Abstract
Leprosy remains a health problem in several countries. Current management of patients with leprosy is complex and requires multidrug therapy. Nonetheless, antibiotic treatment is insufficient to prevent nerve disabilities and control Mycobacterium leprae. Successful infectious disease treatment demands an understanding of the host immune response against a pathogen. Immune-based therapy is an effective treatment option for malignancies and infectious diseases. A promising therapeutic approach to improve the clinical outcome of malignancies is the blockade of immune checkpoints. Immune checkpoints refer to a wide range of inhibitory or regulatory pathways that are critical for maintaining self-tolerance and modulating the immune response. Programmed cell-death protein-1 (PD-1), programmed cell death ligand-1 (PD-L1), cytotoxic T-lymphocyte-associated protein 4, and lymphocyte-activation gene-3 are the most important immune checkpoint molecules. Several pathogens, including M. leprae, are supposed to utilize these mechanisms to evade the host immune response. Regulatory T cells and expression of co-inhibitory molecules on lymphocytes induce specific T-cell anergy/exhaustion, leading to disseminated and progressive disease. From this perspective, we outline how the co-inhibitory molecules PD-1, PD-L1, and Th1/Th17 versus Th2/Treg cells are balanced, how antigen-presenting cell maturation acts at different levels to inhibit T cells and modulate the development of leprosy, and how new interventions interfere with leprosy development.
Keywords: immunotherapy, leprosy, T-regulatory cells, immune checkpoint blockade, PD-1:PD-L1, cytotoxic T-lymphocyte-associated protein 4
Introduction
Leprosy remains a relevant health problem in Brazil and India even after the introduction of multidrug therapy and has spread worldwide (1–3). Leprosy presents different clinical features that are determined by the host immune response against Mycobacterium leprae; at the pole of this spectrum are tuberculoid and lepromatous disease. In tuberculoid leprosy (TT), Th1 polarization, characterized by the production of IFN-γ, which activates CD8 T cells, macrophages and bactericidal mechanisms that control M. leprae growth, is critical for the protective response (2, 4, 5). By contrast, lepromatous leprosy (LL) presents with impaired specific cellular immunity. The immune response often differentiates into a Th2 profile, with abundant production of IL-4 and predominant B cell activation, which allows for evasion by the bacillus. M. leprae shows strategies to limit the host protective immune response leading to chronic infection (6, 7). In chronic infections, T cells are exposed to persistent antigen stimulation as a gradual loss of effector functions and cytokine production as well as persistently increased expression of multiple inhibitory receptors (6, 8). The immunomodulatory properties from mycobacteria have been explored to understand macrophage function (5, 9). In addition, M. leprae antigens interfere with T-cell proliferation (10) and are involved in Treg-cell expansion through HSP-60 (11). Evidence has indicated that Treg cells, besides expression of immune checkpoint molecules with inhibitory activity, such as PD-1, PD-L1, and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), induce specific T-cell anergy, leading to disseminated and progressive disease (7, 12, 13). Immune checkpoint (ICP) molecules play an important role in T-cell activation and determine the functional outcome of T cells, reducing the proliferation and secretion of inflammatory cytokines, such as IL-2, IFN-γ, and TNF-α (14, 15). Those molecules also interfere with dendritic cell (DC) maturation and macrophage effector function (5, 16). ICP, particularly PD-1/PD-L1 and CTLA-4, have been widely explored as therapeutic targets in cancer because these biomarkers are also highly expressed in the tumor microenvironment (14, 15). In infectious diseases, this therapeutic approach has been applied against HIV, HCV, and tuberculosis as an adjuvant of antimicrobial drugs (17–19).
Herein, to discuss new approaches for leprosy monitoring and treatment, we reviewed some of the ICP for leprosy persistence and mechanisms associated with T-cell lymphocyte anergy to M. leprae antigens as well as the role of Treg cells to modulate disease development.
Immune Checkpoints in Leprosy
Although ICP have been studied for approximately two decades, many features of their biology and signaling pathways remain unknown. ICP receptors are associated with autoimmunity, suggesting that these molecules play a critical role in immune tolerance and homeostasis (7, 8). In chronic infections, T lymphocytes are under persistent exposure to antigens, and this stimulus is commonly associated with T exhaustion (20). Various ICP molecules are highly expressed on exhausted T cells (14, 20), and this literature indicates that ICP blockade can restore immunity after reversion of the exhaustion phenotype of T cells (8). In leprosy, some recent data have shown a strict relationship between ICP expression and disease persistence.
