Abstract
We tested the hypothesis that the effects of food restriction on behavioral motivation are mediated by one or both of the RFamides, RFamide-related Peptide-3 (RFRP-3) and kisspeptin (Kp) in female Syrian hamsters (Mesocricetus auratus). Female hamsters fed ad libitum and given a choice between food and adult male hamsters are highly motivated to visit males instead of food on all four days of the estrous cycle, but after 8 days of mild food restriction (75% of ad libitum intake) they shift their preference toward food every day of the estrous cycle until the day of estrus, when they shift their preference back toward the males. In support of a role for RFRP-3 in these behavioral changes, the preference for food and the activation of RFRP-3-immunoreactive (Ir) cells in the dorsomedial hypothalamus (DMH) showed the same estrous cycle pattern in food-restricted females, but no association was observed between behavior and the activation of Kp cells in the hypothalamic arcuate nucleus or preoptic area. Next, we tested the hypothesis that food-restriction-induced activation of RFRP-3-Ir cells is modulated by high levels of ovarian steroids at the time of estrus. In support of this idea, on nonestrous days, mild food restriction increased activation of RFRP-3-Ir cells, but failed to do so on the day of estrus even though this level of food restriction did not significantly decrease circulating concentrations of estradiol or progesterone. Furthermore, in ovariectomized females, food-restriction-induced increases in activation of RFRP-3-Ir cells were blocked by systemic treatment with progesterone alone, estradiol plus progesterone, but not estradiol alone. Central infusion with RFRP-3 in ad libitum-fed females significantly decreased sexual motivation and produced significant increases in 90-minute food hoarding, in support of the hypothesis that elevated central levels of RFRP-3 are sufficient to create the shift in behavioral motivation in females fed ad libitum. Together, these results are consistent with the hypothesis that high levels of ingestive motivation are promoted during the nonfertile phase of the estrous cycle by elevated activation of RFRP-3-Ir cells, and RFRP-3-Ir cellular activation is modulated by ovarian steroids around the time of estrus, thereby diverting attention away from food and increasing sexual motivation.
Key Terms: GnIH, RFRP-3, Estradiol, Food Hoarding, Male Preference, Progesterone, Progesterone Receptor, Leptin, Kp, Ingestive Behavior, Sex Behavior
1. Introduction
The female reproductive system is inhibited under conditions of low energy availability in most species studied (reviewed by [1–5]), but the underlying mechanisms differ depending on the level of energy deficit [6–10]. Severe energetic challenges, such as food deprivation, inhibit estrous cyclicity and the female hypothalamic-pituitary-gonadal (HPG) system, and the phenomenon has been well studied in species from every mammalian order, including Homo sapiens (reviewed by [1–5]). In female Syrian hamsters, total food deprivation induces anestrus [11–13], but even mild food restriction or housing at cold ambient temperatures decrease sexual motivation and increase the hunger for food [6–10]. The effects of mild metabolic challenges on behavior can be significant even when the level of metabolic deficit is insufficient to inhibit HPG function, i.e., when estrous cycles and/or circulating levels of ovarian steroids remain at the levels of ad libitum-fed females [6–10]. It might seem obvious that females would be less interested in courtship when they are hungry, however, the neuroendocrine mechanisms that set behavioral priorities under mild energetic challenges are under-studied despite the fact that they have important implications for survival and reproductive success. The present experiments were designed to test the general hypothesis that food-restriction-induced changes in sexual and ingestive motivation are mediated by changes in the activation of two RFamides, RFamide-related peptide-3 (RFRP-3) and kisspeptin (Kp). Three specific hypotheses were tested. First, we tested the hypothesis that mild food restriction (at levels known to increased ingestive motivation and decreased sexual motivation) activates kisspeptin-immunoreactive cells (Kp-Ir), RFRP-3-immunoreactive cells (RFRP-3-Ir), or both. Second, we tested that hypothesis that the effects of mild food restriction on cellular activation are modulated by ovarian steroids in gonadally-intact females during the estrous cycle and in ovariectomized females treated with estradiol, progesterone, or both estradiol and progesterone. Third we tested the hypothesis that elevated intracerebroventricular (I. C. V.) levels of (RFRP-3) are sufficient for increases in the preference for hoarding food vs. having sex in ad libitum-fed females.
The rationale for these experiments is based on the idea that energy is the most important environmental factor that controls reproduction because, in the environments in which animals live and evolve, energy availability fluctuates seasonally or unpredictably [1]. Furthermore, the energy-reproduction link is reciprocal. Energy availability influences reproductive processes, and reproductive hormones influence body weight, energy expenditure, and ingestive behavior (reviewed by [4, 14–16]). Estradiol, for example, decreases food intake in a wide variety of species (reviewed by [4, 14–16]), including Syrian hamsters [17]. Low circulating levels of estradiol would be expected to facilitate ingestive behaviors during the infertile phase of the estrous cycle, whereas estradiol-induced inhibition of food intake during the most fertile phase of the estrous cycle might facilitate attention toward sexual stimuli and thereby synchronize high levels of reproductive motivation with ovulation. It might be speculated that oscillations in ingestive behavior that are the exact opposite of oscillations in sex behavior might enhance survival and reproductive success in environment in which food availability fluctuates or is unpredictable because. These oscillations in behavioral priorities might allow females to store fuels (as a hoard or in adipose tissue) prior to engaging in reproductive activities. Together, these ideas suggest that the link between ovarian hormones and behavior might be elucidated by manipulation of the availability of food and mating partners and by measurement of the preference for food versus sex in an ecologically relevant context. Thus, in our laboratories, Syrian hamsters are either food restricted or fed ad libitum and housed in a home cage attached by two tubes. One tube leads to a food source box and the other leads to a box that contains a restrained, adult male hamster. Using this apparatus, we examined the effect of energetic status on the duration of time that female Syrian hamsters voluntarily spend with males or with food. When female preference for food versus sex is measured in this apparatus every day of the four-day estrous cycle, mild food restriction creates a fluctuation in motivation that follows fluctuations in ovarian steroid levels: Food restriction stimulates food hoarding and inhibits vaginal scent marking (a precopulatory sex behavior) and the time spent with males on the infertile days of the cycle, but these food-restriction-induced effects are absent at the time of estrus [6–10]. In summary, these foundational experiments showed that under conditions of ad libitum food availability and the absence of opposite-sex conspecifics, the effects of fluctuating ovarian hormones on appetitive sex and ingestive behavior are masked. By contrast, when the female is subjected to mild food restriction and is provided with the option to visit males, the effects of ovarian steroids on appetitive sex and ingestive behavior are unmasked.
Subsequent experiments from our laboratories suggested that the effects of mild food restriction on the preference for spending time with males might be mediated by RFRP-3 [9], the mammalian ortholog of avian gonadotropin-inhibitory hormone (GnIH) [18]. GnIH was first isolated from the brains of Japanese quail and named GnIH because it acted directly on the quail pituitary to inhibit the secretion of gonadotropins [19]. Subsequently, it was discovered that cDNAs of mammalian species encoded RFRP-3, which when injected intracerebroventricularly (I.C.V.) reduces plasma LH in a variety of mammalian species (reviewed by [20]), including ovariectomized Syrian hamsters [21]. In species other than birds and sheep, the effects of RFRP-3 on LH occur indirectly by inhibition of gonadotropin-releasing hormone (GnRH) [21–23] and occur in the context of winter day lengths (reviewed by [24–26]), stressful stimuli [27–32], and energetic deficits [33–36]. With regard to control of ingestive and sex behavior, we are interested in RFRP-3 because administration of this peptide inhibits reproductive behavior [37–39] and stimulates ingestive behavior [34, 38]. Based on these considerations, members of the Schneider and Kriegsfeld laboratories to hypothesize that food-restriction-induced changes in motivation over the estrous cycle are mediated by RFRP-3 in Syrian hamsters.
We are also interested in another RFamide, Kp. This peptide, encoded by the kiss1 gene, is synthesized in the arcuate nucleus of the hypothalamus (Arc) and in the anteroventral periventricular (AVPV) nucleus, and stimulates GnRH secretion and reproductive behavior (reviewed by [40, 41]). Kp-containing cells, like RFRP-3 and GnIH-containing cells, contain receptors for, and are regulated by, estradiol ([42] and reviewed by [41]). In contrast to GnIH and RFRP-3 cell activation, however, Kp cell activation and/or gene expression are downregulated by food restriction [43, 44], and Kp secretion is generally stimulatory for GnRH secretion and reproductive processes and inhibitory for food intake ([45, 46] and reviewed by [47–49]). In the present experiments, we explored the role of Kp and RFRP-3 in control of behavioral priorities in female Syrian hamsters.
Intracerebral RFRP-3 and Kp secretion are difficult to measure, but the function of these peptides are often inferred by measuring the activation of cells that contain the peptide. Using immunohistochemistry, cells are double-labeled for RFRP-3 and Fos or Kp and Fos. Fos is the protein product of the immediate-early gene, c-fos, and the percent of cells double-labeled with Fos (relative to that in a baseline condition) is an established marker for cellular activation correlated with neuropeptide secretion [50–52]. In Syrian hamsters, increasing the duration of food restriction results in increasing levels of food hoarding and RFRP-3 cell activation in the DMH, and these effects on food hoarding and RFRP-3 cell activation are reversed by re-feeding [9]. Given this clear effect of food restriction on activation of RFRP-3 cells, we hypothesized that the food restriction-induced increase in ingestive behavior and decrease in preference for males during the infertile phase of the cycle is mediated by increases in RFRP-3 secretion. Thus, we predicted that during the infertile phase of the cycle, food-restricted females would show elevated activation of RFRP-3-Ir cells. Another possibility is that the effects of food restriction are mediated by decreases in activation of Kp-Ir cells. We also hypothesized that food restriction-induced activation of RFRP-3-Ir cells would be blocked at the time of ovulation in intact females and in ovariectomized females treated with estrus levels of ovarian steroids. In addition, we hypothesized that increases in periovulatory hormones might decrease ingestive behavior and increases sex behavior by increasing secretion of the anorexigenic hormone, leptin, and thus, plasma leptin concentrations were measured. We measured concentrations of plasma leptin and ovarian steroids and the percentage of hypothalamic RFRP-3 and Kp cells labeled with Fos in female Syrian hamsters that were either fed ad libitum or restricted to 75% of their ad libitum daily intake over the estrous cycle and in ovariectomized females treated with estradiol, progesterone, both steroids, or a vehicle. The results showed a close association between behavior and RFRP-3, but not Kp, activity. Thus, to determine whether increases in central RFRP-3 are sufficient for increases in food hoarding or decreases in sexual motivation, we measured ingestive and sex behavior in females that were either food restricted or fed ad libitum and received chronic I.C.V. infusions of RFRP-3 or saline.
2. Materials and Methods
2.1. General Methods
2.1.1. Animals and Housing
Adult, female Syrian hamsters were purchased from Charles River Breeding Laboratory or obtained from a colony bred at the Lehigh University animal facility (previous generations of colony animals were either obtained from a breeding colony from Cornell University (Ithaca, NY, USA), Harlan Laboratories (Indianapolis, IN, USA), or Charles River Breeding Laboratories (Wilmington, MA, USA). Animals were fed Harlan Rodent Chow #2016 ad libitum, unless otherwise noted and water was available ad libitum. Subjects were singly housed in opaque, Nalgene cages (31 × 19 × 18 cm) in a room maintained at 22 ± 1 °C with a 14:10 light-dark cycle (lights on at 2200h, unless otherwise noted). Experiments were conducted according to the guiding principles for research published by the National Institutes of Health, the Lehigh University Institutional Animal Care and Use Committee, and enforced by the United States Department of Agriculture.
2.1.2. Hamster estrous cycles
The Syrian hamster estrous cycle is four days long and ends with estrous behavior and ovulation the afternoon/evening of the fourth day. Day 4, the day of estrus and ovulation, is termed the “periovulatory day” in hamsters (which corresponds to “proestrus” in rats). In our experiments, estrous cycle day 1 is termed the “postovulatory day.” Day 2 is termed “follicular day 1.” Day 3 is termed “follicular day 2.” In Syrian hamsters, appetitive sex behaviors, such as seeking out a male and vaginal scent marking, begin to appear on the evening of the postovulatory day, continue to rise on the next two days of the estrous cycle, and peak on follicular day 2. Lordosis, by contrast, occurs only on the periovulatory day [53].
Prior to being included in the experiment, hamsters were required to show at least two consecutive 4-day estrous cycles as determined by a positive test for sex behavior (the lordosis posture) with a sexually-experienced male hamster. Syrian hamsters, on the evening of estrus and ovulation, stand motionless in the arched-back, tail-elevated lordosis posture beginning seconds to minutes after introduction to a male. The female stands frozen in the posture for 10 or more consecutive minutes, thereby allowing the male to mount and intrommit the penis. The incidence of lordosis was recorded manually by the experimenter. On the other three days of the cycle, the female does not show lordosis. The female was considered “negative” for lordosis if she failed to show lordosis within 10 minutes of introduction to the male. Prior to the start of the experiment, tests for lordosis occurred within 1 hour of the onset of the dark phase of the photoperiod each day. Animals that did not display two consecutive, four-day estrous cycles were removed from the experiment. During the experiment, estrous cycles were monitored by examination of vaginal discharge (according to the method described by Orsini [54]) to ensure that no animals became anestrus as a result of food restriction. The first day of the estrous cycle is designated as the day the experimenter observed the copious, pungent, discharge characteristic of the day after ovulation, which is observed upon palpation of the vagina by the experimenter [54].
2.1.3. Baseline body weight and food intake
For six days before the start of each experiment, daily body weight and food intake were measured (an experimenter weighed the food in the home cage each day at the same time). Animals were fed regular chow pellets ad libitum (18–20 grams of laboratory chow per day) place inside on the floor of the cage.
2.1.4. Mild Food Restriction
For each individual, the daily baseline ad libitum food intake (averaged over four days) was used to calculate each individual animal’s ration during food restriction, which consisted of 75% of its individual daily intake. To limit the period of time subjects were without food, the food-restricted hamsters received half of their daily ration at two time points during the day. These rations were given at irregular intervals at least 8 hours apart so as not to entrain food anticipatory behavior [55]. The last ration was given between 8 and 4 hours before testing. At the beginning of each experiment, animals were divided into groups that did not differ significantly in body weight.
2.2. Experiment 1: Activation of RFamide Cells Over the Estrous Cycle
2.2.1. Experimental Design
The first experiment tested the hypothesis that food-restriction-induced increases in RFRP-3 and Kp cell activation are modulated by natural changes in ovarian steroids during the estrous cycle. Specifically, we tested the hypothesis that food-restriction-induced increases in the activation of RFRP-3-Ir and Kp-Ir cells are modulated at the time of estrus when circulating levels of ovarian steroids are high. We also measured plasma leptin concentrations to test the hypothesis that ovarian steroids modulate circulating concentrations of leptin over the estrous cycle.
Female hamsters (described in the general methods) were either fed ad libitum or food-restricted, as previously described, for eight days. Subjects were further divided into groups representing the four days of the hamster estrous cycle (n=10/group; total of 8 groups), and the onset of food restriction was timed so that the eighth day of food restriction coincided with the designated day of the estrous cycle. Each day that blood and brains were sampled contained hamsters representing each day of the estrous cycle. Blood and brains were collected at the onset of the dark phase of the late-dark cycle on the 8th day of food restriction. The length of food restriction was chosen to mimic those used in previous experiments, in which these energetic manipulations influenced appetitive sex and ingestive behavior [7, 39]. The experimental groups and the timeline for the procedures are illustrated in Figure 1.
Figure 1.
A diagram of the chronology of experiment 3.1. Animals were either fed ad libitum or food restricted (75% of ad libitum baseline food intake) for a period of 8 days. At the end of the dietary treatment, animals were sacrificed in groups representing every day of the hamster four-day estrous cycle.
2.2.2. Blood Collection and Perfusion
At the end of the treatment period, animals were deeply anesthetized with a lethal intraperitoneal (IP) injection of pentobarbital sodium (200 mg/kg of body weight) at the onset of the dark phase of the light-dark cycle. Three mL of blood were collected by cardiac puncture and serum was extracted.
