Abstract
Background
Eosinophils are prominent in some individuals with asthma and are elevated in the submucosa in a subgroup of obese asthmatics. Surfactant protein-A (SP-A) modulates host responses to infectious and environmental insults.
Objective
To determine if SP-A levels are altered in obese asthma patients compared to a control group and to determine the implications of these alterations in SP-A levels in asthma.
Methods
Bronchoalveolar lavage fluid from 23 lean, 12 overweight and 20 obese subjects were examined for SP-A. Mouse tracheal epithelial cells (MTECs) grown at an air-liquid interface were used for mechanistic studies. SP-A−/− mice were challenged in allergen models and exogenous SP-A therapy was given after the last challenge. Eosinophils were visualized and quantitated in the lung parenchyma by immunostaining.
Results
Significantly less SP-A (p=0.002) was detected in samples from obese asthmatics compared to the control group. A univariable regression model found SP-A was significantly negatively correlated with BMI (r= −0.33; p=0.014), while multivariable modeling demonstrated the correlation depended both on asthma status (p=0.017) and the interaction of asthma and BMI (p=0.008). Addition of exogenous TNF-α to MTECs was sufficient to attenuate SP-A and eotaxin secretion. Allergen challenged SP-A−/− mice that received SP-A therapy had significantly less tissue eosinophilia compared to mice receiving vehicle.
Conclusions
SP-A functions as an important mediator in resolving tissue and lavage eosinophilia in allergic mouse models. Decreased levels of SP-A in obese asthmatics, which could be due to increased local TNF-α, may lead to impaired eosinophil resolution and could contribute to the eosinophilic asthma phenotype.
Keywords: Surfactant, SP-A, obesity, asthma, eosinophils, TNF-α, eotaxin, IL-6, epithelial cells, lung function
Introduction
Obesity is a major co-morbidity associated with an increase in the incidence of asthma and is associated with poor asthma control and worsening asthma symptoms (1). Obese asthma is associated with phenotypic heterogeneity similar to that seen in lean asthma (2) and contrary to prior belief, a proportion of obese asthmatics demonstrate the presence of eosinophilic inflammation (3, 4). The underlying mechanism that causes eosinophilia may differ in some obese asthmatics, where allergic sensitization may not be the primary cause of eosinophilia (5). The initial studies aimed at characterizing eosinophilic inflammation in the lungs of obese as opposed to lean asthma subjects relied on sputum measurements (6–9). However, the reliability of sputum to accurately predict sub-mucosal eosinophils in the airways of asthma subjects was called into question following several key publications that demonstrated that in obese asthmatics high levels of tissue eosinophils were identified in subjects with low levels of eosinophils in compartmental fluids (10–13). This discordance was demonstrated in asthmatic subjects of all severities. One mechanism to explain this persistence of tissue eosinophilia in obesity may be related to alterations in eosinophil trafficking, however the underlying etiology of these findings remains unclear.
Leptin and TNF-α are both elevated in serum and BAL in obese patients, relative to non-obese healthy controls (3, 14, 15), and leptin has been associated with elevations in BAL TNF-α levels (16). Leptin is known to mediate eosinophil chemotaxis and therefore may be a causal factor in the obesity-related changes in eosinophil numbers in different lung compartments (17–19). Furthermore, TNF-α and leptin are associated with increased eosinophil activity (17, 18, 20), which may worsen asthma control in patients who demonstrate airway eosinophilia. In addition, studies in mice suggest that restoring normal body weight leads to reduction in TNF-α levels and improvement in lung inflammation and function in asthma models (21).
Surfactant Protein-A (SP-A) is a lung collectin that is thought to be an important mediator in regulating eosinophil activities and maintaining airway homeostasis. Previous studies have been controversial regarding altered SP-A levels in asthmatic patients, with some reporting an increase in SP-A (22) and others a decrease in SP-A (23, 24). Studies from our group and others have found that SP-A extracted from asthmatic patients is itself dysfunctional in regulating immune responses (25, 26). The goal of our study was to determine if SP-A levels are altered in obese patients with asthma compared to normal and overweight patients with and without asthma and to define mechanisms and potential consequences underlying this association.
Materials and Methods
Study Subjects
The Duke University Institutional Review Board approved the protocol. Subjects were recruited from the population in Durham County and the surrounding area. Informed consent was obtained from each subject (18–65 years of age). Lean subjects were defined as having a BMI kg/m2 < 25, while 25 ≥ BMI kg/m2 < 30 was considered overweight and BMI kg/m2 ≥ 30 obese. The BMI category for each subject was further categorized by presence or absence of asthma. This stratification provided 6 groups including lean asthma (LA), lean normal (LN), overweight asthma (OWA), overweight normal (OWN), obese asthma (OA) and obese normal (ON). Asthma subjects met NAEPP criteria for mild and moderate asthma (27). The presence of atopy was determined using skin prick testing. Healthy subjects had normal lung function and no history of atopy. Exclusion criteria included use of antibiotics or oral corticosteroids within four weeks of the study, greater than a 10-pack year history of smoking, and any other significant medical conditions. Research bronchoscopy with BAL was performed on subjects as previously described (25). Please see online repository for additional processing information.
Normal human SP-A extraction
SP-A was purified from the BAL fluid of patients with alveolar proteinosis that were seen at Duke University Medical Center and were under IRB approval using previously described methods (28). Extracted SP-A had final endotoxin concentrations of < 0.01 pg/mg SP-A.
Bronchoalveolar lavage analysis from obese mice
Obese mice (Lepob/ob and Leprdb-9J) were purchased from Jackson labs. All murine experiments were part of protocols approved by the Institutional Animal Care and Use Committee at Duke University, University of Arizona or University of Vermont. For BAL, 0.1 mM EDTA in PBS was used to flush the lungs with 1 ml volume to measure SP-A. The SP-A antibody used to detect mouse SP-A was purified in the lab of Dr. Jo Rae Wright (Duke University) and used as previously described (29).
Mouse models of allergic airways disease
WT and SP-A−/− mice were maintained on a C57BL/6J background (Jackson labs). SP-A−/− mice were generated as described previously (30). Please see online repository for methods of OVA and HDM exposure models.