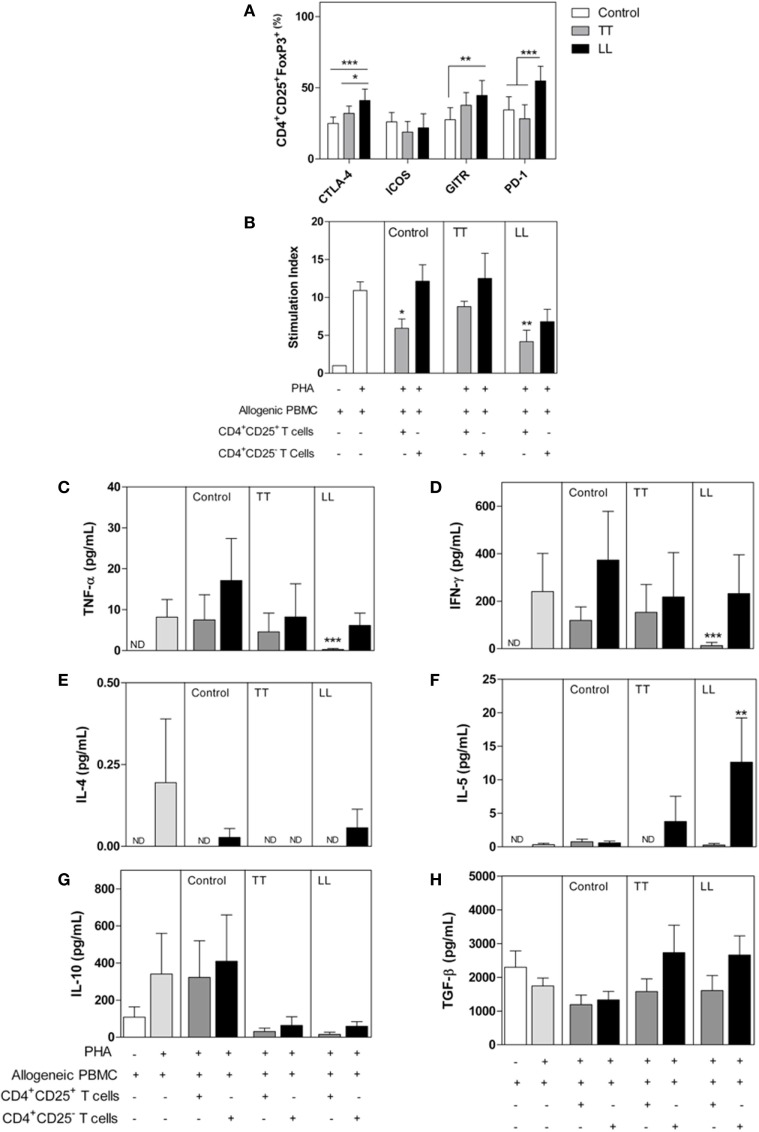
Cytotoxic T-lymphocyte-associated protein 4 is an important molecule that controls lymphocyte activation (21). This molecule binds to CD80/CD86, antagonizing CD28 signaling, on antigen-presenting cell (APC) cells, leading CD4+ and CD8+ T cells to assume an anergic phenotype (14, 22). Some CTLA-4 signaling pathways are still unknown, and it is unclear how this receptor interferes with lymphocyte activation as well as how CD3 phosphorylation, ZAP-70 activation, or tyrosine phosphatase SHP-2 act as intracellular mediators of those pathways (21). Indeed, CTLA-4 is essential for Tregs function. Treg cells highly express CTLA-4, which controls DC maturation, leading to internalization of CD80 and/or CD86 in addition to indoleamine-2,3-dioxygenase (IDO) activation, leading to expression of the immunosuppressive mediator kynurenin (16, 21, 23). These signals can also promote nuclear localization of Foxo, a transcriptional factor that suppresses transcription of the genes encoding IL-6 and TNF-α, both of which are crucial effector cytokines for the control M. leprae infection (6, 22). In LL patients, CTLA-4 has been described as a biomarker in blood and inflammatory infiltrating cells (24, 25). Increased expression of CTLA-4 was detected in LL lesions compared with that in TT lesions (24). Our group has found increased expression of CTLA-4 on lymphocytes and Treg cells from LL patients in contrast to reduced CTLA-4 expression on the same cell populations of TT patients1 (Figure 1). We also observed that CD4+CD25− T cells obtained from LL patients suppressed allogenic proliferation in functional tests (Figure 1). A suppressive role of CTLA-4 has also been demonstrated in FoxP3− T cells, and these data might explain the suppressive profile presented by LL patients (26). We also observed that CD4+CD25− T cells obtained from LL patients suppressed allogenic proliferation on functional tests (Figure 1). In TT patients, in vitro blockade of CTLA-4 restored peripheral blood mononuclear cell (PBMC) proliferation (12), but there is no clinical trial showing those effects on LL patients. Immunotherapy (IT) with CTLA-4 blockade has mostly been conducted against tumoral cells; nonetheless, recent evidence has shown that CTLA-4 expression is associated with reduced secretion of TNF-α and IFN-γ and enhanced frequency of memory CD8+ lymphocytes in experimental L. monocytogenes infection (27). Similarly, CTLA-4 blockade induced higher production of IFN-γ and NO when T cells were stimulated with Trypanosoma cruzi antigens, although it did not restore lymphocyte proliferation (28). Furthermore, despite few clinical trials concerning CTLA-4 blockade to control infectious diseases, HCV patients demonstrated promising results after this therapy (29). Taken together, these data suggest that immunotherapies might modulate the immune system in patients with a latent leprosy infection or active disease, enabling better control of M. leprae replication. Therefore, new discoveries concerning the role of CTLA-4 in the immune response during M. leprae infection could provide critical insight that can be applied to other infectious diseases.
Figure 1.
Phenotype and functional characterization of CD4+CD25+ T cells in leprosy patients. Peripheral blood mononuclear cells (PBMCs) were isolated from patients with tuberculoid (TT, n = 12) and lepromatous leprosy (LL, n = 12), as well as from healthy control subjects (n = 12). (A) The frequency of CD25+ and FoxP3+ cells and expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), GITR, ICOS, and PD-1 were determined by flow cytometry. (B) Allogeneic PBMC (1 × 105 cells/well) was cultured with medium only, PHA, PHA plus CD4+CD25+ T, or CD4+CD25− T cells (1 × 104 cells/well) from patients or control subjects. Proliferation was determined after 4 days of culture by CFSE dilution analyzed by flow cytometry. The results are expressed as the means ± SEM of the stimulation index of proliferation. IFN-γ (C), TNF-α (D), IL-4 (E), IL-5 (F), IL-10 (G), and TGF-β (H) levels were determined in supernatants from cultures of suppression assays. The results are presented as the means ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001, compared with control subjects using ANOVA and the Bonferroni posttest. For the suppressive assay (B), the results are expressed as the means ± SEM; *p < 0.05, **p < 0.01, and ***p < 0.001, compared with the proliferation of allogeneic PBMCs cultured with PHA. ND, not detected.
