Non-melanoma cutaneous squamous and basal cell carcinoma (NMSC) is common, and most patients present with early stage (T1/T2) NMSC, which has an excellent prognosis. However, a minority of NMSC patients present with an advanced stage (T3/T4) primary tumor, which may be surgically unresectable but potentially curable with radiation therapy (RT). Because advanced NMSC is rare, studies1-5 examining the outcomes of RT for patients with T3/T4 tumors are limited. As the outcomes of RT in this patient cohort are unclear, this study was undertaken to examine survival in a large cohort of patients treated at our institution and to explore variables associated with survival outcomes.
With institutional review board approval, we reviewed records of T3/T4 histologically-confirmed NMSC patients treated with RT at our institution between 1990 and 2016. Radiation equivalent dose in 2 Gy fractions (EQD2) were tabulated with an α/β coefficient of 8.5. DSS and OS were determined, and univariate Cox regression examined characteristics and treatment parameters associated with OS and DSS.
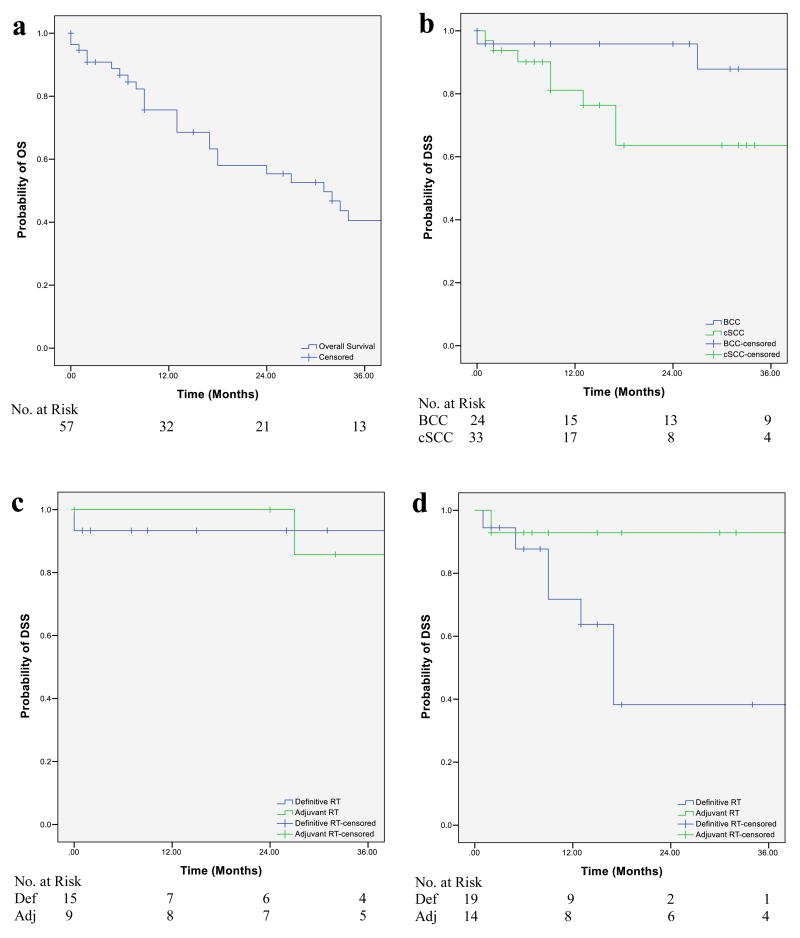
Seventy-one patients (7.2%) harbored a T3/T4 tumor, of which 25 had basal cell carcinoma (BCC) and 46 had cutaneous squamous cell carcinoma (cSCC). 57 underwent curative-intent RT; in 23, RT was adjuvant (post-operative), while in 34, RT was the exclusive definitive therapy (in-lieu of surgery). Median age was 78 years (range: 28-93), with most being white (87.3%) men (69.0%). Baseline characteristics between BCC and cSCC patients were similar except head and neck primary tumors (p=0.055), nodal involvement (p=0.002), and chemotherapy receipt (p=0.001) were more common in cSCC patients. RT was delivered via orthovoltage-photons, megavoltage-photons, electrons, or protons. The median EQD2 was 60 Gy for both the definitive-RT (Range: 3.53-86) and adjuvant-RT (Range: 24.7-76) cohorts. Follow-up for local recurrence and adverse events was median 9 months (Range: 0-86), while follow-up for survival was carried out until death for all patients. All patients were deceased within 10 years of completing RT (Figure 1A). There were no deaths due to NMSC ≥3 years after RT.
Figure 1.
Non-melanoma cutaneous squamous cell and basal cell carcinoma. Kaplan-Meier curves demonstrating the (A), OS of the curative intent cohort, (B) DSS of the curative intent cohort by tumor histology [p=0.084 by log rank test], (C) DSS for BCC patients based on treatment intent [p=0.95 by log rank test], and (D) DSS for cSCC patients based on treatment intent [p=0.036 by log rank test]
Abbreviations: def=definitive; adj=adjuvant; DSS=disease-specific survival; OS=overall survival
Among the curative-intent patients (n=57), the median DSS was not reached, while the median OS was 32 months. Three-year DSS rates for BCC patients was 87.8% (95% CI: 79.3-96.3); 93.3% (95% CI: 86.9-99.7) for patients treated with definitive-RT and 85.7% (95% CI: 72.5-98.9) for adjuvant-RT, respectively. Three-year DSS rate for cSCC patients was 63.6% (95% CI: 52.7-74.5); 38.3% (95% CI: 22.2-54.4) and 92.9% (95% CI: 77.9-95.5) for patients treated with definitive and adjuvant-RT, respectively (Figure 1B-D). Eight of 13 (61.5%) patients treated with palliative-intent experienced symptom relief. On univariate analyses, non-head and neck primary tumors (p=0.016) and nodal metastases (p=0.049) were associated with worse DSS for cSCC. No significant association was observed with RT type or EQD2 and DSS.
