Abstract
Mast cells are first responders to intracerebral hemorrhage. They release potent mediators that can disrupt the blood-brain barrier promoting injury, vasogenic edema formation, and hematoma exacerbation. Also, mast cells recruit other inflammatory cells that maintain and amplify brain damage. Given their early role in the cascade of events in intracerebral hemorrhage, mast cells may offer an alternative target for antichemotactic interventions.
Keywords: Mast cells, intracerebral hemorrhage, blood-brain barrier, inflammation, edema, cytokines
1 Introduction
Intracerebral hemorrhage (ICH) is associated with high mortality and morbidity. Hematoma-induced secondary brain injury can lead to severe neurological deficits and death. This secondary brain injury is precipitated by a cascade of molecular and cellular inflammatory responses in which mast cells (MCs) are believed to play an early and important role. The aim of this article is to present a review of basic and translational research conducted to investigate the role of MCs in cerebrovascular disease with emphasis on ICH and to suggest some potential clinical applications.
2 Mast Cells
MCs are perivascularly located tissue-resident cells of hematopoietic origin recognized as effectors in inflammation and immunity due to their role in a range of processes including atopic diseases, parasitic infections, hematologic malignancies, autoimmune and autoinflammatory diseases, as well as connective tissue remodeling and repair [1, 2]. In humans, they originate in the bone marrow from CD34+/CD117+ pluripotent progenitor cells, then circulate in the blood as committed precursors, and complete their differentiation in peripheral tissues where they reside and are maintained by local cytokines including stem cell factor [3, 4].
2.1 Classification
There is variance in literature concerning the differentiation of MCs. In humans and rodents, they are subcategorized into populations based on their tissue of residence as well as their mediator content [2]. In humans, MCs can be distinguished in general according to their protease content into MCT (tryptase) or MCTC (tryptase and chymase). In rodents, they are differentiated into two broad subtypes: connective tissue MCs and mucosal MCs. Some argue that rodent brain MCs are remarkably differentiated to be another subtype [1, 4].
2.2 Modes of Action
MCs can be activated in response to various stimuli. The most notorious one is the IgE pathway that elicits an anaphylactic reaction through high-affinity receptors on MCs. MCs also exhibit IgG receptors and respond to several other receptor-binding agonists including complement, neuropeptides, microbial products, cytokines, and chemokines. They also respond to physical activators such as temperature and pressure as well as to cell-cell contact [2, 4].
MCs can be activated in three steps, and they respond by releasing mediators that can be divided into: (1) preformed mediators, (2) newly synthesized lipid derived mediators, and (3) cytokines/chemokines. The first step of MC activation is a rapid response within seconds involving generation of reactive oxygen species and extrusion of preformed mediators stored in granules including histamine, serotonin, heparin, proteases, proteoglycans, cathepsin G, and cytokines such as tumor necrosis factor alpha (TNFα). The second step occurs within minutes and involves the generation and release of lipid derived mediators such as prostaglandins, leukotrienes, platelet-activating factor, growth factors, free radicals, substance P, and other cytokines and chemokines. Finally, within hours, MC activation is followed by de novo synthesis and release of more cytokines and chemokines including TNFα which occurs in both forms as preformed and newly synthesized mediator [2, 4, 5].
2.3 Mast Cells in the Brain
MCs reside in the normal brain of many species including humans [6]. They are most numerous in the meninges, thalamus, and hypothalamus [2, 7]. MCs are present in the rat brain from birth and occur in two locations during development, the pia and brain parenchyma. During the development of the rat brain, MC precursors enter the pia and access the thalamus by migrating along the abluminal surface of penetrating blood vessels with which they remain intimately associated [8]. In all vertebrate studies, including humans, MCs can be found on the brain side of the blood-brain barrier (BBB) in contact with the blood vessel wall or its extracellular matrix [4]. MCs preferentially home to branching points along large blood vessels surrounded by astroglial processes suggesting a role in vasculature growth as well as the development and regulation of the BBB permeability [9].
MCs have a complex and intimate relationship with the cellular and extracellular components of the neurovascular unit which encompasses neurons, glia, microglia, and endothelial cells with potential multidirectional signaling [4, 8], and they are believed to participate in the regulation of homeostasis at the level of the microcirculation within the neurovascular unit [5, 7].
3 Mast Cells in Acute Neurological Injury
MCs contribute to the normal physiology as well as the pathology of the brain in conditions including stroke, traumatic brain injury, multiple sclerosis, and brain tumors [2, 10]. They are often called first responders that initiate and amplify immune and nervous responses through their multiphasic response pattern [4, 5].