After blood collection, animals were perfused first with heparin, then 0.9% saline, and then by 4% paraformaldehyde in phosphate buffer (all solutions at room temperature) by intracardiac infusion. Brains were carefully removed and postfixed in 4% paraformaldehyde for 3 hours at 4°C and transferred for cryoprotection in 30% sucrose for 3 days. Tissue was snapfrozen and stored at −80°C until sectioned. Tissue was sectioned at 40 µm using an E505 Microm cryostat and was stored in a polyvinylpyrrolidone (PVP) solution at −20°C [56].
2.2.3. Estradiol and Progesterone Radioimmunoassay
Serum was analyzed for estradiol and progesterone concentrations by radioimmunoassay (RIA) (TKE21 and TKPG2, Simens Medical Solutions Diagnostics, Los Angeles, CA, USA). Samples were run in duplicate. The reportable range for the estradiol assay was 12.3–1700.0 pg/mL. The intra-assay coefficient of variation (CV) was 6.1, and the inter-assay %CV was 8.9. For the progesterone assay, plasma was diluted to a 1:10 concentration in 0.1M PBS with 1% BSA to allow values to lie within the reportable range of 0.07–22.0 ng/mL. The intra-assay %CV was 3.6, and the inter-assay %CV was 9.0. The assays for estradiol and progesterone were conducted by the Ligand Assay and Analysis Core at the Center for Research on Reproduction at the University of Virginia (Charlottesville, VA, USA).
2.2.4. Leptin and Insulin Radioimmunoassay
Serum was analyzed for leptin using the Mouse Leptin enzyme-linked immunosorbent assay (ELISA) kit (Millipore, St. Charles, MO, USA). Plasma samples were run in duplicate. The reportable range for the leptin assay was 0.2–30 ng/mL. The inter-assay %CV was 6.5–8.7 and the intra-assay %CV was 2.8–3.6. The assay for leptin was conducted by EMD Millipore (Millipore, St. Charles, MO, USA).
2.2.5. Immunohistochemistry
For measuring activation in RFRP-3-containing cells, every fourth section was used for fluorescence immunohistochemical (IHC) staining with primary antibodies for RFRP-3 and Fos. Tissue was washed in 0.1M phosphate buffered saline (PBS) and then incubated with a 0.55% hydrogen peroxide (H2O2). Tissue was subsequently incubated in a normal goat serum (1:50 Jackson ImmunoResearch Laboratories; West grove, PA, USA) suspended in 0.1% Triton-X100 (PBT) for a period of 1 hour at room temperature. Sections were then incubated in a 1:40,000 dilution of rabbit anti-Fos (SC-52, Santa Cruz biotechnology; Santa Cruz, CA, USA) for a period of 48 hours at 4°C. To amplify Fos signaling, tissue was washed in phosphate buffered saline with Triton-X (0.1%) (PBT) before it was incubated for 1 hour with biotinylated goat anti-rabbit (BA-1000, 1:300 Vector Laboratories; Burlingame, CA, USA) at room temperature. Afterwards, sections were allowed a 1-hour incubation period with avidin-biotin-horseradish peroxidase complex (PK-6100, ABC Kit, Vector Laboratories, Burlingame, CA, USA) and then subjected to a 0.6% biotinylated tyramide (SAT700B001EA, Perkin Elmer, Waltham, MA) solution for exactly 30 minutes at room temperature. The following steps were performed with as little contact with light as possible. Cy-2 Conjugated Streptavidin (016-220-084, 1:200 Jackson Immunoresearch Laboratories) was used as the fluorophore to label cells containing Fos for a period of 1 hour at room temperature. Tissue was subsequently washed with a PBS solution before being blocked for an hour in normal donkey serum (1:66 Jackson ImmunoResearch Laboratories). The second primary antibody was generated by the Kriegsfeld laboratory and is specifically directed against RFRP-3 of the Syrian hamster and does not show cross reactivity with other RFamides [57]. The sections were incubated in RFRP-3 antiserum (1:5000 PAC1365) for a period of 48 hours at 4°C. The specificity of the PAC1365 anti-RFRP-3 has been confirmed previously in this species [57]. After incubation, tissue was washed in PBT and RFRP-3 was fluorescently labeled with CY-3 Donkey anti-rabbit (711-165-152, 1:500 Jackson Immunoresearch Laboratories) as the secondary anti-body and fluorophore for a period of 1 hour at room temperature. For all double labeling approaches, we did not observe instances where the second secondary detected the first primary. In previous work [57, 58], preadsorption controls for Kp and RFRP-3 confirming specificity. In the present experiments, omission of the primary antibodies eliminated staining. The sections were mounted on gelatin-coated slides and allowed to dry overnight. Slides were coverslipped using a dehydration and clarification protocol using ethanol and xylene.
For measuring activation of Kp-containing cells, every fourth section was used for fluorescence IHC staining with primary antibodies for Kp and Fos. IHC was performed as above but for the following differences. The primary antibody was obtained from the Mikkelsen laboratory and was validated for specificity in Syrian hamsters [59]. Sections were incubated in a 1:2000 dilution of rabbit polyclonal anti-kisspeptin-10 antiserum for a period of 48 hours at 4°C then amplified using the same procedure as above, followed by staining with Fos and slide preparation as above.
2.2.6. Light Microscopy
Sections were examined using a Nikon Eclipse E800 microscope. Labeling was examined using the filters for the fluorophores, CY-2 (488 nm) and Cy-3 (568 nm). Areas of the DMH that contained RFRP-3-Ir were digitally captured by a Diagnostic Instruments 7.2 Color Mosaic digital camera. Without changing the microscope focus, but changing the filter, an additional image was captured for Fos-Ir. The two images were merged and then analyzed using Photoshop to count the total number of RFRP-3-Ir cells (with or without Fos-Ir and RFRP-3-Ir cells containing Fos-Ir). Photoshop allowed independent visualization of the green and red channels. Colocalization of RFRP-3/Fos-Ir was confirmed by two observers blind to the experimental conditions.
Sections labeled for Kp and Fos were visualized using a Zeiss Z1 microscope (Thornwood, NY). Labeling was examined using the filters for CY-2 (488 nm) and Cy-3 (568 nm). Areas of the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus of the hypothalamus (ARC) containing Kp-Ir cells were digitally captured using a cooled CCD camera (Zeiss). Without changing the microscope focus but changing the filter, an additional image was captured for Fos-Ir. The two images were merged and then analyzed with ImageJ to count the total number of Kp-Ir cells and Kp-Ir cells containing Fos-Ir. Colocalization of Kp/Fos-Ir was confirmed by two observers blind to the experimental conditions.
2.3. Experiment 2: Effects of Estradiol and Progesterone on Activation of RFRP-3 Cells
2.3.1 Experimental Design
Given the results of experiment 1 in intact females, we hypothesized that food-restriction-induced activation of RFRP-3-Ir cells would be modulated by one or both of the ovarian steroids in ovariectomized females. To test this hypothesis, ovariectomized females were food restricted and treated with a dose of estradiol alone, progesterone alone, or estradiol plus progesterone and then euthanized and their brains processed for Fos-Ir/RFRP-3-Ir. The treatment doses and timing were selected for their ability to affect behaviors of interest based on the results of our previous experiments.
2.3.2. Experimental Design, Surgery and Treatment
Forty female Syrian hamsters were anesthetized by sodium pentobarbital (100 mg/kg of body weight, IP) and received bilateral ovariectomy (OVX). Two weeks after surgery, animals were either food restricted (75% of their ad libitum daily intake) or fed ad-libitum. Females were further divided into four additional groups (n=8/group) in order to examine the effects of estradiol and progesterone on RFRP-3 cellular activation during conditions of mild energetic challenge. One group of OVX hamsters received a subcutaneous (S.C.) injection of 2.5 µg of estradiol benzoate, dissolved in 0.1 mL canola oil, 48 hours before tissue collection. All other groups received a S.C. injection of the oil vehicle. Six hours before tissue collection, females that were pretreated with estradiol and one group of females pretreated with oil were treated with a S.C. injection of 500 µg of progesterone, dissolved in 1.0 mL canola oil (both steroids purchased from Sigma Aldrich). One group of females that were pretreated with oil received a S.C. injection of estradiol benzoate. The remaining control females received a S.C. injection of oil during this time period. The treatments and timeline are illustrated in Figure 2.
Figure 2.
A diagram of the treatment schedule for experiment 3.2. Animals were either fed ad libitum or food restricted (75% of ad libitum baseline food intake) for a period of 8 days before animals were perfused. Animals were given a S.C. injection of either vehicle or 2.5 µg of estradiol benzoate 48 hours before perfusion. Animals that were treated with estradiol and one group treated with oil were given an injection of 500 µg of progesterone 6 hours before perfusion. The remaining groups received a S.C. injection of either vehicle or estradiol benzoate.
At the end of the treatment period, females were deeply anesthetized with an overdose of sodium pentobarbital (200 mg/kg of body weight, IP), as previously described, at the onset of the dark phase of the light-dark cycle. Perfusions were performed as previously described and brains were section using an E505 Microm cryostat. Sections were stored in a PVP solution at −20°C. Tissue was processed for RFRP-3-Ir and Fos-Ir by IHC, as previously described. Light microscopy was used to determine colocalization of RFRP-3-Ir and Fos-Ir as previously described.
2.4. Experiment 3: Localization of Progestin Receptors on RFRP-3-containing Cells
2.4.1. Experimental Design
This experiment was designed to test the hypothesis that progesterone exerts a direct action on RFRP-3 cellular activation via progesterone receptor (PR). Co-localization of RFRP-3-Ir with PR-Ir was quantified. Five female Syrian hamsters (125– 142 grams) received bilateral OVX as previously described in experiment 2. Females were given a two-week recovery period to allow for clearance of circulating ovarian hormones. Females received a subcutaneous (S.C.) injection with 5 µg of estradiol benzoate in 0.5 mL oil vehicle, a treatment that induces progesterone receptor expression in this species [60]. After 48 hours, hamsters were perfused and brains were collected as previously described. Tissue was sectioned with a cryostat as previously described in experiment 2.
2.4.2. Immunohistochemistry
Every fourth section was used for florescence IHC staining with primary antibodies for PR and RFRP-3. Tissue was washed in 0.01M PBS and then incubated with a 0.55% H2O2. Tissue was subsequently incubated in a normal goat serum (1:120 Jackson ImmunoResearch Laboratories) suspended in PBT for a period of 1 hour at room temperature. Sections were incubated in a 1:10,000 dilution of rabbit polyclonal anti-human PR antiserum (DAKO Inc.; Carpinteria, CA, USA) for a period of 48 hours at 4°C. To amplify PR signaling, tissue was washed in PBT before it was incubated for 1 hour with biotinylated goat anti-rabbit (1:200) at room temperature. Afterwards, sections were allowed a 1-hour incubation period with avidin-biotin-horseradish peroxidase complex and then subjected to a 0.6% biotinylated tyramide solution for exactly 30 minutes at room temperature. The following steps were performed with as little contact with light as possible. Cy-2 Conjugated Streptavidin (1:133) was used to label cells containing PR for a period of 1 hour at room temperature. Tissue was subsequently washed with a PBS solution before being blocked for an hour in normal donkey serum (1:120 Jackson ImmunoResearch Laboratories). The sections were incubated in RFRP-3 antiserum (1:5000 PAC1365) for a period of 48 hours at 4°C. After incubation, tissue was washed in PBT and RFRP-3 was fluorescently labeled with CY-3 Donkey anti-rabbit (1:200 Jackson Immunoresearch Laboratories) for a period of 1 hour at room temperature. The sections were mounted on gelatin coated slides and allowed to dry overnight. Slides were coverslipped using a dehydration and clarification protocol using ethanol and xylene.
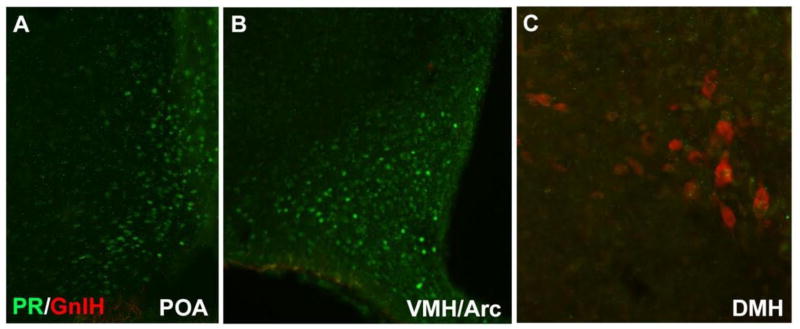
Sections labeled for RFRP-3 and PR were visualized and captured according to the same procedure used for Kp-Fos described above. In addition, areas outside of the DMH, for example the preoptic area (POA), ventromedial hypothalamus (VMH), and Arc, were examined and digitally captured for PR-Ir as a positive control.
2.5. Experiment 4: Intracerebral Infusion of RFRP-3 and Behavior
Given the close association between motivation and activation of RFRP-3-Ir cells and the lack of association with activation of Kp-Ir cells, we hypothesized that food hoarding and preference for spending time with food vs. males would be increased by elevating brain levels of RFRP-3. If so, continuous I.C.V. infusion of RFRP-3 would increase appetitive ingestive behaviors and reduce appetitive sex behaviors in ad libitum-fed female Syrian hamsters.
2.5.1. The Preference Apparatus and Behavioral Testing
A preference apparatus was designed to mimic important aspects of the hamsters' natural habitat and was used to test both appetitive and consummatory aspects of sex and ingestive behavior. In the wild, Syrian hamsters are solitary, live in deep burrows, are active above ground for short time periods, and travel long distances to their food source, filling their large cheek pouches with food, and transporting the food back to their burrows [61]. At least one feral female has been observed in the wild mating near the entrance to her burrow [61]. Thus, the preference apparatus consisted of a home cage that house one female subject with tunnels leading in one direction to the food source box and in the other direction to a box that contained an adult, sexually-experience male hamster. Subject females lived in the home cage when they were not being trained or tested.
Preference tests occurred during the 90 min period that began at the onset of the dark phase of the light-dark cycle. The timing of the preference test was dictated by a combination of the observations made of Syrian hamsters living in the wild and in our laboratory. In their natural habitat, females spend about 90 min per day outside the burrow at dawn and dusk, and their time outside the burrow is thought to be limited due to predators or climate [61]. In the laboratory, they are known to be nocturnal. As determined in previous experiments [7, 10], when males are present, appetitive sex behaviors peak within the first 15 minutes after lights out, and most food hoarding occurs within 90 minutes after the onset of the dark phase of the light-dark cycle.
The home cage had the same dimensions and the same food and water as previously described. When opened, a door at one end of the cage led to a 134 cm-long tube, which led from the home cage to two horizontal tubes 40–50 cm in length. These latter tubes were connected in a T configuration with the home cage at the bottom of the T. At the top of the T, there was a food source box on the left and a box containing a male hamster on the right.
One of the horizontal tubes in the T-configuration was connected to the male box, which was made of clear Plexiglass (27 × 20 × 15 cm). The male box contained an adult, sexually experienced male Syrian hamster, no food or water, plus 50 mL of male bedding. This male bedding was collected prior to the start of the experiment from 6 or more different soiled home cages, mixed, and kept frozen. Just prior to the start of each preference test, 50 mL of this bedding mixture was sprinkled in each of the male boxes to standardize the pheromonal contributions of the male stimulus across all female subjects. Within the male box, the male hamster was restrained within a smaller wire box that permitted olfactory, gustatory, auditory, visual, and limited tactile interaction. The male stimulus hamster could neither leave the male box, nor could he fight or mate with the female.
The second horizontal tube was connected to the food box, a disk-shaped, clear plastic chamber that contained a weighed amount of hoardable pellets but no water. Hoardable pellets were made from standard laboratory chow pellets cut into 2 cm pieces, a size that readily fits into Syrian hamster cheek pouches and allows the females to travel through the tubes with their cheek pouches full.
The apparatus described above could be modified to assess food hoarding independent of the presence of the male by closing off access to the male.
2.5.2. Experimental design and procedures
Female, estrous cycling Syrian hamsters were acclimated to their home cages for two weeks prior to testing to reduce the tendency to sleep, hoard food, or attempt to inhabit any other compartment of the apparatus during testing. After the period of acclimation to the home cage, females were trained to expect food in the food source box and males in the male cage. The timeline of the training period, baseline testing, surgery/treatment, and experimental testing is illustrated in Figure 3. During training, access to only the food source was allowed on the first two days of the estrous cycle and access to only the male cage was allowed on the remaining days of the cycle (day 3 and day 4). The following two days, animals were allowed access to both male and food compartments.
