Summary
Reprogramming of cellular identity using exogenous expression of transcription factors (TFs) is a powerful and exciting tool for tissue engineering, disease modeling, and regenerative medicine. However, generation of desired cell types using this approach is often plagued by inefficiency, slow conversion, and an inability to produce mature functional cells. Here, we show that expression of constitutively active SMAD2/3 significantly improves the efficiency of induced pluripotent stem cell (iPSC) generation by the Yamanaka factors. Mechanistically, SMAD3 interacts with reprogramming factors and co-activators and co-occupies OCT4 target loci during reprogramming. Unexpectedly, active SMAD2/3 also markedly enhances three other TF-mediated direct reprogramming conversions, from B cells to macrophages, myoblasts to adipocytes, and human fibroblasts to neurons, highlighting broad and general roles for SMAD2/3 as cell-reprogramming potentiators. Our results suggest that co-expression of active SMAD2/3 could enhance multiple types of TF-based cell identity conversion and therefore be a powerful tool for cellular engineering.
Keywords: reprogramming, transdifferentiation, direct reprogramming, iPSCs, Smad2, Smad3, induced neuron
Graphical Abstract
Highlights
-
•
Enhancement of iPSC reprogramming by TGF-βR inhibition is independent of SMAD2/3
-
•
Constitutively active SMAD2/3 improves reprogramming efficiency and kinetics
-
•
SMAD3 co-occupies OCT4 targets during reprogramming
-
•
Constitutively active SMAD2/3 also boosts TF-mediated direct lineage reprogramming
Ruetz et al. show that constitutively active SMAD2/3 has a surprising ability to boost the efficiency of cell reprogramming both to iPSCs and across lineages and may therefore be a general factor that can enhance transcription-factor-mediated reprogramming in a variety of contexts.
Introduction
Cell-type-specific transcription factors (TFs), which are responsible for specifying unique cellular identities, are often described as master TFs. Those master TFs not only play critical roles in self-renewal or cell fate specification during normal development but also can be used to drive cell identity conversions in vitro and in vivo (Graf, 2011). The first demonstration of cell identity conversion by an exogenous master TF was in 1987, with overexpression of MyoD in fibroblasts resulting in the generation of myoblasts (Davis et al., 1987). Follow-up studies accomplished TF-mediated transdifferentiation of hematopoietic lineages (Kulessa et al., 1995, Xie et al., 2004), which led to Takahashi and Yamanaka (2006) demonstrating the power of this strategy by generating induced pluripotent stem cells (iPSCs) from differentiated cells with only four TFs (Oct4, Sox2, Klf4, and c-Myc). Inspired by these seminal works, several different cell types have been generated by master-TF-mediated cellular identity conversions (Bussmann et al., 2009, Feng et al., 2008, Kajimura et al., 2009, Pfisterer et al., 2011, Vierbuchen et al., 2010), holding tremendous promise for cellular engineering and disease modeling. While overexpressing cell-type-specific master TFs is a key concept in forced cell identity conversions, the set of TFs, culture conditions, functionality of the resulting cells, and duration of conversion largely vary. Whether common molecular mechanisms are able to potentiate cell fate conversions across different models has remained to be explored. It has recently been demonstrated that CAF-1 is a common roadblock in three different master-TF-mediated cell conversions (Cheloufi et al., 2015). Further investigation of reprogramming mechanisms could unveil common molecular machineries that might similarly be involved in various cell conversion models, ultimately allowing for more efficient and faithful forced cell identity changes.
Among the several TF-mediated cell conversions, the generation of iPSCs is one of the most extensively studied systems. Several small molecules, targeting various epigenetic or signaling pathways, have been identified to enhance the reprogramming process and even replace one or more of the Yamanaka factors, including transforming growth factor β receptor (TGF-βR) inhibitors (Ichida et al., 2009, Li et al., 2010, Maherali and Hochedlinger, 2009). TGF-β signaling counteracts the mesenchymal to epithelial transition (MET) (Xu et al., 2008), an essential early event for fibroblasts to become iPSCs (Li et al., 2010, Redmer et al., 2011, Samavarchi-Tehrani et al., 2010), providing a possible mechanism for how inhibition of this pathway promotes iPSC generation. However, TGF-βR inhibitors also improve reprogramming efficiency of epithelial cells (Li et al., 2010) and when added at post-MET stages of the reprogramming process (Ichida et al., 2009, Li et al., 2010), suggesting additional mechanisms of the reprogramming enhancement exist.
TGF-βRs transmit signals intracellularly through SMAD2/3-dependent and independent pathways (Derynck and Zhang, 2003). When phosphorylated by the activated TGF-βRs, SMAD2/3 translocate to the nucleus with SMAD4 and regulate transcription of hundreds of genes in a highly context-dependent manner (Massagué, 2012). SMAD2/3 interact with a range of TFs, as well as transcriptional activators, silencers, and nucleosome modifiers, including CBP/p300 transcriptional regulators (Feng et al., 1998), switch/sucrose non-fermentable (SWI/SNF) chromatin remodelers (Xi et al., 2008), the mixed lineage leukemia (MLL) histone H3 lysine 4 (H3K4) methyltransferase complex (Bertero et al., 2015), and the Mediator complex (Kato et al., 2002). Because SMAD2/3 have weak binding affinity to DNA (Shi et al., 1998), their robust interaction with the genome relies on other TFs, which can partially explain cell-type and context-dependent cellular responses to TGF-β activation (Derynck and Zhang, 2003, Ikushima and Miyazono, 2010, Massagué et al., 2005). Recent genome-wide chromatin immunoprecipitation followed by sequencing (ChIP-seq) analyses demonstrated that SMAD3 binds almost entirely unique target loci across the genome in a given cell type, co-localizing with OCT4, SOX2, and NANOG in embryonic stem cells (ESCs), PU.1 in pro-B cells, and MYOD1 in myotubes (Mullen et al., 2011). Thus, SMAD2/3 are powerful gene expression regulators, yet their potential positive role in iPSC generation has been disregarded, because the inhibition of the upstream molecule, TGF-βR, enhances reprogramming efficiency, and because their involvement in other master-TF-mediated cell identity conversions has not been explored.
Here, we demonstrate that TGF-βR inhibitor treatment circumvents reprogramming-dependent upregulation of p19ARF, a senescence inducer, independently of the promotion of the MET. Unexpectedly, we found that active SMAD2/3 increases when cells are cultured in the presence of TGF-βR inhibitors for prolonged periods. Following that observation, we assessed the impact of constitutively active SMAD2/3 (Smad2/3CA) on reprogramming and found a remarkable boost in efficiency and acceleration of the process. However, Cas9-mediated double knockout of Smad2/3 revealed that endogenous SMAD2/3 was not responsible for TGF-βR-inhibitor-mediated reprogramming enhancement, suggesting that other receptor downstream targets are involved. Irrespectively, we discovered that overexpressed SMAD3CA physically interacted with reprogramming factors and localized at OCT4 target loci during reprogramming. Moreover, active SMAD3 could also enhance three other master-TF-mediated cell identity conversions. This work highlights SMAD2/3 as common powerful cofactors that potentiate diverse forced cell identity conversions with master TFs.
Results
TGF-βR Inhibition Enhances Reprogramming Independently of the MET
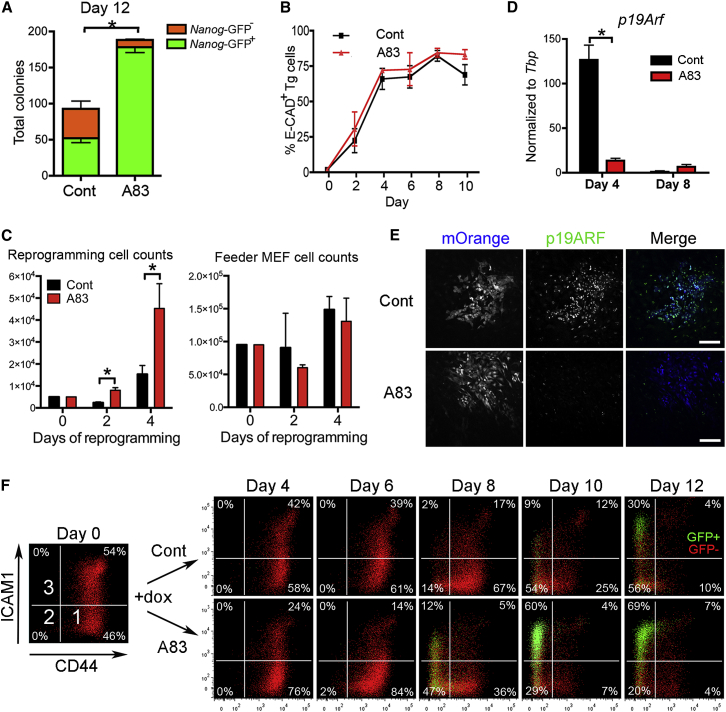
To explore how TGF-βR inhibitors enhance reprogramming (Ichida et al., 2009, Li et al., 2010, Maherali and Hochedlinger, 2009), we first confirmed the beneficial effect of the ALK4/5/7 inhibitor A83-01 (A83) (Tojo et al., 2005) using mouse embryonic fibroblasts (MEFs) with doxycycline (dox)-inducible Yamanaka factors (c-Myc, Klf4, Oct4, and Sox2) and an mOrange reporter of transgene expression (MKOS-ires-mOrange) (Chantzoura et al., 2015). The addition of A83 to reprogramming media resulted in a 3-fold increase in reprogramming efficiency, measured by the number of colonies positive for Nanog-GFP reporter (Figure 1A). TGF-βR inhibitors are thought to facilitate reprogramming, at least in part, by enhancing the transition to an epithelial-like state (Li et al., 2010). However, over 70% of cells demonstrated E-CADHERIN expression after day 4 of reprogramming either in the presence or absence of A83 in this reprogramming system with a polycistronic reprogramming vector containing full-length Klf4, as previously reported (Figures 1B, S1A, and S1B) (Chantzoura et al., 2015, Kim et al., 2015, O’Malley et al., 2013). These results suggest that TGF-βR inhibition facilitates reprogramming independently of the acquisition of epithelial character in this system. During reprogramming, MKOS-expressing transgenic (Tg; mOrange+) MEFs were found to proliferate more in the presence of A83, while the proliferation of surrounding wild-type MEFs was unaffected (Figure 1C). Real-time PCR and immunofluorescence revealed that transient upregulation of p19ARF, a p53-dependent cell-cycle arrest and apoptosis inducer, was suppressed in the Tg cells on day 4 of reprogramming in the presence of A83 (Figures 1D and 1E). Thus, one of the positive effects of A83 is anti-senescence/apoptosis at the early stage of reprogramming, which is consistent with the fact that A83 treatment only at the initial stages of reprogramming is also beneficial (Figure S1C) (Maherali and Hochedlinger, 2009). In addition, analysis of reprogramming progression with the cell-surface markers CD44 and ICAM1 indicated that the speed of reprogramming was also affected by the inhibitor (Figure 1F). MEFs with high CD44 and broad ICAM1 expression reach an iPSC state by going through gates 1, 2, and 3, which are marked by CD44 and ICAM1 expression levels (Figure 1F, day 0) (O’Malley et al., 2013). These CD44 and ICAM1 expression changes were accelerated in the presence of A83; for example, >50% of cells had already downregulated CD44 at day 8 as compared to ∼15% of control cells (Figure 1F). Moreover, with the addition of A83, 33.7% of cells were Nanog-GFP+ at day 8, in contrast to 1.6% in the absence of A83, indicating a robust acceleration of reprogramming. Late reprogramming intermediates generated in the presence of A83 were 2- to 2.5-fold more likely to form Nanog-GFP+ colonies when flow-sorted based on CD44/ICAM1/Nanog-GFP expression profiles at day 10 of reprogramming (Figure S1D). These results confirmed that post-MET populations are also positively affected by TGF-β inhibition, underlining the multimodal nature of TGF-β signaling.
Figure 1.
Reprogramming Enhancement and Acceleration by TGF-β Inhibitors
(A) Nanog-GFP− and GFP+ colony numbers on day 12 of reprogramming.
(B) Flow cytometry analysis of E-CADHERIN (E-CAD) expression during reprogramming.
(C) Numbers of mOrange+ MKOS expressing transgenic (Tg) cells (left) and mOrange− wild-type feeder MEFs (right) during the first 4 days.
(D) Real-time RT-PCR for p19Arf with mOrange+ cells on days 4 and 8.
(E) Immunofluorescence for p19ARF on day 4.
(F) CD44/ICAM1/Nanog-GFP expression throughout reprogramming. Red, Nanog-GFP−; green, Nanog-GFP+ Tg cells.
Cont and A83 indicate in the absence and presence of A83, respectively. See also Figure S1.
