.Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health. 2008 Aug;98:1372–81. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu KS, Chen YS, Lin HS, et al. A nationwide covert observation study using a novel method for hand hygiene compliance in health care. Am J Infect Control. 2016 Nov 09; doi: 10.1016/j.ajic.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Neo JR, Sagha-Zadeh R, Vielemeyer O, Franklin E. Evidence-based practices to increase hand hygiene compliance in health care facilities: An integrated review. Am J Infect Control. 2016 Jun 01;44:691–704. doi: 10.1016/j.ajic.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Castle N, Wagner L, Ferguson J, Handler S. Hand hygiene deficiency citations in nursing homes. J Appl Gerontol. 2014 Feb;33:24–50. doi: 10.1177/0733464812449903. [DOI] [PubMed] [Google Scholar]
- 13.Gould DJ, Moralejo D, Drey N, Chudleigh JH. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev. 2010:CD005186. doi: 10.1002/14651858.CD005186.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Buet A, Cohen B, Marine M, et al. Hand hygiene opportunities in pediatric extended care facilities. J Pediatr Nurs. 2013 Jan;28:72–6. doi: 10.1016/j.pedn.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Eveillard M, Raymond F, Guilloteau V, et al. Impact of a multi-faceted training intervention on the improvement of hand hygiene and gloving practices in four healthcare settings including nursing homes, acute-care geriatric wards and physical rehabilitation units. J Clin Nurs. 2011 Oct;20:2744–51. doi: 10.1111/j.1365-2702.2011.03704.x. [DOI] [PubMed] [Google Scholar]
- 16.Kacelnik O, Forland OJ, Iversen B. Evaluation of the national campaign to improve hand hygiene in nursing homes in Norway. J Hosp Infect. 2011 Apr;77:359–60. doi: 10.1016/j.jhin.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Zomer TP, Erasmus V, Looman CW, et al. Improving hand hygiene compliance in child daycare centres: a randomized controlled trial. Epidemiol Infect. 2016 Sep;144:2552–60. doi: 10.1017/S0950268816000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostini R, Hegney D, Jackson C, et al. Systematic review of interventions to improve prescribing. Ann Pharmacother. 2009 Mar;43:502–13. doi: 10.1345/aph.1L488. [DOI] [PubMed] [Google Scholar]
- 19.Sketris IS, Langille Ingram EM, Lummis HL. Strategic opportunities for effective optimal prescribing and medication management. Can J Clin Pharmacol. 2009 Winter;16:e103–25. [PubMed] [Google Scholar]
- 20.Hysong SJ, Best RG, Pugh JA. Audit and feedback and clinical practice guideline adherence: making feedback actionable. Implement Sci. 2006;1:9. doi: 10.1186/1748-5908-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son C, Chuck T, Childers T, et al. Practically speaking: rethinking hand hygiene improvement programs in health care settings. Am J Infect Control. 2011 Jun 9;39(9):716–724. doi: 10.1016/j.ajic.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Clancy CM. New research highlights the role of patient safety culture and safer care. J Nurs Care Qual. 2011 Jul-Sep;26:193–6. doi: 10.1097/NCQ.0b013e31821d0520. [DOI] [PubMed] [Google Scholar]
- 23.Clancy CM, Berwick DM. The science of safety improvement: learning while doing. Ann Intern Med. 2011 May 17;154:699–701. doi: 10.7326/0003-4819-154-10-201105170-00013. [DOI] [PubMed] [Google Scholar]
- 24.Patel SJ, Saiman L, Duchon JM, Evans D, Ferng YH, Larson E. Development of an antimicrobial stewardship intervention using a model of actionable feedback. Interdiscip Perspect Infect Dis. 2012;2012:150367. doi: 10.1155/2012/150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sax H, Allegranzi B, Uckay I, Larson E, Boyce J, Pittet D. ‘My five moments for hand hygiene’: a user-centred design approach to understand, train, monitor and report hand hygiene. J Hosp Infect. 2007 Sep;67:9–21. doi: 10.1016/j.jhin.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Carter EJ, Cohen B, Murray MT, Saiman L, Larson EL. Using workflow diagrams to address hand hygiene in pediatric long-term care facilities. J Pediatr Nurs. 2015 Jul-Aug;30:e17–21. doi: 10.1016/j.pedn.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son C, Chuck T, Childers T, et al. Practically speaking: rethinking hand hygiene improvement programs in health care settings. Am J Infect Control. 2011 Nov;39:716–24. doi: 10.1016/j.ajic.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Conway LJ, Riley L, Saiman L, Cohen B, Alper P, Larson EL. Implementation and impact of an automated group monitoring and feedback system to promote hand hygiene among health care personnel. Jt Comm J Qual Patient Saf. 2014 Sep;40:408–17. doi: 10.1016/s1553-7250(14)40053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loyland B, Wilmont S, Cohen B, Larson E. Hand-hygiene practices and observed barriers in pediatric long-term care facilities in the New York metropolitan area. Int J Qual Health Care. 2016 Feb;28:74–80. doi: 10.1093/intqhc/mzv097. [DOI] [PubMed] [Google Scholar]