Figure 3.
A diagram of the chronology of experiment 4.3. Subjects were implanted with osmotic minipumps that infused either Saline or RFRP-3 into the lateral ventricles two days later. Animals were further divided into groups that were either fed ad libitum (AD) or food restricted (FR) (75% of ad libitum baseline food intake) starting 3 days after surgery. Eight days later, animals were tested in the choice apparatus (on day 3 of the estrous cycle) and in the hoarding apparatus (on day 1 of the estrous cycle).
Females were tested for baseline behavior first in the choice apparatus with a stimulus male, and then in the hoarding apparatus without a male for assessment of long-term food hoarding (24 hours). Animals were tested within the choice apparatus on follicular Day 2 of their estrous cycle. According to previously published experiments, vaginal scent marking and male preference is highest during this period in this species [62]. The subjects were tested in the choice apparatus for 90 minutes starting at the beginning of the dark phase of the light-dark cycle. For the first 15 minutes of this period, observers recorded the females’ behaviors every 5 seconds. Animals were allowed access to both the food source and the male for an additional 75 minutes. After the 90-minute choice test, access to the choice apparatus was removed and the weight of the food was measured in the home cage and the food cage to determine the amount of food eaten and/or hoarded.
Two days later, animals were tested within the hoarding apparatus during the postovulatory phase of the estrous cycle. Previous work has demonstrated that the effects of food restriction on food hoarding are highest during this this period. At the onset of the dark phase of the light-dark cycle, animals were only allowed access to the food compartment. Ingestive behaviors were measured 90 minutes and 24 hours after access was allowed. After behavioral baseline testing, entrance to the choice/hoarding apparatus was blocked until animals are ready for experimental testing.
2.5.3. Preparation of Osmotic Pump and Surgery
All animals were implanted with cannula (Plastics One, Roanoke, VA) aimed at their lateral ventricle two days after baseline testing. The osmotic pump (Alzet, Cupertino, CA: Model 2002), which was implanted SC in the intrascapular region, is connected to the cannula by 2 cm of polyethylene 50 clear (PE50) plastic tubing (Alzet, Cupertino, CA). Using a specialized filling tube, the osmotic pump, flow modulator and catheter tubing were filled with either 0.9% saline, or a concentration of RFRP-3 (Rat RFamide-related peptide; ANMEAGTMSHFPSLPQRF; [63]) that allowed us to deliver 50 ng/hr, resulting in 600 ng/day. This treatment was found to inhibit reproductive behavior without significant effect on luteinizing hormone concentrations in Syrian hamsters [39]. To prevent contamination and to prime the osmotic pumps, the cannula were soaked in an aqueous solution of 70% ethanol and then incubated in a 0.9% sterile saline solution at 37°C for a period of 4–6 hours before surgery.
Prior to cannulation surgery, hamsters were given Metacam, the nonsteroidal postoperative analgesic, and were anesthetized with isoflurane vapors and placed in a stereotaxic apparatus (David Kopf Instruments, Tujanga, CA). Cannulas were aimed at the following coordinates: 1.1 mm anterior to bregma, 1.0 mm lateral to the midline, and 4.0 mm ventral to bregma. Cannulas were permanently fixed with dental cement (Stoelting, Wood Dale, IL) and 3 screws.
2.5.4. Behavior Testing
Three days after surgeries, animals were further divided into groups (n = 8/group) that were either fed ad libitum or food restricted as described for 8 days. As previously described, estrous cycles were examined during the duration of the experiment. After 8 days, female hamsters were tested in the choice apparatus during follicular 2 phase of the estrous cycle in the choice apparatus. After testing, the amount of food consumed during the testing period was removed from their daily ration and food restriction continued until the 24 hour hoarding test two days later (Figure 3).
2.5.5. Verification Cannulation placement
After testing, animals were injected with an overdose of sodium pentobarbital (200 mg/kg of body weight, IP). Cannula placement was tested by an I.C.V. injection of 0.1 µL of fluorescent retrobeads (Lumafluor, Durham, NC). Brains were sectioned and examined for fluorescent labeling within the ventricular system. Animals absent of labeling were removed from the experiment. Osmotic pumps were extracted and tested for infusion rate by examining pump volume displacement.
2.6. Statistical Analysis
In experiments 1 and 2, data were analyzed using a two-way analysis of variance (ANOVA). The main effects were day of estrous cycle and feeding regimen (food restriction or fed ad libitum) in Experiment 1 and hormone infusion (RFRP-3 or saline) and feeding regimen in Experiment 2. This two-way ANOVA was used to determine the differences in immunoreactivity, circulating hormone concentrations, and body weight. The percent of cellular activation of RFRP-3-Ir cells was calculated by (total number of cells double labeled with RFRP-3-Ir and Fos-Ir divided by the total number of RFRP-3-Ir cells) multiplied by 100. When main effects were significant, a Tukey’s post hoc comparison was conducted. Differences were considered significant if P was less than 0.05.
In experiment 4, data were analyzed using a two-way ANOVA on behavioral data collected during the choice test and white adipose tissue (WAT) pad mass. Behavioral data scores were transformed to the natural log of raw score + 1, so that data would meet the assumption of normal variance and homoscedasticity. Male preference was calculated as ((the time spent with males minus the time spent with food) divided by the total time). To analyze long-term food hoarding after infusion of RFRP-3 or saline, we used a mixed-model ANOVA (feeding regimen × infusion × time) with time as a repeated measure. If there was an interaction of all three factors, a two-way ANOVA (feeding regimen × time) was performed at both time points. A one-way ANOVA with repeated measures was used to examine treatment effects on body weight and food intake. A one-way ANOVA with two groups was used to analyze change in body weight (final body weight minus initial body weight). When there was a significant main effect, a Tukey’s post hoc comparison was conducted to determine whether there were differences between two groups. If equal variance was not assumed, a Games-Howell post hoc comparison was performed [64]. Differences were considered significant if P was less than 0.05.
For all of the experiments, the effect sizes were calculated using eta-squared (η2), and the Pearson’s correlation coefficient (r) are shown for correlations between two measured variables.
3. Results
3.1. Experiment 1: Activation of RFamide Cells Over the Estrous Cycle
3.1.1. Body weight and serum leptin concentrations
Body weight significantly decreased after food restriction, but the body weight loss did not vary over the estrous cycle (Table 1). The two-way ANOVA performed on body weight yielded a significant main effect of feeding regimen (75% food restriction vs. ad libitum access to food) (F (1, 61) = 30.291, P < 0.001; η2 = 0.331) (Table 1). The analysis yielded no significant effect of estrous cycle day and no significant body weight X estrous cycle day interaction. When the change in body weight was calculated by subtracting the terminal body weight from the body weight at the start of restriction, a two-way ANOVA showed a significant main effect of feeding regimen on the body weight change (F (1, 62) = 128.034, P < 0.001; η2 = 0.676). Hamsters that were food restricted for 8 days lost significantly more weight than animals that were fed ad libitum. The analysis yielded no significant effect of estrous cycle day and no significant body weight X estrous cycle day interaction.
Table 1.
(A) Mean and standard error of the mean for terminal body weight and body weight change in female Syrian hamsters either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days from animals in Experiment 1. (B) Mean and standard error of the mean for (left) terminal body weight (right) and body weight change in female Syrian hamsters that were either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days and either treated with estradiol, progesterone, or a combination of both in Experiment 2.
| Ad libitum-fed | Food-restricted | |||||||
|---|---|---|---|---|---|---|---|---|
| A. Variable/Estrou s Cycle Day | Follicular 1 | Follicular 2 | Periovulatory | Postovulatory | Follicular 1 | Follicular 2 | Periovulatory | Postovulatory |
| Terminal Body Weight (g) | 141.16 ± 4.86 | 134.92 ± 2.51 | 139.61 ± 9.69 | 137.19 ± 7.26 | 125.09 ± 7.8 | 125.24 ± 9.37 | 130.01 ± 5.72 | 122.48 ± 9.7 |
| Body Weight Change (g) | 2.66 ± 2.29 | 3.34 ± 0.59 | 3.98 ± 2.72 | 6.07 ± 0.95 | −11.36 ± 1.60 | −11.31 ± 0.66 | −7.54 ± 2.39 | −11.26 + 1.81 |
| Ad libitum-fed | Food-restricted | |||||||
|---|---|---|---|---|---|---|---|---|
| B. Variable/Hormone Treatment | Vehicle | Estradiol | Estradiol + Progesterone | Progesterone | Vehicle | Estradiol | Estradiol + Progesterone | Progesterone |
| Terminal Body Weight (g) | 153.24 ± 4.88 | 156.11 ± 4.22 | 144.33 ± 4.41 | 147.59 ± 1.78 | 133.95 ± 4.46 | 136.88 ± 5.18 | 132.11 ± 3.54 | 130.29 ± 2.78 |
| Body Weight Change (g) | 8.92 ± 1.74 | 5.17 ± 1.84 | 6.62 ± 1.47 | 6.74 ± 1.74 | −8.87 ± 2.67 | −10.25 ± 1.85 | −9.64 ± 1.51 | −9.65 + 1.53 |
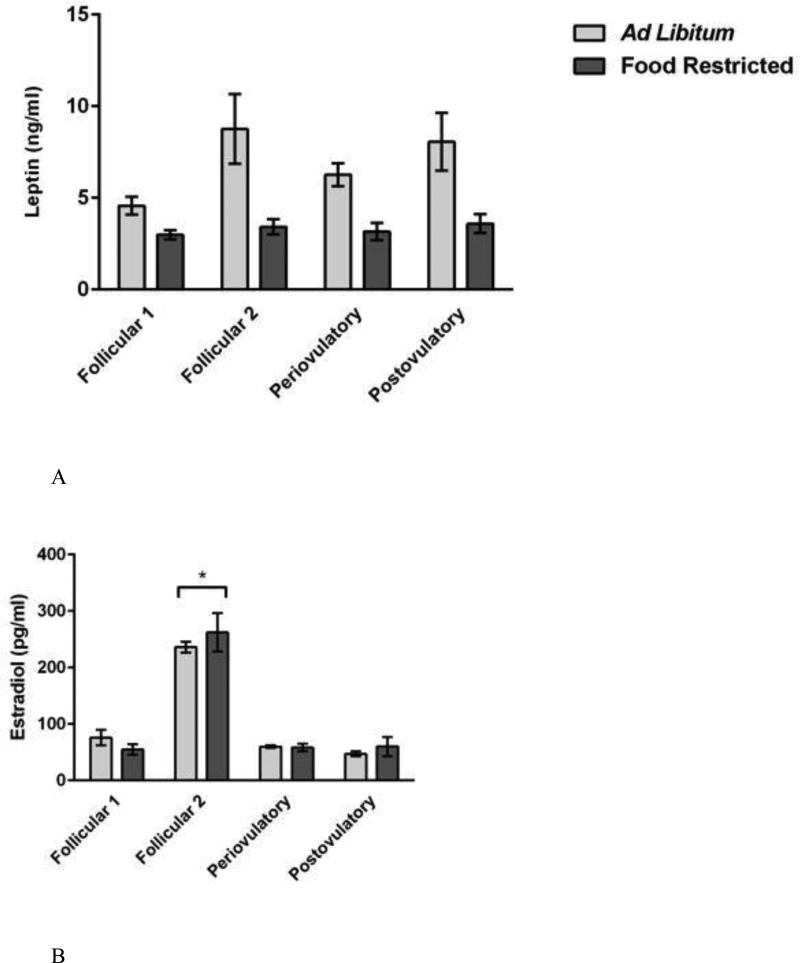
Leptin concentrations were significantly decreased by food restriction, but the results did not vary over the estrous cycle (Figure 4A). The two-way ANOVA performed on serum leptin concentrations yielded a significant main effect of feeding regimen (F (1, 61) = 33.158, P < 0.001; η2 = 0.322) and of estrous cycle day (F (3, 59) = 3.225, = P < 0.05; η2 = 0.094) (Figure 4A). Post hoc comparisons shows that serum leptin concentrations were significantly lower in food-restricted compared to ad libitum-fed females on each day of the estrous cycle (P < 0.05) (Figure 4A). Post hoc comparisons failed to find significant differences in serum leptin concentrations among groups sampled on different days of the estrous cycle. This analysis yielded no significant feeding regimen X estrous cycle day interaction.
Figure 4.
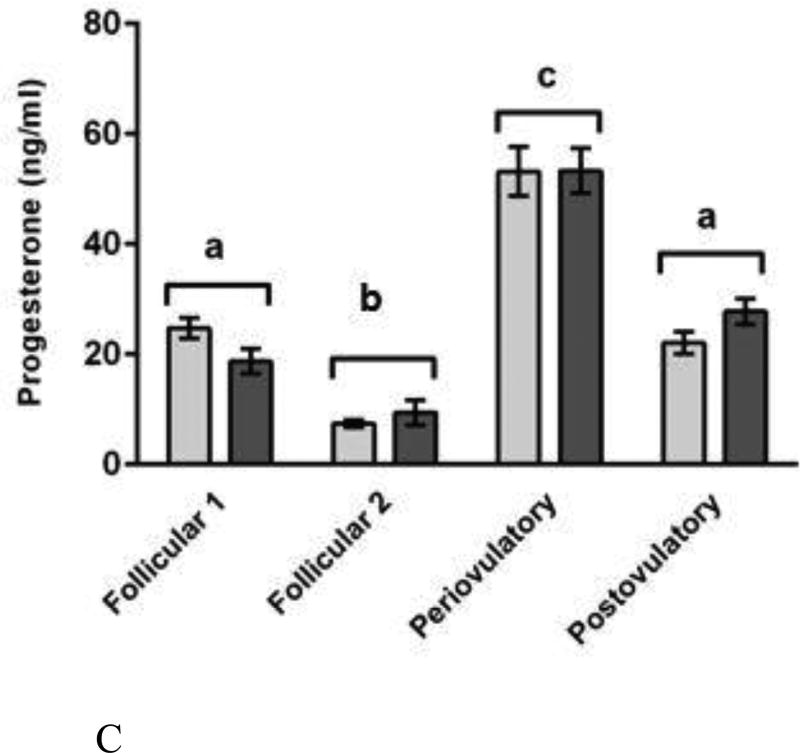
Mean and standard error of the mean for serum concentrations of (A) leptin (top), (B) estradiol (bottom-left), and (C) progesterone (bottom-right) in female Syrian hamsters either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days. Terminal blood was collected from groups representing every day of the four-day estrous cycle. Plasma was analyzed for estradiol and progesterone by radioimmunoassay (RIA). Plasma was analyzed for leptin using an enzyme-linked immunosorbent assay (ELISA) kit. * = significantly different at P < 0.05.
Serum leptin concentrations were positively correlated with body weight (r = 0.596; p < 0.001) and change in body weight (r = 0.392; p < 0.01), and these correlations were statistically significant. The correlations among serum leptin concentrations and the levels of other hormones were not significant (estradiol, progesterone, total number of RFRP-3-Ir cells, and total number of RFRP-3-Ir cells with Fos-Ir). In one experimental subject, the volume of serum was insufficient to measure leptin concentrations. As a result, the sample sizes were as follows: ad libitum/follicular 1 (n = 8), ad libitum/follicular 2 (n = 8), ad libitum/periovulatory (n = 8), ad libitum/postovulatory (n = 7), food-restricted/follicular 1 (n = 8), food-restricted/follicular 2 (n = 8), food-restricted/periovulatory (n = 8), food-restricted/postovulatory (n = 8).
3.1.2. Serum estradiol and progesterone concentrations
Ovarian hormone concentrations changed significantly over the estrous cycle but were not significantly decreased by food restriction (Figure 4B and 4C). The two-way ANOVAs on ovarian steroid hormone concentrations yielded a significant main effect of estrous cycle day on serum concentrations of estradiol (F (3, 59) = 72.445, P < 0.001; η2 = 0.79) and progesterone (F (3, 59) = 90.252, P < 0.001; η2 = 0.819) (Figure 4B and 4C, respectively), and the effect sizes were large and the mean values were as expected based on the known levels in estrous cycling females of this species [65]. Post hoc analysis showed that mean estradiol concentrations were significantly higher on the night before estrus than on any other night of the estrous cycle (P < 0.05), and those of progesterone were significantly higher on the night of ovulation and significantly lower on the night before ovulation (both P < 0.05) compared to all other days of the estrous cycle (Figure 4B and 4C). This analysis yielded no significant effect of the feeding regimen on circulating concentrations of estradiol (F (1, 61) = 0.134, P > 0.05; η2 = 0.001) or progesterone (F (1, 61) = 0.048, P > 0.05; η2 = 0.001). We found no significant feeding regimen X estrous cycle day interaction on circulating concentrations of estradiol (F (3, 59) = 0.835, P > 0.05; η2 = 0.009) or progesterone (F (3, 59) = 1.546, P > 0.05; η2 = 0.14).