Programmed cell-death protein-1 (PD-1) and its ligands PD-L1/L2 have also been identified as relevant ICP that promote immune evasion of tumor cells and infected cells (8, 14, 15). Those molecules are promising targets in anticancer therapy and are implicated in dysfunctional acquired immune responses, reducing the TCR signal to lymphocyte proliferation through ITIM (immune receptor tyrosine-based inhibition) motifs (30). The PD-1 signaling axis has been strongly related to T-cell anergy, pathogen persistence, and peripheral immune tolerance (14, 30). Although not yet targeted clinically, PD-1 is a promising target for leprosy IT. In leprosy, patients have presented with increased expression of PD-1 and PD-L1 on CD4+, B cells, and CD11+ cells (12, 13, 24, 31, 32), and in vitro blockade of PD-1 increased IFN-γ and IL-17 production by T cells (33). In accordance, our group found increased expression of PD-1 and GITR on lymphocytes and Tregs from LL patients (Figure 1). Blockade of PD-1 signaling in infectious disease has been associated with pathogen control in animal models for HBV, HIV, Plasmodium spp., Leishmania spp., Trypanossoma spp., and M. tuberculosis infection (34–38). These results suggest that ICP might be an important mechanism to regulate the immune response of LL patients. Thus, antibodies targeting the PD-1 pathways may improve the clinical outcome by restoring T-cell-mediated M. leprae immunity. However, in the infectious diseases context, immunotherapies based on ICP have not been tested or developed to the same extent as they have in cancer (39).
More recently, the signaling pathways and inhibitory mechanisms of lymphocyte-activation gene-3 (LAG-3) and TIGIT (T-cell immunoglobulin and ITIM domain) have also been explored as suitable new targets for immune blockade (14, 40, 41). TIGIT, a member of the CD28 family, is expressed on effector and memory T cells, Tregs, and natural killer (NK) cells, and its ligands, CD155 and CD122, are expressed on APC, T cells, and non-hematopoietic cell types, such as tumor cells (14, 40). TIGIT blockade might influence both adaptive and innate immune responses. TIGIT+ Treg cells seem to control the Th1/Th17 ratio through enhanced IL-10 secretion, leading to a Th2 phenotype in animal models (42). In addition, TIGIT also controls NK cell function, limiting IFN-γ secretion and granule production (43–45); however, there are no available data concerning the role of this molecule on NK cells during leprosy. In HIV-infected subjects, PD-1, TIGIT and LAG-3 are considered to be biomarkers of persistent infection because CD4+ T cells expressing these molecules present viral markers, even under antiretroviral therapy (41). Analysis of TIGIT expression on Th2 lymphocytes and Tregs from LL patients as well as its correlation with disease progression are highly warranted.
Regarding LAG-3, this molecule is also expressed on Treg cells and has been associated with increased suppressive events, such as in the immune response against HIV and Plasmodium spp., as well as many types of cancers (14, 41, 46). In leprosy patients, the role of LAG-3 remains unknown, although LAG-3+CD8+ T cells were detected when human PBMC cells were cultured with M. leprae as well as in human mycobacteria-induced granulomas (47). Some clinical trials have explored LAG-3 blockade to achieve tumor reduction and control cancer progress. Although the LAG-3 signaling pathway is not completely understood in M. leprae immunity, its homology to CD4 and cross-linking with MHC class II lead to impaired maturation of DC and Treg development (48, 49), suggesting LAG-3 as a new target for therapeutic intervention. Therefore, new discoveries concerning the role of this molecule in the immune response during M. leprae infection could provide insights that can be applied to other infectious diseases.
Recently, some evidence has indicated that combined ICP blockade might be a better strategy to explore the synergic effect of multiple immune checkpoints. Single-agent ICP approaches seem to induce compensatory upregulation of other ICPs as a cell mechanism to evade IT effects, and its failure index has been observed in one-half of oncology patients under this therapy (50, 51). One possibility would be to associate independent and non-redundant inhibitory pathways of these molecules, as observed in the CTLA-4 or PD-1 combination (50, 51). In addition, each infectious disease has its own ICP pattern of expression, as observed in HBV patients whose PD-1 expression is higher than that of CTLA-4. Therefore, combined ICP blockade might be a relevant mechanism for immune response regulation in leprosy and, likely, a feasible pathway to be explored as therapeutic targets.