Increased Phosphorylated Smad3 Levels Correlate with Successful Reprogramming
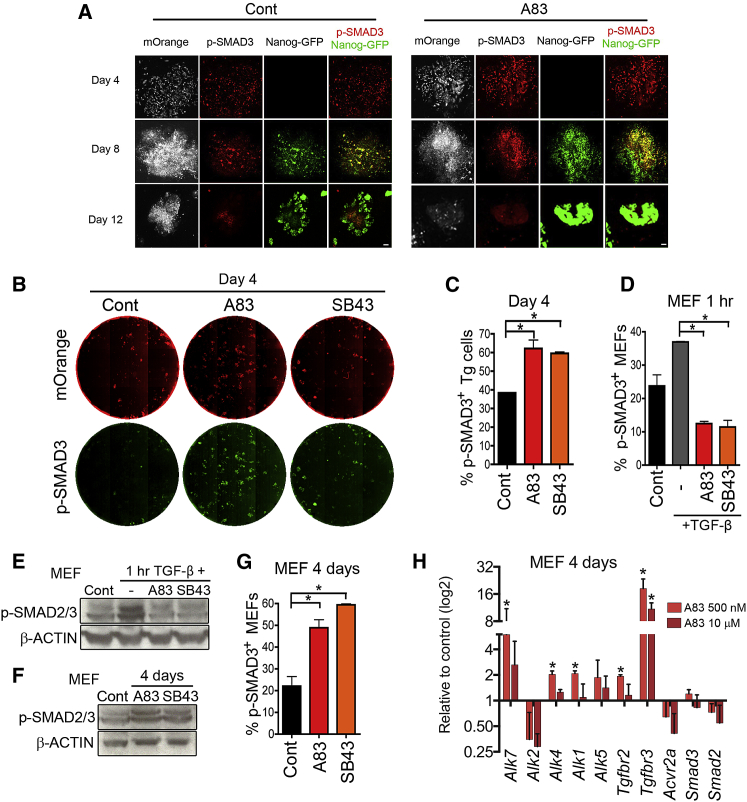
Downstream targets of TGF-βRs include SMAD2/3, mitogen activated protein kinase (MAPK), Rho GTPase, and the phosphatidylinositol 3-kinase (PI3K)/AKT pathways (Derynck and Zhang, 2003, Zhang, 2009). TGF-βRs phosphorylate the C terminus of SMAD3, which causes complex formation with SMAD4 and translocation to the nucleus to regulate gene expression (Shi and Massagué, 2003). In the absence of A83 some, but not all, cells in reprogramming colonies demonstrated phosphorylated SMAD3 (p-SMAD3) at day 4 (Figure 2A, Cont Day4). p-SMAD3 was often observed in cells with Nanog-GFP expression at day 8 (Cont Day 8, Figure 2A), in contrast to day 12 when p-SMAD3 was barely detectable in Nanog-GFP+ cells (Cont Day 12, Figure 2A). Unexpectedly, we observed p-SMAD3+ cells more frequently within the colonies in the presence of A83 on both day 4 and day 8 of reprogramming (Figure 2A, A83). The finding that p-SMAD3+ cells increase in the presence of A83 contradicts the knowledge that ALK4/5/7 inhibitors dampen SMAD3 signaling (Tojo et al., 2005). However, the increase in p-SMAD3+ cells was also observed at day 4 of reprogramming with another ALK4/5/7 inhibitor, SB-431542 (SB43), which likewise enhances reprogramming (Ichida et al., 2009) (Figure 2B). When we quantified the percentage of mOrange+ Tg cells with p-SMAD3 foci in the whole-well images, there was a 1.5-fold increase in the presence of either A83 or SB43 (Figures 2C and S2A). Notably, both the image analysis and western blotting demonstrated that either A83 or SB43 could block SMAD2/3 phosphorylation in MEFs treated with TGF-β for 1 hr (Figures 2D, 2E, and S2B). These data demonstrate that these inhibitors can work as predicted for short-duration treatments. However, when wild-type MEFs were cultured in the presence of inhibitors for 4 days, we observed a clear increase in p-SMAD2/3 levels (Figures 2F, 2G, and S2C) similar to that observed in Tg cells during reprogramming (Figures 2A–2C). Many TGF-βR (Alk1, Alk4, Alk7, Tgfbr2, and Tgfbr3) were upregulated in MEFs after 4 days-treatment with A83 (Figure 2H). This may be a feedback mechanism that contributes to the unexpected cellular response of increased p-SMAD2/3 levels during prolonged exposure to TGF-βR inhibitors.
Figure 2.
Increased Phosphorylated SMAD3 in the Presence of TGF-β Inhibitors
(A) Immunofluorescence for phosphorylated SMAD3 (p-SMAD3) on days 4, 8, and 12 of reprogramming in combination with the mOrange and Nanog-GFP reporters. Scale bar, 100 μm.
(B) Whole-well images of 6-well plates at day 4 of reprogramming. Red, mOrange+ Tg cells; green, p-SMAD3 staining.
(C) Quantification of cells with strong p-SMAD3 foci in mOrange+ Tg cells from the day 4 whole-well images.
(D) Whole-well p-SMAD3 image analysis with wild-type MEFs cultured for 1 hr with no treatment (Cont) or with the addition of 10 ng/mL TGF-β without inhibitors (−) or with A83-01 (A83) or SB431542 (SB43).
(E) p-SMAD2/3 western blotting in wild-type MEFs treated with 10 ng/mL TGF-β for 1 hr without inhibitors (−) or with A83 or SB43.
(F) p-SMAD2/3 western blotting in wild-type MEFs cultured for 4 days without inhibitors (Cont) or with A83 or SB43.
(G) Whole-well p-SMAD3 image analysis of wild-type MEFs cultured for 4 days without inhibitors (Cont) or with A83 or SB43.
(H) Real-time RT-PCR for TGFβRs and Smad2/3 after 4 days culture of MEFs in the presence of A83. Each expression value was normalized to Tbp and then compared to DMSO-(carrier)-treated control samples.
All graphs represent averages of 3 independent experiments, with 2 technical replicates. Error bars indicate SD. ∗p < 0.05 based on a two-sided t test. See also Figure S2.
Constitutively Active SMAD2/3 Boost Reprogramming
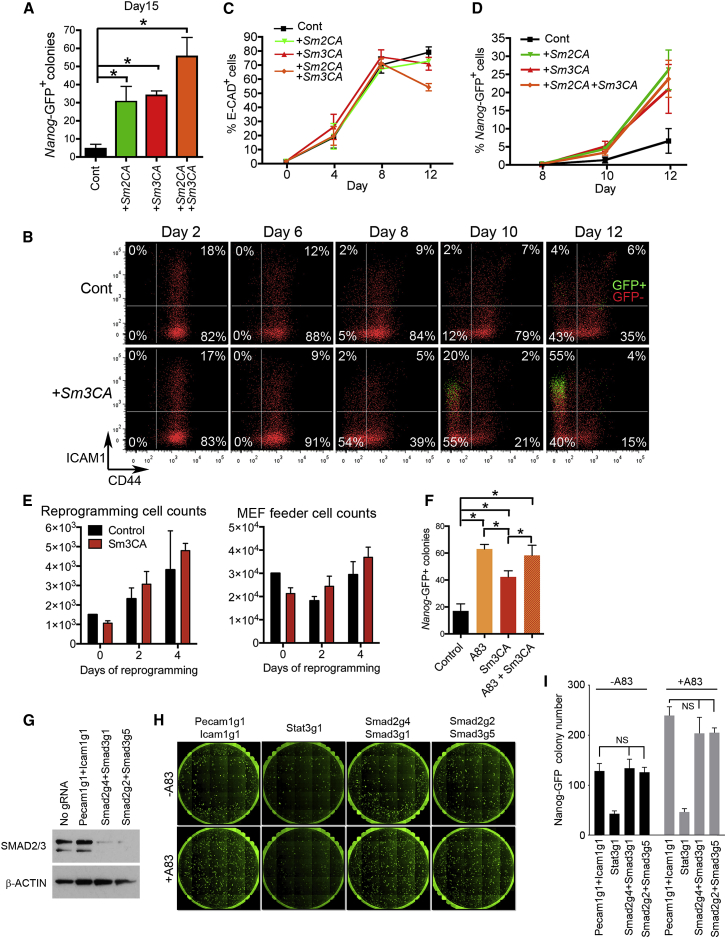
It was previously shown that SMAD3 is recruited to target loci by cell-type-specific master TFs, including by OCT4 to pluripotency gene loci in mouse ESCs (Mullen et al., 2011). Furthermore, SMAD3 interacts with several TFs, chromatin remodelers, and transcriptional regulators in a number of diverse cell types (Gaarenstroom and Hill, 2014). Our observations that the majority of cells becoming Nanog-GFP+ are p-SMAD3+ (Figure 2A) and prolonged treatment of cells with TGF-βR inhibitors increased the number of p-SMAD3+ cells (Figures 2B–2G) prompted us to investigate roles for SMAD3 during reprogramming. We first tested whether constitutively active forms of SMAD2 (SMAD2CA) or SMAD3 (SMAD3CA) (Chipuk et al., 2002, Funaba and Mathews, 2000) could boost reprogramming. Interestingly, retroviral overexpression of Smad2CA and/or Smad3CA in our MKOS reprogramming system resulted in an over 6-fold increase in Nanog-GFP+ colonies, and the combined effect of Smad2CA and 3CA resulted in a 10-fold increase in efficiency (Figures 3A and S3A). Flow cytometry analysis revealed that expression changes of CD44, ICAM1, and Nanog-GFP were accelerated in the presence of Smad3CA, while E-CAD expression was not affected (Figures 3B–3D). Analyses of other reprogramming cell surface markers including MEFSK4, CD47, CD73 and CD104 (Lujan et al., 2015) also demonstrated accelerated reprogramming phenotypes at later time points beyond day 6 (Figure S3B). Smad3CA did not enhance the proliferation of cells undergoing reprogramming at the early stages (Figure 3E), different from A83 treatment (Figure 1C). When directly compared, reprogramming efficiency with A83 was higher than that of Smad3CA overexpression, and treatment with A83 and Smad3CA together did not further improve reprogramming efficiency (Figures 3F and S3C). The strong effect of A83, including its anti-senescence action, is potentially masking the effect of Smad3CA and/or their downstream mechanisms of facilitating reprogramming overlap. To address whether A83-mediated reprogramming enhancement is attributed to the unexpected increase of p-SMAD2/3, we performed reprogramming after knocking out both Smad2 and Smad3 in dox-inducible MKOS MEFs with constitutive Cas9 expression by infection of lentiviral guide RNA (gRNA) expression vectors (Figure S3D) (Tzelepis et al., 2016). Efficient double knockout (KO) was confirmed by western blotting 3 days after gRNA vector infection (Figure 3G). Unexpectedly, Smad2/3 double KO did not have obvious effects on reprogramming efficiency in either the presence or absence of A83 (Figures 3H and 3I). This indicated that reprogramming enhancement by A83 was largely SMAD2/3 independent and that endogenous SMAD2/3 is not required for mouse iPSC generation. Nevertheless, SMAD2/3CA also enhanced the generation of human iPSCs within an episomal reprogramming system (Okita et al., 2011) (Figure S3E). Independently, Yamakawa et al. (2016) also identified SMAD2 as one factor that can enhance human iPSC generation in a cDNA overexpression screen. These results solidified the notion that exogenous SMAD2/3 facilitates reprogramming and warranted further mechanistic analysis.
Figure 3.
Constitutively Active Smad2/3 Boosts Reprogramming
(A) Nanog-GFP+ colony numbers on day 15 of reprogramming with retroviral infection of control vector (Cont), constitutive active Smad2 (+Sm2CA), Smad3 (+Sm3CA), or Smad2CA plus Smad3CA (+Sm2CA+Sm3CA).
(B) CD44/ICAM1/Nanog-GFP expression during reprogramming with control (top) and Smad3CA (bottom) expression vector infection. Red, Nanog-GFP−; green, Nanog-GFP+ Tg cells.
(C and D) E-CAD (C) and Nanog-GFP (D) expression during reprogramming with control, Smad2CA, Smad3CA, or Smad2CA plus Smad3CA expression vector infection.
(E) Numbers of Tg (left) and wild-type feeder MEFs (right) with control (Cont) or Sm3CA vectors during reprogramming.
(F) Nanog-GFP+ colony numbers on day 15 of reprogramming in the presence of A83, Sm3CA, or A83 plus Sm3CA.
(G) Efficient double knockout (dKO) of Smad2 and Smad3 was observed 72 hr after co-infection of lentiviruses encoding gRNA targeting Smad2 and Smad3 (Smad2g2+Smad3g1, Smad2g4+Smad3g5). Controls included samples with no gRNA infection and with co-infection of gRNA viruses against Pecam1 (Pecam1g1) and Icam1 (Icam1g1), respectively.
(H and I) Smad2/3 double KO did not affect reprogramming efficiency in the presence or absence of A83. Pecam1g1+Icam1g1 and Stat3g1 (gRNA against Stat3) were used as negative and positive controls, respectively.