Estradiol and progesterone concentrations were not significantly correlated with any other variables (leptin concentration, body weight, total number of RFRP-3-Ir cells, and total number of RFRP-3-Ir cells with Fos-Ir, and percent of RFRP-3-Ir cells with Fos-Ir). In one experimental subject, the volume of serum was insufficient to measured estradiol and progesterone concentrations. As a result, the sample sizes were as follows: ad libitum/follicular 1 (n = 8), ad libitum/follicular 2 (n = 7), ad libitum/periovulatory (n = 8), ad libitum/postovulatory (n = 8), food-restricted/follicular 1 (n = 8), food-restricted/follicular 2 (n = 8), food-restricted/periovulatory (n = 8), food-restricted/postovulatory (n = 8).
3.1.3. RFRP-3 immunoreactivity and cellular activation
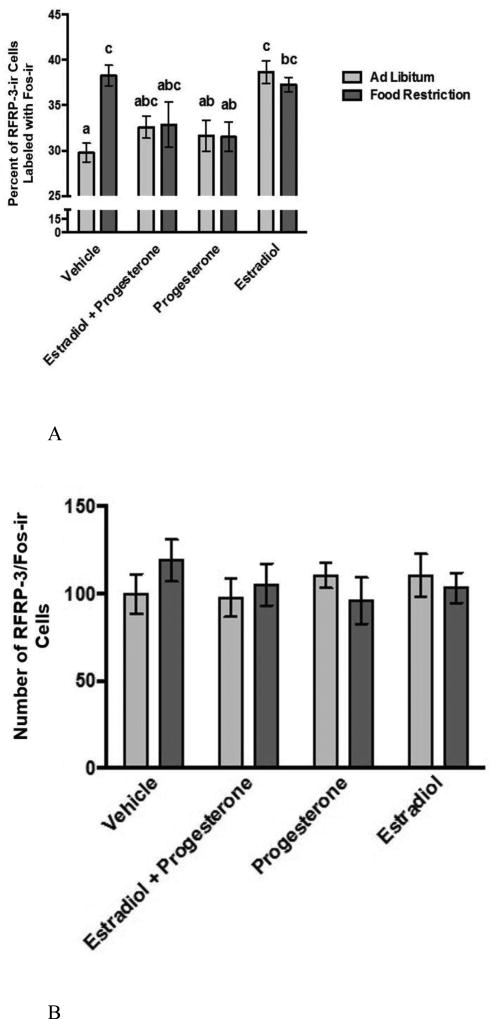
The activation of RFRP-3-Ir cells was significantly increased by food restriction, but the effect varied significantly according to the day of the estrous cycle (Figure 5). Specifically, on the day of ovulation and estrous behavior, there was no food-restriction-induced increase in this variable. A two-way ANOVA performed on the percent of RFRP-3-Ir cells labeled with Fos-Ir, a measure of cellular activation, showed a significant main effect of feeding regimen (food restriction or ad libitum feeding) (F (3, 52) = 81.082, p < 0.001; η2 = 0.504) and estrous cycle day (F (3, 52) = 4.191, P < 0.05; η2 = 0.078) (Figure 5A). In addition, there was a significant feeding regimen X estrous cycle day interaction (F (7, 48) = 6.369, P < 0.05; η2 = 0.118) (Figure 5A). A post hoc analysis showed that food restriction significantly increased the percentage of RFRP-3-Ir cells with Fos-Ir on three of the four estrous cycle days (P < 0.05). In contrast to the significant food-restriction-induced increases on the infertile days of the estrous cycle, on the periovulatory day, there was no significant food-restriction-induced increase in RFRP-3 cell activation (Figure 5A and 5B). The percent of RFRP-3-Ir cells labeled with Fos-Ir was negatively correlated with leptin concentration (r = −0.432; p < 0.01), terminal body weight (r = −0.474; p < 0.001), and change in body weight (r = −0.464; p < 0.001), and these correlations were statistically significant.
Figure 5.
Mean and standard error of the mean for (A) the percent of RFRP-3-Ir cells labeled with Fos-Ir across the 4-day estrous cycle and in female Syrian hamsters either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days. Tissue was collected from groups representing every day of the four day estrous cycle. (B) Photomicrograph of cells in the DMH labeled with Fos-Ir cells (green) and RFRP-3-Ir cells (red). * = significantly different from ad libitum at P < 0.05.
The total number of RFRP-3-Ir cells was slightly but significantly decreased by food restriction, but the effect did not differ according to estrous cycle day (Table 2B). The two-way ANOVA performed on the total number of RFRP-3-Ir cells revealed a significant main effect of feeding regimen (F (1, 54) = 4.3, p < 0.05; η2 = 0.079) (Table 2B). The mean number of RFRP-3 cells was slightly lower in food-restricted compared to ad libitum-fed females on three out of the four days of the estrous cycle, but post hoc analysis revealed no statistically significant differences between the ad libitum-fed vs. the food-restricted groups within days of the estrous cycle. The two-way ANOVA yielded no significant effect of the estrous cycle (Table 2B). Food restriction significantly decreased the total number of RFRP-3-Ir cells. The feeding regimen X estradiol cycle day interaction was not statistically significant.
Table 2.
(A) Mean and standard error of the mean for the total number of cells labeled with RFRP-3-Ir or with Kp-Ir across the 4-day estrous cycle and in female Syrian hamsters either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days. Tissue was collected from groups representing every day of the four-day estrous cycle. (B) Mean and standard error of the mean for the total number of cells labeled with RFRP-3-Ir in separate groups of female Syrian hamsters that were either fed ad libitum or food restricted to 75% of their ad libitum food intake for eight days. Half of each group was infused intracerebroventricularly with either saline or RFRP-3.
| Ad libitum-fed | Food-restricted | |||||||
|---|---|---|---|---|---|---|---|---|
| A. Variable/Estrou s Cycle Day | Follicular 1 | Follicular 2 | Periovulatory | Postovulatory | Follicular 1 | Follicular 2 | Periovulatory | Postovulatory |
| Number of RFRP-3-Ir Cells (DMH) | 306 ± 47.3 | 283 ± 18.4 | 290.6 ± 42.2 | 303.4 ± 35.8 | 224.7 ± 27.5 | 274.6 ± 36.6 | 221.9 ± 35.1 | 258.5 ± 22.6 |
| Number of Kp-Ir Cells (Arc) | 407.6 ± 35.2 | 394.9 ± 40.1 | 381.0 ± 23 | 505.8 ± 46.5 | 440.6 ± 89.5 | 431.0 ± 43.3 | 423.7 ± 41.9 | 404.1 ± 38.2 |
| Number of Kp-Ir Cells (AVPV) | 145 ± 21.0 | 187.9 ± 32.7 | 167.1 ± 28.5 | 140.6 ± 41.9 | 199.9 ± 54.7 | 146.7 ± 39.4 | 103.2 ± 12.3 | 114.0 ± 34.8 |
| Ad libitum-fed | Food-restricted | |||||||
|---|---|---|---|---|---|---|---|---|
| B. Variable/Hormone Treatment | Vehicle | Estradiol | Estradiol + Progesterone | Progesterone | Vehicle | Estradiol | Estradiol + Progesterone | Progesterone |
| Number of RFRP-3 Cells | 335.1 ± 31.1 | 282.9 ± 30.1 | 297.1 ± 26.1 | 351.6 ± 22.8 | 317.9 ± 25.9 | 275.9 ± 21.8 | 324.8 ± 36.5 | 299.4 ± 27.6 |
Eight females were not included in the analysis due to poor staining or damaged tissue from sectioning. As a result, the sample sizes were as followed: ad libitum-fed/follicular 1 (n = 7), ad libitum-fed/follicular 2 (n = 6), ad libitum-fed/periovulatory (n = 7), ad libitum/postovulatory (n = 7), food-restricted/follicular 1 (n = 7), food-restricted/follicular 2 (n = 7), food-restricted/periovulatory (n = 7), food-restricted/postovulatory (n = 8).
3.1.4. Kisspeptin immunoreactivity and cellular activation
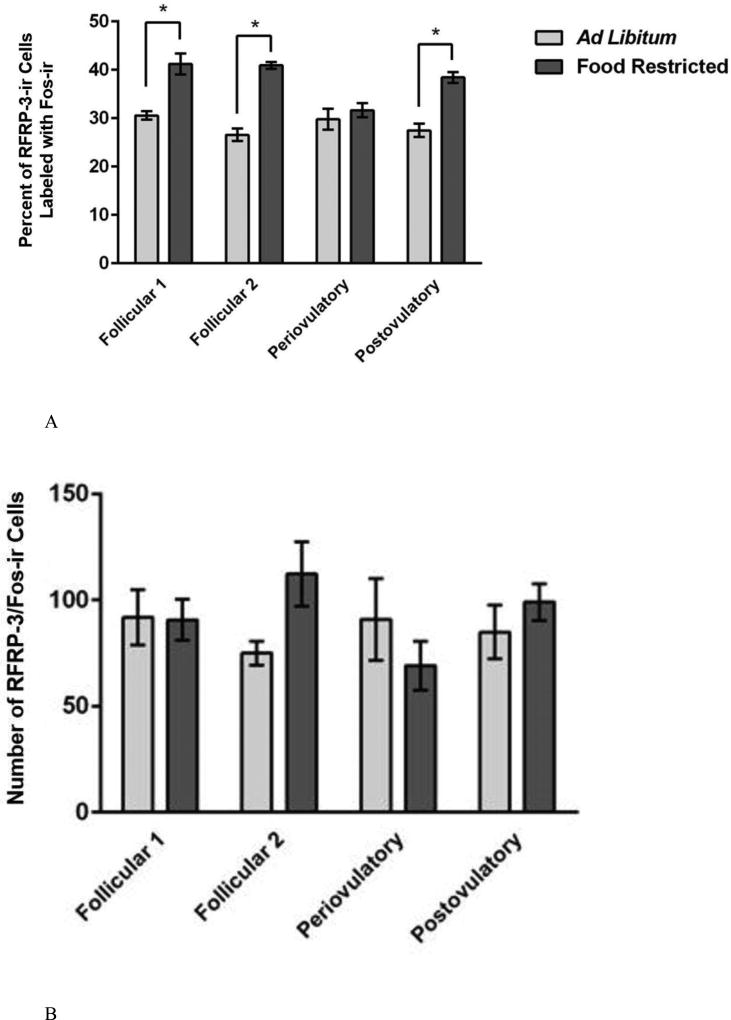
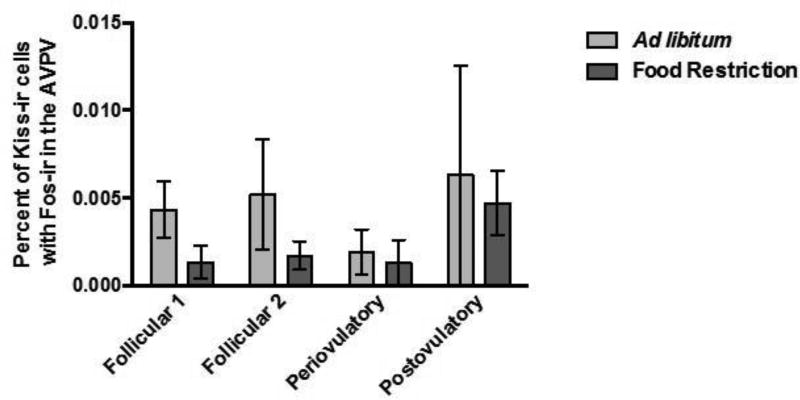
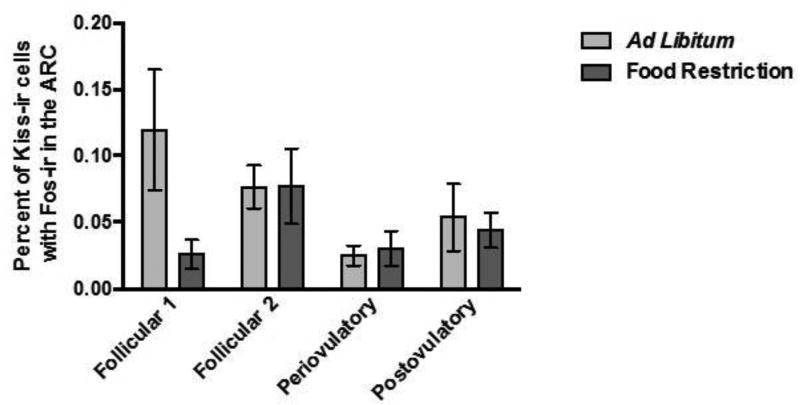
The effects of food restriction and estrous cycle day on activation of Kp-Ir cells were not significant in either of the two brain areas. First, in the AVPV, two-way ANOVA performed on the percent of Kp-Ir labeled with Fos-Ir in the AVPV yielded no significant main effects of feeding regimen (F (1, 53) = 1.606, P > 0.05; η2 = 0.029), no significant effect of the day of the estrous cycle (F (3, 51) = 0.835, P > 0.05; η2 = 0.045), and no significant feeding regimen X estrous cycle day interaction (F (7, 48) = 0.152, P > 0.05; η2 = 0.008) (Figure 6A). There was a positive correlation between the total number of Arc Kp-Ir cells labeled with Fos (r = 0.29; p < 0.05) and the serum concentration of estradiol, and the percent of Arc Kp-Ir cells labeled with Fos and serum estradiol concentrations (r = 0.31; p < 0.05), and these correlations were statistically significant. Second, in the Arc, there was no significant effect of food restriction or day of the estrous cycle on the activation of Kp-Ir cells. The two-way ANOVA performed on the percentage of Kp-Ir cells with Fos-Ir in the Arc yielded no significant main effect of feeding regimen (F (1, 54) = 2.218, P > 0.05; η2 = 0.036), day of the estrous cycle (F (3, 52) = 1.959, P > 0.05; η2 = 0.095), and no significant feeding regimen X day of the estrous cycle interaction (F (7, 49) = 2.081, P > 0.05; η2 = 0.104) (Figure 7A).
Figure 6.
Mean and standard error of the mean for the percent of Kp-ir cells labeled with Fos-ir [(the number of double-labeled Fos-Ir and Kp-Ir cells divided by the total number of Kp-Ir cells) multiplied by 100] across the 4 day estrous cycle in the AVPV of female Syrian hamsters either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days. Tissue was collected from groups representing every day of the four day estrous cycle.
Figure 7.
Mean and standard error of the mean for the percent of Kp-Ir cells labeled with Fos-ir [(the number of double-labeled Fos-ir and Kp-Ir cells divided by the total number of Kp-Ir cells) multiplied by 100] across the 4 day estrous cycle in the Arc of female Syrian hamsters either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days. Tissue was collected from groups representing every day of the four-day estrous cycle.
The effects of food restriction and estrous cycle day on the total number of Kp-Ir cells were not significant in either of the two brain areas. First, in the AVPV, the two-way ANOVA performed on the total number of Kp-Ir cells revealed no significant main effect of feeding regimen (F (1, 53) = 0.571, P > 0.05; η2 = 0.167), day of the estrous cycle (F (3, 51) = 0.784, P > 0.05; η2 = 0.228), and no significant feeding regimen X day of the estrous cycle interaction (F (7, 48) = 1.076, P > 0.05; η2 = 0.314) (Table 2). The total number of Kp-Ir cells in the AVPV was positively correlated with estradiol serum concentrations (r = 0.31; p < 0.05), and this correlation was significant. Second, in the Arc, the ANOVA performed on the total number of Kp-Ir cells yielded no significant the main effect of feeding regimen (F (1, 54) = 0.159, P > 0.05; η2 = 0.003), day of the estrous cycle (F (3, 52) = 0.657, P > 0.05; η2 = 0.037), and no significant feeding regimen X day of the estrous cycle interaction (F (7, 49) = 1.277, P > 0.05; η2 = 0.072) (Table 2).