Histological analysis of tissue eosinophilia
Left lung lobes were dissected and immersed in 10% buffered formalin for fixation. Embedded sections (4 μm) were mounted on Fisher-PLUS slides and immunostained as previously described by using a well-characterized rabbit anti-mouse MBP polyclonal antisera (31).
Mouse tracheal epithelial cells cultures
Mouse tracheal cells were isolated from WT, SP-A−/− and TNF-R−/− (Tnrfsf1atm1/mx;Jackson Labs) mice and cultured as previously described (32). On the day of challenge, 50 μl of sterile media was added to the apical surface and flushed several times to remove the pre-secreted SP-A. MTECs were challenged with vehicle (media), TNF (100 ng/ml), or Leptin (100 ng/ml) apically for either 24 hours or 5 days, after which the apical and basolateral secretions were collected for analysis of SP-A and eotaxin. Mouse eotaxin (eBioscience) was assessed by ELISA via manufacturer’s instructions. A set of MTECs was assessed every other day with a Voltmeter to determine epithelial integrity with the various challenges. Some MTECs were collected into TRI Reagent® (Sigma) and RNA was isolated using standard methods. RT-PCR was performed using Bioline 2x SensiFAST SYBR no-ROX mix for expression levels of mouse SP-A using primers (see online repository).
Statistical Analysis
Please see the online repository for detailed description of statistical analysis.
Results
Demographics and Baseline Characteristics
Fifty-five subjects with analyzable BMI, asthma status and SP-A were included in the study and were categorized as 11 obese asthma and 44 control subjects. The control group included 10 lean patients with asthma, 13 lean normal, 5 overweight asthma, 7 overweight normal, and 9 obese normal patients (Table 1). A significantly higher percentage of obese patients were female (p=0.01). There was no significant difference in age between the groups (p=0.495); however, obese subjects were slightly older (33.2 ± 13.0) than the control subjects (30.8 ± 9.7). Significant imbalances were not found in race between the obese and control groups (p=0.509); however, a higher percentage of the patients in the OA (63.6%) were Caucasian compared to the control group (50.0%). Obese asthmatics demonstrated a significantly older age at asthma diagnosis (p=0.046) with a higher percentage of OA subjects (55.6%) having a later onset asthma (≥ 12 yr.) compared to 8.3% in the LA and OWA groups combined. Baseline inhaled corticosteroid use was also trending higher in obese asthma subjects (45.4%) compared to 7.1% in the LA and OWA groups (p=0.056). No significant differences in the presence of allergic sensitization as characterized by skin prick testing were found between obese asthma (57.1%) and the control group (39.0%) (p=0.433); however, LA patients demonstrated a higher percentage of positive skin tests (83.3%) than OA subjects.
Table 1.
Demographic Data of Participants by BMI Group and Asthma Status.
| Control Group | All Control | Study Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Weight Group | Lean (BMI < 25) | Overweight (25 ≤ BMI < 30) | Obese (BMI ≥ 30) | Obese | ||||
| Asthma Status | Asthma | Normal | Asthma | Normal | Normal | Asthma | P value | |
| N | 10 | 13 | 5 | 7 | 9 | 44 | 11 | |
| Female (%) | 7 (70.0%) | 7 (53.8%) | 2 (40.0%) | 2 (28.6%) | 7 (77.8%) | 25 (56.8%) | 11 (100.0%) | 0.010* |
| Age (yr.) | 27.9 (7.1) | 27.8 (7.5) | 36.8 (13.6) | 37.4 (13.4) | 29.8 (7.0) | 30.8 (9.7) | 33.2 (13.0) | 0.495 |
| # Age at Asthma Onset ≥12 yr. (%) | 1/7 (14.3%) | N/A | 0/5 (0.0%) | N/A | N/A | 1/12 (8.3%) | 5/9 (55.6) | 0.046* |
| # Caucasian Race (%) | 4/8 (50.0%) | 8/13 (61.5%) | 5/5 (100.0%) | 3/7 (42.9%) | 1/9 (11.1%) | 21/42 (50.0%) | 7/11 (63.6%) | 0.509 |
| BMI (kg/m2) | 21.7 (1.5) | 22.2 (1.9) | 26.4 (0.9) | 26.9 (1.6) | 34.6 (3.0) | 25.8 (5.2) | 36.5 (8.0) | <0.001* |
| ICS Use (%) | 1/9 (11.1%) | N/A | 0/5 (0.0%) | N/A | N/A | 1/14 (7.1%) | 5/11 (45.4%) | 0.056 |
| # Atopy (%)(skin prick test) | 5/6 (83.3%) | 6/11 (54.6%) | 0/1 (0.0%) | 3/7 (42.9%) | 2/9 (22.2%) | 16/34 (39.0%) | 4/7 (57.1%) | 0.697 |
| # Hx Allergic Rhinitis (%) | 5/7 (71.4%) | 2/5 (40.0%) | 3/5 (60.0%) | 0/3 (0.0%) | 0/3 (0.0%) | 10/23 (43.5%) | 6/9 (66.7%) | 0.433 |
Continuous data are presented as mean (standard deviation) and categorical data are presented as n (%) or as n/N (%). BMI=body mass index. ICS=inhaled corticosteroid.
Denotes that demographics were only available for a subgroup of subjects.
Denotes a statistically significant difference between Control and Obese/Asthma groups.
Lung function as related to BMI and asthma
The mean FEV1 (% predicted) was significantly different between groups (p=0.013) and was higher in the control group (96.8 ± 12.5) than in obese asthma (85.2 ± 16.3), as shown in Table 2. The mean FVC (% predicted) was trending higher in the control (103.3 ± 13.0) than in the OA group (95.1 ± 18.1) but was not statistically different (p=0.089). The mean SP-A was significantly lower (p=0.002) in the obese asthma group (0.4 ± 0.6) than the control group (1.5 ± 1.0). No differences were observed in airway hyperresponsiveness as determined by methacholine PC20 between the control and obese asthma subjects (p=0.839).
Table 2.