Graphs represent averages of 3 (A, C, D–F, F) or 2 (I) independent experiments with 2 technical replicates. Error bars indicate SD. ∗p < 0.05; NS, not significant (two-sided t test). See also Figure S3.
Accelerated Reprogramming Process by Smad3CA
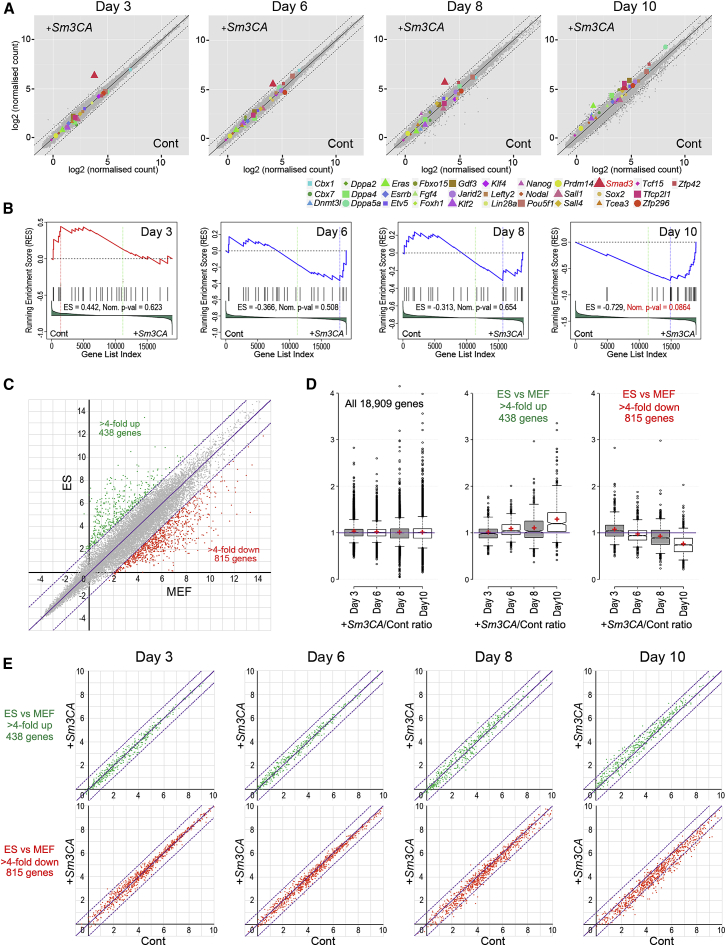
First, we performed time-course global gene expression profiling. Day 15 Nanog-GFP+ cells generated from reprogramming with the addition of Smad3CA were similar to ESCs (R2 = 0.968), indicating that Smad3CA expression does not alter the final outcome of reprogramming (Figure S4A). When we compared samples on days 3, 6, 8, and 10 of reprogramming with and without Smad3CA expression, higher Smad3 expression was clearly detectable in +Smad3CA samples, especially at earlier time points before viral vector silencing occurs (Figure 4A, red triangles). Unexpectedly, global gene expression was very similar between control and +Smad3CA samples across these time points (Figure 4A, gray dots). However, expression of almost all pluripotency-associated genes became higher in +Smad3CA samples by day 10, as further illustrated by gene set enrichment analysis (GSEA) (Figures 4A, 4B, and S4B; all read counts are available in Table S1) (Subramanian et al., 2005). Similarly, when we investigated expression patterns of genes >4-fold up- or downregulated in ESCs compared to MEFs (438 and 815 genes, respectively) during reprogramming, a small but global acceleration of up- and downregulation by Smad3CA was apparent (Figures 4C–4E). These results indicated that SMAD3CA expression did not cause outstanding gene expression changes compared to those induced by Yamanaka factors alone but globally boosted the required gene expression changes, resulting in a clear enhancement of reprogramming.
Figure 4.
Accelerated Global Gene Expression Changes toward iPSCs by Smad3CA
(A) Global gene expression comparison between reprogramming with control (Cont) and +Smad3CA (+Sm3CA) vector expression on days 3, 6, 8, and 10. The diagonal dashed lines represent 2-fold differences.
(B) Gene set enrichment analysis (GSEA) for the pluripotency genes highlighted in (A). ES, enrichment score.
(C) Genes >4-fold up- and downregulated in ESCs compared to MEFs. Solid and dotted diagonal lines represent 1-fold and 4-fold changes, respectively. The x and y axes show log2 read counts.
(D) Read count ratios (+Sm3CA/Cont) of all 18,909 genes, 438 ESC upregulated genes, and 815 ESC downregulated genes at days 3, 6, 8, and 10 are shown with boxplots. Red crosses represent mean.
(E) Scatterplots (+Sm3CA versus Cont) of ESC up- and downregulated genes in (C) and (D). Solid and dotted diagonal lines represent 1-fold and 2-fold changes, respectively.
To further investigate how SMAD3CA altered the gene expression changes caused by Yamanaka factors, we first performed pattern clustering with control and +Smad3CA samples (Figure S4C). We classified 3,750 genes with above-background expression levels (mean count ≥2) and high variation (coefficient of variation >100) into 8 distinct gene expression patterns (scaled expression profiles are shown in Figure S4C, and classification of genes is available in Table S1), of which 7 cluster patterns in control and +Smad3CA sample series were very similar: downregulated by day 3 (Dwn), transiently upregulated at day 3 (D3Up), transiently upregulated at day 8 (D8Up), highly (D10Up) or slightly (D10sUp) upregulated at day 10 but low expression in ESCs, expected to be upregulated after day 10 (Up), and finally a down and up cluster (DwnUp). Additionally, the control series had a cluster of transiently upregulated genes on day 6 (D6Up), and the +Smad3CA series had a group of genes upregulated earlier than the Up genes (eUp). A comparison of the genes classified in each category revealed that genes in Dwn, D3Up, Up, and DwnUp clusters were very similar between control and +Smad3CA samples as shown with pale colors in the cord diagram (Figure S4D). Most of the genes in other categories were cross-classified between the control and +Smad3CA series. Among the cross-classified gene groups, 5 had over 100 genes (Figure S4D, ∗E-I). Most of the control D8Up genes were classified into +Smad3CA D10sUp genes (108 genes), with their upregulation minimized in the presence of +Smad3CA when medians of all the 108 genes from the control (blue) and +Smad3CA (red) series were plotted along the time points (Figure S4E). These 108 genes were enriched in nervous-system-related genes, potentially indicating unnecessary upregulation of SOX2 targets reflecting its critical roles in neurons (Figure S4J). Approximately 24% and 45% of control D10Up genes were cross classified into D8Up (124 genes) or D10sUp (233 genes) +Smad3CA clusters, where the peak of the transient upregulations of the median line in +Smad3CA occurred earlier at day 8 (Figure S4F) or was minimized (Figure S4G), respectively. These two groups of genes were enriched in immune-response-associated genes (Figure S4J). Most of the control D10sUp genes fell into +Smad3CA D10Up cluster (102 genes), but those had relatively low upregulation in the +Smad3CA series (compare +Sm3CA [red] median line in Figure S4H with the Cont [blue] median lines in Figures S4F and S4G). Finally, almost all genes that belonged to eUp, a unique cluster in the +Smad3CA series, were classified in the control Up cluster (120 genes) (Figure S4I), indicating an accelerated upregulation for these genes. Altogether, these gene expression cluster analyses suggested a global and swifter transition to a pluripotent state with less transient upregulation of miscellaneous genes and/or less production of divergent cells that are unable to become iPSCs in the presence of Smad3CA.
SMAD3 Interacts with Both Nucleosome Remodelers and Reprogramming Factors and Co-occupies OCT4 Targets throughout the Genome
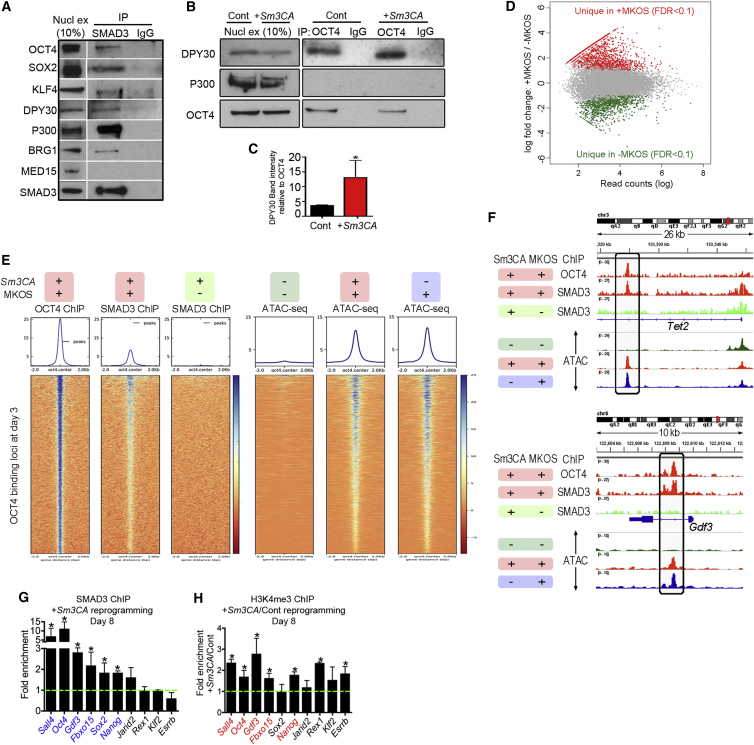
SMAD3 is ubiquitously expressed and interacts with many different proteins, including TFs and co-activators, in various cell types (Massagué et al., 2005). Its DNA-binding affinity is weak compared to other TFs, to the extent that its interaction with other TFs determines where SMAD3 binds throughout the genome (Massagué et al., 2005, Mullen et al., 2011, Shi et al., 1998). We therefore hypothesized that SMAD3, in combination with nucleosome remodelers or co-activators, is recruited by OCT4, SOX2, and/or KLF4 to their binding sites, which augments expression of target genes during reprogramming. Indeed, SMAD3 immunoprecipitation pulled down OCT4, SOX2, and KLF4 (mildly) at day 4 of reprogramming when expressing Smad3CA (Figure 5A). We could also detect interactions between SMAD3 and histone modifiers and transcriptional regulators, including DPY30 (part of the MLL complex), p300, and BRG1 (part of the SWI/SNF complex), but not MED15 (part of the Mediator complex) (Figure 5A). To investigate the possibility that SMAD3 bridges reprogramming factors with co-activators, we assessed interactions between OCT4 and the co-activators at day 4 of reprogramming in the presence or absence of Smad3CA. Interaction between OCT4 and DPY30 (MLL complex), but not p300, was readily detectable at day 4 of reprogramming in the control conditions (Figure 5B). However, when SMAD3CA was overexpressed, the amount of DPY30 pulled down with OCT4 increased nearly 3-fold (Figures 5B and 5C), indicating that SMAD3CA facilitated the interaction between OCT4 and the MLL complex component. Furthermore, ChIP-seq analysis revealed that just 3 days of MKOS induction largely altered SMAD3-binding sites across the genome, yielding 1,176 novel binding sites and losing the 904 binding sites observed in the starting MEF population (false discovery rate [FDR] < 0.1) (Figure 5D). The Oct4-binding sites on day 3 of reprogramming were clearly co-occupied by SMAD3, while SMAD3 binding was hardly detectable in the absence of Yamanaka factor expression at those loci, suggesting a recruitment of SMAD3 by MKOS (Figures 5E and 5F). Chromatin at those OCT4-binding loci was closed in MEFs, but open on day 3 of reprogramming either in the presence or absence of exogenous Smad3CA (Figures 5E and 5F), confirming OCT4 as a pioneering factor (Soufi et al., 2012). We then performed an assay for transposase accessible chromatin with high-throughput sequencing (ATAC-seq), which demonstrated that chromatin at early OCT4-binding loci was similarly accessible regardless of either the presence or absence of SMAD3CA co-expression on day 3 (Figures 5E and 5F). It has been reported that histone marks of OCT4 target loci dynamically change during reprogramming (Chronis et al., 2017). Considering the ability of SMAD3 to interact with histone modifiers, such as Dpy30 and p300 (Figure 5A), SMAD3CA might contribute to such changes and facilitate gene expression changes toward iPSCs. In fact, we could detect increased H3K4me3 at promoters of 5 out of 6 pluripotency genes (Figure 5H, red), near which SMAD3 binding was observed on day 8 of reprogramming (blue, Figure 5G), although it could be either a cause or a consequence of enhanced reprogramming by SMAD3CA. In summary, these results support a model whereby Smad3 interacts with reprogramming factors and is directed to their target loci, which potentially facilitates the recruitment of histone modifiers and/or nucleosome remodelers, facilitating the cell identity change.
Figure 5.