3.1 In Vitro Studies
In vitro experiments showed that MCs can increase the BBB permeability via different mechanisms. MC-derived histamine, tryptase, chymase, and cathepsin G can bind to specific receptors on endothelial cells and increase the phosphorylation of the adherens junction which loosens vascular endothelial cadherin-mediated adhesion [7]. Also, MC activation can increase the redox stress through the production of reactive oxygen species which cause peroxidation of lipid-rich structures of the BBB resulting in its disruption [11]. In addition, TNFα and IL-1β produced by MCs can alter endothelial cell-matrix interactions by downregulating the expression of integrins and their receptors leading to decreased adhesion of endothelial cells to laminin and contributing to BBB increased permeability [12, 13].
The communication between MCs and glia was also explored in vitro. Histamine and CD40/CD40-ligand regulate the interaction between MCs and astrocytes [14, 15]. Glia release Interleukin-33 (IL-33) and IL-1β which stimulate MCs to produce large amounts of inflammatory cytokines. MC-derived IL-13 and tryptase activate microglia through protease-activated receptor 2 (PAR2) to produce other pro-inflammatory mediators including arginase, IL-6, monocyte chemotactic protein-1, and TNFα [16–19].
MC-derived mediators can have direct toxic effects on neurons. MC proteases such as tryptase cause neuronal hyperexcitability and inflammation through protease-activated receptors [19]. When co-cultured with hippocampal neurons, activated MCs potentiated glutamate excitotoxicity, presumably by histamine release, suggesting a role for MCs under conditions of cerebral ischemia with enhanced glutamatergic neurotransmission [20].
3.2 In Vivo Studies
3.2.1 Mast Cells, the Blood-Brain Barrier Breakdown, and Vasogenic Edema
In vivo experiments support the idea that MC degranulation increases the BBB permeability and promotes edema formation. Intramuscular injection of MC degranulator compound 48/80 causes vastly increased Evans Blue dye leakage in MC-rich brain areas with fenestrated capillaries, while this effect is blocked by MC stabilizers or absent in MC-deficient mice [21]. In a rat model of transient middle cerebral artery occlusion, Strbian et al. reported that ischemic swelling was reduced by 39% after cromoglycate administration and increased by 89% after compound 48/80 administration [22]. Early ischemic blood-brain barrier leakage was reduced by 51% after cromoglycate and increased by 50% after compound 48/80. Also, MC-deficient rats responded to focal ischemia with 58% less brain swelling than did their wild-type littermates. Blood-brain barrier damage was 47% lower in MC-deficient rats than in wild type [22].
In ischemic stroke, the basal lamina of the BBB loses its integrity very early after the onset of ischemia as demonstrated by the rapid disappearance of its three main constituents: laminin, fibronectin, and collagen type IV [23, 24]. In a rat model of transient cerebral ischemia, MCs can degrade the components of the basal lamina, especially collagen type IV, and disrupt tight junction proteins through proteolytic gelatinase enzymes, matrix metalloproteinases MMP2 and MMP9 [25]. In this experiment, compound 48/80 increased gelatinase activity which was correlated with increased brain edema, while these effects were significantly less in MC-deficient rats and with MC stabilization using cromoglycate. MCs not only secrete gelatinases but also can activate tissue progelatinases and degrade tissue inhibitor of metalloproteinase-1 through released chymase and tryptase which also can degrade most components of the blood-brain barrier. MCs stimulate neighboring cells to release gelatinases through TNFα. They also promote neutrophil infiltration leading to more accumulation of gelatinases [25].
3.2.2 Mast Cells and the Inflammatory Response
Preclinical studies showed that MCs participate in the inflammatory response and recruitment of immune cells to the CNS under different pathologic conditions.
In a study of hypoxia-ischemia in 9-day-old mice, an upregulation of expression of MC-associated inflammatory genes was observed in the injured brain hemisphere [26]. Also, MC-derived histamine was shown to contribute to ischemia-induced inflammation and neuronal death in new-born rats at postnatal day 7 [27]. On the other hand, MC stabilization limited hypoxic-ischemic brain damage in the immature rat [28].
In the rat model of transient middle cerebral artery occlusion, the cromoglycate treated group showed 37% less postischemic neutrophil infiltration than controls. Cromoglycate reduced the number of transmigrated neutrophils and that of those still within the intravascular space. This indicates that MC blocking can reduce passive neutrophil trafficking through BBB breaches and probably attenuate the perivascular chemotactic gradient up which neutrophils migrate. Neutrophil infiltration was also reduced by 47% in MC-deficient rats compared to wild type [22].