Biomarker and Lung Function Data by BMI Group and Asthma Status.
| Control Group | All Control | Study Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Weight Group | Lean (BMI < 25) | Overweight (25 ≤ BMI < 30) | Obese (BMI ≥ 30) | Obese | ||||
| Asthma Status | Asthma | Normal | Asthma | Normal | Normal | Asthma | P value | |
| N | 10 | 13 | 5 | 7 | 9 | 44 | 11 | |
| FVC (%) | 108.2 (16.5) | 101.5 (12.3) | 107.2 (9.7) | 96.4 (10.0) | 103.9 (12.8) | 103.3 (13.0) | 95.1 (18.1) | 0.089 |
| FEV1 (%) | 91.0 (14.3) | 102.8 (13.8) | 94.0 (11.7) | 92.6 (6.9) | 99.3 (9.6) | 96.8 (12.5) | 85.2 (16.3) | 0.013* |
| FEV1 /FVC | 72.1 (10.3) | 84.6 (6.2) | 71.2 (5.8) | 78.9 (4.9) | 81.7 (6.2) | 78.7 (8.7) | 75.3 (10.2) | 0.260 |
| MCH PC20 | n=9 0.2 (0.1, 4.0) |
N/A | n=4 0.6 (0.5, 2.1) |
N/A | N/A | n=13 0.5 (0.1,4.0) |
n=11 0.5 (0.0,13.4) |
0.839 |
| IL-6 (ng/ml) | n=8 8.8 (6.7) |
n=7 10.9 (7.5) |
n=3 4.6 (2.9) |
n=5 11.2 (8.3) |
n=6 11.3 (7.6) |
n=29 9.8 (7.0) |
n=10 10.6 (7.6) |
0.777 |
| SP-A (RD/ug protein) | 1.9 (1.2) | 1.5 (1.2) | 1.6 (0.9) | 1.0 (0.8) | 1.2 (0.9) | 1.5 (1.0) | 0.4 (0.6) | 0.002* |
| TNF-α (ng/ml) | n=8 6.2 (6.4) |
n=7 7.8 (6.7) |
n=3 2.0 (1.6) |
n=5 5.0 (3.2) |
n=6 11.9 (9.6) |
n=29 7.1 (6.9) |
n=10 6.5 (6.1) |
0.799 |
Data are presented as mean (standard deviation) or as median (minimum, maximum). FEV1 =forced expiratory volume in the first second of expiration pre-bronchodilator. FVC=forced vital capacity pre-bronchodilator. MCH=Methacholine. PC20=provocative concentration of methacholine causing a 20% drop in FEV1. SP-A=surfactant protein-A. RD=relative density. TNF-α=Tumor necrosis factor-alpha.
Denotes a statistically significant difference between all control and obese/asthma groups.
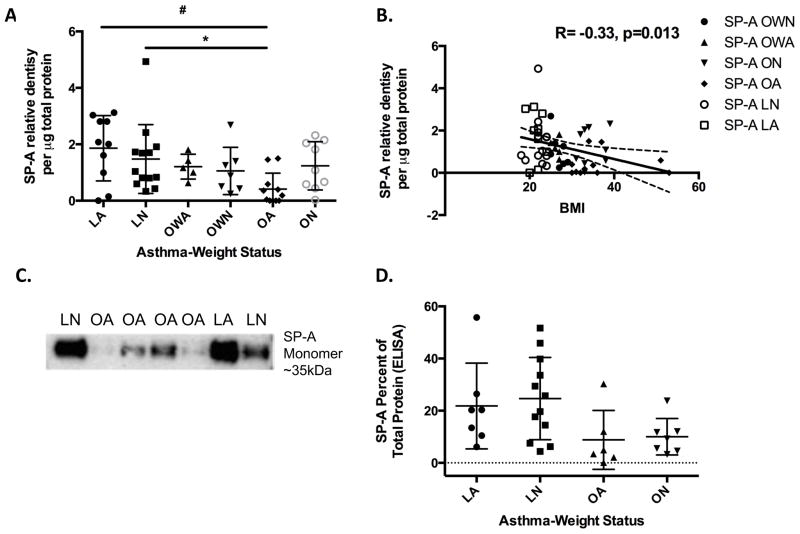
SP-A levels differ on the basis of obesity
Overall, statistically significant correlations were observed among the markers of inflammation and lung function, including r=0.61 [FVC (%) and FEV1 (%)] and r=0.82 (IL-6 and TNF-α) (Table E1). Pearson’s correlations were then determined among biomarkers within each weight category. We did not observe correlations between SP-A expression and other biomarkers overall or within each weight category. In contrast, obese asthmatic subjects had the lowest levels of SP-A with significantly lower mean levels (0.4 ± 0.6) in comparison to both the LA (1.9 ± 1.2; p=0.002) and LN (1.5 ± 1.2; p=0.014) subjects (Figure 1A and 1C). In a subset of available samples, SP-A levels were confirmed by ELISA as demonstrated in Figure 1D. A significant positive correlation between IL-6 and TNF-α was found within the lean (r=0.90), overweight (r=0.82) and obese (r=0.82) BMI groups (Table E1). Conversely, SP-A levels were modestly but positively correlated with FVC (% predicted) (r=0.21; p=0.126) and FEV1 (% predicted) (r=0.17; p=0.203) demonstrating that increased levels of SP-A may have some correlation with improved lung function; however, continuous BMI was the only statistically significant variable correlated with SP-A after considering all other biomarkers (TNF-α, and IL-6), lung function measures [FVC (%) and FEV1 (%)], and asthma status. If we treat BMI as a categorical variable (lean, overweight and obese), then we find a significant negative correlation with SP-A, and an interaction with asthma status that is trending towards significance (p=0.065).
Figure 1.
(A) SP-A expression as detected by Western blot is significantly decreased in obese asthma (OA) as compared to lean asthma (LA) (#p=0.002) and lean normal (LN) (*p=0.014) subjects. (B) SP-A levels are negatively correlated with increasing BMI, R= −0.33, p=0.013. (C) Representative Western blot of lean normal (LN), lean asthma (LA), obese normal (ON) and obese asthma (OA) samples. (D) SP-A levels measured via ELISA in a subset of samples demonstrate similar trends among groups (p=0.034).
Relationship between TNF-α and SP-A levels in pelleted BAL cells
TNF-α is elevated in serum and BAL in obese patients, relative to non-obese healthy controls (15, 33). Due to the nature of our retrospective study, we were unable to measure TNF-α in cell-free lavage or in serum of our subjects. We analyzed TNF-α in a subset of our pelleted BAL samples from which SP-A was also measured. There was negative correlation, albeit non-significant, between SP-A and TNF-α levels overall and within the lean and overweight weight groups however, the correlation was positive within the obese group (Table E1). Hypothesizing an interaction with asthma, we considered the relationship in only asthmatic subjects and found a negative correlation overall within each of the lean, overweight and obese groups. While our sample size was too small to make any definitive conclusions, the correlation between SP- A and TNF-α appeared stronger within the asthma subjects if a subject was overweight or obese.