SMAD3 Interacts with Reprogramming Factors and Chromatin Remodelers and Is Localized at OCT4-Binding Sites during Reprogramming
(A) Co-immunoprecipitation (co-IP) of SMAD3 and reprogramming factors or chromatin remodelers at day 4 of reprogramming with Smad3CA expression. Nucl ex, 10% of nuclear extract used for co-IP; IgG, immunoglobulin G (negative control for co-IP).
(B) Co-IP of OCT4 and DPY30 at day 4 of reprogramming with control (Cont) or Smad3CA (+Sm3CA) vector expression. An interaction between OCT4 and P300 was not observed.
(C) DPY30 band intensity analysis from 2 independent experiments shown in (B).
(D) Comparison of SMAD3-binding peaks in MEFs with overexpression of Sm3CA or Sm3CA plus Yamanaka factors for 3 days.
(E and F) OCT4, SMAD3 ChIP-seq, and ATAC-seq heatmaps at OCT4 bound loci (E) and tracks at the Tet2 and Gdf3 loci (F) with (+) and without (−) MKOS induction in the presence (+) or absence of (−) Sm3CA expression for 3 days.
(G) SMAD3 ChIP-qPCR at day 8 of reprogramming with Smad3CA expression at known Oct4-binding loci in ESCs associated with pluripotency genes. Fold enrichment refers to enrichment over the averaged value of binding to known unbound regions of the Oct4 and Nanog genes.
(H) H3K4me3 ChIP-qPCR at day 8 of reprogramming with control (Cont) and Smad3CA (+Sm3CA) vector expression. Data are shown as relative enrichment in +Sm3CA samples against control samples.
Graphs represent averages of 2 (H3K4 ChIP) or 3 (Smad3 ChIP) independent experiments with 2 technical replicates. Error bars indicate SD. ∗p < 0.05 (one-sided t test).
Smad3CA Potentiates Other Master-TF-Mediated Cell Identity Conversions
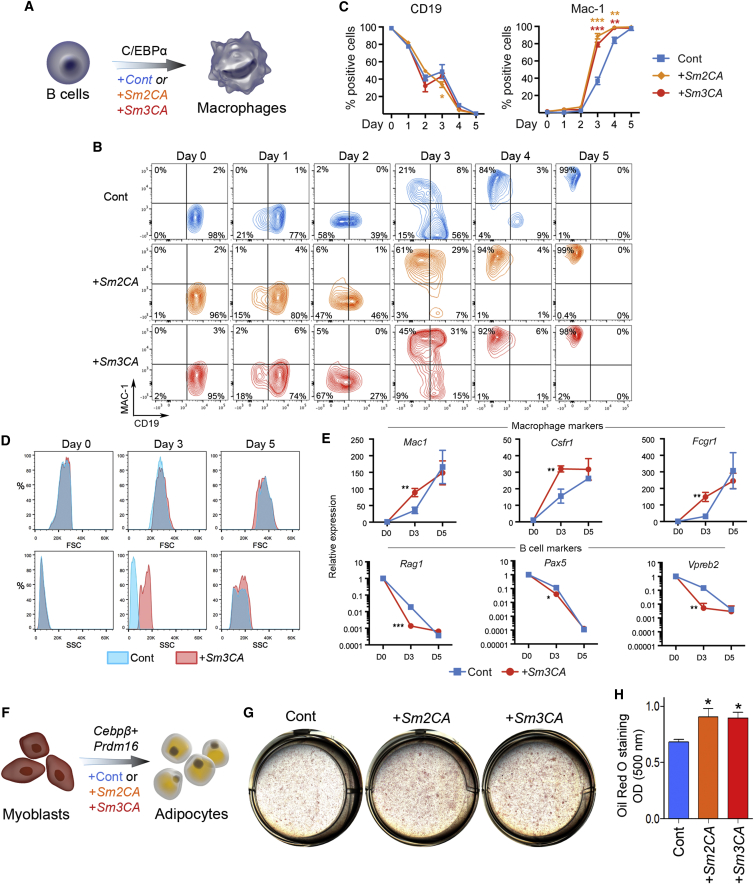
Before and after the initial generation of iPSCs in 2006, several cell types have been generated by overexpression of cell-type-specific master TFs (Bussmann et al., 2009, Davis et al., 1987, Feng et al., 2008, Kajimura et al., 2009, Pfisterer et al., 2011, Vierbuchen et al., 2010, Xie et al., 2004). SMAD3 is known to interact with a multitude of cell-type-specific master TFs (Massagué, 2012, Mullen et al., 2011). We asked whether Smad2/3CA could boost other master-TF-mediated cell identity conversions. B cells can be converted to macrophages by overexpression of Cebpα (Bussmann et al., 2009, Fukuchi et al., 2006, Xie et al., 2004) (Figure 6A). The conversion is near 100% efficient, occurring over a 6-day period, and can be monitored by downregulation of the B cell surface marker CD19 and upregulation of the macrophage marker MAC-1. Expression of Smad2/3CA hardly affected CD19 downregulation; however, more than 75% of cells became MAC-1+ by day 4 of conversion with Smad2/3CA compared to less than 30% of cells converted with Cebpα alone (Figures 6B and 6C). Furthermore, in the Smad2/3CA expressing samples, cells with very high granularity, a feature of mature myeloid cells, could be observed from day 3 (Figure 6D). Quantitative PCR (qPCR) detected accelerated upregulation of the macrophage markers Mac1, Csfr, and Fcgr1 and downregulation of the pre-B cell markers Rag1, Pax5, and Vpreb2 (Figure 6E). By analyzing CEBPα ChIP-seq data 18 hr after initiation of the B cell to macrophage conversion, we also found that 1,255 out of 4,211 CEBPα-binding peaks had SMAD-binding motifs (Figures S5A and S5B) (Di Stefano et al., 2016). Genes associated with those peaks were highly enriched in gene ontology terms related to macrophage functions (Figure S5C). ChIP-qPCR confirmed that SMAD3 indeed bound to these loci during the B cell to macrophage conversion (Figures S5D and S5E). In summary, SMAD2/3CA significantly accelerated and enhanced the CEBPα-mediated B cell to macrophage conversion, likely via its recruitment to CEBPα targets.
Figure 6.
Smad2/3CA Potentiate Macrophage and Adipocyte Conversions
(A) Conversion of B cells to macrophages by retroviral transduction of Cebpα with or without Smad2CA (Sm2CA) or Smad3CA (Sm3CA).
(B) Expression changes of the B cell marker CD19 and the macrophage marker MAC-1 during the Cebpα-mediated transdifferentiation process with additional control (Cont), Sm2CA, or Sm3CA vector expression.
(C) Quantification of CD19 and MAC-1 expression.
(D) Changes in forward scatter (FSC) and side scatter (SSC).
(E) qPCR analysis of macrophage and pre-B cell markers.
Graphs in (C) and (E) represent averages of 2 independent experiments with 2 technical replicates, respectively. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (two-sided t test).
(F) Conversion of C2C12 myoblasts to adipocytes with expression of Cebpβ and Prdm16 with or without Sm2CA or Sm3CA.
(G) Oil red O staining of cells 6 days post induction of Cebpβ and Prdm16 with an additional control (Cont), Sm2CA, or Sm3CA vector.
(H) Quantification of oil red O staining. Graphs represent averages of 2 independent experiments, with 3 technical replicates, one of those images is presented in G. ∗∗p < 0.01; ∗p < 0.05 (two-sided t test).
See also Figure S5.
Next, we investigated the process of converting myoblasts to adipocytes with Cebpβ and Prdm16 (Kajimura et al., 2009) (Figure 6F). Successful conversion of C2C12 myoblasts to adipocytes can be assessed by oil red O staining, which identifies the triglycerides and lipids in converted adipocytes. Expression of either Smad2CA or Smad3CA in conjunction with Cebpβ and Prdm16 resulted in an ∼20% increase in conversion efficiency (Figures 6G and 6H).
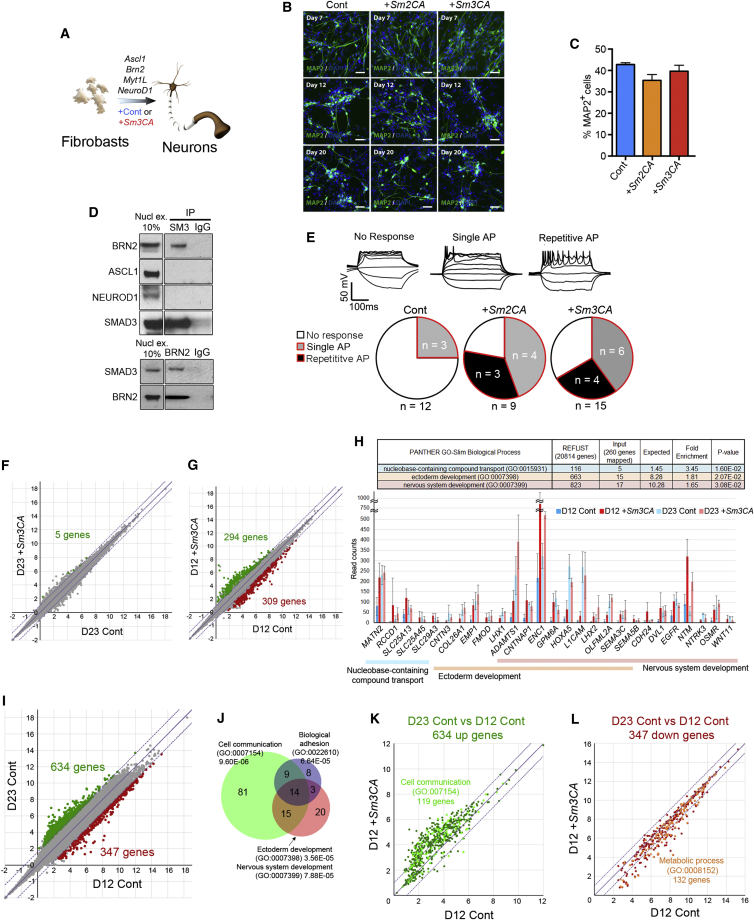
Finally, we addressed whether Smad2/3CA can accelerate conversion of human fibroblasts to neurons by Ascl1, Brn2a, MytIl, and NeuroD1 (ABMN) (Figure 7A) (Pang et al., 2011). The proportion of cells with neuronal morphology and expressing the neuronal lineage marker MAP2 was not affected by the overexpression of Smad2/3CA, as assessed at day 7, 12, or 20 after initiation of ABMN transgene expression (Figures 7B and 7C). However, we could observe a clear interaction between SMAD3 and one of the induced neuron (iN) factors, BRN2 (Figure 7D), and when we assessed neuronal maturity by patch-clamp electrophysiology, marked differences emerged (Figure 7E). On days 23–25 of the neural conversion, 25% (3/12) of human iNs (hiNs) generated with ABMN showed only immature single action potentials (APs) after step-wise current injections, and 75% (9/12) were not functional at this time point (Figure 7E, Cont). In contrast, with the addition of Smad2CA or Smad3CA, 44% (4/9) or 40% (6/15) of hiNs had single APs, and 33% (3/9) or 26.7% (4/15) showed a more mature phenotype with repetitive APs, respectively, which was consistent with their significantly greater membrane capacitance and Na+, K+ currents (Figures 7E, S6A, and S6B). To reveal molecular signatures that explain this accelerated maturation, we performed RNA sequencing (RNA-seq) analysis. To our surprise, gene expression was very similar on day 23, with only 5 differentially expressed genes (USH1C, C12orf29, KIAA0391, SEZ6, and COMMD8 with fold change [Fc] >2, FDR <0.1) between control and +Smad3CA iNs (Figure 7F; all reads counts are available in Table S2). There was also no difference in expression of glutamatergic- and GABAergic-neuron-associated genes, which are the main neural subtypes generated by ABMN (Figure S6C) (Pang et al., 2011, Vierbuchen et al., 2010). However, earlier phenotypes were evident in 294 and 309 genes up- and downregulated, respectively, on day 12 in iNs generated with Smad3CA (Figure 7G; Fc >2, FDR <0.1). The most enriched gene ontology (GO) terms in the upregulated genes were “nucleobase-containing compound transport,” “ectoderm development,” and “nervous system development” genes (Figure 7H). Intriguingly, many of those genes are further upregulated on day 23 in both control and +Smad3CA (Figure 7H, pale blue and pink). Thus, we hypothesized that SMAD3CA expression accelerated gene expression changes caused by ABMN in a global manner, similar to what was observed in iPSC generation, and resulted in faster neural maturation. To test this hypothesis, we generated a list of differentially expressed genes between day 12 and day 23 control samples and identified 634 up- and 347 downregulated genes at day 23 (>3-fold, FDR <0.1) (Figure 7I). The most enriched GO terms in these upregulated genes were “cell communication,” “biological adhesion,” “ectoderm development,” and “nervous system development” (Figure 7J). Strikingly, the majority of the 634 late iN upregulated genes were more highly expressed in +Smad3CA iNs on day 12 (Figure 7K). Global, faster downregulation of the late iN downregulated genes was also evident in day 12 +Smad3CA iNs, including multiple “metabolic process”-related genes (Figure 7L). These results revealed that when co-expressed with neural master TFs, SMAD3CA could remarkably accelerate global gene expression changes, resulting in faster iN maturation and formation of neural connectivity as revealed by patch-clamp recordings.