3.2.3 Meningeal Mast Cells in Acute Brain Injury
Some studies focused on the role of meningeal MCs in acute neurological injury suggesting a more important pathological contribution for them compared to parenchymal MCs.
In experimental autoimmune encephalomyelitis in mice, Christy et al. showed evidence that meningeal MCs are activated within 24 h of disease induction but do not directly compromise CNS vascular integrity [29]. However, through production of TNFα, MCs elicit an early influx of neutrophils into meninges promoting early breakdown of the local BBB and cerebrospinal fluid-blood barrier allowing immune cell access to the CNS. C-kit-mutant MC-deficient mice exhibit a mild disease course associated with significantly decreased immune cell infiltration to the CNS and failure to breach the BBB compared to WT. Selective reconstitution of meningeal MCs to c-kit-mutant MC-deficient mice via intracranial injection restores WT disease phenotype. Reconstitution of meninges with TNFα-knocked out (KO) MCs fails to restore severe disease indicating TNFα is essential for mediating MC effects. The numbers and percentage of infiltrating neutrophils in mice reconstituted with TNFα-KO MCs was similar to that of c-kit-mutant MC-deficient mice and consistently lower than WT mice or c-kit-mutant mice reconstituted with WT MCs. Also, MC-deficient mice repleted with TNFα-KO MCs failed to develop frank BBB permeability. These data demonstrate a direct influence of TNFα produced by meningeal MCs on meningeal neutrophil influx and alterations in BBB permeability [29].
MCs can influence the numbers of brain leukocytes in mice after stroke as well [30]. In a study on the role of meningeal MCs in a mouse model of stroke, Arac et al. (2014) showed that meningeal MCs contribute to key features of stroke pathology including infiltration of granulocytes and activated macrophages, brain swelling, and infarct size. MC-deficient mice exhibited significantly less brain swelling at 3 days after stroke as well as significantly smaller infarcts at 3 days and 2 weeks after stroke compared to WT mice. However, MC-deficient mice engrafted intravenously with MCs exhibited brain swelling and infarct sizes similar to those of the WT mice. It was noticed that after engraftment the meninges had near normal numbers of MCs while the brain parenchyma had no or few MCs. Targeted repair of meningeal MC deficiency produced significantly more brain swelling, larger infarcts, and more granulocyte and activated macrophage infiltration at 3 days after stroke in MC-engrafted mice compared to MC-deficient mice. These results were similar to the WT pathology suggesting that meningeal MCs alone may be sufficient to exacerbate injury after stroke. Given the perivascular location of MCs and that blood flow to the brain traverses the meninges, meningeal MCs are ideally located to function as potential gatekeepers for modulating immune cell trafficking to the brain in stroke [31].
4 Mast Cells in Intracerebral Hemorrhage
Patient with large ICH (>100 ml) have poor prognosis. In smaller ICH, most patients survive the initial ictus, but hematoma-induced secondary brain injury can lead to severe neurological deficits and death. Hematoma expansion occurs during the first 24 hours in 20–40% of patients contributing to the mass effect and predicting poor outcome. Edema forms rapidly around the hematoma adding to the mass effect and brain injury and peaking in the second week. The hematoma resolves gradually over several weeks usually leaving a cavity where brain tissue was damaged [32–36].
Hemorrhagic stroke is a disorder that starts at the vascular level and involves the neurovascular unit. It is hypothesized that the resulting sudden disruption in homeostasis in the neurovascular unit due to the cessation of normal blood flow and the accumulation of noxious stimuli such as blood and waste products along with the drop in pH would all activate MCs that act as a “fast response force” by rapid degranulation and release of preformed and stored mediators [8]. Within hours of their activation, MCs continue to synthesize and release pro-inflammatory mediators maintaining and increasing the inflammatory response.
Several in vivo preclinical studies were conducted to investigate the role of MCs in ICH using pharmacologic MC stabilization and MC-deficient animals. The results of these experiments are summarized below.