- 30.Loyland B, Wilmont S, Hessels AJ, Larson E. Staff knowledge, awareness, perceptions, and beliefs about infection prevention in pediatric long-term care facilities. Nurs Res. 2016 Mar-Apr;65:132–41. doi: 10.1097/NNR.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008 Jun;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Murray MT, Pavia M, Jackson O, et al. Health care-associated infection outbreaks in pediatric long-term care facilities. Am J Infect Control. 2015 Jul 1;43:756–8. doi: 10.1016/j.ajic.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saffari S, Adnan R, Greene W. Hurdle negative binomial regression model with right censored count data. Sort. 2012;36:181–94. [Google Scholar]
- 34.Sepkowitz KA. Why doesn’t hand hygiene work better? Lancet Infect Dis. 2012 Feb;12:96–97. doi: 10.1016/S1473-3099(11)70349-8. [DOI] [PubMed] [Google Scholar]
- 35.Pittet D. Improving adherence to hand hygiene practice: a multidisciplinary approach. Emerg Infect Dis. 2001;7:234–40. doi: 10.3201/eid0702.010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. The Lancet. 2000;356:1307–12. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 37.Whitby M, Pessoa-Silva CL, McLaws ML, et al. Behavioural considerations for hand hygiene practices: the basic building blocks. J Hosp Infect. 2007 Jan;65:1–8. doi: 10.1016/j.jhin.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Allegranzi B, Conway L, Larson E, Pittet D. Status of the implementation of the World Health Organization multimodal hand hygiene strategy in United States of America health care facilities. Am J Infect Control. 2014 Mar;42:224–30. doi: 10.1016/j.ajic.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kingston L, O’Connell NH, Dunne CP. Hand hygiene-related clinical trials reported since 2010: a systematic review. J Hosp Infect. 2016 Apr;92:309–20. doi: 10.1016/j.jhin.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Ellingson K, Polgreen PM, Schneider A, et al. Healthcare personnel perceptions of hand hygiene monitoring technology. Infect Control Hosp Epidemiol. 2011 Nov;32:1091–6. doi: 10.1086/662179. [DOI] [PubMed] [Google Scholar]
- 41.Assanasen S, Edmond M, Bearman G. Impact of 2 different levels of performance feedback on compliance with infection control process measures in 2 intensive care units. Am J Infect Control. 2008 Aug;36:407–413. doi: 10.1016/j.ajic.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Boyce JM. Hand hygiene compliance monitoring: current perspectives from the USA. J Hosp Infect. 2008 Oct;70:2–7. doi: 10.1016/S0195-6701(08)60003-1. [DOI] [PubMed] [Google Scholar]
- 43.Boyce JM. Measuring healthcare worker hand hygiene activity: current practices and emerging technologies. Infect Control Hosp Epidemiol. 2011 Oct;32:1016–28. doi: 10.1086/662015. [DOI] [PubMed] [Google Scholar]
- 44.Boyce JM, Cooper T, Dolan MJ. Evaluation of an electronic device for real-time measurement of alcohol-based hand rub use. Infect Control Hosp Epidemiol. 2009 Nov;30:1090–5. doi: 10.1086/644756. [DOI] [PubMed] [Google Scholar]
- 45.Morgan DJ, Pineles L, Shardell M, et al. Automated hand hygiene count devices may better measure compliance than human observation. Am J Infect Control. 2012 May 25;40(10):955–959. doi: 10.1016/j.ajic.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 46.Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009 Mar;47:356–363. doi: 10.1097/MLR.0b013e3181893f6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas JP, Larson EL. Measurement of compliance with hand hygiene. J Hosp Infect. 2007 May;66:6–14. doi: 10.1016/j.jhin.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Diller T, Kelly JW, Blackhurst D, Steed C, Boeker S, McElveen DC. Estimation of hand hygiene opportunities on an adult medical ward using 24-hour camera surveillance: validation of the HOW2 Benchmark Study. Am J Infect Control. 2014 Jun;42:602–7. doi: 10.1016/j.ajic.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Kelly JW, Blackhurst D, McAtee W, Steed C. Electronic hand hygiene monitoring as a tool for reducing health care-associated methicillin-resistant Staphylococcus aureus infection. Am J Infect Control. 2016 Aug 1;44:956–7. doi: 10.1016/j.ajic.2016.04.215. [DOI] [PubMed] [Google Scholar]
- 50.Steed C, Kelly JW, Blackhurst D, et al. Hospital hand hygiene opportunities: where and when (HOW2)? The HOW2 Benchmark Study. Am J Infect Control. 2011 Feb;39:19–26. doi: 10.1016/j.ajic.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Larson EL, Early E, Cloonan P, Sugrue S, Parides M. An organizational climate intervention associated with increased handwashing and decreased nosocomial infections. Behav Med. 2000 Spring;26:14–22. doi: 10.1080/08964280009595749. [DOI] [PubMed] [Google Scholar]
- 52.Marra AR, Noritomi DT, Westheimer Cavalcante AJ, et al. A multicenter study using positive deviance for improving hand hygiene compliance. Am J Infect Control. 2013 Nov;41:984–8. doi: 10.1016/j.ajic.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Conway LJ. Challenges in implementing electronic hand hygiene monitoring systems. Am J Infect Control. 2016 May 2;44:e7–e12. doi: 10.1016/j.ajic.2015.11.031. [DOI] [PubMed] [Google Scholar]