Figure 7.
Smad3CA Accelerates the Generation of Mature iNs
(A) Conversion of human fibroblasts to iNs by lentiviral transduction of Ascl1, Brn2a, Myt1L, and NeuroD1 (ABMN) with or without Smad2CA (Smad2CA) or Smad3CA (Sm3CA).
(B) Confocal imaging of the neural marker MAP2 7, 12, and 20 days after viral infection with ABMN (Cont) or ABMN+Sm2CA or Sm3CA. Scale bars, 50 μm.
(C) Quantification of MAP2+ cells (% of total cells) on day 12. Data represent averages of 2 independent experiments with 2 technical replicates.
(D) Co-immunoprecipitation of BRN2 and SMAD3 when overexpressed in HEK293 cells.
(E) Electrophysiological measurements using whole-cell patch clamp at day 23–25 post-conversion initiation using ABMN (Cont) or ABMN+Sm2CA or Sm3CA. Top panels represent traces of current-induced action potentials (APs) of different phenotypes.
(F and G) Gene expression comparison between flow-sorted NCAM1+ABMN (Cont) and ABMN+Sm3CA iNs on day 23 (F) and day 12 (G). The x and y axis show log2 read counts; green and red genes, fold change (Fc) >2, FDR <0.1.
(H) Expression of genes that have the top 3 most enriched gene ontology (GO) terms in the 294 upregulated genes in day 12 +Sm3CA iNs.
(I) Differentially expressed genes between day 12 and day 23 iNs with ABMN (Cont) (Fc >3, FDR < 0.1).
(J) Top 4 most enriched GO terms in the 634 upregulated genes on day 23, and number of genes with these GO terms.
(K and L) Expression comparison of up-regulated (K) and down-regulated (L) genes highlighted in (I) between ABMN (Cont) and ABMN+Sm3CA iNs on day 12.
In summary, in addition to iPSC generation with Yamanaka factors, SMAD2/3CA potentiated three other master-TF-mediated cell identity conversions, showing that SMAD2/3 are partners of multiple TFs and chromatin modifiers in various contexts.
Discussion
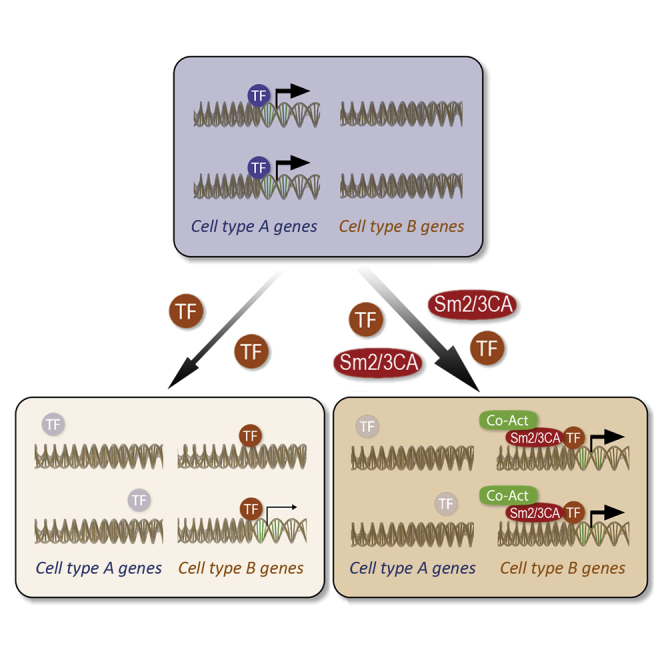
Cell identity conversions by the exogenous expression of master TFs offer powerful tools for tissue engineering, disease modeling, and regenerative medicine, yet a deeper understanding of the underlying molecular mechanisms is necessary to make such extraordinary processes more efficient and prompt. This study identified that exogenous, constitutive active SMAD2/3 can act as a part of the core cellular machinery that potentiates forced cell identity changes with master TFs. Mechanistically, during iPSC generation, SMAD3 co-occupied OCT4-binding sites across the genome and facilitated cellular conversion, likely providing a platform to recruit chromatin remodelers and transcriptional activators.
Our study commenced with the investigation of how TGF-βR inhibitors enhance reprogramming efficiency. One of the mechanisms involved suppression of p19ARF upregulation. While we observed that prolonged TGF-βR inhibitor treatment unexpectedly increased p-SMAD2/3 levels, double KO of endogenous Smad2/3 did not affect reprogramming efficiency in the presence or absence of A83, indicating that the positive effects of A83 are likely independent of SMAD2/3. However, overexpression of constitutively active SMAD2/3 boosted iPSC generation through recruitment to Oct4 target loci and markedly enhanced three other master-TF-mediated cell conversions.
In addition to widely expressed co-activators and co-repressors, a multitude of cell-type-specific TFs interact with SMAD2/3 (Massagué et al., 2005), partially explaining cell-type-dependent responses to TGF-β signaling (Mullen et al., 2011). During cell fate specification, it is thought that TFs might compete to recruit active SMAD2/3-co-activator complexes to target loci and activate their targets (Massagué, 2012, Mullen et al., 2011). Analogously, the amount of active SMAD2/3 could be a rate-limiting factor to fully realize the exogenous TF’s potential during forced cell identity changes, resulting in inefficient and incomplete cellular conversion, even when the best possible combinations of master TFs are used. In such cases, SMAD2/3CA overexpression might overcome these limitations and enhance cell conversions.
The rapid and efficient generation of functionally mature adult cell types represents a major challenge in the direct cellular conversion and ESC differentiation fields. For example, most of the protocols used to generate fully functional human neurons, whether derived from ESCs or by direct conversion of differentiated cells, require months (Brennand et al., 2011, Kriks et al., 2011, Pereira et al., 2014). The use of SMAD2/3CA in the generation of mature neurons and potentially other challenging cell types represents an exciting opportunity for disease modeling and regenerative medicine. Various cell types share multiple TFs, co-activators, and co-repressors, and they contribute to the establishment of unique cellular character during normal development. Our findings propose that other shared co-factors could also be powerful tools to achieve more efficient and precise cell conversions.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD19 (1D3) | BD Pharmigen | 550992, RRID: AB_398483 |
| MAC-1 (M1/70) | BD Pharmigen | 52850, RRID: AB_394491 |
| ICAM1-biotin conjugate | Thermo Fisher | 13-0541, RRID: AB_466480 |
| CD44-allophycocyanin (APC) conjugate | Thermo Fisher | 17-0441, RRID: AB_469390 |
| E-CADHERIN-eFluor 660, | Thermo Fisher | 50-3249-82, RRID: AB_11040003 |
| MEFSK4-biotin conjugate | Miltenyi | 130-101-875, RRID: AB_2660622 |
| CD73-Alexa Fluor 647 | BD Biosciences | 561543, RRID: AB_10896329 |
| CD104-APC | Miltenyi | 130-106-924, RRID: AB_2654418 |
| CD47-biotin conjugate | BioLegend | 127505, RRID: AB_1134125 |
| Strepdavidin PE-Cy7 | Thermo Fisher | 25-4317, RRID: AB_10116480 |
| p19ARF | Abcam | Ab80, RRID: AB_306197 |
| E-CADHERIN-eFluor 660 | Thermo Fisher | 50-3249-82, RRID: AB_11040003 |
| CD44-allophycocyanin (APC) conjugate | Thermo Fisher | 17-0441, RRID: AB_469391 |
| pSmad3 | Cell Signaling | C25A9, RRID: AB_2193207 |
| pSmad2/3 | Santa Cruz Biotechnology | 11769, RRID: AB_2193189 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| A83-01, Alk4/5/7 inhibitor | Tocris | #2939 |
| SB431542, Alk4/5/7 inhibitor | Tocris | #1614 |
| FGF2 | peprotech | 100-18B |
| heparin | Sigma | 1304016 |
| ATCC-formulated F-12K Medium | ATCC | 30-2004 |
| N2B27 | Stem Cell Sciences | SCS-SF- NB-02 |
| BDNF | R&D systems | P23560 |
| GDNF | R&D systems | P39905 |
| NT3 | R&D systems | 267-N3-005 |
| db-cAMP | Sigma | D0627 |
| CHIR99021 | Axon | 1386 |
| Noggin | R&D systems | 6057-NG-025 |
| LDN-193189 | Axon | 1509 |
| Doxycycline hyclate | Sigma | D9891 |
| Ascorbic acid (Vitamin C) | Sigma | PHR1008 |
| Human IL-7 | Peprotech | 200-07 |
| human colony-stimulating factor 1 (hCSF-1) | Peprotech | 300-25 |
| Flt3 ligand | Peprotech | 300-19 |
| Stem cell factor | Peprotech | 300-07 |
| Deposited Data | ||
| RNA-Seq, ChiP-Seq, and ATAC-Seq data | This study | GEO: GSE85178 |
| Experimental Models: Cell Lines | ||
| TNG MKOS mouse line | Keisuke Kaji lab | N/A |
| PreB cells from CebpαER mice | Thomas Graf lab | N/A |
| C2C12 | ATCC | CRL-1772 |
| Human fetal lung (hFL1) | ATCC | CCL-153 |
| Oligonucleotides | ||
| See Table S3 for guide RNA sequences | This study | N/A |
| See Table S3 for iPSC ChIP qPCR primers | This study | N/A |
| See Table S3 for B cell to macrophage transdifferentiation primers | This study | N/A |
| See Table S3 for ChIP-qPCR primers for C10 pre-B cells | This study | N/A |
| Recombinant DNA | ||
| pMX-Smad3CA | This study | N/A |
| pMX-Smad2CA | This study | N/A |
| pMXs-dsRed | (Hong et al., 2009) | Addgene #22724 |
| pMXs-BFP | This study | N/A |
| pCXLE-hSmad2CA | This study | N/A |
| pCXLE- hSmad3CA | This study | N/A |
| pCXLE-hOct3/4-shp53-F | (Okita et al., 2011) | Addgene #27077 |
| pCXLE-hSK(Sox2/Klf4) | (Okita et al., 2011) | Addgene #27078 |
| pCXLE-hUL(L-Myc/Lin28) | (Okita et al., 2011) | Addgene #27080 |
| pMIG-CEBPβ | (Di Stefano et al., 2014) | N/A |
| pMSCV-PRDM16 | (Kajimura et al., 2009) | N/A |
| TET-Ascl1 | (Vierbuchen et al., 2010) | Addgene #27150 |
| TET-Brn2 | (Vierbuchen et al., 2010) | Addgene #27151 |
| TET-Myt1L | (Vierbuchen et al., 2010) | Addgene #27152 |
| TET-NeuroD1 | (Vierbuchen et al., 2010) | Addgene #30129 |
| FUW-M2rtTA | (Hockemeyer et al., 2008) | Addgene #20342 |
| FUW-Smad3CA | This study | N/A |
| pKLV-U6gRNA(BbsI)-PGKpuro2ABFP | (Koike-Yusa et al., 2014) | N/A |
| Software and Algorithms | ||
| DESeq2 (Version 1.6.3) package of Bioconductor | Love et al., 2014 | N/A |
| SAMtools | (Li et al., 2009) | N/A |
| Bowtie 2 | (Langmead and Salzberg, 2012) | N/A |
| BEDTools | (Quinlan and Hall, 2010) | N/A |
| See also RNA-sequencing and data analysis section | This study | N/A |
| See also ChIP-seq and ATAC-seq data analysis sections | This study | N/A |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Keisuke Kaji, at keisuke.kaji@ed.ac.uk
Experimental Model and Subject Details
All animal experiments for the iPSC generation were approved by the University of Edinburgh Animal Welfare and Ethical Review Body, performed at the University of Edinburgh, and carried out according to regulations specified by the Home Office and Project License. All experiments for the B-to-M conversion were approved by the Ethics Committee of the Barcelona Biomedical Research Park (PRBB) and performed according to Spanish and European legislation. The use of human fetal fibroblasts is in compliance with Lund University ethical guidelines and practices.
Primary cell lines
The TNG MKOS reprogramming line harbors a targeted Nanog-GFP (TNG) reporter at the AUG start site of exon 1 of the Nanog locus, thereby creating a reporter of the endogenous promoter activity, while also producing a heterozygous KO of the targeted allele (Chambers et al., 2007). The TNG line was targeted with an MKOS-ires-mOrange dox inducible reprogramming cassette that also harbored a CAG promoter driven rtTA, targeted to the Sp3 locus (Chantzoura et al., 2015). The resulting TNG MKOS ESC line was used to generate TNG MKOS MEFs from E12.5 chimeric embryos (mixture of male and female embryos) via morula aggregation, and a reprogrammable TNG MKOS mouse line via blastocyst injection. Percentages of Tg MEFs from each chimeric embryo were assessed by mOrange expression after culturing a small portion of the MEFs in the presence of 1 μg/ml doxycycline (dox) for 48 hours before freezing down the rest of the MEFs. The TNG MKOS mouse line was backcrossed with CD1 mice ∼3-6 generations before used to generate TNG MKOS MEFs from mixture of male and female E12.5 embryos.