BMI and asthma as predictors of lung function biomarkers
A univariable linear regression analysis found a statistically significant negative correlation between SP-A levels and continuous BMI (r=−0.33; p=0.014) (Figure 1B). When adjusting for both BMI and asthma status in a multivariable model, both asthma (p=0.017) and the interaction of asthma and BMI (p=0.008) significantly predicted SP-A (Table E2). Similar results were found when predicting FVC (%) on the basis of BMI and asthma status. These results indicate that SP-A and FVC (%) values depend on asthma status (different intercepts for each group) and that the slope of the relationship is dependent on BMI if subjects have asthma with obese subjects having lower values. However, only asthma (r=0.36; p=0.006) was predictive of FEV1 (%). BMI and asthma group did not correlate with either TNF-α or IL-6.
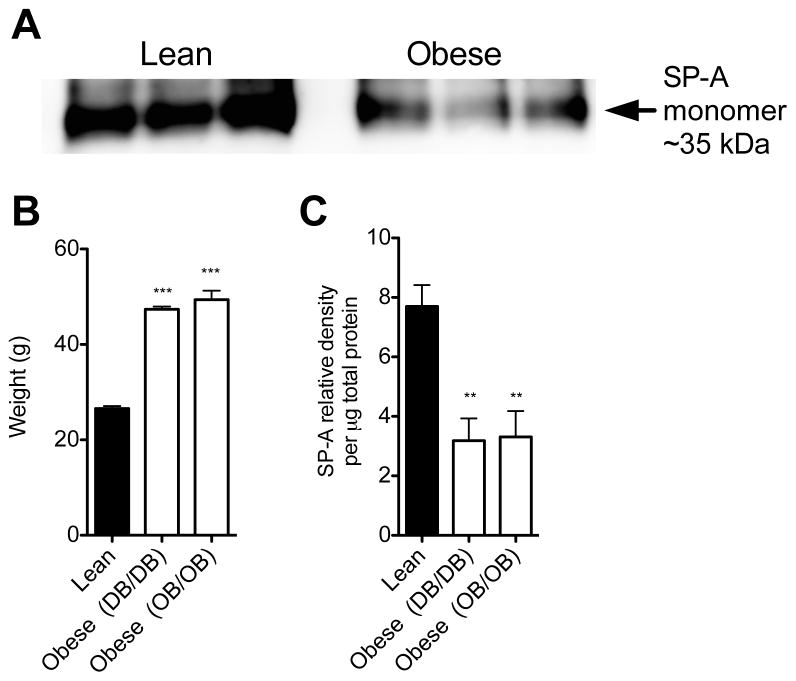
Obese mice demonstrate decreased SP-A levels in lavage fluid
In order to determine if further mechanistic studies were possible in mouse models, we sought to determine if differences in BAL SP-A levels exist in obese as compared to lean mice of the same age and sex. We examined BAL SP-A levels from two common strains of obese mice: db/db (leptin receptor deficient) and ob/ob (leptin deficient). Both db/db and ob/ob mice weighed significantly more than lean mice at the time of BAL harvest (Figure 2B). We determined that naïve obese mice have significantly decreased levels of SP-A in cell-free BAL as compared to naïve WT (lean) mice (Figure 2A, 2C).
Figure 2.
(A) Representative Western blot for SP-A expression in cell-free BAL from lean and obese mice. (B) Obese mice that are either leptin deficient (Ob/Ob) or leptin receptor deficient (Db/Db) were significantly heavier than WT mice (lean) and (C) have significantly lower SP-A levels in BAL than lean mice (p<0.01).
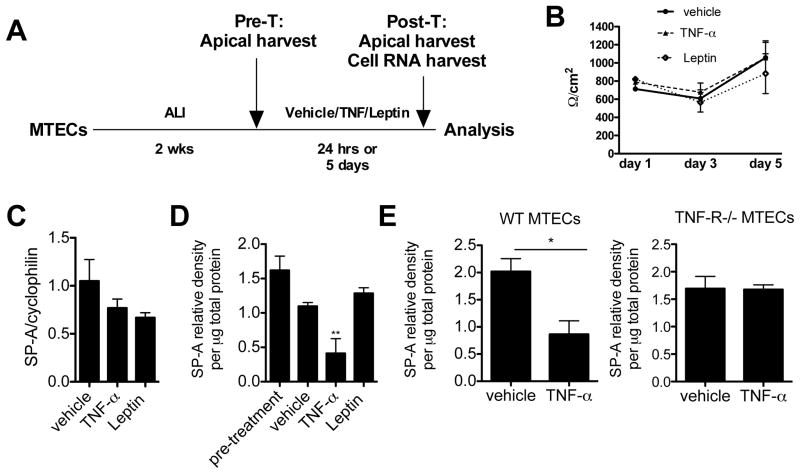
TNF-α represses SP-A secretion from mouse tracheal epithelial cells (MTECs)
Previous experiments in cell lines showed that TNF- α can reduce SP-A mRNA levels (34–36). However, no studies in primary pulmonary cells have verified this finding nor examined the levels of SP-A secretion. We sought to determine if TNF-α could also repress SP-A secretion from WT primary mouse tracheal epithelial cells, which are known to secrete SP-A (32). There were no significant differences in the levels of SP-A secreted in each individual transwell pre-challenge. Thereafter, the apical surface was challenged with either TNF-α, leptin or vehicle (Figure 3A). In order to verify the epithelial tight junction integrity of the transwells remained the same throughout the challenges, transepithelial resistance (TER) was assessed. There were no significant changes in TER in either vehicle or TNF and leptin challenged conditions for up to 5 days of challenge (Figure 3B). We detected a slight decrease in SP-A mRNA levels after treatment of MTECs with either TNF or leptin as compared to vehicle only (Figure 3C). TNF-α treatment, but not leptin, resulted in a significant reduction in SP-A secretion into the apical supernatants at 24 hours (Figure 3D). Similar results were observed for up to 5 days of challenge (data not shown).
Figure 3.