3.2. Experiment 2: Effects of Estradiol and Progesterone Treatment on Activation of RFRP-3 Cells in Ovariectomized Females
3.2.1.Body weight
Body weight was significantly decreased by food restriction, but there was no significant effect of hormone treatment. The two-way ANOVA on body condition parameters yielded a significant main effect of feeding regimen on terminal body weight (F (1, 55) = 34.546, P < 0.01; η2 = 0.359) (Table 1) and body weight change (F (1, 55) = 161.572, P < 0.001; η2 = 0.738) (Table 1). Food-restricted females weighed significantly less and lost more weight than females fed ad libitum for eight days (P < 0.001). This analysis yielded no significant main effect of the hormone treatment and no significant feeding regimen X hormone treatment interaction on terminal body weight or on the change in body weight.
3.2.2. RFRP-3 immunoreactivity and cellular activation after hormone treatment
In ovariectomized females, effect of food restriction on the activation of RFRP-3-Ir cells differed significantly according to hormone treatment (Figure 8). The two-way ANOVA on the percentage of RFRP-3-Ir cells labeled with Fos-Ir, the measure of cellular activation, yielded a significant main effect of hormone treatment (F (3, 52) = 6.979, P < 0.001; η2 = 0.225), but no significant main effect of feeding regimen. The analysis yielded a significant feeding regimen X hormone treatment interaction (F (3, 52) = 4.649, P < 0.01; η2 = 0.15). Within the vehicle-treated, ovariectomized hamsters, a post hoc analysis revealed that food-restricted hamsters had a significantly higher level of RFRP-3 cell activation compared to ad libitum-fed controls (P < 0.05). Within the groups treated with progesterone alone or with estradiol plus progesterone, differences between food-restricted and ad libitum-fed hamsters were not statistically significant. Similarly, within the group treated with estradiol alone, food-restricted females did not differ significantly from ad libitum-fed females. In ovariectomized females treated with estradiol alone, both food-restricted and ad libitum-fed females had significantly higher activation than those treated with progesterone or estradiol plus progesterone, and the ad libitum-fed females treated with estradiol had a significantly higher level of activation than the ad libitum-fed vehicle-treated group (P < 0.05). Though the percentage of RFRP-3 neurons labeled with Fos differed according to treatment, the total number of RFRP-3 neurons did not (Figure 8).
Figure 8.
Mean and standard error of the mean for (A) across in female Syrian hamsters either fed ad libitum or food-restricted to 75% of their ad libitum intake for eight days and treated with either estradiol, estradiol and progesterone, or progesterone. Fos-Ir cells (green) and RFRP-3-Ir (red) cells in the DMH. * = significantly different from ad libitum at P < 0.05.
3.3. Experiment 3
Double labeling for RFRP-3-Ir and PR-Ir revealed a dense population of PR-Ir cells in the POA, VMH and Arc, and no labeling (zero cells labeled) with RFRP-3-Ir in those brain areas. In the DMH, we saw a substantial number of cells labeled with RFRP-3-Ir, but very little staining with PR and no cells were co-localized (zero labeled cells) with both PR-Li and RFRP-3-Li at 40× light microscopy (n=5) (Figure 9).
Figure 9.
Progesterone receptor (PR)-ir cells (green) and GnIH-ir cells (red) in the POA, VMH, Arc and DMH.
3.4. Experiment 4: I.C.V. Infusion of RFRP-3 and Behavior
3.4.1. Choice Test: Reproductive behavior during RFRP-3 infusion and food restriction
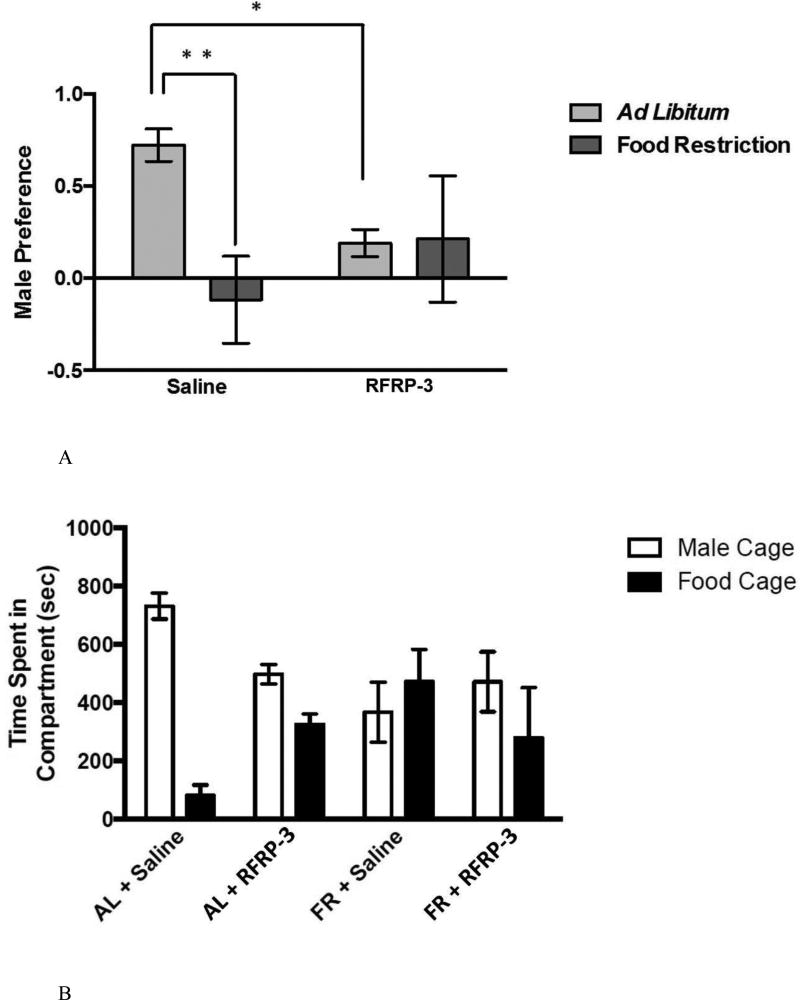
Infusion of RFRP-3 inhibited the preference for spending time with males, but only in ad libitum-fed not food-restricted females (Figure 10A). A two-way ANOVA performed on male preference ((time spent with males minus time spent with food) divided by the total time) yielded no significant main effect of feeding regimen and no significant main effect of RFRP-3 infusion, but revealed a statistically significant feeding regimen X infusion interaction (F (3, 23) = 4.628, p < 0.05; η2 = 0.145). A post-hoc analysis revealed that within the ad libitum-fed group, male preference was significantly decreased by RFRP-3 infusion (P < 0.05), but within the food-restricted group the RFRP-3-infused females did not differ significantly from the ad libitum-fed group (Figure 10A).
Figure 10.
Behavioral results from the 90-minute choice test. Mean and standard error of the mean for (A) male preference, calculated as the (time spent with males minus the time spent with food) divided by the total time) (top), and the (B) spent in either the male or food cage (bottom) during the first 15 minutes of testing. Animals were either fed ad libitum or a food restriction diet for 8 days and either centrally infused with 0.9% saline or RFRP-3 (500 ng/day for 10 days. * = significantly different from ad libitum fed saline treated animals at p < 0.05 and ** = significantly different at p < 0.01. Difference in the time spent with either the male or food are indicated by *** and are significantly different at p < 0.001.
Male preference was negatively correlated with aspects of body condition. There was a negative correlation between male preference and body weight change (r = −0.443; p < 0.05) and between male preference and weight of the subcutaneous WAT pad weight (r = −0.467; p < 0.05), and these correlations were statistically significant. Male preference was not correlated with any of the weights of any of the other WAT pads that we examined.
Two-way ANOVA performed on the number of vaginal scent marks (sexual solicitation) and flank marks (territorial behavior) revealed no significant main effect of feeding regimen, no significant effect of infusion, and no significant feeding regimen X infusion interaction. (Table 3). The total number of vaginal scent marks during the testing period was positively correlated with male preference, and this correlation was statistically significant (r = 0.474; p < 0.012).
Table 3.
In experiment 4, mean and standard error of the means for the number of vaginal scent markings (VM) or flank markings (FM) in the 15-minute preference test. Mean and standard error of the mean and the standard error of the mean for the total time spent sniffing a male, grooming, digging, biting the barrier separating the male from the female, eating food, and hoarding food.
| Treatment/Behavior | #Vaginal Marks |
#Flank Marks |
Time Sniffing the Male (sec) |
Time Grooming (sec) |
Time Digging (sec) |
Time Biting (sec) |
Time Eating Food (sec) |
Time Hoarding Food (sec) |
|---|---|---|---|---|---|---|---|---|
| Ad libitum-fed Saline-treated | 8.33 ± 3.66 | 0.83 ± 0.54 | 190 ± 61.5 | 114.2 ± 33.8 | 54.2 ± 19.9 | 25 ± 21.1 | 6.67 ± 6.67 | 0.305 ± 0.305 |
| Ad libitum-fed RFRP-3-treated | 3.63 ± 1.27 | 1 ± 0.5 | 65.6 ± 19.9 | 68.8 ± 34.1 | 168.8 ± 27.9 | 66.3 ± 17.2 | 69.7 ± 21.9* | 0.613 ± 0.403 |
| Food-restricted Saline-treated | 3.13 ± 2.17 | 0.37 ± 0.18 | 70.6 ± 23.5 | 55 ± 33.9 | 216.9 ± 53.4 | 28.1 ± 18.4 | 125.6 ± 32.6* | 32.69 ± 8.09* |
| Food- restricted RFRP3-treated | 9 ± 5.23 | 0 | 111 ± 64.8 | 35 ± 13.8 | 116 ± 70.6 | 80 ± 63 | 123 ± 65.8* | 19.75 ± 7.93* |
The two-way ANOVA performed on the other behavioral variables that we measured yielded no significant main effects of feeding regimen and no main effect of infusion. These variables included the time spent grooming, biting the male barrier, digging, and anogenital investigations. With regard to digging, the feeding regimen X infusion interaction of was statistically significant (F (3, 24) = 5.495, p < 0.05; η2 = 0.183). With regard to anogenital investigation, the feeding regimen X infusion interaction was not significant (F (3, 24) = 4.043, P < 0.056; η2 = 0.585) (Table 3).
3.4.2. Choice Test: Ingestive behavior during RFRP-3 infusion and food restriction
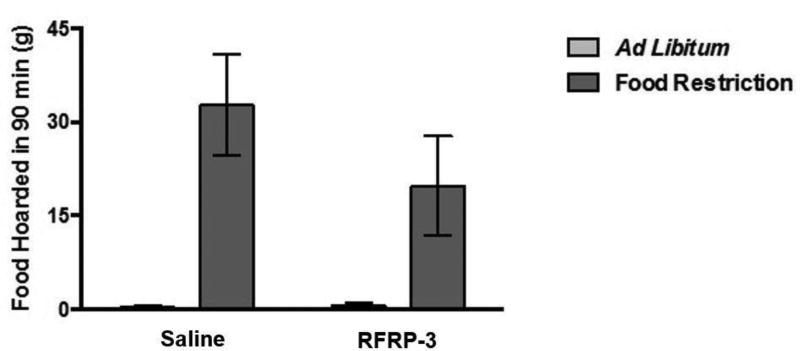
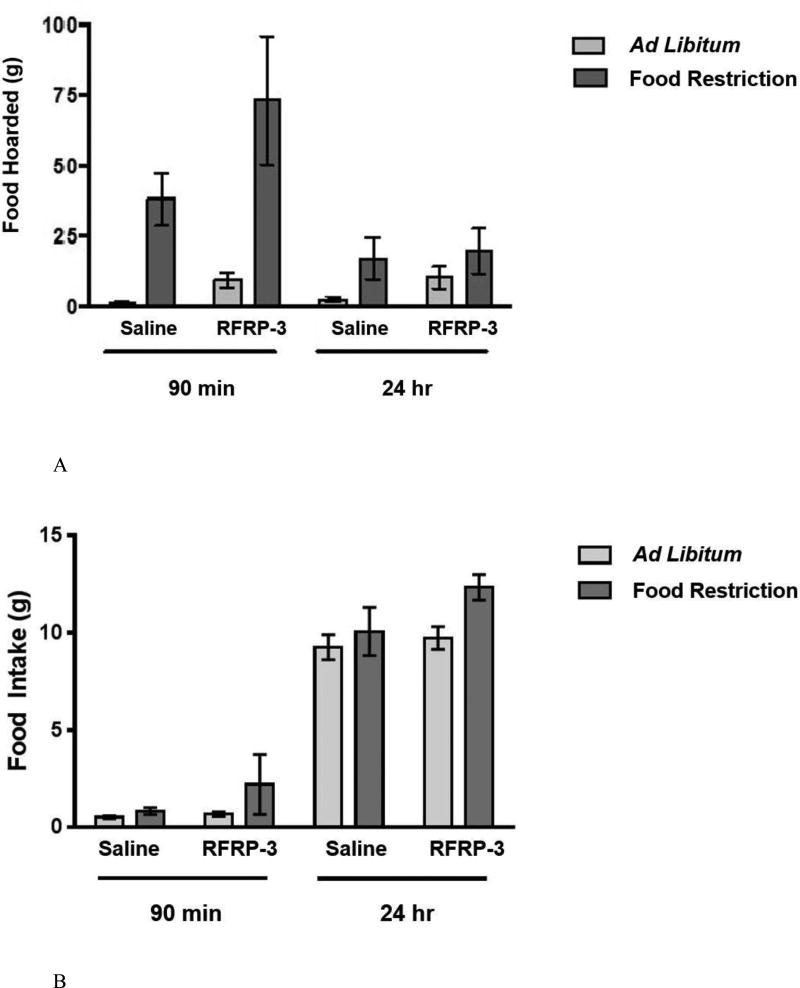
During the 90-minute preference test (the preference for spending time with males), there were no effects of infusion on food hoarding. A two-way ANOVA performed on the amount of food hoarded during the 90-minute choice test yielded a statistically significant main effect of feeding regimen (food restriction vs. ad libitum feeding) on the total weight of food pellets hoarded during the choice test (F (1, 26) = 20.066, p < 0.001; η2 = 0.145) (Figure 11), such that food-restricted females hoarded more food that ad libitum-fed females. The analysis yielded no significant main effect of RFRP-3 infusion and no significant feeding regimen X infusion interaction.
Figure 11.
Mean and standard error of the mean for total food hoarded during the 90 minute choice test. Animals were either fed ad libitum or a food restricted for 8 days and either centrally infused with 0.9% saline or RFRP-3 (500 ng/day for 10 days).
The two-way ANOVA performed on food intake, time spent hoarding food, and time spent eating yielded no significant main effect of feeding regimen, no significant main effect of infusion and no significant feeding regimen X infusion interaction (Table 3).
The amount of hoarding during the choice test was associated with changes in body condition. The change in body weight at testing and the weights of the visceral and subcutaneous WAT pads were positively correlated with the total amount of food hoarded (r = 0.437, 0.386, 0.386; p < 0.05, body weight change, visceral WAT, and subcutaneous WAT, respectively) and these correlations were statistically significant (Table 3).
3.4.3. Hoarding Test: Ingestive behavior during RFRP-3 infusion and food restriction
In a separate test in which food hoarding was measured over time outside the preference apparatus (a tube leading to a food source was connected to the home cage), there were significant stimulatory effects of RFRP-3 infusion on the amount of food hoarded in 90 min, but not the amount of food hoarded in 24 hours (Figure 12A). A mixed-model, two-way ANOVA performed on the amount of food hoarded over time yielded a significant time X feeding regimen interaction (F (1, 26) = 29.434, p < 0.001; η2 = 0.441). Most food hoarding occurred in the first 90 min after the onset of dark, and less food hoarding occurred in the following 24 hours (Figure 12A). In addition, the analysis yielded a significant effect of infusion (F (3, 24) = 5.325, p < 0.05; η2 = 0.08), reflecting an increase in food hoarding in the RFRP-3-infused relative to the saline-infused, ad libitum-fed females. This analysis yielded a significant main effect of feeding regimen (F (3,24) = 16.876, p < 0.001; η2 = 0.388), reflecting large increases in food hoarding in food-restricted females. The analysis yielded a significant feeding regimen X infusion interaction (F (3, 24) = 5.092, p < 0.05; η2 = 0.076), reflecting a greater increase in food hoarding in the ad libitum-fed, RFRP-3-infused than in the food-restricted, RFRP-3-infused. Post hoc analysis showed that within the ad libitum-fed group RFRP-3-infused females had significantly higher levels of food hoarding at 90 min compared to ad libitum-fed saline infused females (P < 0.05).