MEFs were cultured at 37°C, 5% CO2 in MEF medium (Glasgow’s Minimum Essential Medium (GMEM), 10% FCS, non-essential amino acids, 2 mM L-Glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin/streptomycin, all from Life technologies) supplemented with 5 ng/ml FGF2 (Peptrotech) and 1 ng/ml heparin (Sigma-Aldrich). The TNG MKOS ESC line was maintained on gelatin in ESC medium (MEF medium without FGF2 and heparin, supplemented with human LIF 100 U/ml).
Pre-B cells (a mixture of both male and female) were isolated from bone marrow of inducible CebpαER mice (Fukuchi et al., 2006) using monoclonal antibodies to CD19 (1D3, BD Pharmigen), using MACS (Miltenyi Biotech).
C2C12 cells (female) were obtained from ATCC and cultured in DMEM supplemented with 10% FBS, L-glutamine and penicillin/streptomycin (Life Technologies).
Human (female) fetal lung (hFL1, ATCC- CCL- 153) fibroblasts were cultured in ATCC-formulated F-12K Medium, supplemented with 10% fetal bovine serum at 37°C, 5% CO2.
Method Details
MEF Reprogramming
Reprogramming was performed on gelatin-coated plates with ESC medium containing doxycycline (dox) (300 ng/ml, Sigma-Aldrich), 10 μg/ml Vitamin C (Sigma-Aldrich), with or without 500 nM A83-01 (A83) (Tocris, #2939) or 10 μM SB431542 (SB43) (Tocris, #1614,). Reprogramming experiments, except immunoprecipitation and ChIP, utilized a mixture of Tg MEF harvested from E12.5 chimeric embryos generated by morula aggregation as described above (5%) and wild-type MEFs (95%) in order to avoid saturating the number of iPSC colonies in the assays. MEFs were defrosted at high confluence (> 70%) in MEF media 2-3 days prior to plating for reprogramming. One day prior to initiating reprogramming, the cells were seeded on gelatin pre-coated plates at 1x105 total cells for 1 well of a 6-well plate, or 7x105 total cells for a 10cm plate, which is 5x103 or 3.6x104 Tg cells, respectively. One day after plating, MEF media is replaced with reprogramming media containing 300 ng/ml dox to activate expression of MKOS reprogramming factors. Reprogramming media is replenished every 2 days until the end of the experiment.
For immunoprecipitation and ChIP, which required a large amount of cells undergoing reprogramming, we used Tg MEFs derived from a TNG MKOS mouse line (Nanog+/GFP and Sp3+/tetO-MKOS-ires-mOrange, CAG-rtTA) with 129 and CD1 mixed genetic background and perform the experiments with 100% Tg cells with 5x104/well (6 well plate) or 5x105 cells/10 cm dish.
B cell to macrophage transdifferentiation
Pre-B cells were isolated from bone marrow of inducible CebpαER mice (Fukuchi et al., 2006) using monoclonal antibodies to CD19 (1D3, BD Pharmigen), using MACS (Miltenyi Biotech). After infection with Smad3CA or control retroviral vectors, pre-B cells were plated on S17 feeder cells in RPMI medium supplemented with 10% FBS (Life technologies), 10 ng/mL IL-7 (Peprotech). Transdifferentiation was induced by treating the cells with 1 μM 4-OHT (Sigma), supplemented with 10 ng/mL of IL-7 (Peprotech), IL-3 (Peprotech), human colony-stimulating factor 1 (hCSF-1), FLT3 ligand (Peprotech) and stem cell factor (SCF). Cells stained with antibodies against MAC-1 (44, BD Pharmigen) and CD19 (1D3, BD Pharmigen) were analyzed with a FACS LSR fortessa flow cytometer (BD Biosciences) and FlowJo software (Tree Star, V10). S17 feeder cells were maintained in DMEM supplemented with 10% FBS, L-glutamine and penicillin/streptomycin (Life Technologies).
Myoblast to adipocyte transdifferentiation
C2C12 cells were obtained from ATCC and cultured in DMEM supplemented with 10% FBS, L- glutamine and penicillin/streptomycin (Life Technologies). C2C12 were infected with Cebpβ, Prdm16 and either Smad3CA or control retroviral vectors for 48 hours. After the infection, cells were cultured in adipogenic-induction medium (DMEM containing 10% FBS, 0.5 mM isobutylmethylxanthine, 125 nM indomethacin, 5M dexamethasone, 850 nM insulin, 1 nM T3 and 1 M rosiglitazone (Santa Cruz) for 2 days. Subsequently, medium was switched to DMEM containing 10% FBS, 850 nM insulin, 1 nM T3 and 1 μM rosiglitazone, and cells were maintained for 4 days in this culture condition before Red Oil O staining was performed. All the reagents were purchased from Sigma unless otherwise indicated. For Oil Red O staining, cultured cells were washed with PBS and fixed in formalin for 10 min at room temperature. Cells were incubated for 30 min at room temperature with 60% filtered Oil Red O stock solution (0.3 g/100 mL of isopropanol) (Sigma). Cells were washed with 60% isopropanol and then water before visualization. Lipid levels were quantified by extracting Oil Red O stained cells with isopropanol and measuring absorbance at 510 nm.
Generation of human iNs
Human fetal lung (hFL1, ATCC- CCL- 153) fibroblasts were purchased from the American Type Culture Collection (ATCC) and were expanded in MEF medium and grown at 37°C in 5% CO2. hFL1 were converted to hiNs by transduction of the cells with lentiviral vectors (LVs) encoding for the conversion factors Ascl1, Brn2a, Myt1L, NeuroD1 with or without Smad3CA (MOI = 5) and co- transduction of the transactivator (FUW-rtTA-SM2, Addgene) (MOI = 10). Transgene expression was initiated by doxycycline (2 μg/ mL, Saveen & Werner) administration on day 5 after infection. On day 3 of transgene expression, cells received neuronal induction medium (N2B27 + doxycycline 2 μg/ mL) supplemented with CHIR99201 (2 μM, Axon), noggin (100 ng/ mL, R&D systems), LDN (0.5 μM, Axon), LM4A22 (2 ng/ mL, R&D system), GDNF (2 ng/ mL, R&D system), NT3 (10 ng/ mL, R&D Systems) and db-cAMP (0.5 mM, Sigma). For immunocytochemistry with mouse anti-MAP2 antibodies (1:500, Sigma), hiNs were fixed in PFA (4%, 15 min at room temperature) 7, 12 and 23 days after transgene activation. Neuronal conversion efficiency was assessed by quantification of DAPI+/ MAP2+ hiNs using Cellomics Array Scan (Array Scan VTI, Thermo Fischer). Using the program “Target activation,” 20 fields (10x magnification) were automatically captured in a spiral fashion (from center to outside) and conversion efficiency was determined as the ratio of total number of hiNs present at time of analysis and the number of input fibroblasts.
For RNA analysis, hiNs were detached using Accutase at days 12 and 23 after transgene expression and after gentle trituration, filtered through cell strainer caps in order to obtain a single cell suspension. Cells were spun down and resuspended in the staining buffer (HBSS supplemented with 45% BSA) for blocking (15 min at room temperature) before staining with hNCAM-APC antibody (1:50) for 15 minutes at room temperature. A final centrifugation step and resuspension in HBSS 45% BSA, supplemented with the secondary antibody, Nuclear Orange (ATT Bioquest) and DNase, were perfomed and viable, NCAM+ cells were isolated by flow cytometry for RNA-seq library preparation.
For electrophysiological recordings, hFL1s were converted to iN on glass coverslips. Total 3 coverslips each on 3 different days, from 2 independent cell conversion experiments were used. Patch-clamp electrophysiology was performed on hiNs at day 23-25 post-conversion. Cultured hiNs were grown on coverslips and transferred to a recording chamber and submerged in a continuously flowing Krebs solution gassed with 95% O2 - 5% CO2 at 28°C. The composition of the standard solution was: 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 2.5 mM CaCl2, 25 mM Glucose and 26 mM NaHCO3. Recordings were made with a Multiclamp 700B amplifier (Molecular Devices), using borosilicate glass pipettes (3–7 MOhm) filled with the following intracellular solution: 122.5 mM potassium gluconate, 12.5 mM KCl, 0.2 mM EGTA, 10 mM HEPES, 2 mM MgATP, 0.3 mM Na3GTP and 8 mM NaCl adjusted to pH 7.3 with KOH. Data were acquired with pClamp 10.2 (Molecular Devices); current was filtered at 0.1 kHz and digitized at 2 kHz. Cells with neuronal morphology with round cell body were selected for whole-cell patch clamp. Resting membrane potentials were monitored immediately after breaking-in in current-clamp mode. Thereafter, cells were kept at a membrane potential of −60 mV to −80 mV, and 500 ms currents were injected from −20 pA to +90 pA with 10 pA increments to induce action potentials. For sodium and potassium current measurements cells were clamped at −70mV and voltage-depolarizing steps were delivered for 100 ms at 10 mV increments.
Western Blotting
For pSmad3 blots, to confirm TGF-β inhibitors performed as expected, cells were serum starved for 1 hour in GMEM then placed in fibroblast media containing 10 ng/ml TGF-β (R&D Systems) with or without TGF-β inhibitors, then western blotting was performed as for all experiments, as follows. Cells were harvested in Trypsin-EDTA, lysed with 1x Nupage LDS lysis buffer (Life Technologies), with or without phosphatase inhibitors (HALT, Life Technologies), heated to 95°C for 10 minutes followed by sonication of 3 cycles of 15 s on the Misonix XL2000 sonicator on setting 2. Protein concentration was measured by BCA assay (Life Technologies), and 1-100 μg lysate was mixed with DTT (final 100 μM), then run at 150 V and transferred at 50 V for 3-hours with BioRad Mini-PROTEAN and Mini Trans-Blot Cell tanks respectively. Antibodies used included pSMAD2/3 antibody (Santa Cruz sc-11769), and B-ACTIN-HRP antibody (Abcam, ab20272l).
Plasmids
For Smad2CA/3CA expression constructs, PCR primers were designed to amplify Smad2, Smad3 cDNA with base pair mismatches at the 3′ end, to exchange serine codons for glutamic acid (SSMS→SEME) in Smad2 or aspartic acid (SSVS→DDVD) in Smad3 in the C terminus of the proteins (Chipuk et al., 2002, Funaba and Mathews, 2000). The primers used for mouse Smad2CA were forward- CTAGGGTAGATTTACCGGGC, Reverse-CGAGTCTTTGATGGGTTTACTCCATCTCTGAGCATCGCACTGAA, and for Smad3CA forward- GCTGGCGCCGGAACCAATTCAGTCGACGTGACCCTTCGGTGCCAG, reverse-CTAATCCACATCGTCACAGCGGATGCTCGGGGAACCCATCTGGGT. The PCR products were then cloned into the TOPO Blunt vector and subsequently cloned into pMXs or FUW expression vectors. A control vector pMXs-BFP was generated by inserting EBFP2 cDNA in the pMXs-gw (Addgene #18656). pCXLE-hSmad2CA and pCXLE- hSmad3CA were generated by inserting human Smad2CA and Smad3CA coding sequence into pCXLE-gw (Addgene #37626). Other pCXLE vectors were obtained from Addgene (Okita et al., 2011). The Cebpβ expression vector has been described previously (Di Stefano et al., 2014). The PRDM16 expression vector was kindly provided by Dr Kajimura (Kajimura et al., 2009). The lentiviral vectors used for hiN generation were described before (Pang et al., 2011, Pfisterer et al., 2011, Vierbuchen et al., 2010). Plasmids used in this work are listed in the Key Resource Table.
Fluorescence activated cell sorting
Preparation of cells for sorting was performed in PBS with 2% FCS at 4°C. The following antibodies were used with indicated dilution: ICAM1-biotin conjugate (1:100, eBioscience, #13-0541), CD44-allophycocyanin (APC) conjugate (1:300, eBioscience, #17-0441), E-CADHERIN-eFluor 660 (1:300, eBioscience, #50-3249-82), MEFSK4-biotin conjugate (1:100, Miltenyi, 130-101-875), CD47-biotin conjugate (1:100, BioLegend, 127505), CD73-Alexa Fluor 647 (1:300, BD Biosciences, 561543) and CD104-APC (1:300, Miltenyi, 130-106-924) and Strepdavidin PE-Cy7 (1:1500, eBioscience, #25-4317).