(A) Model in which murine tracheal epithelial cells were cultured at ALI for 2 weeks and then treated with vehicle, TNF-α, or leptin for either 24 hr. or 5 days. (B) Trans-epithelial resistance was not altered by TNF-α or leptin challenges. TNF-α did not affect (C) SP-A mRNA levels by MTECs at 24 hr. but did cause a significant reduction in (D) secretion of SP-A into the apical supernatants. **p<0.001 versus vehicle, pre-treatment and leptin treated. Leptin did not have any effect on SP-A transcription or secretion. (E) TNF-R−/− MTECs did not have altered levels of SP-A secretion upon TNF challenge.
Since TNF-α is known to have pleiotropic functions, which may not require specific receptor interactions, we sought to determine if TNF-α repression of SP-A secretion was independent of the TNF receptor. MTECs were grown from WT and TNF-R−/− mice and after 2 weeks at ALI were challenged with TNF-α for 24 hours. Again, we demonstrated significant repression of SP-A in WT MTECs that were challenged with TNF-α versus vehicle, however, there was no appreciable decrease in SP-A secretion in TNF-α challenged MTECs that lacked the TNF-R (Figure 3E).
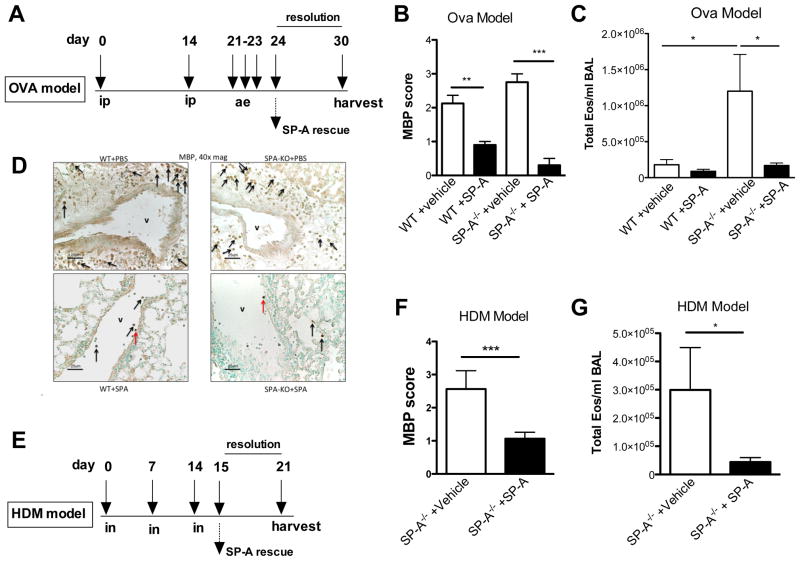
Exogenous SP-A therapy reduces tissue eosinophilia in allergic mouse models
We determined in the human study that obese asthmatics had significantly decreased levels of SP-A, and prior publications have shown that obese asthmatics have significantly increased tissue eosinophilia regardless of asthma severity. Therefore, we sought to determine if SP-A might play a role in the resolution of eosinophilia in lung tissue. In order to determine the role of SP-A in eosinophil migration, we instilled exogenous human SP-A into the lungs of allergic SP-A−/− (non-obese) mice and examined tissue eosinophilia in the “SP-A rescue” mice as compared to the vehicle control mice. In both the OVA (Figure 4A) and HDM models (Figure 4E), SP-A was given 1 day after the terminal challenge, a peak inflammatory time in both models. Lungs were examined for the presence of eosinophils 7 days after the last challenge. No BAL or tissue eosinophilia was observed in unchallenged control mice (data not shown). In the OVA and HDM models, significantly fewer BAL eosinophils (Figure 4C, 4G) and MBP+ tissue eosinophils (Figure 4B, 4F) were detected in the SP-A rescue group as compared to the vehicle treated groups.
Figure 4.
Mice lacking SP-A were challenged in a standard (A) OVA or (E) HDM protocol. Twenty-four hours after the last challenge in either model, mice were given an SP-A rescue treatment (or vehicle) and lung tissue was harvested 6 days later for analysis of tissue eosinophilia by anti-MBP staining. In both the OVA (B,C) and HDM (F,G) models, SP-A rescue treatment led to significantly lower tissue and BAL eosinophilia as compared to the vehicle treated. *p<0.05, ***p<0.001. (D) Representative lung sections from OVA mice that received vehicle (upper) or SP-A treatment (lower). MBP+ stained eosinophils denoted by arrows; V denotes a vessel.
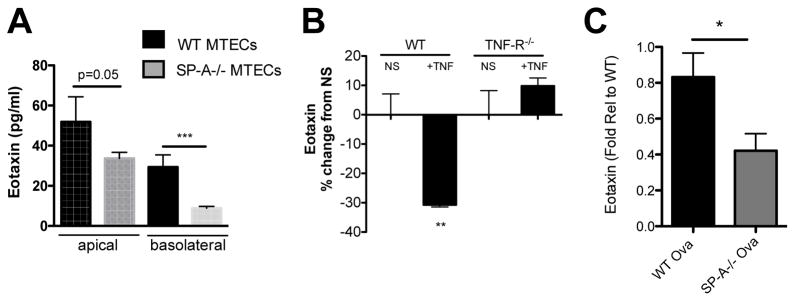
SP-A deficient MTECS have attenuated eotaxin secretion
MTECs from WT mice and SP-A−/− mice were grown at an ALI for 2 weeks and baseline measurements of eotaxin production by naïve epithelial cells were assessed. We found significantly less eotaxin secretion into the apical and basolateral compartments from SP-A−/− MTECs as compared to WT MTECs (Figure 5A). We have shown that TNF-α is sufficient to decrease SP-A secretion from epithelial cells in culture (Figure 3D) and that SP-A−/− MTECs secrete significantly less eotaxin at baseline as compared to WT MTECs. Taken together, this suggested that TNF-α exposure would also result in decreased eotaxin secretion. Figure 5B shows a 30% reduction in basolateral eotaxin secretion from the TNF-α treated WT MTECs as compared to the non-stimulated control wells. There was no significant repression of eotaxin secretion in TNF-α challenged MTECs that lacked the TNF-R, suggesting this response is also receptor dependent. In support of these findings, we examined BAL from OVA treated WT and SP-A−/− mice and found that mice lacking SP-A have significantly less eotaxin in BAL 7 days after the last challenge as compared to WT mice (Figure 5C).