Strbian et al. investigated the effect of MC stabilization on brain edema, hematoma volume, and neurological outcome in an autologous blood injection rat model of ICH [37]. MC stabilization with cromoglycate resulted in highly significant better neurologic scores, decreased mortality, less brain swelling, and smaller hematoma volume after 24 hours. Depending on the route of administration of cromoglycate, brain edema was reduced by 50% (intravenous) and 75% (intracerebroventricular). MC degranulating compound 48/80 led to opposite effects. The degree of brain edema corresponded with mortality. All rats treated with cromoglycate survived and showed good outcome, whereas those treated with saline or compound 48/80 had mortality rates of 45% and 55% respectively. All MC-deficient rats survived, while 25% of their wild-type littermates died. Also, MC-deficient rats had significantly better neurologic scores, less brain swelling, and smaller hematoma growth [37].
Strbian et al. also investigated the effect of MC stabilization on hemorrhage formation and mortality after the administration of thrombolytics in experimental ischemic stroke [38]. In vitro, tPA induces strong MC degranulation as shown by histamine release. In a focal cerebral ischemia/reperfusion rat model, postischemic tPA administration showed 70- to 100-fold increase in hemorrhagic formation. MC stabilization with cromoglycate led to significant decrease in tPA-mediated hemorrhagic formation at 3 (97%), 6 (76%), and 24 hours (96%) compared with controls. Also, MC-deficient rats showed decrease in tPA-mediated hemorrhagic transformation at 6 (92%) and 24 hours (89%) compared with wild-type littermates. MC stabilization and deficiency also significantly reduced other hallmarks of reperfusion injury such as brain swelling and neutrophil infiltration. This translated into significantly better neurological outcome and lower mortality after 24 hours [38].
In a study that investigated the effect of hydrogen inhalation on MC-mediated brain injury in a collagenase mouse model of ICH, Manaenko et al. (2013) reported BBB disruption, brain edema, and neurological deficits at 24 and 72 hours post-ICH accompanied by the phosphorylation of Lyn kinase and release of tryptase, which are markers of MC activation, at 6 hours post-ICH. Hydrogen inhalation significantly diminished ICH-induced MC activation, reduced redox stress, enhanced BBB preservation, ameliorated edema, and diminished neurological deficits at 24 hours post-ICH [11].
MC stabilization with cromoglycate significantly decreased perihematomal edema with reduced hemispheric expansion without changes in hematoma volume following intravenous tPA in a collagenase rat model of ICH [39].
The pathophysiological basis of MC-dependent brain injury during ICH is uncertain, but it is believed that many mechanisms proposed in MC-dependent brain injury during ischemic stroke and other forms of acute brain injury could be applied in ICH as well. MCs can induce BBB breakdown, vasogenic edema, prolonged extravasation, hemorrhage, and inflammation. Further studies are needed to prove these suggested mechanisms in ICH.
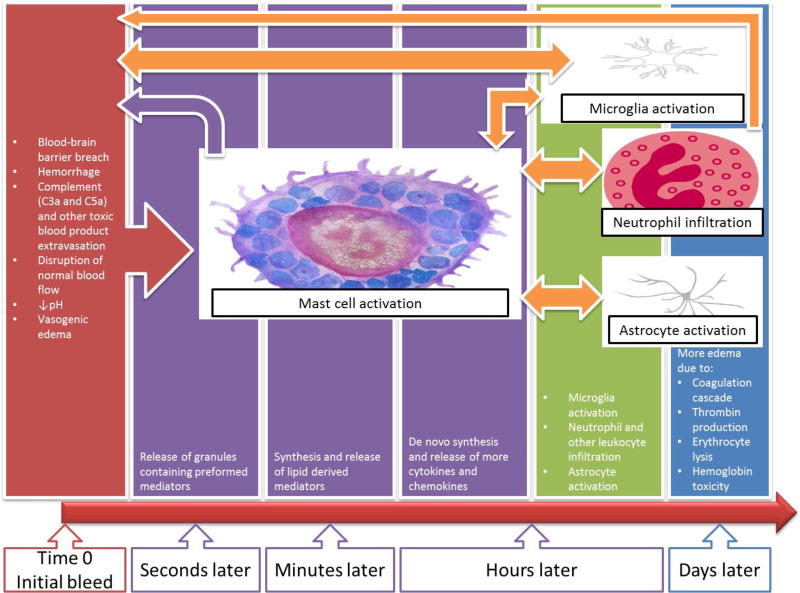
An important aspect of MC role in ICH is their early onset of action and recruitment of other effectors. MCs are believed to respond very rapidly even before microglia. Microglia are activated later within the first hours of injury [32, 33, 40]. Figure 1 is a simplified diagram showing a timeline of events in ICH with an emphasis on the very early role of MCs and their complex interactions with other elements.
Figure 1.