Figure 12.
Mean and standard error of the mean for (A) total food hoarded (top) and (B) food intake (bottom) during the 24-hour hoarding test. Females were allowed access to the hoarding apparatus and food intake and food hoarding were measured at 90 minutes and 24 hours. Animals were either fed ad libitum or food restricted for 10 days and either centrally infused with 0.9% saline or RFRP-3 (500 ng/day for 12 days).
A two-way ANOVA performed on the total amount of food hoarded in 90 minutes (omitting the food hoarded at 24 hours) revealed a significant main effect of feeding regimen (F (1,26) = 16.876, p < 0.001, η2 = 0.388) and a significant main effect of infusion (F (3,24) = 4.456, p < 0.05, η2 = 0.083), and no significant feeding regimen X infusion interaction.
A two-way ANOVA performed on the total amount of food hoarded in 24 hours yielded no significant main effects and no significant interaction (Figure 12A).
The amount of food hoarded was associated with changes in body condition. The change in body weight at the time of testing was negatively correlated with hoarding at 90 minutes and 24 hours respectively (r = −0.626, −0.511; p < 0.001, 0.01,), and these correlations were statistically significant. The amount of food hoarded at 90 minutes was negatively correlated with the three different WAT pad weights (r = −0.453, −0.525, −0.614; p < 0.05, 0.01, 0.01; visceral, parametrial, and subcutaneous WAT pads, respectively) and uterine weight (r = −0.385; p < 0.05), and these correlations were statistically significant (Table 4).
Table 4.
Mean and standard error of the mean for A) visceral B) parametrial C) subcutaneous fat pad weights. Mean and standard error of the mean for D) uterine weight. Animals were either fed ad libitum or a food restriction diet for 11 days and either centrally infused with 0.9% saline or RFRP-3 (500 ng/day for 13 days) in Experiment 3.
| Treatment/Behavior | Visceral WAT (g) |
Parametrial WAT (g) |
Subcutaneous WAT (g) |
Uterine Weight (g) |
|---|---|---|---|---|
| Ad libitum-fed Saline-treated | 1.61 ± 0.29 | 0.35 ± 0.2 | 1.02 ± 0.1 | 0.62 ± 0.03 |
| Ad libitum-fed RFRP-3-treated | 2.20 ± 0.41 | 0.34 ± 0.05 | 1.0 ± 0.07 | 0.64 ± 0.02 |
| Food-restricted Saline-treated | 0.52 ± 0.09 | 0.05 ± 0.02 | 0.3 ± 0.07 | 0.47 ± 0.02 |
| Food- restricted RFRP3-treated | 0.71 ± 0.16 | 0.09 ± 0.04 | 0.41 ± 0.14 | 0.57 ± 0.06 |
3.4.4. Food intake, body weight, white adipose tissue (WAT) pads and uterine weight
A one-way, repeated measures ANOVA performed on food intake in ad libitum-fed females yielded no significant main effect of infusion (Figure 12B). The two-way ANOVA was not performed on food intake because the food-restricted group received a predetermined amount of food.
A student’s T-test on the final change in body weight and final change in average daily food intake yielded no significant main effect of RFRP-3 infusion. Animals that were ad libitum-fed generally gained weight during infusion regardless of the type of infusion (RFRP-3 or saline). A one-way, repeated measures ANOVA performed on body weight yielded no significant main effect of infusion (Table 1).
Food-restricted females lost a significant amount of body weight and white adipose tissue (WAT) pad mass regardless of the type of infusion (RFRP-3 or saline). The one-way ANOVA performed on the change in body weight in the food-restricted group yielded a significant main effect of feeding regimen (food restriction vs. ad libitum feeding) at the time of the choice test (F (1, 25) = 73.563, p < 0.001; η2 = 0.76), at the time of the hoarding test (F (1. 25) = 122.745, p < 0.001; η2 = 0.841), and at the end of the experiment (F (1, 25) = 102.532, p < 0.001; η2 = 0.805). Food restriction significantly decreased the weight of the visceral (F (1, 25) = 19.448, p < 0.05; η2 = 0.435) (Figure 11C), parametrial (F (1, 25) = 30.545, p < 0.001; η2 = 0.567), and subcutaneous WAT pads (F (1, 25) = 52.934, p < 0.001; η2 = 0.699). The analysis of food intake, body weight, WAT pad weight, and uterine weight revealed no significant main effect of infusion. The analysis yielded no significant regimen X infusion interaction (Table 4).
4. Discussion
The present findings provide new support that RFRP-3-steroid interactions orchestrate the changes in behavioral priorities that occur over the hamster estrous cycle. Specifically, we hypothesized that for most of the estrous cycle, when circulating concentrations of ovarian steroids are low, RFRP-3 cells are activated and GnRH action is increased to promote food hoarding. Furthermore, we hypothesized that RFRP-3 cell activation is modulated by high levels of ovarian steroids during the hours before ovulation, and this inhibits the urge to forage and hoard food and stimulates sexual motivation, thereby synchronizing mating with the time of highest fertility. In previous experiments, eight days of 25% food restriction increased food hoarding (but not food intake) and decreased sexual motivation (but not lordosis) and this effect was reversed by re-feeding [7–9]. In the present experiments, I.C.V. RFRP-3 treatment mimicked these effects in ad libitum-fed females by decreasing the preference for spending time with males on the day before ovulation (when plasma estradiol concentrations are known to be elevated in this species) and by increasing food hoarding on a day of the estrous cycle when plasma estradiol concentrations are known to be low (Figures 10–12). In previous experiments, food restriction-induced increases in food hoarding and decreases in sexual motivation occurred only on the non-estrous days of the estrous cycle [7], and in the present experiment, food restriction-induced increases in activation of RFRP-3-Ir cells occurred on only the three non-estrous days of the estrous cycle; food restriction-induced RFRP-3-Ir cell activation did not occur on the day of ovulation (Figure 5A). No such pattern occurred in the activation of Kp cells (Figures 6A and 7A). Furthermore, the food restriction-induced activation of RFRP-3-Ir that was seen in ovariectomized females was blocked by pre-treatment with progesterone alone or estradiol plus progesterone (Figure 8C). These results add to a growing body of data (e.g., [39]) implicating RFRP-3 in the neural circuitry that underlies natural changes in behavioral motivation over the estrous cycle in animals with limited energy availability and access to potential mating partners. It is particularly notable that RFRP-3 is so closely associated with the behaviors that are responsive to mild food restriction even when lordosis and food intake are not affected. This suggests that the effect of energy on reproduction can act via the choice between hoarding and approaching males long before the same energetic challenge affects the choice between copulation and eating food [9, 66].
Most critical in these experiments were the environmental conditions and experimental methods that included attention to appetitive behaviors and “integrating the animal with its environment” [67, 68]. First, females were subjected to mild food restriction (75% of daily ad libitum intake) that did not delay estrous cyclicity and did not decrease the plasma levels of ovarian steroids (Figures 4B and 4C). The levels of plasma estradiol and progesterone fluctuated over the estrous cycle as shown in other experiments [65], but were not significantly different between the food-restricted and ad libitum-fed females, and thus, the present results are not directly relevant to understanding the inhibition of the HPG system by severe metabolic challenges. The effects of food restriction on RFRP-3 cell activation are, however, closely linked to food-restriction induced changes in behavioral priorities (Figures 10–12). Second, our experimental subjects were tested in key conditions relevant to their natural habitat. For example, they were tested for a 90-minute period at the onset of the dark phase of the light-dark cycle, a time when hamsters in their native habitat are known to engage in most of their daily activity [61]. Third, we included measurements of appetitive aspects of behavior. Appetitive aspects of behavior have been defined as those pre-current behaviors that lead to the attainment of a goal [69–73]. Appetitive sex behaviors such as preference for visiting a male and vaginal scent marking, differ from consummatory behavior in that they are more sensitive to food restriction ([7, 39] and see Figures 12A and 12B). Furthermore, the preference for spending time with a male vs. food represents a decision, whereas lordosis is a reflexive, rapid motor response to a stimulus. Male preference provides a window into sexual motivation relatively unconfounded by sexual ability or performance. Similarly, changes in the appetitive behavior, food hoarding, provides a window into ingestive motivation without the confounding influence of the post-intestinal effects that accompany changes in food intake. In general, appetitive behaviors tend to be controlled by brain areas and neurohormones/neurotransmitters that overlap with but are different from those that control consummatory behaviors [69, 70]. We therefore measured both appetitive and consummatory aspects of behavior.
To understand the importance of appetitive behavior, it is important to consider the habitats in which Syrian hamsters live and evolved. Syrian hamsters are native to harsh desert environments near the boarder of Syrian and Turkey where summer days are hot and winter nights are near freezing. During most of the estrous cycle, female hamsters are solitary and live alone in deep burrows hundreds of yards from the fields where they gather food, available mainly in the summer [61]. The burrows are few and far between, and field researchers find them with considerable difficulty. It is suspected that the burrows are highly coveted because they provide a safe haven in which to store food, hide from predators and competitors, and escape from extreme ambient temperatures (Robert Johnston, personal communication). Competition for these burrows might be fierce. Wild Syrian hamsters implanted with tracking devices are observed to spend most of the day and night in the burrow. They emerge for only 90-minutes per day at dawn and dusk [61]. Virtually all of their daily 90-minute above-ground time period is spent traveling long distances to gather and hoard food (Robert Johnston, personal communication). Given the seasonal changes in food availability and the energetic costs of lactation, a vigilant predisposition toward food hoarding rather than courtship during the nonfertile phases of the estrous cycle might be important for winter survival and spring reproductive success. A decision to forego the 90-minutes of food hoarding to spend time in search of a male is likely to be risky. So far, mating has been observed only near the female burrow (Robert Johnston, personal communication). Given these observations, we speculated that Syrian hamster survival and reproductive success depends upon vigilant foraging and hoarding, decisions that are made during the 90-min period at the onset of darkness. Thus, we hypothesized that Syrian hamsters have a critical time window in which sexual motivation is higher than ingestive motivation. We further hypothesize that the duration of this time window of high sexual motivation is decreased when energy availability is limited, even though consummatory behavior, lordosis duration, is not affected. The duration of this time window of sexual motivation is increased when food availability is unlimited. This idea was first supported by the work of Klingerman et al., [7] in which 25% food restriction decreased the time window of appetitive sex behavior (vaginal scent marking and preference for spending time with males) but had no significant effect on the consummatory sex behavior (lordosis frequency or duration) and increased the time window of high levels of food hoarding (appetitive behavior), but had no effect on daily food intake (consummatory behavior). The present experiments confirm that changes in the activation of RFRP-3 cells is more closely associated with appetitive, than with consummatory behaviors, i.e., there were significant effects of food restriction on food hoarding (Figure 12A) but not food intake (Figure 12B), and effects on the preference for spending time with males vs. food (Figure 10), but not on lordosis.
The effects of food restriction on RFRP-3 cell activation and behavior are modulated by ovarian steroids (Figure 8A). Previous experiments showed a decrease in RFRP-3 gene expression at the time of the Syrian hamster LH surge, but these results were not examined in relation to sex and ingestive behavior [57]. It is important to note that if we had measured only food intake and lordosis frequency (consummatory behaviors), the connection between appetitive sex and ingestive behavior, RFRP-3 cell activation, and plasma progesterone concentrations would have been missed.
Almost two thirds of the RFRP-3-Ir cells remained inactive after food deprivation. Thus, it is possible that the “inactive” RFRP-3 cells are not activated unless the animal experiences more severe energetic challenges, cold temperature, and/or short day photoperiods. First, remember that the food restriction in the present experiment is mild and it is known that the HPG system was not inhibited and steroid levels were not significantly lower after 8 days of restriction. Unactivated cells might be normally quiescent during pulsatile LH secretion and/or the approaching LH surge. Of course, these cells might mediate effects of other stressors. Given the wide distribution of RFRP-3 cells beyond projections to GnRH cells and the median eminence, RFRP-3 cells could perform functions unrelated to ingestive behavior and reproductive processes. Likewise, it is possible that other Fos-related antigens, or other immediate early genes, might have revealed additional activated cells.
In the present analysis, because 40 µm sections were analyzed using conventional rather than confocal microscopy level, it is possible, that the number of double-labeled cells was overestimated. However, given that we confined our analysis to cells with a clear nucleus and only characterized cells as double-labeled when Fos was localized to the nuclear compartment without extending beyond the nuclear border, this concern is minimal, and would not be expected to affect differences among the groups. We have published at least three other peer-reviewed papers using conventional microscopy to measure double-labeling of RFRP-3 and Fos [57, 58] and one has revealed differences caused by food restriction [9]. Another methodological issue is that in mildly food-restricted females, there is a small but significant decrease in the number of RFRP-3 cells along with a large increase in the percent of cells activated. This might be interpreted as either 1) an increase in secretion of the peptide not accompanied by increases in translation and/or transcription, or 2) a decrease in translation/transcription and secretion of the peptide, perhaps reflecting increased activaton of RFRP-3 cells when secretion falls. Thus, we interpretation the increase in activation of RFRP-3 cells with caution. The first interpretation is favored because infusion of the peptide decreases sexual motivation and increases food hoarding.
In the present study, the inhibitory effect of food restriction on male preference was mimicked in ad libitum-fed females by infusion with RFRP-3 (Figure 10). This inhibitory effect on sexual motivation is partially-consistent with our previous studies in which continuous infusion of RFRP-3 in ad libitum-fed female Syrian hamsters decreases the number of vaginal scent marks in response to cues from an intact male compared to a castrated male [39]. Whereas RFRP-3 inhibits sexual motivation, but not copulation in female Syrian hamsters, this peptide affects copulatory behaviors in other species of one or the other sex. For example, treatments that increase circulating or I.C.V. RFRP-3 or treatments that increase the expression of RFRP-3 RNAi induce rapid inhibition of consummatory sex behavior in female white-crowned sparrows, male rats, and male quail [37, 74, 75]. Continuous infusion of RFRP-3, however, does not affect sexual performance in all species studied, including male macaque, sheep, and mice [34]. Appetitive aspects of sex behavior were not examined in these latter studies, and thus, it is possible that sexual motivation is affected by RFRP-3 in species other than Syrian hamsters. One small difference between the present data and those of Piekarski et al. [39] is that Piekarski et al. reported RFRP-3-inhibition of vaginal scent marking whereas in this experiment we did not. The differences between the two studies might be related to differences in the methodology (the previous study measured scent marking in a neutral arena whereas we measured scent marking in the subjects’ territory), or possibly related to low levels of baseline marking in our particular population of hamsters (Table 3).
The present results extend the findings of Piekarski et al. [39] to show that I.C.V. RFRP-3 treatment decreased preference for males vs. food and increased appetitive ingestive behavior (food hoarding), but not consummatory aspects of ingestive behavior (food intake, Figure 12B). The effects of RFRP-3 on food hoarding were only significant when tested on Day 1 of the estrous cycle (when plasma estradiol concentrations are lowest), but were not significant when tested on Day 3 of the estrous cycle (when plasma estradiol concentrations are highest), even though the females spent less time with males during this test. The increases in RFRP-3-stimulated food hoarding were restricted to ad libitum-fed females; levels of food hoarding were elevated by food restriction in saline-treated females and did not increase further in RFRP-3-treated, food-restricted females (Figure 12A). The stimulatory effect of RFRP-3 on food hoarding in the present experiments are partially consistent with effects of RFRP-3 on ingestive behavior in other species. For example, acute and continuous central treatment with RFRP-3 produces small increases food intake in mice, rats, sheep, non-human primates, drakes and chicks [34, 74, 76–79]. In these other studies, measurements were restricted to the consummatory aspects of ingestive behavior, and it is not known whether RFRP-3 would have increased the appetitive aspects of ingestive behavior. The lack of effect of RFRP-3 on food intake in Syrian hamsters is likely linked to the lack of postfast hyperphagia in Syrian hamsters [80–82]. In Syrian and Siberian hamsters and in human subjects, food restriction or deprivation increases food hoarding or grocery shopping more than it increase daily food intake (reviewed by [83, 84]). Many such species were vulnerable to cold ambient temperatures and predators during their evolution, and might have adapted to these selection pressures by storing and hoarding food and limiting their food intake to relatively safe and warm homes or burrows. It is therefore not surprising that treatment with RFRP-3 increases food hoarding but not food intake in hamsters, and the mechanisms that contribute to this phenomenon are worthy of future study. Together, the results of the RFRP-3 I.C.V. infusion experiment are consistent with the idea that elevated levels of intracerebral RFRP-3 is sufficient to increase food hoarding and decrease the preference for spending time with males versus food in Syrian hamsters.