To our knowledge, this is the first study to characterize survival outcomes by histopathology, treatment intent, and demographic factors in advanced NMSC. Consistent with the literature, we illustrate superior outcomes in BCC (3-year DSS: 87.8%) compared to cSCC (3-year DSS: 63.6%). Our study overcomes a criticism of other reports3-6 by conducting analyses according to histopathology, given the distinct differences between BCC and cSCC.
Interestingly, survival was not associated with tumor stage or treatment intent for BCC, whereas survival was associated with these factors in cSCC. The comparable outcomes of definitive and adjuvant-RT suggest surgery may not improve DSS in BCC, while surgery and adjuvant-RT appears to be associated with significantly improved DSS in cSCC. Thus, the treatment approach requires further study. Our data suggests that a selection bias for definitive versus adjuvant-RT was present, as more patients treated with definitive-RT were older (median age 81 years versus 67), node positive (21% versus 13%), and had immune dysfunction (15% versus 4%), supporting the need for prospective clinical trials.
Patient characteristics seem associated with outcome and have not been well-characterized. For example, cSCC patients has poorer outcomes than BCC patients, of which 20% had immune dysfunction, and worse outcomes in immunosuppressed cSCC patients treated with RT have been observed7. Race may be associated with outcomes; the 2 black patients in our cohort died of disease, consistent with prior studies8 demonstrating higher mortality in NMSC minority patients. There are complex psychosocial factors present in this patient population, which require further exploration. Some presented for medical attention many years after their first tumor manifestation; several discontinued treatment prematurely. Many patients with advanced NMSC also decline follow up, as evidenced by our median follow-up of 9 months. This was not surprising given the delays in diagnosis and initiation of therapy. Additionally, one-third of all curative-intent patients died within 12 months of RT, either from skin cancer or other causes. All patients in our study were deceased within ten years of treatment, likely due to advanced age (median age 78 years), possibly obviating concerns about late effects of RT.
There are several limitations to our study. The retrospective, single-institution nature of the study with associated selection biases and the heterogeneity of our cohort restrict the strength of the conclusions, which could be overcome by a multi-center prospective trial.
Our study gathered survival data on an unusual patient population over a 26-year time period, which has otherwise not been reported in the contemporary literature. Advanced BCC carries a better prognosis than cSCC. In cSCC, but not BCC, RT intent and tumor stage are more strongly associated with survival. Outcomes in advanced T3/T4 NMSC vary and may be associated with histopathology, RT intent, and patient characteristics, suggesting that individualized approaches are necessary.
Acknowledgments
This research study was supported in part through a National Institutes of Health/National Cancer Institute Cancer Center support grant (P30 CA008748) awarded to Memorial Sloan Kettering Cancer Center (Principal investigator: Craig Thompson).
Footnotes
Disclosures: None declared
References
- 1.Matthiesen C, Thompson JS, Forest C, et al. The role of radiotherapy for T4 non-melanoma skin carcinoma. J Med Imaging Radiat Oncol. 2011;55:407–16. doi: 10.1111/j.1754-9485.2011.02277.x. [DOI] [PubMed] [Google Scholar]
- 2.Kwan W, Wilson D, Moravan V. Radiotherapy for locally advanced basal cell and squamous cell carcinomas of the skin. Int J Radiat Oncol Biol Phys. 2004;60:406–11. doi: 10.1016/j.ijrobp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Parsons JT, Mendenhall NP, et al. T2-T4 carcinoma of the skin of the head and neck treated with radical irradiation. Int J Radiat Oncol Biol Phys. 1987;13:975–81. doi: 10.1016/0360-3016(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 4.Al-Othman MO, Mendenhall WM, Amdur RJ. Radiotherapy alone for clinical T4 skin carcinoma of the head and neck with surgery reserved for salvage. Am J Otolaryngol. 2001;22:387–90. doi: 10.1053/ajot.2001.28083. [DOI] [PubMed] [Google Scholar]
- 5.Lee WR, Mendenhall WM, Parsons JT, et al. Radical radiotherapy for T4 carcinoma of the skin of the head and neck: a multivariate analysis. Head Neck. 1993;15:320–4. doi: 10.1002/hed.2880150409. [DOI] [PubMed] [Google Scholar]
- 6.Landthaler M, Braun-Falco O. Use of the TDF factor in soft roentgen radiotherapy. Hautarzt. 1989;40:774–7. [PubMed] [Google Scholar]
- 7.Manyam BV, Garsa AA, Chin RI, et al. A multi-institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer. 2017 doi: 10.1002/cncr.30601. [DOI] [PubMed] [Google Scholar]
- 8.Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748–62. doi: 10.1016/j.jaad.2013.11.038. [DOI] [PubMed] [Google Scholar]