Graphical description of the time sensitive mast cell activation process and the inflammatory cascade after intracerebral hemorrhage.
It is also plausible that MCs in brain areas such as the meninges that are away from the site of the initial bleed get activated as well after ICH and participate in the inflammatory response through collateral circulation that delivers MC-derived mediators to the site of injury.
4.1 Mast Cells, the Blood-Brain Barrier Breakdown, and Vasogenic Edema
The initial insult in ICH is a result of a breach in the BBB. The subsequent disruption of homeostasis and leakage of blood products especially complement C3a and C5a into the brain activates MCs that possess an armamentarium of mediators capable of targeting most of the components of the BBB immediately [8]. De novo synthesis of these mediators and the recruitment of other inflammatory cells maintain and potentiate this effect. Consequently, more areas of the BBB are compromised and the extravasation of blood cells, serum proteins, and plasma is prolonged resulting in the aggravation of the hemorrhage and vasogenic edema.
The pathophysiologic basis of MC-dependent brain edema and swelling during ICH has not been fully illustrated yet, but it is thought to depend on the release of several MC-derived vasoactive substances (histamine, bradykinin) and proteolytic enzymes (tryptase, chymase, cathepsin G, and gelatinases) which increase vascular permeability and disrupt the basal lamina of the vasculature along with the surrounding extracellular tissue matrix leading to extravasation of blood cells and serum proteins [37].
4.2 Mast Cells and Hematoma Expansion
Hematoma expansion after ICH has been attributed to continued bleeding from the primary source and to mechanical disruption of the surrounding vessels [41]. On activation, MCs release heparin, an endogenous anticoagulant synthesized and released only by MCs. This may lead to impaired hemostasis and plugging of BBB breaches which prolongs the extravasation of red blood cells and promotes primary hematoma growth as well as secondary hemorrhage formation. This hypothesis is consistent with the data showing a significant growth of hematoma in the saline and compound 48/80 groups in the rat model of ICH. In contrast, a lack of hematoma growth was observed in both the cromoglycate-treated group and MC-deficient rats [37].
This antihemostatic effect of MC-derived heparin superimposed on the vasculopathic proteolytic capacity of other MC-derived mediators reviewed in the previous subsection makes MCs a potential effector in hematoma and edema evolution in ICH.
4.3 Mast Cells and the Inflammatory Response
The infiltration of neutrophils after ICH is believed to compromise the microcirculation and BBB integrity and to contribute to the release of free radicals [42]. One of the most potent mediators involved in the recruitment of neutrophils is TNFα [43], which represents a distinct type of MC mediators since it is both acutely released from preformed granules and then continuously synthesized de novo and secreted. Being probably the only cell type containing preformed stores of TNFα, MCs are likely to represent a critical initial cellular source of this mediator during inflammation in ICH. Later, TNFα is also produced by infiltrating neutrophils and macrophages. In addition to neutrophils, MCs can also recruit macrophages from the circulation and activate brain-resident inflammatory cells such as microglia. MCs produce IL-6 and activate microglia to produce more IL-6. The cumulative effect of all these inflammatory cells and their products following ICH aggravates the primary damage and precipitates secondary injury.
It is believed that following ICH, MCs, other brain-resident inflammatory cells, and immune cells recruited from the circulation interact to orchestrate a cascade of responses resulting in secondary brain injury. After the hematoma size stabilizes, the surrounding edema may continue to expand. Hemoglobin produced by erythrocyte lysis and coagulation products such as thrombin maintain the inflammatory response for days after ICH aggravating the brain damage and toxicity.
In fact, several human studies investigated levels of molecular markers of inflammation and injury in ICH and disclosed a significant correlation with clinical outcomes. Plasma concentration of TNFα was fond to correlate with degree of brain edema in ICH patients [44]. An increased serum levels of IL-6 and IL-10 in ICH patients significantly correlated with Glasgow Coma Scale score. Also, IL-6 level correlated with blood volume and mass effect [45]. Plasma concentrations of IL-6, TNFα, MMP9, and cellular fibronectin were significantly higher in patients with early growth of ICH [46]. In a human postmortem study, an upregulation was noted in the expression of nuclear factor-kappa B/p65 subunit, macrophage inflammatory protein-2, and MMP9 on the injured side of the hippocampus at times ranging from 2 hours to 5 days post-ICH [47].