Increases in RFRP-3 cell activation were seen in ovariectomized females but were not seen in females treated with estradiol followed by progesterone and in females treated with progesterone alone. In contrast, estradiol alone did not decrease RFRP-3 cell activation, although at other doses, the result might have been different. As progesterone alone was effective in preventing the food restriction-induced increase in RFRP-3 cell activation (Figure 8A), we therefore examined whether RFRP-3 cells contain progestin receptors. Whereas plentiful progestin receptor immunoreactivity was observed in the VMH and basal medial hypothalamus, including the Arc, no PR-Ir was observed in the DMH and no PR-Ir was colocalized with RFRP-3-Ir (Figure 9). Thus, if progesterone modulates RFRP-3 cell activation it might act independently of progestin receptors via rapid membrane action or via intermediate projections from progesterone receptor containing cells located in other brain areas (such as the Arc or the VMH). For example, we have documented neuropeptide Y-immunoreactive (NPY-Ir) projections in close apposition to RFRP-3-Ir cells, and NPY cells are known to contain progestin receptors [9].
We observed no food restriction-induced decreases in ovarian steroids, but there might be changes in steroid receptors. In Syrian hamsters, severe food deprivation, treatments that block oxidation of metabolic fuels, and prolonged cold exposure decrease the number of estrogen receptor α-Ir (ERα) cells within the VMH but increased the number of cells in the medial preoptic area (mPOA) [85, 86]. It is not known whether mild food restriction would decrease steroid receptors, however, if so, food restriction might change the sensitivity of RFRP-3 to ovarian hormones, such that mild energetic challenge changes the expression of ERα in RFRP-3 cells.
With regard to the effects of mild food restriction, there is little information about how a small drop in energy availability reaches RFRP-3 cells. Candidate mediators of energy status include peripheral hormones (e.g., leptin, ghrelin, insulin) or intracellular detectors of metabolic fuel availability. Plasma leptin concentrations are a plausible candidate because circulating levels of this hormone are proportional to the amount of adipose tissue [87–89] and are greatly reduced in laboratory models and humans during conditions of short-term fasting [90–92]. Plasma leptin levels fall rapidly with food restriction and are lower in lean compared to fat Syrian hamsters [93]. Furthermore, treatment with leptin inhibits food restriction-induced increases in food hoarding in Syrian [94] and Siberian hamsters [95]. Falling levels of circulating leptin concentrations might mediate the effects of food restriction on cellular activation of RFRP-3-Ir cells in the DMH. In the present experiments, body weights, WAT pad weights, and serum concentrations of leptin were significantly lower in food-restricted compared to ad libitum fed animals (Figure 4A) and this reduction is similar to other conditions of mild energetic challenge, such as housing at 4°C [6]. Concentrations of leptin were negatively correlated with the percent of RFRP-3 cellular activation. In a choice test, systemic leptin treatment modulated the time food-restricted females spent with food and increased the time spent with males (Schneider et al., unpublished data). Together, these results suggest that effects of food restriction on appetitive behavior and activation of RFRP-3 cells might be affected by decreases in plasma leptin, although other signals (e.g., ghrelin) might also be involved. Effects of leptin on RFRP-3 cells have provided conflicting evidence depending on the model system [96–98], and effects of leptin on RFRP-3 cells in Syrian hamsters are under investigation in our laboratories. The fall in leptin might not be necessary as leptin-deficient Lepob/Lepob mice do not show differences in RFRP-3 expression or total number of RFRP-3 cells compared to wild type mice [99]. In some studies in wild type rodents, leptin receptor (LepRb) expression is absent from RFRP-3 cells, and leptin treatment does not induce pSTAT3 in these cells [99]. By contrast, in Lepob/Lepob mice the gene that encodes LepRb has been shown to be co-expressed with RFRP-3 in some parts of the hypothalamus [100]. Furthermore, leptin increases intracellular calcium concentrations in immortalized RFRP-3 cells [101]. We found a decrease in plasma leptin concentrations in food-restricted females, and yet, the plasma leptin concentrations, unlike food-restriction-induced RFRP-3 activation, did not fluctuate across the estrous cycle. Thus, Figure 4 provides no evidence that ovarian steroid modulation of RFRP-3 cells is mediated by ovarian steroid action on plasma leptin concentrations. One possibility is that changes in metabolic fuel availability have direct effects on both leptin and RFRP-3 cells, producing a correlation without the causal effect of leptin on RFRP-3 synthesis and secretion.
In addition to control of food intake, the DMH and RFRP-3 have been implicated in control of energy expenditure, adiposity, and body weight gain. For example, adenoviral knock down of DMH NPY gene expression results in decreased food intake, “browning” of WAT, increases in thermogenesis and whole body energy expenditure and body weight loss [102]. In addition, RFRP-3 receptor knockout mice lose significantly more body weight than wild type mice during fasting [103]. In mice, RFRP-3 cells are located in areas other than the DMH, but, nevertheless, the greater body weight loss in RFRP-3 receptor knockout mice that had the same level of food intake as wild-type mice suggests that RFRP-3 increases energy expenditure. The inhibitory effects of RFRP-3 on energy expenditure might not generalize to all species, however, as continuous infusion of RFRP-3 does not inhibit energy expenditure in sheep and rats [34], and in the present experiment, I.C.V. treatment with the short-term RFRP-3 treatment (24 hours) had no significant effect on body weight or WAT pad mass (Table 4). As expected, food restriction significantly reduced body weight and WAT pad weight, but RFRP-3 infusion did not affect either of these variables. In female Syrian hamsters, we saw no preferential loss of WAT, i.e., visceral, parametrial and subcutaneous pads all were reduced by food restriction (Table 4). By contrast, we observed a preferential depletion of the parametrial WAT pad weight in female Syrian hamsters housed with running wheels or at cold ambient temperatures [6]. In summary, these data emphasize a direct effect of RFRP-3 and steroids on motivation rather than energy expenditure and storage, at least in the short term (less than one day of RFRP-3 infusion).
A number of other chemical messengers might be involved in effects of energy availability on sexual motivation and hunger for food, and some of these might interact with the RFRP-3 system. In this experiment, no evidence linked food-restriction effects on behavior to Kp-Ir (Figures 6 and 7). This alone should not be taken as strong evidence against a role for this peptide in control of behavior, and, of course, other evidence supports a role for Kp in metabolic control of the HPG system and behavior [43–49]. Kp might be less important in effects of mild food restriction in Syrian hamsters on long day photoperiods, but might play a significant role in the interaction between food availability and photoperiod in seasonally breeding hamsters [104]. In addition, another hypothalamic peptide, NPY increases the preference for mating vs. drinking a sucrose solution (appetitive behavior) in rats [105]. Chronic infusion of RFRP-3 increases activation of NPY cells in sheep and increases expression of NPY in rats [34]. Elevated levels of intracerebral NPY are both necessary and sufficient for food deprivation-induced increases in food hoarding behavior in Siberian hamsters. In ad libitum-fed Siberian hamsters, central injection of NPY and an agonist for the NPY YI receptor stimulates food hoarding [106]. Furthermore, administration of the Y1 receptor antagonist, BIBO3304, restricted to the perifornical area prevents food deprivation-induced food hoarding in the Siberian hamster [107]. In Syrian hamsters, NPY terminals project to the DMH and show close apposition to RFRP-3 cells [9]. Together, these results suggest that RFRP-3 might work downstream from other peptides that communicate information about energetic status.
There are many other plausible mediators of the effects of energy availability on motivation, including factors that changes with energetic status, including melanocortin receptor agonists and antagonists (including agouti-related protein (AgRP), α-melanocyte-stimulating hormone, and melanin concentrating hormone (MCH)) (e.g., [108, 109]. reviewed by [2]). They are well-studied with regard to the consummatory ingestive behavior, but few of these have been studied with respect to appetitive ingestive and sex behaviors. One notable exception is AgRP, an endogenous melanocortin agonist, which is present in a large proportion NPY cells, and in rats, AgRP and Kp interact to modulate hunger-induced risk-taking [108, 109]. Furthermore, MCH, NPY, and vasoactive-intestinal peptide send projections to GnRH cells in mice [110], and at least some GnRH cells might have direct stimulatory effects on sexual behavior.
Energetic challenges can be considered to be stressors, and the “stress hormones” are obvious candidates for influencing RFRP-3 action on behavior. Energetic stressors increase synthesis and secretion of glucocorticoids, which can inhibit reproduction by inhibiting secretion of hormones of the HPG system [111, 112], and it is plausible that mild energetic challenges might modulate the choice between food hoarding and courtship. In rats, RFRP-3 cells are colocalized with glucocorticoid receptor (GR) [113] and the promoter region of the RFRP-3 gene contains two glucocorticoid response elements (GREs) [114]. Immobilization stress, which increases circulating glucocorticoid concentrations, increases the total number of RFRP-3 cells in birds and rats [115, 116]. Furthermore, this treatment induces RFRP-3 expression in rats but this effect is removed by adrenalectomy [113], consistent with the idea that glucocorticoids are necessary for stress-induced expression of RFRP-3. It is not known, to the best of our knowledge, whether the level of food restriction used in the present experiment significantly increases levels of circulating glucocorticoids in Syrian hamsters. Previous work, however, shows that the effects severe energetic challenges on Syrian hamster estrous behavior are more directly related to increases in intracerebral corticotropin releasing hormone than to adrenal steroids [117, 118].
Intracerebral treatment with corticotropin releasing hormone (CRH) facilitates food-deprivation-induced food hoarding in rats [119], although it should be noted that rats do not have cheek pouches and do not hoard food as prodigiously as hamsters (discussed by Bartness [84]). In Syrian hamsters, antagonists to CRH are potent facilitators of lordosis in response to estradiol and progesterone [117], and this might reflect a role for CRH in sexual motivation in Syrian hamsters that would influence the choice between food hoarding and sex. Together, these studies are consistent with a role for CRH in the relative motivation for engaging in food hoarding or sexual behavior. There is no doubt that mild decreases in the level of energy availability alter RFRP-3 cellular activation and behavioral priorities, but research in earlier stages compared to the mechanisms whereby low energy availability inhibits the HPG system.
Highlights.
Mild food restriction or ICV RFRP-3 infusion magnifies the preference for food vs. sex.
Mild food restriction increases the activation of RFRP-3 cells, but not Kp cells.
Both effects are modulated by ovarian steroids.
Acknowledgments
The authors thank Jenifer Golley for help with animal care. This work was funded by IOS-1257638 and IOS-1257876 from the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose
References
- 1.Bronson FH. Mammalian Reproductive Biology. 1. Chicago and London: The University of Chicago Press; 1989. [Google Scholar]
- 2.Schneider JE, Wise JD, Benton NA, Brozek JM, Keen-Rhinehart E. When do we eat? Ingestive behavior, survival, and reproductive success. Horm Behav. 2013;64:702–28. doi: 10.1016/j.yhbeh.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16:235–72. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- 5.Tena-Sempere M. Neuroendocrinology in 2016: Neuroendocrine control of metabolism and reproduction. Nat Rev Endocrinol. 2017;13:67–8. doi: 10.1038/nrendo.2016.216. [DOI] [PubMed] [Google Scholar]
- 6.Abdulhay A, Benton NA, Klingerman CM, Krishnamoorthy K, Brozek JM, Schneider JE. Estrous cycle fluctuations in sex and ingestive behavior are accentuated by exercise or cold ambient temperatures. Hormones and Behavior. 2014;66:135–47. doi: 10.1016/j.yhbeh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Klingerman CM, Krishnamoorthy K, Patel K, Spiro AB, Struby C, Patel A, et al. Energetic challenges unmask the role of ovarian hormones in orchestrating ingestive and sex behaviors. Hormones and Behavior. 2010;58:563–74. doi: 10.1016/j.yhbeh.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Klingerman CM, Patel A, Hedges VL, Meisel RL, Schneider JE. Food restriction dissociates sexual motivation, sexual performance, and the rewarding consequences of copulation in female Syrian hamsters. Behavioural Brain Research. 2011;223:356–70. doi: 10.1016/j.bbr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klingerman CM, Williams WP, 3rd, Simberlund J, Brahme N, Prasad A, Schneider JE, et al. Food Restriction-Induced Changes in Gonadotropin-Inhibiting Hormone Cells are Associated with Changes in Sexual Motivation and Food Hoarding, But not Sexual Performance and Food Intake. Front Endocrinol (Lausanne) 2011;2:101. doi: 10.3389/fendo.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider JE, Casper JF, Barisich A, Schoengold C, Cherry S, Surico J, et al. Food deprivation and leptin prioritize ingestive and sex behavior without affecting estrous cycles in Syrian hamsters. Hormones and Behavior. 2007;51:413–27. doi: 10.1016/j.yhbeh.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Morin LP. Environment and hamster reproduction: responses to phase-specific starvation during estrous cycle. American Physiological Society. 1986:R663–R9. doi: 10.1152/ajpregu.1986.251.4.R663. [DOI] [PubMed] [Google Scholar]
- 12.Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science. 1989;244:1326–8. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- 13.Schneider JE, Wade GN. Decreased availability of metabolic fuels induces anestrus in golden hamsters. The American journal of physiology. 1990;258:R750–5. doi: 10.1152/ajpregu.1990.258.3.R750. [DOI] [PubMed] [Google Scholar]
- 14.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–63. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–93. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- 16.Clegg DJ. Minireview: the year in review of estrogen regulation of metabolism. Molecular Endocrinology. 2012;26:1957–60. doi: 10.1210/me.2012-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin LP, Fleming AS. Variation of food intake and body weight with estrous cycle, ovariectomy, and estradiol benzoate treatment in hamsters (Mesocricetus auratus) J Comp Physiol Psychol. 1978;92:1–6. doi: 10.1037/h0077435. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui K, Osugi T. Evolutionary origin and divergence of GnIH and its homologous peptides. General and comparative endocrinology. 2009;161:30–3. doi: 10.1016/j.ygcen.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Comm. 2000;275:661–7. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 20.Bentley GE, Tsutsui K, Kriegsfeld LJ. Recent studies of gonadotropin-inhibitory hormone (GnIH) in the mammalian hypothalamus, pituitary and gonads. Brain Res. 2010;1364:62–71. doi: 10.1016/j.brainres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103:2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150:2799–804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. The Journal of physiology. 2009;587:1401–11. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henningsen JB, Gauer F, Simonneaux V. RFRP Neurons - The Doorway to Understanding Seasonal Reproduction in Mammals. Front Endocrinol (Lausanne) 2016;7:36. doi: 10.3389/fendo.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriegsfeld LJ, Ubuka T, Bentley GE, Tsutsui K. Seasonal control of gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Front Neuroendocrinol. 2015;37:65–75. doi: 10.1016/j.yfrne.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke IJ, Smith JT. The role of kisspeptin and gonadotropin inhibitory hormone (GnIH) in the seasonality of reproduction in sheep. Society of Reproduction and Fertility supplement. 2010;67:159–69. [PubMed] [Google Scholar]
- 27.Geraghty AC, Muroy SE, Zhao S, Bentley GE, Kriegsfeld LJ, Kaufer D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. Elife. 2015;4 doi: 10.7554/eLife.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Niki H, et al. Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Horm Behav. 2014;66:309–16. doi: 10.1016/j.yhbeh.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Son YL, Ubuka T, Narihiro M, Fukuda Y, Hasunuma I, Yamamoto K, et al. Molecular basis for the activation of gonadotropin-inhibitory hormone gene transcription by corticosterone. Endocrinology. 2014;155:1817–26. doi: 10.1210/en.2013-2076. [DOI] [PubMed] [Google Scholar]