T Regulatory Cells (Tregs)
T regulatory cells (Tregs) are a heterogeneous subset of T CD4+ lymphocytes that might be developed at the thymus or on peripheral tissues under the control of many different signals from the microenvironment, such as TGF-β and IL-10 cytokines, retinol, and pathogen-associated molecular patterns (52, 53). Treg cells control many innate and adaptative immunological events, limiting tissue damage and maintaining homeostasis. Tregs explore many different mechanisms that control the immune response, such as increasing expression of CD25, to reduce lymphocyte proliferation through IL-2, increasing secretion of anti-inflammatory cytokines and increasing expression of granzyme and perforin, as well as ICPs, such as PD-1, CTLA-4, GITR, TIGIT, and LAG-3 (14, 40, 52–54). Treg cells in the infection site seems to be associated with the immune hyporesponsiveness observed after infection with many parasites, including T. cruzi, P. brasiliensis, L. brasiliensis, and S. mansoni (52–58). Some studies have shown the potential of Treg-cell depletion to augment antitumor immune responses (59) and infectious disease outcome (60) and have indicated that Treg-mediated T-cell suppression is an important mechanism by which pathogens evade immune responses (39, 53). In leprosy, an increased frequency of Treg cells was observed, and those lymphocyte subsets seem to contribute to pathogen persistence (13, 31–33, 61, 62). Our group assessed the suppressor features of Treg cells isolated from leprosy patients (Figure 1). Functional suppressive assays demonstrated impaired proliferation of allogenic PBMCs that were CFSE-labeled when cocultured with CD25+ T cells isolated from LL patients. In addition, the IFN-γ and TNF-α production levels were reduced in the presence of CD4+CD25+ T cells from LL patients. Moreover, our results showed that Treg cells (Foxp3+CD25+ cells) express high levels of CTLA-4 and PD-1. Such regulatory features were not hallmarks of Treg cells from TT patients (Figure 1). Expression of CTLA-4 by Treg cells serves as a mechanism of Treg cells to suppress excessive T-cell responses. Blocking CTLA-4 in vivo has been shown to inhibit Treg cells and promote antitumor immunity (63).
Activated Tregs produce IL-10, IL-35, and TGF-β, which act to suppress the immune response (64). Tregs downregulate the immune reactions through production of anti-inflammatory cytokines, lowering the antigen-presenting function in DCs, and macrophages with correspondingly decreased counts of Th1, Th2, Th17 CD4+ T cells, and cytotoxic CD8+ T cells, as well as the cytokines produced by them, and induction of apoptosis [reviewed in Ref. (65)]. High levels of TGF-β and IL-10 producing Foxp3+ T cells were reported to be increased in the lepromatous state in the circulation and skin lesions (61). Recent work has demonstrated that Tregs play a role in M. leprae-specific Th1 unresponsiveness during lepromatous disease (33). In LL, Th2/Treg polarization seems to be important to disease progression, and multiple factors may be responsible for these events, such as antigen exposure and innate immune activation (7). Otherwise, TT patients present a cellular immune response polarized to Th1/Th17 (31–33). Recent work has shown that in patients with a type 2 reaction, downmodulation of Tregs favors the development of Th17 responses (66, 67). The FoxP3+ Treg phenotype seems to be reverted into Th17, exploring the signaling through IL-12 and IL-23 (31). Th17 and Treg cells are new players associated with immunopathology in leprosy and its reactions (62). In this context, the ideal treatment for LL patients seems to require modulation of the T lymphocytes subsets to expand Th17 lymphocytes and control Treg cells, favoring the cellular immune response (5, 33). This strategy to shift the immune response to Th1/Th17 probably might achieve better outcomes in leprosy treatment; however, it might be associated with an increased risk of developing reactional states, such as erythema nodosum (66, 67). Reactional episodes have been associated with immune stimulation and can occur at any moment during leprosy infection and represent one of the most adverse events associated with disease (2, 5). Future work will need to confirm the efficacy of Treg cells for IT of infectious diseases. In addition, it is important to analyze the combinations of Treg cell targeting with ICP blockade to make IT more effective.
Antigen-Presenting Cells
Because leprosy is an intracellular infection, T-cell activation and responses are important for protective immunity. It is well known that macrophages and DCs regulate the activity of lymphocytes in adaptive immune responses, which could allow them to play important roles in IT (68). This capacity makes them potent adjuvants for the induction of antigen-specific T cells in infected hosts. In leprosy, data have shown that a delicate balance of costimulatory pathways between T-cell and APCs is essential for T-cell activation.
Dendritic cells play important roles in both innate and acquired immunity responses to M. leprae infection. These cells induce Th1 immunity and CTL responses (2, 69). However, M. leprae have evolved mechanisms to inhibit the ability of DCs to present antigens, thereby promoting a protective immune response. Exposure of DCs to M. leprae impairs its maturation and inhibits CD80, CD86, HLA-DR and CD40 expression (70–72). Recognition of M. leprae antigens, such as LAM, through DC-SIGN has also been described as an important signaling pathway to control DC maturation, leading to increased IL-10 secretion, increased lipid metabolism and bacterial persistence (73, 74). Furthermore, IDO is another molecule associated with DC maturation and its tolerogenic phenotype. IDO has also been detected at high levels in LL patients (75). IDO catalyzes the conversion of tryptophan to N-formyl-kynurenine, and this molecular messenger controls cell proliferation, induces apoptosis, and shifts T-naïve cells to develop into Tregs (52, 75). DC-based IT has been used to improve the immune response against tumor cells using DC vaccines and blocking ICP associated with DC tolerogenic phenotypes (76). To improve the immune response in chronic infectious diseases, such as HBV infection, PD-L1 blockade has been used to restore the production of Th1 cytokines, such as TNF-α, IL-2, and IFN-γ (34). On the other hand, DC vaccines have also been used to improve the immune response to the Th17 profile against Leishmania spp. infection (77). Application of DCs in IT against M. leprae has not been explored despite the potential for the stimulation of an efficient antibacterial immunity. In other disease, the results indicate that DC-based IT might be more effective in combination with conventional treatments because the association should modulate the immune system in a way that helps the host control or eliminate pathogens (68, 77, 78). Therefore, exploring strategies to shift the immune response to Th1 might achieve better outcomes in leprosy treatment, leading to reduced expression of M. leprae virulence factors, such as LAM, PGL-I, and lipid metabolism (9, 10). Future studies should also address the possible advantage of combining DC-based IT with ICP blockade or other therapeutic approaches, such as antimicrobial and anti-inflammatory drugs.