4.4 Summary of the Role of Mast Cells in Intracerebral Hemorrhage
The disruption of homeostasis after the initial bleed in ICH and the leakage of blood products such as complement into the brain activate MCs rapidly resulting in the release of preformed and stored proteolytic enzymes, histamine, heparin, and TNFα. These mediators are capable of disrupting the BBB leading to more extravasation and contributing to the hematoma growth and vasogenic edema formation. Through TNFα and additional cytokines such as IL-6, MCs can activate other brain-resident inflammatory cells and contribute to the chemotactic gradient that recruits immune cells from the blood. Activated glia and infiltrating neutrophils and macrophages add to the already beginning damage by further compromising the integrity of the BBB and accumulating more toxic pro-inflammatory products. Infiltrating immune cells and glia can activate MCs again maintaining and snowballing the inflammatory response. This might help explain the growth of microbleeds surrounding the primary hematoma source and the secondary bleeding and edema development over time following ICH.
5 Clinical Applications
Although ICH is associated with a high mortality and morbidity, a treatment remains elusive [48]. The mass effect created by the hematoma and later by the perihematomal edema in addition to the subsequent neuroinflammation are major determinants of clinical outcome in ICH patients [35, 49–51]. Hence, new therapeutic modalities targeting major effectors in these processes may be helpful in developing novel treatment protocols in the clinical setting [36].
Recent ICH clinical trials with different therapeutic targets some of which were supported by animal data have failed. The trials involved both surgical and medical approaches including critical care measures such as blood pressure and hyperglycemia control, reversal of antiplatelet and anticoagulation agents, and cerebral edema management in addition to the use of antiinflammatory agents like statins and Fingolimod [52, 53].
Recent research has not been focused on brain-resident MCs that are capable of immediate host responses following ICH even before microglia. On the other hand, inhibition of the inflammatory cascade of blood-borne neutrophil and macrophage infiltration after cerebral injury has been the subject of trials to establish new therapies in stroke [8, 54]. In ischemic stroke, clinical trials based on the inhibition of neutrophils did not succeed which might be explained by the fact that leukocytes arrive relatively late to influence secondary damage propagation [7]. The same rationale is probably applicable in ICH. Hence, MCs may offer an alternative target for antichemotactic interventions. MC stabilization may be an efficient strategy for blocking simultaneously several molecular and cellular cascades that are involved in the pathophysiology of ICH. Also, meningeal MCs may be relatively more accessible for pharmacologic intervention given that meningeal vasculature is less restrictive than that of the BBB [31].
Hemorrhagic transformation following thrombolysis in ischemic stroke is associated with worse clinical outcome. The mechanisms underlying post-thrombolytic hemorrhagic transformation are not fully understood. An important observation is that hemorrhage may be further aggravated by heparin acutely released by MCs [8]. Endogenously released heparin may impair hemostasis and prevent plugging of BBB breaches [7]. Thus, targeting MCs may improve the safety of thrombolysis.
6 Conclusion
The pathophysiology of ICH is complex, encompasses various effectors, and awaits further exploration. A better understanding of the pathological processes underlying the development of ICH would help in evolving new therapeutic interventions. MC blocking agents may prove to be neuroprotective in ICH in light of the very early role of MCs, their influence on other effectors, and their impact on major determinants of clinical outcome such as hematoma and edema expansion as well as neuroinflammation. The multiphasic response of MCs to injury may offer a window period for intervention. Further research on the mechanisms of MC-mediated brain damage following ICH is necessary.
Acknowledgments
Disclosure:
Dr. Torbey is funded through NIH Award U10NS086484 and U10NS080368. Dr. Yehya is a StrokeNET fellow funded under NIH Award U10NS086484.