- 30.Ernst DK, Lynn SE, Bentley GE. Differential response of GnIH in the brain and gonads following acute stress in a songbird. Gen Comp Endocrinol. 2016;227:51–7. doi: 10.1016/j.ygcen.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Clarke IJ, Bartolini D, Conductier G, Henry BA. Stress Increases Gonadotropin Inhibitory Hormone Cell Activity and Input to GnRH Cells in Ewes. Endocrinology. 2016;157:4339–50. doi: 10.1210/en.2016-1513. [DOI] [PubMed] [Google Scholar]
- 32.Kim JS, Brownjohn PW, Dyer BS, Beltramo M, Walker CS, Hay DL, et al. Anxiogenic and Stressor Effects of the Hypothalamic Neuropeptide RFRP-3 Are Overcome by the NPFFR Antagonist GJ14. Endocrinology. 2015;156:4152–62. doi: 10.1210/en.2015-1532. [DOI] [PubMed] [Google Scholar]
- 33.Fraley GS, Coombs E, Gerometta E, Colton S, Sharp PJ, Li Q, et al. Distribution and sequence of gonadotropin-inhibitory hormone and its potential role as a molecular link between feeding and reproductive systems in the Pekin duck (Anas platyrhynchos domestica) Gen Comp Endocrinol. 2013;184:103–10. doi: 10.1016/j.ygcen.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, et al. Gonadotropin-Inhibitory Hormone Is a Hypothalamic Peptide That Provides a Molecular Switch between Reproduction and Feeding. Neuroendocrinology. 2012;95:305–15. doi: 10.1159/000332822. [DOI] [PubMed] [Google Scholar]
- 35.Lynn SE, Perfito N, Guardado D, Bentley GE. Food, stress, and circulating testosterone: Cue integration by the testes, not the brain, in male zebra finches (Taeniopygia guttata) Gen Comp Endocrinol. 2015;215:1–9. doi: 10.1016/j.ygcen.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Leon S, Garcia-Galiano D, Ruiz-Pino F, Barroso A, Manfredi-Lozano M, Romero-Ruiz A, et al. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: studies in the NPFF1 receptor null mouse. Endocrinology. 2014;155:2953–65. doi: 10.1210/en.2014-1030. [DOI] [PubMed] [Google Scholar]
- 37.Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–5. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Hormones and Behavior. 2007;51:171–80. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piekarski DJ, Zhao S, Jennings KJ, Iwasa T, Legan SJ, Mikkelsen JD, et al. Gonadotropin-inhibitory hormone reduces sexual motivation but not lordosis behavior in female Syrian hamsters (Mesocricetus auratus) Horm Behav. 2013;64:501–10. doi: 10.1016/j.yhbeh.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–30. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 41.Kauffman AS. Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol. 2010;22:682–91. doi: 10.1111/j.1365-2826.2010.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 43.Ladyman SR, Woodside B. Food restriction during lactation suppresses Kiss1 mRNA expression and kisspeptin-stimulated LH release in rats. Reproduction. 2014;147:743–51. doi: 10.1530/REP-13-0426. [DOI] [PubMed] [Google Scholar]
- 44.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089–97. doi: 10.1111/j.1365-2826.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- 45.Stengel A, Wang L, Goebel-Stengel M, Tache Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. NeuroReport. 2011;22:253–7. doi: 10.1097/WNR.0b013e32834558df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–25. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 47.De Bond JA, Smith JT. Kisspeptin and energy balance in reproduction. Reproduction. 2014;147:R53–63. doi: 10.1530/REP-13-0509. [DOI] [PubMed] [Google Scholar]
- 48.Clarke IJ. Interface between metabolic balance and reproduction in ruminants: Focus on the hypothalamus and pituitary. Hormones and Behavior. 2014 doi: 10.1016/j.yhbeh.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, et al. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006;254–255:127–32. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 50.Curran T, Morgan JI. Memories of fos. Bioessays. 1987;7:255–8. doi: 10.1002/bies.950070606. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Frontiers in Neuroendocrinology. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman GE, Lee WS, Smith MS, Abbud R, Roberts MM, Robinson AG, et al. c-Fos and Fos-related antigens as markers for neuronal activity: perspectives from neuroendocrine systems. NIDA Res Monogr. 1993;125:117–33. [PubMed] [Google Scholar]
- 53.Takahashi LK, Lisk RD. Organization and expression of agonistic and socio-sexual behavior in golden hamsters over the estrous cycle and after ovariectomy. Physiol Behav. 1983;31:477–82. doi: 10.1016/0031-9384(83)90069-0. [DOI] [PubMed] [Google Scholar]
- 54.Orsini MW. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster, Mesocricetus auratus Waterhouse. Proc Anim Care Panel. 1961:193–206. [Google Scholar]
- 55.Rusak B, Mistlberger R, Losier B, Jones C. Daily hoarding opportunity entrains the pacemaker for hamster activity rhythms. Journal of Comparative Physiology A. 1988;164:165–71. doi: 10.1007/BF00603948. [DOI] [PubMed] [Google Scholar]
- 56.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–9. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 57.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, et al. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–69. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams WP, 3rd, Gibson EM, Wang C, Tjho S, Khattar N, Bentley GE, et al. Proximate mechanisms driving circadian control of neuroendocrine function: Lessons from the young and old. Integr Comp Biol. 2009;49:519–37. doi: 10.1093/icb/icp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams WP, III, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2010;152:595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mangels RA, Powers JB, Blaustein JD. Effect of photoperiod on neural estrogen and progestin receptor immunoreactivity in female Syrian hamsters. Brain Research. 1998;796:63–74. doi: 10.1016/s0006-8993(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 61.Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Ozkurt S, et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett. 2008;4:253–5. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnston RE. The causation of two scent-marking behavior patterns in female hamsters (Mesocricentus auratus) Animal Behaviour. 1977;25:317–27. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- 63.Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Letters. 2002;512:255–8. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- 64.Morgan GA, Leech NL, Gloeckner GW, Barrett KC. SPSS for Introductory and Intermediate Statistics: IBM SPSS for Introductory Statistics Use and Interpretation. Routledge: 2012. [Google Scholar]
- 65.Siegel HI. The hamster-reproduction and behavior. Plenum Press; 1985. [Google Scholar]
- 66.Schneider JE, Klingerman CM, Abdulhay A. Sense and Nonsense in Metabolic Control of Reproduction. Front. Endocrinol. 2012;3:3–26. doi: 10.3389/fendo.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider JE, Buckley CA, Blum RM, Zhou D, Szymanski L, Day DE, et al. Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. The European journal of neuroscience. 2002;16:377–9. doi: 10.1046/j.1460-9568.2002.02118.x. [DOI] [PubMed] [Google Scholar]
- 68.Bartness TJ. Species-specific changes in the metabolic control of food intake: integrating the animal with its environment. Int J Obes. 1990;14(Suppl 3):115–23. discussion 23–4. [PubMed] [Google Scholar]
- 69.Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm Behav. 2008;53:307–11. doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 71.Craig W. Appetites and aversions as constituents of instinct. Proc. Natl. Acad. Sci. 1917;3:685–8. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lorenz K. The comparative method in studying innate behavior patterns. Symp. Soc. Exp. Biol. 1950;1950:221–68. [Google Scholar]
- 73.Sherrington CS. The Integrative Action of the Nervous System. New York: Scribner; 1906. [Google Scholar]
- 74.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Hormones and behavior. 2007;51:171–80. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ubuka T, Haraguchi S, Tobari Y, Narihiro M, Ishikawa K, Hayashi T, et al. Hypothalamic inhibition of socio-sexual behaviour by increasing neuroestrogen synthesis. Nature communications. 2014;5 doi: 10.1038/ncomms4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tachibana T, Sato M, Takahashi H, Ukena K, Tsutsui K, Furuse M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005;1050:94–100. doi: 10.1016/j.brainres.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 77.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, et al. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. The Journal of endocrinology. 2008;199:105–12. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 78.Fraley GS, Coombs E, Gerometta E, Colton S, Sharp P, Li Q, et al. Distribution and sequence of gonadotropin-inhibitory hormone and its potential role as a molecular link between feeding and reproductive systems in the Pekin duck (<i>Anas platyrhynchos domestica</i>) General and comparative endocrinology. 2013;184:103–10. doi: 10.1016/j.ygcen.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 79.Tachibana T, Masuda N, Tsutsui K, Ukena K, Ueda H. The orexigenic effect of GnIH is mediated by central opioid receptors in chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2008;150:21–5. doi: 10.1016/j.cbpa.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 80.Borer KT, Rowland N, Mirow A, Borer RC, Jr, Kelch RP. Physiological and behavioral responses to starvation in the golden hamster. Am J Physiol. 1979;236:E105–12. doi: 10.1152/ajpendo.1979.236.2.E105. [DOI] [PubMed] [Google Scholar]
- 81.Rowland N. Failure by deprived hamsters to increase food intake: some behavioral and physiological determinants. J Comp Physiol Psychol. 1982;96:591–603. doi: 10.1037/h0077905. [DOI] [PubMed] [Google Scholar]
- 82.Silverman HJ, Zucker I. Absence of post-fast food compensation in the golden hamster (Mesocricetus auratus) Physiology & Behavior. 1976;17:271–85. doi: 10.1016/0031-9384(76)90076-7. [DOI] [PubMed] [Google Scholar]
- 83.Keen-Rhinehart E, Ondek K, Schneider JE. Neuroendocrine regulation of appetitive ingestive behavior. Front Neurosci. 2013;7:213. doi: 10.3389/fnins.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartness TJ, Keen-Rhinehart E, Dailey MJ, Teubner BJ. Neural and hormonal control of food hoarding. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;301:R641–55. doi: 10.1152/ajpregu.00137.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H, Wade GN, Blaustein JD. Manipulations of metabolic fuel availability alter estrous behavior and neural estrogen receptor immunoreactivity in Syrian hamsters. Endocrinology. 1994;135:240–7. doi: 10.1210/endo.135.1.8013358. [DOI] [PubMed] [Google Scholar]
- 86.Early AH, Wade GN, Lempicki RL. Effects of cold exposure on estrous behavior and neural estrogen receptor in Syrian hamsters. Physiology & behavior. 1998;65:763–8. doi: 10.1016/s0031-9384(98)00224-8. [DOI] [PubMed] [Google Scholar]
- 87.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 88.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proceedings of the National Academy of Sciences. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedman JM. Leptin at 14 y of age: an ongoing story. The American journal of clinical nutrition. 2009;89:973S–9S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. 1996 doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 91.Kolaczynski J, Ohannesian J, Considine R, Marco C, Caro J. Response of leptin to short-term and prolonged overfeeding in humans. Journal of Clinical Endocrinology & Metabolism. 1996;81:4162–5. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 92.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. Journal of Clinical Investigation. 2003;111:1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider JE, Blum R, Wade GN. Long-term rather than short-term changes in adiposity and plasma leptin levels are associated with fasting induced anestrous. Hormones and Behavior. 1999 submitted. [Google Scholar]
- 94.Buckley CA, Schneider JE. Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. American journal of physiology. Regulatory, integrative and comparative physiology. 2003;285:R1021–9. doi: 10.1152/ajpregu.00488.2002. [DOI] [PubMed] [Google Scholar]
- 95.Keen-Rhinehart E, Bartness TJ. Leptin inhibits food-deprivation-induced increases in food intake and food hoarding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1737–46. doi: 10.1152/ajpregu.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poling MC, Shieh MP, Munaganuru N, Luo E, Kauffman AS. Examination of the influence of leptin and acute metabolic challenge on RFRP-3 neurons of mice in development and adulthood. Neuroendocrinology. 2014;100:317–33. doi: 10.1159/000369276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rizwan MZ, Harbid AA, Inglis MA, Quennell JH, Anderson GM. Evidence that hypothalamic RFamide related peptide-3 neurones are not leptin-responsive in mice and rats. Journal of Neuroendocrinology. 2014;26:247–57. doi: 10.1111/jne.12140. [DOI] [PubMed] [Google Scholar]
- 98.Ozcan M, Saatci T, Ayar A, Canpolat S, Kelestimur H. Leptin activates cytosolic calcium responses through protein kinase-C dependent mechanism in immortalized RFamide-related peptide-3 neurons. Brain Res. 2015;1601:8–14. doi: 10.1016/j.brainres.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 99.Rizwan M, Harbid A, Inglis M, Quennell J, Anderson G. Evidence That Hypothalamic RFamide Related Peptide-3 Neurones are not Leptin-Responsive in Mice and Rats. Journal of neuroendocrinology. 2014;26:247–57. doi: 10.1111/jne.12140. [DOI] [PubMed] [Google Scholar]
- 100.Poling MC, Shieh MP, Munaganuru N, Luo E, Kauffman AS. Examination of the influence of leptin and acute metabolic challenge on RFRP-3 neurons of mice in development and adulthood. Neuroendocrinology. 2014;100:317–33. doi: 10.1159/000369276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozcan M, Saatcı T, Ahmet A, Canpolat S, Kelestımur H. Leptin Activates cytosolic calcium Responses through protein kinase-C dependent mechanism in immortalized RFamide-related peptide-3 Neurons. Brain Research. 2015 doi: 10.1016/j.brainres.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 102.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, et al. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29:179–90. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.León S, García-Galiano D, Ruiz-Pino F, Barroso A, Manfredi-Lozano M, Romero-Ruiz A, et al. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: studies in the NPFF1 receptor null mouse. Endocrinology. 2014;155:2953–65. doi: 10.1210/en.2014-1030. [DOI] [PubMed] [Google Scholar]
- 104.Paul MJ, Pyter LM, Freeman DA, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. Journal of Neuroendocrinology. 2009;21:1007–14. doi: 10.1111/j.1365-2826.2009.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ammar AA, Sederholm F, Saito TR, Scheurink AJ, Johnson AE, Sodersten P. NPYleptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1627–33. doi: 10.1152/ajpregu.2000.278.6.R1627. [DOI] [PubMed] [Google Scholar]
- 106.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding and food intake by Siberian hamsters. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2005 doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 107.Dailey MJ, Bartness TJ. Appetitive and consummatory ingestive behaviors stimulated by PVH and perifornical area NPY injections. Am J Physiol Regul Integr Comp Physiol. 2009;296:R877–92. doi: 10.1152/ajpregu.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114:2413–8. doi: 10.1073/pnas.1621065114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci. 2016;19:734–41. doi: 10.1038/nn.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ward DR, Dear FM, Ward IA, Anderson SI, Spergel DJ, Smith PA, et al. Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. PLoS One. 2009;4:e5322. doi: 10.1371/journal.pone.0005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and behavior. 1994;28:464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 112.Tilbrook A, Clarke I. Neuroendocrine mechanisms of innate states of attenuated responsiveness of the hypothalamo-pituitary adrenal axis to stress. Frontiers in neuroendocrinology. 2006;27:285–307. doi: 10.1016/j.yfrne.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 113.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11324–9. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Son YL, Ubuka T, Narihiro M, Fukuda Y, Hasunuma I, Yamamoto K, et al. Molecular basis for the activation of gonadotropin-inhibitory hormone gene transcription by corticosterone. Endocrinology. 2014;155:1817–26. doi: 10.1210/en.2013-2076. [DOI] [PubMed] [Google Scholar]
- 115.Calisi RM, Rizzo NO, Bentley GE. Seasonal differences in hypothalamic EGR-1 and GnIH expression following capture-handling stress in house sparrows (Passer domesticus) General and comparative endocrinology. 2008;157:283–7. doi: 10.1016/j.ygcen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 116.Kaewwongse M, Takayanagi Y, Onaka T. Effects of RFamide-related peptide (RFRP)-1 and RFRP-3 on oxytocin release and anxiety-related behaviour in rats. Journal of neuroendocrinology. 2011;23:20–7. doi: 10.1111/j.1365-2826.2010.02077.x. [DOI] [PubMed] [Google Scholar]
- 117.Jones JE, Pick RR, Davenport MD, Keene AC, Corp ES, Wade GN. Disinhibition of female sexual behavior by a CRH receptor antagonist in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2002;283:R591–7. doi: 10.1152/ajpregu.00233.2002. [DOI] [PubMed] [Google Scholar]
- 118.Seymour PL, Dettloff SL, Jones JE, Wade GN. Corticotropin-releasing factor receptor subtypes mediating nutritional suppression of estrous behavior in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;289:R418–R23. doi: 10.1152/ajpregu.00168.2005. [DOI] [PubMed] [Google Scholar]
- 119.Cabanac M, Richard D. Acute intraventricular CRF lowers the hoarding threshold in male rats. Physiol Behav. 1995;57:705–10. doi: 10.1016/0031-9384(94)00322-x. [DOI] [PubMed] [Google Scholar]