Figure 5.
MTECs were cultured at ALI for 2 weeks prior to experimental analysis. (A) MTECs from SP-A−/− mice demonstrated a significant reduction in eotaxin secretion in both the apical and basolateral supernatants as compared to WT MTECs. n=3 independent experiments, 3–6 transwells/experiment, ***p<0.01. (B) WT and TNF-R−/− were challenged with TNF-α or vehicle for 24 hr. and Eotaxin was assessed in the basolateral compartment. **p<0.01, n=3–4 transwells/group. C) Eotaxin levels in BAL from OVA treated WT and SP-A−/− mice as determined by ELISA. N=2 experimental sets combined and represented as fold relative to WT, *p<0.05.
Discussion
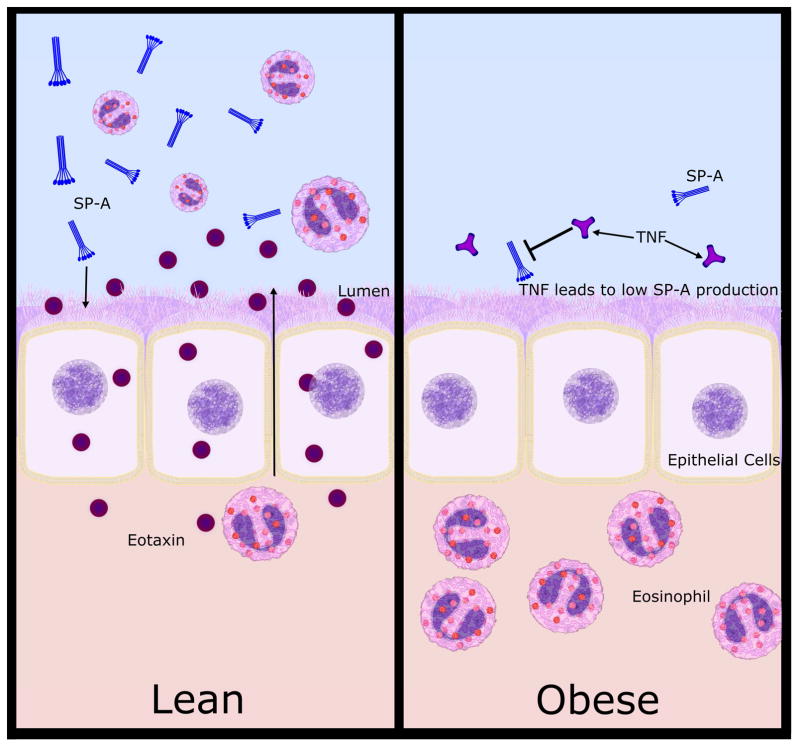
In the human studies presented here, we found that SP-A levels were negatively correlated with increased BMI. We demonstrated that obese asthmatics have significantly lower levels of BAL SP-A as compared to lean asthma and lean normal subjects. Mechanistically, we also found that a common cytokine up-regulated in obesity, TNF-α, was sufficient to repress SP-A secretion and eotaxin production in a receptor dependent manner. Using SP-A−/− mice and epithelial cell cultures grown at an ALI, our findings suggest a novel role for SP-A in promoting the resolution of tissue eosinophilia by enhancing eotaxin production by epithelial cells. Taken together, we propose a model (Figure 6) in which elevated levels of TNF-α in obese individuals may result in an attenuation of SP-A secretion. While the loss of SP-A could affect many aspects of lung function and host defense, here we propose that loss of functional SP-A is a contributing factor to the enhanced tissue eosinophilia that has been observed in several studies of obese asthmatics (10, 13).
Figure 6.
Proposed model includes elevated levels of TNF-α in obese individuals, in part leads to an attenuation of SP-A secretion. Loss of functional SP-A is a contributing factor to the enhanced tissue eosinophilia, which has been observed in several studies of obese asthmatics. In vitro studies support the concept that SP-A promotes movement of eosinophils from the tissue indirectly by enhancing eotaxin production from airway epithelial cells.
Our studies in vitro support the notion that SP-A promotes movement of eosinophils from the tissue indirectly by enhancing eotaxin production from airway epithelial cells. Additionally, we were able to verify in the same MTEC culture wells in which TNF-α challenge attenuated SP-A secretion that eotaxin secretion was also significantly decreased, both of which were dependent on the presence of the TNF-receptor. Taken together these results support the concept that increased levels of TNF-α can lead to decreased SP-A secretion, which results in decreased eotaxin production and impairment in eosinophil movement out of the tissue. In further support of our proposed model, we show that in two different mouse models of allergic airways disease exogenously administered human “SP-A therapy” leads to significant reductions in tissue eosinophilia during the resolution phase as compared to vehicle control therapy.
The epithelial lining fluid SP-A concentration has been reported to be as high as ~800μg/mL, while that measured in lung lavages was typically in the 30 μg/mL range (37). A better understanding of SP-A levels in asthma has been an ongoing topic of research for over a decade that has led to contradictory conclusions, likely based on heterogeneous characteristics of asthmatic subjects and sampling protocols (22–24). Our studies proposed an alternative novel concept, that within the asthmatic populations, SP-A levels are significantly altered based on obesity and inversely correlated with BMI.
Several limitations of our study warrant consideration. First, all obese asthmatic participants recruited were female, therefore no conclusions can be made with regards to male obese asthmatic participants. Second, there were differences in the use of inhaled corticosteroids by obese and lean asthma subjects. We do not believe that this had a major impact on SP-A levels as steroids have been shown to result in an increase in SP-A levels both in mouse models and premature infants (38, 39). Third, high-speed pellets were obtained from BAL that were then analyzed for SP-A, TNF and IL-6 expression. This leads to the possibility that BAL macrophages that may differentially bind SP-A in obese and lean asthmatic patients and, could impact the amount of SP-A measured in our study. We observed lower SP-A levels in cell-free BAL obtained from obese mice as compared to lean mice, suggesting that macrophage populations are unnecessary to detect SP-A differences in the context of obesity. We also acknowledge that increased airway closure that occurs in obese asthmatics could affect sampling of these individuals and thus alter the readout of SP-A in BAL. While SP-A is predominantly produced by alveolar type II cells in the distal airway, SP-A is present in the central large airways and is produced by club cells and submucosal cells. Future studies should be conducted to determine if increased compressive forces generated in the distal airways of obese individuals alters SP-A production. Due to the retrospective study, we did not completely phenotype our patients using state of the art biomarkders, which will be the approach taken in future studies to validate this observation. Likewise, we were unable to measure IL-6 and TNF levels in serum or cell-free BAL or examine tissue eosinophilia in our obese subjects to compare with the previous reports. We also acknowledge that our sample size was small, especially when creating 6 different subgroups based on BMI and asthma status. However, we had approximately 88% power to detect differences across means in a one-way analysis of variance (ANOVA) design with 55 subjects, assuming a 0.05 significance level and a common standard deviation within groups of 0.90.