References
- 1.Ghably J, Saleh H, Vyas H, Peiris E, Misra N, Krishnaswamy G. Paul Ehrlich's mastzellen: a historical perspective of relevant developments in mast cell biology. Methods in molecular biology. 2015;1220:3–10. doi: 10.1007/978-1-4939-1568-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Nelissen S, Lemmens E, Geurts N, Kramer P, Maurer M, Hendriks J, Hendrix S. The role of mast cells in neuroinflammation. Acta neuropathologica. 2013;125(5):637–650. doi: 10.1007/s00401-013-1092-y. [DOI] [PubMed] [Google Scholar]
- 3.Collington SJ, Williams TJ, Weller CL. Mechanisms underlying the localisation of mast cells in tissues. Trends in Immunology. 2011;32(10):478–485. doi: 10.1016/j.it.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends in neurosciences. 2013;36(9):513–521. doi: 10.1016/j.tins.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Ribatti D. The crucial role of mast cells in blood-brain barrier alterations. Experimental cell research. 2015;338(1):119–125. doi: 10.1016/j.yexcr.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Dropp JJ. Mast cells in the human brain. Acta anatomica. 1979;105(4):505–513. doi: 10.1159/000145157. [DOI] [PubMed] [Google Scholar]
- 7.Strbian D, Kovanen PT, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. An emerging role of mast cells in cerebral ischemia and hemorrhage. Annals of medicine. 2009;41(6):438–450. doi: 10.1080/07853890902887303. [DOI] [PubMed] [Google Scholar]
- 8.Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30(4):689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalil M, Ronda J, Weintraub M, Jain K, Silver R, Silverman AJ. Brain mast cell relationship to neurovasculature during development. Brain research. 2007;1171:18–29. doi: 10.1016/j.brainres.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dines KC, Powell HC. Mast cell interactions with the nervous system: relationship to mechanisms of disease. Journal of neuropathology and experimental neurology. 1997;56(6):627–640. [PubMed] [Google Scholar]
- 11.Manaenko A, Lekic T, Ma Q, Zhang JH, Tang J. Hydrogen inhalation ameliorated mast cell-mediated brain injury after intracerebral hemorrhage in mice. Critical care medicine. 2013;41(5):1266–1275. doi: 10.1097/CCM.0b013e31827711c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defilippi P, Silengo L, Tarone G. Alpha 6.beta 1 integrin (laminin receptor) is down-regulated by tumor necrosis factor alpha and interleukin-1 beta in human endothelial cells. Journal of Biological Chemistry. 1992;267(26):18303–18307. [PubMed] [Google Scholar]
- 13.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23(8):879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 14.Kim DY, Jeoung D, Ro JY. Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. Journal of immunology (Baltimore, Md: 1950) 2010;185(1):273–283. doi: 10.4049/jimmunol.1000991. [DOI] [PubMed] [Google Scholar]
- 15.Kim DY, Hong GU, Ro JY. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J Neuroinflammation. 2011;8:25. doi: 10.1186/1742-2094-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. The FASEB Journal. 2012;26(8):3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- 17.Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. Journal of leukocyte biology. 2008;84(3):631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic signalling. 2010;6(1):3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Zeng X, Yang H, Hu G, He S. Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;29(5–6):931–940. doi: 10.1159/000171029. [DOI] [PubMed] [Google Scholar]
- 20.Skaper SD, Facci L, Kee WJ, Strijbos PJ. Potentiation by histamine of synaptically mediated excitotoxicity in cultured hippocampal neurones: a possible role for mast cells. J Neurochem. 2001;76(1):47–55. doi: 10.1046/j.1471-4159.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 21.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain research Brain research reviews. 2005;49(1):65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26(5):605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- 23.Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26(11):2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- 24.Tagaya M, Haring HP, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21(7):835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Mattila OS, Strbian D, Saksi J, Pikkarainen TO, Rantanen V, Tatlisumak T, Lindsberg PJ. Cerebral mast cells mediate blood-brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke. 2011;42(12):3600–3605. doi: 10.1161/STROKEAHA.111.632224. [DOI] [PubMed] [Google Scholar]
- 26.Hedtjarn M, Mallard C, Hagberg H. Inflammatory gene profiling in the developing mouse brain after hypoxia-ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24(12):1333–1351. doi: 10.1097/01.WCB.0000141559.17620.36. [DOI] [PubMed] [Google Scholar]