Immunofluorescence microscopy
Cells were reprogrammed in 6-well plates on 18 mm circular coverslips (Fisher Scientific) for confocal imaging, or directly on the plastic for whole well imaging, both coated with gelatin. Cells were fixed with 4% Paraformaldehyde for 10 minutes, permeabilized in 0.1% Triton-X in PBS for 45 minutes, blocked in 5% normal goat serum in PBS for 1 hour at room temperature, and then stained in blocking solution with primary antibody overnight at 4°C. The next day, secondary antibody was applied in blocking solution for 1 hour at room temperature. Slides where then mounted with prolong gold with or without DAPI (Life technologies). The following antibodies were used with indicated dilution: p19ARF (1:300, Abcam, Ab80), E-CADHERIN-eFluor 660 (1:300, eBioscience, #50-3249-82), CD44-allophycocyanin (APC) conjugate (1:300, eBioscience, #17-0441), pSmad3 (1:100, Cell Signaling, C25A9). For confocal microscopy, all imaging was performed with a Leica TSC SP2 and processed using Adobe Photoshop. For whole well colony and cell counting, the sistched whole well images were taken and analyzed using the Celigo S Cell Cytometer (Nexcelom).
Smad2/3 double KO MEF reprogramming
An EF1α promoter-driven Cas9 expression cassette was targeted into the Rosa26 locus of TNG MKOS ES cell line, which has a doxycycline (dox)-inducible MKOS-ires-mOrange reprogramming and a CAG promoter-driven rtTA expression cassette in the Sp3 locus and a Nanog-GFP reporter (Chantzoura et al., 2015). The resulting Cas9 TNG MKOS ES cells were used to generate chimeric embryos via morula aggregation. Each E12.5 embryo was dissociated with trypsin/EDTA and 1/20 and 19/20 of the cells were seeded in one well of a 12-well plate in the presence of dox and a 10 cm dish in the absence of dox, respectively. Two days later, the cells in the 12-well plate were used to determine contribution of the Tg cell by measuring percentage of mOrange+ cells with flow cytometry. MEFs with high (> 90%) Tg cell contribution were harvested from the 10 cm dishes, pooled and cryopreserved at 5x106 cells/vial as passage 0. Lentiviral gRNA expression vectors were constructed by inserting double strand oligos encoding the following gRNA sequencing into pKLV-U6gRNA(BbsI)-PGKpuro2ABFP. Defrosted cells were seeded into a T25 flask/vial (passage 1) and became confluent within 2 days. The MEFs were harvested and seeded into a 6-well plate at 150,000 cells/well to make samples for western blotting. A portion of remaining MEFs were mixed with wild-type (WT) MEFs (129 strain) as the percentage of the Tg MEFs became 5%, and the same amount (150,000 cells/well) of the mixed MEFs were seeded in a 6 well plate for reprogramming experiments (passage 2). 24 hours later, cells for both experiments were infected with the same amount of gRNA viruses (MOI3) for 4 hours. For western blotting, cells were harvested 72 hours after infection, and 3 μg of cell lysate was used to detect SMAD2/3 (1:1000, Cell Signaling, #5678) and β-ACTIN (1:2500, Abcam, #ab20272). For reprogramming, dox was administrated 24 hours infection, and number of Nanog-GFP+ colonies was assessed on day 14 of reprogramming. gRNA sequences are in Table S3.
Retroviral mediated gene transduction for iPSC reprogramming enhancement
Ten micrograms of pMXs retroviral vector with BFP, dsRed (Addgene, 22724) (Hong et al., 2009), Smad2CA or Smad3CA, were transfected into 1.7x106 Plat-E packaging cells in a 10 cm dish (Morita et al., 2000), in 8 mL of ES media, using the CaCl2-HBS transfection method. The cells were cultured for 24 hours at 32°C, and the viral supernatant was collected. Filtrated supernatant with 0.45 μm Whatman acetate filters was then mixed with 8 μg/ml polybrene, of which 2 mL was added to each well containing 1x105 MEFs. After 6-hours of infection at 32°C, media was changed to initiate reprogramming and cells were placed back at 37°C.
Human fibroblast reprogramming to iPSCs
Commercially available neonatal human dermal fibroblasts (nHDFs, Life technologies) were cultured as per manufacturer’s instructions. Reprogramming was accomplished by transfection of episomal plasmids as previously published (Okita et al., 2011). Briefly 5x105 passage 4 nHDFs were nucleofected (Amaxa, Human Dermal Fibroblast Nucleofector Kit, program U023) with 5 μg total plasmids containing equal amounts pCXLE-hOct3/4-shp53 with pCXLE-hSK and pCXLE-hUL, +/− pCXLE-Smad3CA or pCXLE-Smad2CA. After 5 days of expansion in nHDF media, cells were lifted with EDTA and 1.9x105 were plated on matrigel coated plates, media was then changed 2 days later to E8 media (Life Technologies) supplemented with pen/strep (100 U/ml) and exchanged every 2 days until cells reached ∼70% confluence, at which point media was changed daily. Number of NANOG+ colonies was assessed on day 21 of reprogramming immunofluorescence with anti-human NANOG antibody (1:200, BD PharMingen, #560482).
RNA-sequencing and data analysis
RNA-seq was performed with the single-cell tagged reverse transcription (STRT) platform using 500 cells/sample, which had been snap frozen in PBS with RNase inhibitor, then processed as previously described (Islam et al., 2012, Islam et al., 2014). The RNA-seq reads were processed by an automated pipeline as outlined in a recent publication (Islam et al., 2014). Counts data, based on both read and molecule counts, were processed with the DESeq2 (Version 1.6.3) package of Bioconductor(Love et al., 2014). However we found that the processing steps used to produce molecule count data lead to the loss of read counts for low-abundance genes, so proceeded with analysis on read count data, analogous to standard RNA-seq. 2-3 technical replicates per sample tightly clustered and therefore summed across columns prior to further data processing. As per the standard DESeq2 protocol, normalized expression estimates were obtained by adjusting columns by a size factor corresponding to library size. For read counts, for example, this adjustment ranged from 0.57 to 0.71. Data were transformed to log2 scale by use of DESeq2′s rlog() command, which also minimizes differences at very low count levels. The clustering patterns of genes in the two series were assessed based on a matrix of the mean of biological replicate samples. Two matrices were constructed containing the Cont and +SmCA3 series. The mean of MEF and ES samples were included in both series. To reduce noise, genes with background level expression (no mean count > = 2) or low variation (coefficient of variation < 100) in both series were removed from this matrix. The matrix was clustered by use of the clara() function in R, which approximates the Partitioning Around Medoids algorithm. A K value of 8 clusters was chosen as 7 out of 8 clustered patterns were similar between the Cont and +SmCA3 series. Mean normalized counts of each cross-classified gene group (with > 100 genes) identified in the chord diagram were shown. Gene ontology statistical overrepresentation test was performed using PANTHER (http://pantherdb.org/) with GO-Slim Biological Process with (Figure S4J) and without the Bonferroni correction for multiple testing (Figures 7I and 7K) (Thomas et al., 2003).
Co-immunoprecipitation
Cells were suspended in hypotonic solution (10 mM HEPES pH7.9, 1.5 mM MgCl2, 10 mM KCl) containing proteinase inhibitors (cOmplete protease inhibitor cocktail, Roche) and phosphatase inhibitors (HALT, Life Technologies) with 0.5% NP40, briefly vortexed and left on ice for 5 minutes prior to centrifugation at 500 g to pellet nuclei. The pellet was suspended in high salt solution (HEPES pH 7.9, 1.5 mM MgCl2, 0.2 mM EDTA, 400 mM NaCl, 25% Glycerol) to extract nuclear protein. The corrected nuclear lysates were pre-cleared in IP-buffer (20 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 20% Glycerol, 100mM NaCl, protease and phosphates inhibitors) overnight at 4°C with 25 μL Dynabeads-Protein G (Life Technologies, #10003D) that were pre-blocked blocked in 2% milk with no-salt buffer (20 mM HEPES pH 7.9, 1.5 mM MgCl2, 0.2 mM EDTA, 20% Glycerol, phosphatase and protease inhibitors). Bead-antibody complexes were generated by incubating 2 μg of antibody or control IgG with 25 μL blocked beads in IP buffer rotating overnight at 4°C. Antibody bound beads were then magnetically isolated and added to 200 μg pre-cleared lysate for a 3-hour incubation, rotating at 4°C. Bead-protein complexes were then washed 3 times in IP buffer and processed for western in LDS buffer as describe above. Antibodies used for co-IP experiments included Rb-SMAD3 (Abcam, #ab28379), ms-SMAD3 (Abcam, #AF9F7), OCT4 (Santa Cruz Biotechnology, #sc8628), SOX2 (Santa Cruz Biotechnology, #sc17320), KLF4 (Santa Cruz Biotechnology, #sc20691), DPY30 (Sant Cruz Biotechnology, #sc167677), p300 (Thermo Scientific, #MS-586-PO), BRG1 (Santa Cruz Biotechnology, #sc17796), MED15 (Sigma Aldrich, SAB2500761), and IgG (Millipore).
Chromatin Immunoprecipitation followed by high-throughput DNA sequencing (ChIP-seq)
Fixation
Cells were grown to 80% confluency and fixed by addition of formaldehyde (final 1%) for 12 minutes with gentle shaking at room temperature. Formaldehyde was quenched with glycine (final 0.125 M) for 5 minutes shaking at room temp. Samples where then washed once with cold PBS containing protease inhibitors (cOmplete protease inhibitor cocktail, Roche) collected with silicon scrapers and centrifuged at 300 g for 5 minutes. Cells were re-suspended in PBS with protease inhibitor, and ∼2x106 cells (based on sacrificed plate cell counts) were aliquoted into 1.5 mL tubes, centrifuged at 500 g and pellets were snap frozen on dry ice.
Nuclear isolation and sonication
A pellet of ∼2x106 cells was thawed and nuclei were isolated by re-suspension in lysis buffer (5 mM Pipes pH 8.0, 85 mM KCl, 1% NP40) with fresh protease inhibitors (cOmplete protease inhibitor cocktail, Roche) for 20 minutes on ice followed by brief vortexing and centrifugation at 500 g for 10 minutes at 4°C. The nuclear pellet was re-suspended in 300 ul IP buffer (0.5% SDS, 1% Triton, 2 mM EDTA, 20 mM Tris-HCl pH 8.0, 150 mM NaCl, fresh protease inhibitors) and sonicated to produce ∼200-300 bp fragments in a BioRuptor Sonicator with a total of 5-8 cycles of: 10 pulses of sonication on the “high” setting, each followed by 30 s off. Thus, each sample received between 50-80 pulses of 30 s ‘high’ sonication. Samples were then diluted in IP buffer without SDS (to final 0.1% SDS), then 8 μg antibody was added for overnight incubation, rotating at 4°C.
Immunoprecipitation and DNA isolation
Dynabeads-ProteinG were blocked for 1-hour in 0.5% BSA in IP buffer with protease inhibitors, prior to incubation with the sonicated antibody bound chromatin suspensions for 4-hours rotating at 4°C. Bead-chromatin complexes were then serially washed for 5 minutes each with the following solutions: IP buffer (150 mM NaCl), followed by IP buffer with high salt concentration (500 mM NaCl), then 1 wash with washing-buffer (10 mM Tris-HCl pH 8.0, 0.25 M LiCl, 0.5% NP40, 0.5% Na-Deoxycholate, 1 mM EDTA), followed by 2 washes with TE (10 mM Tris, 1 mM EDTA). The DNA-protein complex was then eluted from beads by incubation with 100 μl of elution buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl pH 8.0) for 30 minutes at 65°C, vortexing every 10 minutes, followed by magnet extraction of beads. The beads were re-washed with 150 μl TE with 1% SDS, magnet extracted, and the TE with remaining DNA solution was added to the eluted samples, followed by 65°C overnight incubation to reverse crosslink the DNA-protein complexes. The dissociated DNA and protein solution was then treated with 4 units proteinase K (NEB) at 37°C for 2 hours. DNA was isolated with SeraMag beads, using 450 μl SeraMag bead solution and 225 μl of 30% PEG in 1.25 M NaCl (Sera-Mag DNA isolation methods below) (Rohland and Reich, 2012). After bead purification, DNA was re-suspended in 30 μl DNase and RNase free H2O and stored at −80°C prior to library preparation.
ChIP library preparation for sequencing
Blunt end repair
The ChIP’d DNA from ∼2x106 cells, ∼6 ng total on average, was prepared in the following reaction mix: 5 μl T4 DNA ligase buffer (NEB), 2 mL 10 mM dNTP’s, 0.5 μl end repair mix (0.72 U T4 DNA polymerase, 0.24 U Klenow Fragment, 2.4 U T4 DNA Polynucleotide Kinase), up to 50 μl with ddH2O (DNase/RNase free). Samples were incubated for 30 minutes at 20°C, and then purified with addition of 50ul SeraMag bead solution and 50 μl 30% PEG solution (in 1.25 M NaCl) as per bead protocol outlined below. The final bead:DNA complexes were re-suspended in 18 μl TE, and 16.5 μl were transferred to new tube.