Concluding Remarks and Perspectives
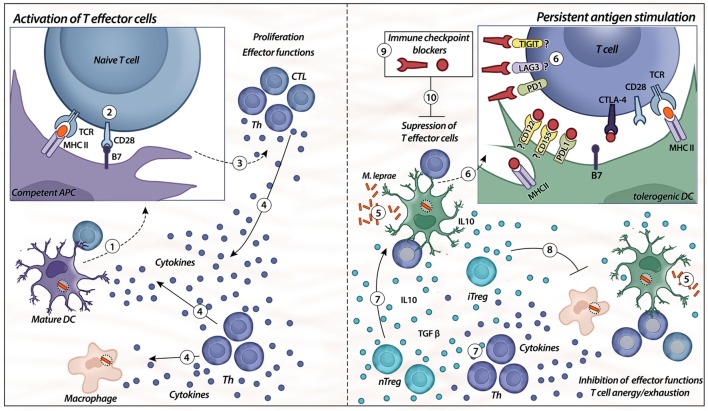
Altogether, evidence indicates that multiple factors are responsible for antigen-specific unresponsiveness in leprosy. We summarized some of these features and showed how ICP interfere with T-cell activation. We suggest that ICP blockade might interfere with leprosy pathogenesis (Figure 2). In our opinion, leprosy has been shown to have many interesting features concerning regulation that can be explored to better understand immunological mechanisms (11).
Figure 2.
Immune checkpoints. Activation of T effector cells is initiated with competent/mature antigen-presenting cells (APCs), such as mature dendritic cells (DC) (1, 2). For the first signal, APC displays the antigen to the naïve T cell via a complex with MHC II on their surfaces that is recognized by TCR on the surface of T cells; the second signal is nonspecific, resulting from the binding of B7 ligand on the APC with its receptor, CD28, on the T cell (2). When both signals are provided (3), T cells (different types of T helper and CTLs) exert their effector functions, such as release of cytokines by different Th cells (IL-6, IL-2, IFN-γ, IL-12, and TNF-α) and cytotoxicity from CTL (4). The presence of chronic immune stimulation due to persistent microbial antigens impairs specific cellular immunity (5, 6). Expression of co-inhibitory molecules, such as PD-1, TIGIT, lymphocyte-activation gene-3 (LAG-3), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), on lymphocytes and their respective ligands on the APC surface (PD-L1, CD122/155, MHC class II, and B7) induce specific T-cell anergy, leading to disseminated and progressive disease. In addition, there is higher differentiation of natural and induced types of Treg cells (nTreg/iTreg), as well as an imbalance of Th cells (7). The release of IL-10 and TGF-β from heterogeneous Treg cell subsets controls the immune response by the inhibition of effector functions, as well as induces tolerogenic phenotypes in DCs (8). The blockade of immune checkpoints, such as PD-1, CTLA-4, LAG-3, and TIGIT, might be a strategy to control the tolerogenic features observed in lepromatous leprosy patients (9, 10).
Immune checkpoint blockade has been widely applied in oncology as an adjuvant to chemotherapy and radiotherapy. In infectious diseases, ICP blockade is still a recent approach. In leprosy, it is even more critical because it is a neglected disease and probably ICP blockade might not be used as a large-scale therapy. There are some different strategies that can be used to achieve better treatment outcomes and improve the cellular response against M. leprae. In this context, BCG (re)vaccination for LL patients has been fulfilled without predictive results (79). For refractory patients, IT might be an additional strategy to control chronic disabilities.
Despite these promising results, IT based on ICP blockade has been associated with autoimmune and inflammatory events, such as oral mucositis and hepatitis (80, 81). In leprosy, increased immune stimulation has been associated with reactional states in LL patients and might be a disadvantage of this therapy. Certainly, more studies and clinical trials are needed to determine the role of Treg cells, ICPs and DCs as therapeutic targets to control M. leprae and leprosy progression.
Ethics Statement
The experimental protocol used was approved by Ethical Committee of Bauru School of Dentistry, University of São Paulo and acquainted by Lauro of Souza Lima Institute Research Ethics Committee (protocol #148/2009). All subjects assigned informed consent that was obtained before performing the studies.
Author Contributions
Conception and design: AC, JS, and GG. Development of methodology and formal analysis: HL, TG, TM, VM, MV, MN, FS, EM, and JB. Acquisition of data (provided animals, provided facilities, etc.): AC, JS, VS, and GG. Analysis and interpretation of data (e.g., statistical analysis) and writing of original draft: HL. Writing, review, and/or editing: HL, TG, VS, and AC. Funding acquisition and study supervision: AC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Cláudia Bigguetti for the designed figure.
Funding. This study was supported by a grant (#2010/18142-8) from The State of São Paulo Research Foundation (FAPESP), a grant (#441762/2014-0) from the National Council for Scientific and Technological Development (CNPq), and The State of São Paulo Foundation against Leprosy (Fundação Paulista conta a Hanseníase).