- 27.Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Heron A. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain pathology (Zurich, Switzerland) 2008;18(1):1–9. doi: 10.1111/j.1750-3639.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Silverman AJ, Vannucci SJ. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Developmental neuroscience. 2007;29(4–5):373–384. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- 29.Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. Journal of autoimmunity. 2013;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 30.McKittrick CM, Lawrence CE, Carswell HV. Mast cells promote blood brain barrier breakdown and neutrophil infiltration in a mouse model of focal cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35(4):638–647. doi: 10.1038/jcbfm.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arac A, Grimbaldeston MA, Nepomuceno ARB, Olayiwola O, Pereira MP, Nishiyama Y, Tsykin A, Goodall GJ, Schlecht U, Vogel H, et al. Evidence that Meningeal Mast Cells Can Worsen Stroke Pathology in Mice. The American Journal of Pathology. 2014;184(9):2493–2504. doi: 10.1016/j.ajpath.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. The Lancet Neurology. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 33.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. The Lancet Neurology. 2012;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. The Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rincon F, Lyden P, Mayer SA. Relationship Between Temperature, Hematoma Growth, and Functional Outcome After Intracerebral Hemorrhage. Neurocritical Care. 2013;18(1):45–53. doi: 10.1007/s12028-012-9779-9. [DOI] [PubMed] [Google Scholar]
- 36.Xi G, Fewel ME, Hua Y, Gregory Thompson B, Hoff JT, Keep RF. Intracerebral hemorrhage. Neurocritical Care. 2004;1(1):5–18. doi: 10.1385/ncc:1:1:5. [DOI] [PubMed] [Google Scholar]
- 37.Strbian D, Tatlisumak T, Ramadan UA, Lindsberg PJ. Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27(4):795–802. doi: 10.1038/sj.jcbfm.9600387. [DOI] [PubMed] [Google Scholar]
- 38.Strbian D, Karjalainen-Lindsberg ML, Kovanen PT, Tatlisumak T, Lindsberg PJ. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007;116(4):411–418. doi: 10.1161/CIRCULATIONAHA.106.655423. [DOI] [PubMed] [Google Scholar]
- 39.Marinkovic I, Mattila OS, Strbian D, Meretoja A, Shekhar S, Saksi J, Abo-Ramadan U, Rantanen V, Lindsberg PJ, Tatlisumak T. Evolution of intracerebral hemorrhage after intravenous tPA: reversal of harmful effects with mast cell stabilization. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(1):176–181. doi: 10.1038/jcbfm.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40(9):3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous Intracerebral Hemorrhage. New England Journal of Medicine. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 42.Lok J, Leung W, Murphy S, Butler W, Noviski N, Lo EH. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta neurochirurgica Supplement. 2011;111:63–69. doi: 10.1007/978-3-7091-0693-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? Journal of immunology (Baltimore, Md: 1950) 2010;184(12):6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 44.Castillo J, Davalos A, Alvarez-Sabin J, Pumar JM, Leira R, Silva Y, Montaner J, Kase CS. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58(4):624–629. doi: 10.1212/wnl.58.4.624. [DOI] [PubMed] [Google Scholar]
- 45.Dziedzic T, Bartus S, Klimkowicz A, Motyl M, Slowik A, Szczudlik A. Intracerebral Hemorrhage Triggers Interleukin-6 and Interleukin-10 Release in Blood. Stroke. 2002;33(9):2334–2335. doi: 10.1161/01.str.0000027211.73567.fa. [DOI] [PubMed] [Google Scholar]
- 46.Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Davalos A Stroke Project CDGotSNS. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36(1):86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]
- 47.Wu H, Zhang Z, Hu X, Zhao R, Song Y, Ban X, Qi J, Wang J. Dynamic changes of inflammatory markers in brain after hemorrhagic stroke in humans: a postmortem study. Brain research. 2010;1342:111–117. doi: 10.1016/j.brainres.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rincon F, Mayer SA. The Epidemiology of Intracerebral Hemorrhage in the United States from 1979 to 2008. Neurocritical Care. 2013;19(1):95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Tsirka SE. Contribution of extracellular proteolysis and microglia to intracerebral hemorrhage. Neurocritical Care. 2005;3(1):77–85. doi: 10.1385/NCC:3:1:077. [DOI] [PubMed] [Google Scholar]
- 50.Volbers B, Willfarth W, Kuramatsu JB, Struffert T, Dörfler A, Huttner HB, Schwab S, Staykov D. Impact of Perihemorrhagic Edema on Short-Term Outcome After Intracerebral Hemorrhage. Neurocritical Care. 2016;24(3):404–412. doi: 10.1007/s12028-015-0185-y. [DOI] [PubMed] [Google Scholar]
- 51.Sun W, Pan W, Kranz PG, Hailey CE, Williamson RA, Sun W, Laskowitz DT, James ML. Predictors of Late Neurological Deterioration After Spontaneous Intracerebral Hemorrhage. Neurocritical Care. 2013;19(3):299–305. doi: 10.1007/s12028-013-9894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mittal MK, LacKamp A. Intracerebral Hemorrhage: Perihemorrhagic Edema and Secondary Hematoma Expansion: From Bench Work to Ongoing Controversies. Frontiers in Neurology. 2016;7(210) doi: 10.3389/fneur.2016.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim-Hing K, Rincon F. Secondary Hematoma Expansion and Perihemorrhagic Edema after Intracerebral Hemorrhage: From Bench Work to Practical Aspects. Frontiers in Neurology. 2017;8(74) doi: 10.3389/fneur.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in neurobiology. 2010;92(4):463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]