Previous studies have reported decreased FVC in obese adult asthmatics as compared to lean asthmatics (40), but no difference in FEV1. We observed decreased FEV1 (% predicted) in our obese asthmatic cohort, which weakly correlated with SP-A levels. One study has suggested mechanical stress is sufficient to induce TNF-α production via Ca2+ release and receptor signaling (41). This leads to the possibility that the increased compression inherently present in the lungs of obese asthmatics stimulates TNF-α production, and thereby initiates the process that results in decreased SP-A levels and the potential subsequent alterations in eosinophil trafficking. Future studies should determine if increased compression of lung cells is sufficient to provoke TNF-α production and inhibit SP-A secretion. Our finding that TNF-α was unable to inhibit SP-A secretion when TNF-R was absent in epithelial cells, suggests that this process is not a pleiotropic effect of TNF-α but rather a receptor mediated pathway that should be further explored. Further studies are warranted to determine the impact of weight loss on SP-A secretion and tissue eosinophilia and furthermore, whether targeted anti-TNF therapies may play a role in the management of obese asthma patients with refractory eosinophilic asthma.
In conclusion, our study demonstrated a novel role for SP-A in modulating tissue eosinophilia in a manner that may be affected by obesity. This obese asthma endotype is poorly understood and the mechanisms that modulate tissue eosinophilia on the basis of obesity have not been well explained. We propose that obesity results in alterations in TNF-α levels due to either systemic inflammation or mechanical compression of the lung and that changes in TNF-α result in downstream decreases in SP-A levels and eotaxin secretion with subsequent changes in eosinophil migration and lack of resolution of lung eosinophilia.
Supplementary Material
Key Messages.
Obese asthmatics have significantly lower levels of SP-A as compared to lean asthmatics and BMI is significantly negatively correlated with SP-A levels in BAL.
TNF-α is sufficient to repress SP-A and eotaxin secretion from epithelial cells in a receptor dependent manner.
SP-A−/− epithelial cells secrete less eotaxin as compared to WT cells and exogenous SP-A promotes resolution of tissue and BAL eosinophilia in allergen challenged mouse models.
Acknowledgments
The authors would like to acknowledge Julia Nugent, Nizar Nikam, Anays Murillo and Michael Ghio for providing technical assistance and to Dr. Scott Boitano for assistance in measuring trans-epithelial resistance. We also wish to recognize the late Dr. Jo Rae Wright, as these studies were initiated in collaboration with her laboratory. Grant support was provided by HL111151, HL125602, HL065228.
Grant support: HL111151, HL125602, HL065228.
Abbreviations
- SP-A
surfactant protein-A
- BAL
bronchoalveolar lavage
- TNF-α
Tumor necrosis factor-alpha
- MTECs
mouse tracheal epithelial cells
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- BMI
body mass index
- Ova
Ovalbumin
- HDM
house dust mite
- OA
Obese asthma
- ON
Obese normal
- OWA
Overweight asthma
- OWN
Overweight normal
- LA
Lean asthma
- LN
Lean normal
- ICS
Inhaled Corticosteroid
- MCH
Methacholine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7(5):325–35. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 2.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. American journal of respiratory and critical care medicine. 2010;181(4):315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, et al. Cluster analysis of obesity and asthma phenotypes. PloS one. 2012;7(5):e36631. doi: 10.1371/journal.pone.0036631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127(6):1486–93. e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44(1):239–41. doi: 10.1183/09031936.00220413. [DOI] [PubMed] [Google Scholar]
- 6.Westerhof GA, Korevaar DA, Amelink M, de Nijs SB, de Groot JC, Wang J, et al. Biomarkers to identify sputum eosinophilia in different adult asthma phenotypes. Eur Respir J. 2015;46(3):688–96. doi: 10.1183/09031936.00012415. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178(5):469–75. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 8.Todd DC, Armstrong S, D'Silva L, Allen CJ, Hargreave FE, Parameswaran K. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clin Exp Allergy. 2007;37(7):1049–54. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 9.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63(5):570–4. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 10.van der Wiel E, Ten Hacken NH, van den Berge M, Timens W, Reddel HK, Postma DS. Eosinophilic inflammation in subjects with mild-to-moderate asthma with and without obesity: disparity between sputum and biopsies. Am J Respir Crit Care Med. 2014;189(10):1281–4. doi: 10.1164/rccm.201310-1841LE. [DOI] [PubMed] [Google Scholar]
- 11.Lemiere C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, et al. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006;118(5):1033–9. doi: 10.1016/j.jaci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013;132(1):72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. American journal of respiratory and critical care medicine. 2013;188(6):657–63. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holguin F, Rojas M, Brown LA, Fitzpatrick AM. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J Asthma. 2011;48(3):217–23. doi: 10.3109/02770903.2011.555033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulet LP. Influence of obesity on the prevalence and clinical features of asthma. Clinical and investigative medicine Medecine clinique et experimentale. 2008;31(6):E386–90. doi: 10.25011/cim.v31i6.4926. [DOI] [PubMed] [Google Scholar]