1Peripheral mononuclear blood cells were obtained from untreated patients diagnosed with leprosy at the Dermatology Clinic of the Lauro de Souza Lima (ILSL) in Bauru, São Paulo, Brazil. The experimental protocol used was approved by Ethical Comitee of Bauru School of Dentistry of Bauru (protocol number #148/2009), University of São Paulo, and informed, and written consent was obtained from all subjects before performing the studies.
References
- 1.Britton WJ, Lockwood DN. Leprosy. Lancet (2004) 363(9416):1209–19. 10.1016/S0140-6736(04)15952-7 [DOI] [PubMed] [Google Scholar]
- 2.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev (2006) 19(2):338–81. 10.1128/CMR.19.2.338-381.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Global leprosy update, 2015: time for action, accountability and inclusion. Wkly Epidemiol Rec (2015) 91(35):405–20. [PubMed] [Google Scholar]
- 4.Modlin RL. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol (1994) 102(6):828–32. 10.1111/1523-1747.ep12381958 [DOI] [PubMed] [Google Scholar]
- 5.Fonseca AB, Simon MD, Cazzaniga RA, de Moura TR, de Almeida RP, Duthie MS, et al. The influence of innate and adaptative immune responses on the differential clinical outcomes of leprosy. Infect Dis Poverty (2017) 6(1):5. 10.1186/s40249-016-0229-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D, Shui T, Miranda JW, Gilson DJ, Song Z, Chen J, et al. Mycobacterium leprae-infected macrophages preferentially primed regulatory T cell responses and was associated with lepromatous leprosy. PLoS Negl Trop Dis (2016) 10(1):e0004335. 10.1371/journal.pntd.0004335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park AJ, Rendini T, Martiniuk F, Levis WR. Leprosy as a model to understand cancer immunosurveillance and T cell anergy. J Leukoc Biol (2016) 100(1):47–54. 10.1189/jlb.5RU1215-537RR [DOI] [PubMed] [Google Scholar]
- 8.Tsai HF, Hsu PN. Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J Biomed Sci (2017) 24(1):35. 10.1186/s12929-017-0341-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moura AC, Mariano M. Lipids from Mycobacterium leprae cell wall are endowed with an anti-inflammatory property and inhibit macrophage function in vivo. Immunology (1996) 89(4):613–8. 10.1046/j.1365-2567.1996.d01-786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moura AC, Mariano M. Lipids from Mycobacterium leprae cell wall suppress T-cell activation in vivo and in vitro. Immunology (1997) 92(4):429–36. 10.1046/j.1365-2567.1997.00366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelho V, Faria AM. HSP60: issues and insights on its therapeutic use as an immunoregulatory agent. Front Immunol (2011) 2:97. 10.3389/fimmu.2011.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palermo Mde L, Trindade MA, Duarte AJ, Cacere CR, Benard G. Differential expression of the costimulatory molecules CD86, CD28, CD152 and PD-1 correlates with the host-parasite outcome in leprosy. Mem Inst Oswaldo Cruz (2012) 107(Suppl 1):167–73. 10.1590/S0074-02762012000900024 [DOI] [PubMed] [Google Scholar]
- 13.Bobosha K, Wilson L, van Meijgaarden KE, Bekele Y, Zewdie M, van der Ploeg-van Schip JJ, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis (2014) 8(4):e2773. 10.1371/journal.pntd.0002773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity (2016) 44(5):989–1004. 10.1016/j.immuni.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni L, Dong C. New checkpoints in cancer immunotherapy. Immunol Rev (2017) 276(1):52–65. 10.1111/imr.12524 [DOI] [PubMed] [Google Scholar]
- 16.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science (2011) 332(6029):600–3. 10.1126/science.1202947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao M, Valentini D, Dodoo E, Zumla A, Maeurer M. Anti-PD-1/PD-L1 therapy for infectious diseases: learning from the cancer paradigm. Int J Infect Dis (2017) 56:221–8. 10.1016/j.ijid.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 18.Zumla A, Rao M, Dodoo E, Maeurer M. Potential of immunomodulatory agents as adjunct host-directed therapies for multidrug-resistant tuberculosis. BMC Med (2016) 14:89. 10.1186/s12916-016-0635-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology (2015) 12:14. 10.1186/s12977-015-0144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol (2015) 15(8):486–99. 10.1038/nri3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol (2015) 36(2):63–70. 10.1016/j.it.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassirer-Costa F, Medeiros NI, Chaves AT, Lyon S, Coelho-Dos-Reis JGA, Ribeiro-Junior AF, et al. Cytokines as biomarkers to monitoring the impact of multidrug therapy in immune response of leprosy patients. Cytokine (2017) 97:42–8. 10.1016/j.cyto.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 23.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol (2010) 11(1):7–13. 10.1038/ni.1818 [DOI] [PubMed] [Google Scholar]
- 24.Palermo ML, Pagliari C, Trindade MA, Yamashitafuji TM, Duarte AJ, Cacere CR, et al. Increased expression of regulatory T cells and down-regulatory molecules in lepromatous leprosy. Am J Trop Med Hyg (2012) 86(5):878–83. 10.4269/ajtmh.2012.12-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Naqvi RA, Khanna N, Pathak P, Rao DN. Th3 immune responses in the progression of leprosy via molecular cross-talks of TGF-beta, CTLA-4 and Cbl-b. Clin Immunol (2011) 141(2):133–42. 10.1016/j.clim.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Manzotti CN, Burke F, Dussably L, Qureshi O, Walker LS, et al. Acquisition of suppressive function by activated human CD4+ CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol (2008) 181(3):1683–91. 10.4049/jimmunol.181.3.1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci U S A (2011) 108(1):266–71. 10.1073/pnas.1016791108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martins GA, Tadokoro CE, Silva RB, Silva JS, Rizzo LV. CTLA-4 blockage increases resistance to infection with the intracellular protozoan Trypanosoma cruzi. J Immunol (2004) 172(8):4893–901. 10.4049/jimmunol.172.8.4893 [DOI] [PubMed] [Google Scholar]
- 29.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol (2013) 59(1):81–8. 10.1016/j.jhep.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 30.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev (2010) 236:219–42. 10.1111/j.1600-065X.2010.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarique M, Saini C, Naqvi RA, Khanna N, Sharma A, Rao DN. IL-12 and IL-23 modulate plasticity of FoxP3+ regulatory T cells in human leprosy. Mol Immunol (2017) 83:72–81. 10.1016/j.molimm.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 32.Tarique M, Saini C, Naqvi RA, Khanna N, Rao DN. Increased IL-35 producing Tregs and CD19+IL-35+ cells are associated with disease progression in leprosy patients. Cytokine (2017) 91:82–8. 10.1016/j.cyto.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 33.Sadhu S, Khaitan BK, Joshi B, Sengupta U, Nautiyal AK, Mitra DK. Reciprocity between regulatory T cells and Th17 cells: relevance to polarized immunity in leprosy. PLoS Negl Trop Dis (2016) 10(1):e0004338. 10.1371/journal.pntd.0004338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One (2014) 9(8):e105703. 10.1371/journal.pone.0105703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen L, Gao Y, Liu Y, Zhang B, Liu Q, Wu J, et al. PD-1/PD-L pathway inhibits M.tb-specific CD4+ T-cell functions and phagocytosis of macrophages in active tuberculosis. Sci Rep (2016) 6:38362. 10.1038/srep38362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez FR, Mariano FS, Oliveira CJ, Pavanelli WR, Guedes PM, Silva GK, et al. Regulation of Trypanosoma cruzi-induced myocarditis by programmed death cell receptor 1. Infect Immun (2011) 79(5):1873–81. 10.1128/IAI.01047-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage plasmodium infection. Nat Immunol (2011) 13(2):188–95. 10.1038/ni.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J Immunol (2013) 191(11):5542–50. 10.4049/jimmunol.1301810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol (2017) 47(5):765–79. 10.1002/eji.201646875 [DOI] [PubMed] [Google Scholar]
- 40.Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol (2015) 6:418. 10.3389/fimmu.2015.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, et al. CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog (2016) 12(7):e1005761. 10.1371/journal.ppat.1005761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity (2014) 40(4):569–81. 10.1016/j.immuni.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol (2015) 45(10):2886–97. 10.1002/eji.201545480 [DOI] [PubMed] [Google Scholar]
- 44.de la Barrera S, Finiasz M, Fink S, Ilarregui J, Aleman M, Olivares L, et al. NK cells modulate the cytotoxic activity generated by Mycobacterium leprae-hsp65 in leprosy patients: role of IL-18 and IL-13. Clin Exp Immunol (2004) 135(1):105–13. 10.1111/j.1365-2249.2004.02334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia VE, Uyemura K, Sieling PA, Ochoa MT, Morita CT, Okamura H, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol (1999) 162(10):6114–21. [PubMed] [Google Scholar]
- 46.Doe HT, Kimura D, Miyakoda M, Kimura K, Akbari M, Yui K. Expression of PD-1/LAG-3 and cytokine production by CD4(+) T cells during infection with plasmodium parasites. Microbiol Immunol (2016) 60(2):121–31. 10.1111/1348-0421.12354 [DOI] [PubMed] [Google Scholar]
- 47.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A (2007) 104(19):8029–34. 10.1073/pnas.0702257104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, et al. Lymphocyte activation gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS One (2014) 9(11):e109080. 10.1371/journal.pone.0109080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buisson S, Triebel F. LAG-3 (CD223) reduces macrophage and dendritic cell differentiation from monocyte precursors. Immunology (2005) 114(3):369–74. 10.1111/j.1365-2567.2004.02087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun (2016) 7:10501. 10.1038/ncomms10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology (2017) 6(1):e1249561. 10.1080/2162402X.2016.1249561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol (2010) 10(7):490–500. 10.1038/nri2785 [DOI] [PubMed] [Google Scholar]
- 53.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol (2007) 7(11):875–88. 10.1038/nri2189 [DOI] [PubMed] [Google Scholar]
- 54.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol (2012) 3:51. 10.3389/fimmu.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mariano FS, Gutierrez FR, Pavanelli WR, Milanezi CM, Cavassani KA, Moreira AP, et al. The involvement of CD4+CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect (2008) 10(7):825–33. 10.1016/j.micinf.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 56.Moreira AP, Cavassani KA, Massafera Tristao FS, Campanelli AP, Martinez R, Rossi MA, et al. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J Immunol (2008) 180(5):3049–56. 10.4049/jimmunol.180.5.3049 [DOI] [PubMed] [Google Scholar]