- 16.Mai XM, Chen Y, Krewski D. Does leptin play a role in obesity-asthma relationship? Pediatr Allergy Immunol. 2009;20(3):207–12. doi: 10.1111/j.1399-3038.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Cheung PF, Lam CW. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur J Immunol. 2007;37(8):2337–48. doi: 10.1002/eji.200636866. [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Ueki S, Kamada R, Kihara J, Yamauchi Y, Suzuki T, et al. Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int Arch Allergy Immunol. 2011;155(4):335–44. doi: 10.1159/000321195. [DOI] [PubMed] [Google Scholar]
- 19.Takeda M, Ueki S, Kato H, Konno Y, Chihara M, Itoga M, et al. Obesity and eosinophilic inflammation: does leptin play a role. Int Arch Allergy Immunol. 2012;158(Suppl 1):87–91. doi: 10.1159/000337799. [DOI] [PubMed] [Google Scholar]
- 20.Grotta MB, Squebola-Cola DM, Toro AA, Ribeiro MA, Mazon SB, Ribeiro JD, et al. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm Med. 2013;13:39. doi: 10.1186/1471-2466-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JY, Sohn JH, Lee JH, Park JW. Obesity increases airway hyperresponsiveness via the TNF-alpha pathway and treating obesity induces recovery. PLoS One. 2015;10(2):e0116540. doi: 10.1371/journal.pone.0116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng G, Ueda T, Numao T, Kuroki Y, Nakajima H, Fukushima Y, et al. Increased levels of surfactant protein A and D in bronchoalveolar lavage fluids in patients with bronchial asthma. Eur Respir J. 2000;16(5):831–5. doi: 10.1183/09031936.00.16583100. [DOI] [PubMed] [Google Scholar]
- 23.Erpenbeck VJ, Schmidt R, Gunther A, Krug N, Hohlfeld JM. Surfactant protein levels in bronchoalveolar lavage after segmental allergen challenge in patients with asthma. Allergy. 2006;61(5):598–604. doi: 10.1111/j.1398-9995.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.van de Graaf EA, Jansen HM, Lutter R, Alberts C, Kobesen J, de Vries IJ, et al. Surfactant protein A in bronchoalveolar lavage fluid. J Lab Clin Med. 1992;120(2):252–63. [PubMed] [Google Scholar]
- 25.Wang Y, Voelker DR, Lugogo NL, Wang G, Floros J, Ingram JL, et al. Surfactant protein A is defective in abrogating inflammation in asthma. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):L598–606. doi: 10.1152/ajplung.00381.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohlfeld JM, Ahlf K, Enhorning G, Balke K, Erpenbeck VJ, Petschallies J, et al. Dysfunction of pulmonary surfactant in asthmatics after segmental allergen challenge. Am J Respir Crit Care Med. 1999;159(6):1803–9. doi: 10.1164/ajrccm.159.6.9806145. [DOI] [PubMed] [Google Scholar]
- 27.NHLBI Asthma Treatment Guidelines. 2007 [Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 28.McIntosh JC, Mervin-Blake S, Conner E, Wright JR. Surfactant protein A protects growing cells and reduces TNF-alpha activity from LPS-stimulated macrophages. Am J Physiol. 1996;271(2 Pt 1):L310–9. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- 29.Ledford JG, Lo B, Kislan MM, Thomas JM, Evans K, Cain DW, et al. Surfactant protein-A inhibits mycoplasma-induced dendritic cell maturation through regulation of HMGB-1 cytokine activity. J Immunol. 2010;185(7):3884–94. doi: 10.4049/jimmunol.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, Jobe AH, et al. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci U S A. 1996;93(18):9594–9. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stelts D, Egan RW, Falcone A, Garlisi CG, Gleich GJ, Kreutner W, et al. Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am J Respir Cell Mol Biol. 1998;18(4):463–70. doi: 10.1165/ajrcmb.18.4.2957. [DOI] [PubMed] [Google Scholar]
- 32.Ledford JG, Voelker DR, Addison KJ, Wang Y, Nikam VS, Degan S, et al. Genetic variation in SP-A2 leads to differential binding to Mycoplasma pneumoniae membranes and regulation of host responses. J Immunol. 2015;194(12):6123–32. doi: 10.4049/jimmunol.1500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen XJ, Zhang YH, Wang DH, Liu YL. Effects of body mass index and serum inflammatory cytokines on asthma control in children with asthma. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(7):698–701. [PubMed] [Google Scholar]
- 34.Wispe JR, Clark JC, Warner BB, Fajardo D, Hull WE, Holtzman RB, et al. Tumor necrosis factor-alpha inhibits expression of pulmonary surfactant protein. J Clin Invest. 1990;86(6):1954–60. doi: 10.1172/JCI114929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miakotina OL, Snyder JM. TNF-alpha inhibits SP-A gene expression in lung epithelial cells via p38 MAPK. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L418–27. doi: 10.1152/ajplung.00470.2001. [DOI] [PubMed] [Google Scholar]
- 36.Whitsett JA, Clark JC, Wispe JR, Pryhuber GS. Effects of TNF-alpha and phorbol ester on human surfactant protein and MnSOD gene transcription in vitro. Am J Physiol. 1992;262(6 Pt 1):L688–93. doi: 10.1152/ajplung.1992.262.6.L688. [DOI] [PubMed] [Google Scholar]
- 37.Baughman RP, Sternberg RI, Hull W, Buchsbaum JA, Whitsett J. Decreased surfactant protein A in patients with bacterial pneumonia. Am Rev Respir Dis. 1993;147(3):653–7. doi: 10.1164/ajrccm/147.3.653. [DOI] [PubMed] [Google Scholar]
- 38.Kwong MS, Egan EA. Reduced incidence of hyaline membrane disease in extremely premature infants following delay of delivery in mother with preterm labor: use of ritodrine and betamethasone. Pediatrics. 1986;78(5):767–74. [PubMed] [Google Scholar]
- 39.Sims MW, Tal-Singer RM, Kierstein S, Musani AI, Beers MF, Panettieri RA, et al. Chronic obstructive pulmonary disease and inhaled steroids alter surfactant protein D (SP-D) levels: a cross-sectional study. Respir Res. 2008;9:13. doi: 10.1186/1465-9921-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters JI, McKinney JM, Smith B, Wood P, Forkner E, Galbreath AD. Impact of obesity in asthma: evidence from a large prospective disease management study. Ann Allergy Asthma Immunol. 2011;106(1):30–5. doi: 10.1016/j.anai.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Kim HG, Kim JY, Gim MG, Lee JM, Chung DK. Mechanical stress induces tumor necrosis factor-{alpha} production through Ca2+ release-dependent TLR2 signaling. Am J Physiol Cell Physiol. 2008;295(2):C432–9. doi: 10.1152/ajpcell.00085.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.