- 57.Cavassani KA, Campanelli AP, Moreira AP, Vancim JO, Vitali LH, Mamede RC, et al. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J Immunol (2006) 177(9):5811–8. 10.4049/jimmunol.177.9.5811 [DOI] [PubMed] [Google Scholar]
- 58.Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, Brodskyn CI, et al. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis (2006) 193(9):1313–22. 10.1086/502980 [DOI] [PubMed] [Google Scholar]
- 59.Fisher SA, Aston WJ, Chee J, Khong A, Cleaver AL, Solin JN, et al. Transient Treg depletion enhances therapeutic anti-cancer vaccination. Immun Inflamm Dis (2017) 5(1):16–28. 10.1002/iid3.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol (2005) 174(7):4407–14. 10.4049/jimmunol.174.7.4407 [DOI] [PubMed] [Google Scholar]
- 61.Saini C, Ramesh V, Nath I. Increase in TGF-beta secreting CD4(+)CD25(+) FOXP3(+) T regulatory cells in anergic lepromatous leprosy patients. PLoS Negl Trop Dis (2014) 8(1):e2639. 10.1371/journal.pntd.0002639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saini C, Tarique M, Rai R, Siddiqui A, Khanna N, Sharma A. T helper cells in leprosy: an update. Immunol Lett (2017) 184:61–6. 10.1016/j.imlet.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 63.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med (2009) 206(8):1717–25. 10.1084/jem.20082492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson BM, Sullivan JA, Burlingham WJ. Interleukin 35: a key mediator of suppression and the propagation of infectious tolerance. Front Immunol (2013) 4:315. 10.3389/fimmu.2013.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garib FY, Rizopulu AP. T-regulatory cells as part of strategy of immune evasion by pathogens. Biochemistry (Mosc) (2015) 80(8):957–71. 10.1134/S0006297915080015 [DOI] [PubMed] [Google Scholar]
- 66.Vieira AP, Trindade MA, Pagliari C, Avancini J, Sakai-Valente NY, Duarte AJ, et al. Development of type 2, but not type 1, leprosy reactions is associated with a severe reduction of circulating and in situ regulatory T-cells. Am J Trop Med Hyg (2016) 94(4):721–7. 10.4269/ajtmh.15-0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saini C, Siddiqui A, Ramesh V, Nath I. Leprosy reactions show increased Th17 cell activity and reduced FOXP3+ Tregs with concomitant decrease in TGF-beta and increase in IL-6. PLoS Negl Trop Dis (2016) 10(4):e0004592. 10.1371/journal.pntd.0004592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer (2012) 12(4):265–77. 10.1038/nrc3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinheiro RO, de Souza Salles J, Sarno EN, Sampaio EP. Mycobacterium leprae-host-cell interactions and genetic determinants in leprosy: an overview. Future Microbiol (2011) 6(2):217–30. 10.2217/fmb.10.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray RA, Siddiqui MR, Mendillo M, Krahenbuhl J, Kaplan G. Mycobacterium leprae inhibits dendritic cell activation and maturation. J Immunol (2007) 178(1):338–44. 10.4049/jimmunol.178.1.338 [DOI] [PubMed] [Google Scholar]
- 71.Braga AF, Moretto DF, Gigliotti P, Peruchi M, Vilani-Moreno FR, Campanelli AP, et al. Activation and cytokine profile of monocyte derived dendritic cells in leprosy: in vitro stimulation by sonicated Mycobacterium leprae induces decreased level of IL-12p70 in lepromatous leprosy. Mem Inst Oswaldo Cruz (2015) 110(5):655–61. 10.1590/0074-02760140230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinsimer D, Fallows D, Peixoto B, Krahenbuhl J, Kaplan G, Manca C. Mycobacterium leprae actively modulates the cytokine response in naive human monocytes. Infect Immun (2010) 78(1):293–300. 10.1128/IAI.00816-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar S, Naqvi RA, Bhat AA, Rani R, Ali R, Agnihotri A, et al. IL-10 production from dendritic cells is associated with DC SIGN in human leprosy. Immunobiology (2013) 218(12):1488–96. 10.1016/j.imbio.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 74.Montoya D, Cruz D, Teles RM, Lee DJ, Ochoa MT, Krutzik SR, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe (2009) 6(4):343–53. 10.1016/j.chom.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Sousa JR, de Sousa RP, Aarao TL, Dias LB, Jr, Carneiro FR, Fuzii HT, et al. In situ expression of M2 macrophage subpopulation in leprosy skin lesions. Acta Trop (2016) 157:108–14. 10.1016/j.actatropica.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 76.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity (2013) 39(1):38–48. 10.1016/j.immuni.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jawed JJ, Majumder S, Bandyopadhyay S, Biswas S, Parveen S, Majumdar S. SLA-PGN-primed dendritic cell-based vaccination induces Th17-mediated protective immunity against experimental visceral leishmaniasis: a crucial role of PKCbeta. Pathog Dis (2016) 74(5):ftw041. 10.1093/femspd/ftw041 [DOI] [PubMed] [Google Scholar]
- 78.Leal L, Lucero C, Gatell JM, Gallart T, Plana M, Garcia F. New challenges in therapeutic vaccines against HIV infection. Expert Rev Vaccines (2017) 16(6):587–600. 10.1080/14760584.2017.1322513 [DOI] [PubMed] [Google Scholar]
- 79.Duthie MS, Balagon MF. Combination chemoprophylaxis and immunoprophylaxis in reducing the incidence of leprosy. Risk Manag Healthc Policy (2016) 9:43–53. 10.2147/RMHP.S76058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vigarios E, Epstein JB, Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer (2017) 25(5):1713–39. 10.1007/s00520-017-3629-4 [DOI] [PubMed] [Google Scholar]
- 81.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol (2014) 11(2):91–9. 10.1038/nrclinonc.2013.245 [DOI] [PubMed] [Google Scholar]