Adding A bases to 3′ end of DNA fragment
The 16.5 μl blunt end repaired DNA fragments were mixed in the following reaction: 2 μl 10X NEB Buffer 2, 1 μl 4 mM dATP, 0.5 μl Klenow 3′ to 5′ exonuclease minus (NEB). Reaction was incubated at 37°C for 30 minutes.
Adaptor ligation
The 20 μl A-tailed DNA was added to the following mix: 25 μl Quick Ligase Buffer, 1 μl Annealed TruSeq Adaptors (note below), 1.5 μl Quick Ligase (2,000 U/μl NEB) 2.5 μl H2O. The reaction was incubated 20 minutes at room temperature and then 5 μl of 0.5 M EDTA, pH 8.0. The adaptor ligated DNA fragments were then purified with addition of 50 μl SeraMag bead mix and 50 μl 30% PEG (in 1.25 M NaCl) as per bead protocol below. The final bead:DNA mixture was re-suspended in 15.5 μl TE, and 14 μl was taken to a new tube. TruSeq adaptor annealing was performed by re-suspending adaptors at 100 μM (in 10 mM Tris-HCl pH7.8, 0.1 mM EDTA pH 8.0, 50 mM NaCl), mixing 1:1 with the universal adaptor (100 μM), and annealed using a program of: 2 min at 95°C, 70 cycles of 30 s (95°C decreasing by 1°C each cycle), hold at 4°C. Annealed adaptors were diluted 1:200 (0.25 μM final) and then used for ligation or stored at −20°C and used for up to 5 freeze/thaw cycles.
Library pre-amplification
The adaptor ligated DNA was amplified using the following protocol: 14 μl Adaptor-ligated DNA, 1 μl TruSeq primer cocktail (0.25 μM), 15 μl 2X Kapa HiFi HotStart ready mix. The Libraries were amplified with the following protocol: 45 s at 98°C, 5 cycles of (15 s at 98°C, 30 s at 63°C, 30 s at 72°C), 1 minute of 72°C, hold at 4°C.
Library size selection
After pre-amplification of the library, a size selection step was performed to eliminate free adaptors in solution, and ensure amplification of only the desired size ChIP DNA fragments (300-500bp). First, the volume of DNA solution from pre-amplification step was adjusted to have 50 μl total (added 20 ul ddH2O). Then, 0.9X (45 μl) beads were added. After 15 minute incubation and 10 minutes magnet extraction, the supernatant (92 μl) was transferred to new tube and beads discarded (containing fragments over ∼500 bp). Then 10μl beads (∼0.2X) were added to the solution, incubated 15 minutes followed by 10 minute magnet extraction of beads, 2 times 80% EtOH washes, and re-suspension in 11.5 μl TE. Then 10 μl pre-amplified and size selected DNA was transferred to a new tube. From the final library, 1 μl was used to identify the number of additional cycles required, and then the remaining 9 μl were amplified an additional ∼11-13 cycles as required. The final library was amplified as follows: 9 μl DNA, 1 μl TruSeq primer cocktail, 20 μl 2X Kapa HiFi HotStart ready mix, and 10 μl H2O. The library was amplified with a program of 45 s at 98°C, 11-13 cycles of (15 s 98°C, 30 s 63°C, 30 s 72°C), 1-minute 72°C, hold at 4°C. Therefore, each library was amplified a total of 16-18 PCR cycles. After amplification, the DNA was isolated by bead purification by adding 60 μl SeraMag beads following described protocol below. Libraries were mixed at equal concentration and sequenced with Edinburgh Genomics on the Illumina HiSeq 2500 platform.
Determining ChIP final library amplification cycle number required
We took 1 μl of pre-amplified, size-selected DNA, and performed a quantitative PCR reaction as follows: 1 μl library, 2 μl SybrGreen fluorophore (10X), 1 μl TruSeq PCR primer cocktail, 10 μl 2X Kapa HiFi HotStart ready mix, split into 2 wells for qPCR analysis on a lightcycler 480. Amplification conditions were: 3 minutes at 95°C, 20 cycles of (30 s at 95°C, 30 s at 63°C, 30 s at 72°C). Upon identifying the cycle number required to reach 50% amplification, we subtracted 2 from that number (based on optimization experience), and used that as the number of cycles required for the rest of the library.
SeraMag bead isolation of DNA from solution
We produce SeraMag beads solution for DNA extraction in house, based on (Rohland and Reich, 2012). The final bead solution is as follows: 0.1% SeraMag Magnetic Speed-beads (FisherSci, cat.#: 09-981-123), 18% PEG-800 (w/v) (Sigma Aldrich cat.#: 89510), 1M NaCl, 10 mM Tris-HCl pH 8.0, 1 mM EDTA, 0.05% Tween 20. The beads are stored at 4°C, and their efficiency was tested using the Fermentas ladder (Fisher #FERSM1211).
For DNA isolation, the concentration of PEG can be adjusted by adding different volumes of the above SeraMag bead solution to DNA, creating different stringency criteria for DNA-bead interaction, allowing for size selection of DNA fragments (Rohland and Reich, 2012). For example, adding the SeraMag bead solution to a DNA containing solution at 1.2X (ie. 60 μl SeraMag bead mix to 50 μl DNA), for a final concentration of 9.8% PEG, only DNA fragments greater than 100 bp in size bind to the beads. Increasing the ratio of beads to DNA solution (resulting in increased PEG concentration) results in smaller sized fragments binding the beads.
The general protocol for bead mediated DNA purification is to add the SeraMag bead solution directly to the DNA solution, mix well, and let stand for 15 minutes. Then the DNA bound beads are magnet extracted for 5-10 minutes (depending on PEG concentration), at which point the PEG solution is removed leaving ∼5 μl in bottom of tube so as not to lose any sample, prior to 2 washes with 80% EtOH with tubes on magnet, air-dried for 2 minutes, and then re-suspended in TE (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA) at desired final volume plus 1.5 μl. After 2-minute incubation, the DNA solution is transferred to a new tube, leaving 1.5 μl remaining so as not to take any beads.
ChIP-seq data analyses
Reads were quality-checked using FastQC and trimmed using Trimmomatic to remove adapters and low quality bases (Bolger et al., 2014). Reads were aligned to the mm10 assembly of the mouse genome using Bowtie 2 with the very-sensitive option (Langmead and Salzberg, 2012). Duplicate reads were removed using Picard Tool’s MarkDuplicates command and were filtered for MAPQ > = 40 using SAMtools (Li et al., 2009). Reads aligned to ENCODE blacklist regions were removed using BEDTools (Quinlan and Hall, 2010). Peaks were called using MACS 2 on both individual and merged sample replicates against merged control replicates (Zhang et al., 2008). For narrow peak factors, such as OCT4, peaks were called with the -q 0.01 and —call-summits specified. For broad peak factors, such as SMAD3, peaks were called with the–broad and–broad-cutoff 0.1 options specified. Differentially-bound sites were identified using the DiffBind software package from the Bioconductor project (Ross-Innes et al., 2012). A FDR ≤ 0.1 and absolute FC > = 1 were used as the significance threshold. Merged control replicates were used for contrast against each individual sample replicate. The deepTools suite was used to generate normalized input-subtracted read coverage and heatmap scores using the bamCompare and plotHeatmap commands, respectively. Normalized input-subtracted read coverage was generated from merged sample and control replicates with the–ratio subtract and–normalizeTo1x 2150570000 option specified. Heatmaps were generated using the normalized input-subtracted read coverage and peaks called from the merged sample replicates.
ATAC-seq
ATAC-seq was performed according to the published protocol (Buenrostro et al., 2013). For library preparation, 50,000 cells were flow-sorted into PBS, and then nuclei were isolated by re-suspending the cells in lysis buffer (10 mM Tris-HCl pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% NP-40) for 15 minutes on ice, followed by centrifugation at 500 g for 10 minutes at 4°C. Supernatant was removed and nuclei were tagmented by re-suspension in the Nextera transposase mix (22.5 μL ddH2O, 25 μL 2x Tagment DNA buffer, 2.5 μl Tagment DNA enyme) (Nextera DNA sample prep kit, FC-121-1030 and FC-121-1011) and incubated at 37°C for 30 minutes. The tagmentation reactions were terminated and cleaned-up using QIAGEN Minelute enzyme cleanup kit, eluting in a final volume of 10 μL ddH2O. After tagmentation and cleanup, the DNA was then PCR amplified using Nextera primers, indexes and polymerase mix according to the Nextera DNA sample prep kit protocol. The number of PCR cycles required was determined as follows: the full library was amplified for five cycles, after which a 3 μl aliquot of the 30ul PCR reaction was removed and added to 6 μl of the complete PCR cocktail with addition of 1 μL SybrGreen fluorophore (10,000x). The PCR optimization reaction was run for 20 cycles on the LightCycler480 instrument (same program as master library) to determine the additional number of cycles needed for the remaining 27 μL reactions. The final cycle number was chosen to ensure the PCR was in linear phase growth and had not hit the plateau phase, thus minimizing PCR biases. On average, libraries were produced with ∼16 PCR cycles total. To the final 30 μl amplified library, 20 μl ddH2O was added prior to SeraMag bead cleanup. A ratio of 1.2X Bead:PCR reaction was used to isolate library fragments larger than 100 bp, thus leaving the PCR primers/adaptors out of the final solution sent for sequencing. ATAC libraries were sequenced with Edinburgh Genomics on the Illumina HiSeq 2500 platform.
ATAC-seq data analysis
Reads were quality-checked using FastQC and trimmed using Trimmomatic to remove adapters and low quality bases (Bolger et al., 2014). Reads were aligned to the mm10 assembly of the mouse genome using Bowtie 2 (Langmead and Salzberg, 2012) with the–very-sensitive and -X 2000 options specified. Duplicate reads were removed using Picard Tool’s MarkDuplicates command and were filtered using SAMtools for MAPQ > = 30 and the properly-paired (0x2) flag (Li et al., 2009). Reads aligned to the ENCODE blacklist and mitochondrial blacklist regions were also removed using BEDTools (Quinlan and Hall, 2010). The deepTools suite was used to generate normalized read coverage and heatmap scores using the bamCoverage and plotHeatmap commands, respectively (Ramírez et al., 2014). Normalized read coverage was generated from merged sample replicates with the–normalizeTo1x 2150570000 option specified. Heatmaps were generated using the normalized read coverage and peaks called from the merged sample replicates. The data of one of the ATAC-seq replicates had low read counts and demonstrated abnormal features as assessed by PCA and hierarchical clustering, clustering independent of all other samples including the starting MEF population and resulting iPSC cells, and was therefore removed from the analysis.
ChIP-qPCR for reprogramming to iPSCs
The ChIPed DNA was treated with 2 μL RNase (Life Technologies) at 37°C for 20 minutes, and then stored at −20°C. Each ChIP-qPCR reaction used 0.25-0.5 μL of DNA suspension per reaction. The qPCR was normalized to regions upstream of Oct4 and Nanog, which are devoid of SMAD3 or OCT4 binding based on previously published works (Chen et al., 2008, Mullen et al., 2011). Primers are listed in Table S3.
qPCR in B cell to macrophage transdifferentiation
Total RNA was isolated using RNeasy Mini Kit (QIAGEN) according to manufacture’s instruction. Double strand complementary DNA (cDNA) was synthesized using the High Capacity RNA-to-cDNA kit (Applied Biosystems). Real-time Polymerase Chain Reaction (PCR) was performed on ViiATM 7 system using Power SYBR® Green PCR Master Mix (Applied Biosystems). The relative gene-expression levels were calculated by 2ΔΔCt method. Primers are listed in Table S3.
Bioinformatics analysis of B cell to macrophage transdifferentiation ChIP-seq data
All sequencing data were mapped onto the mouse genome assembly mm10 (Ensembl GRCm38.78) using STAR, then analyzed with R (3.1.0) using packages from the bioconductor suite (v3.0). Cebpα ChIP-seq data were from GSE71218 (Di Stefano et al., 2016) and GSE53362 (van Oevelen et al., 2015). Peak calling was performed using macs2 (2.1.0.20140616) with default parameters and a threshold of qValue < 1e-2. Motif analyses were performed using sequences 500bp around Cebpα peak summits with RSAT peaks-motif (defaut parameters). Smad-binding motifs enrichment in Cebpα ChIP-seq was analyzed with matrixScan using a threshold of pValue < 1e-3 and the best hits for each peak were reported. We used as background a Marlov model of order1. Gene ontology enrichments were analyzed using GREAT (McLean et al., 2010).
ChIP-qPCR with C10 pre-B cell line
C10 cells were cultured in PRMI medium (Life Technologies) complemented with 10% FBS (Life Technologies) and were induced to transdifferentiate into macrophage (treatment with 100 nM β-estradiol and grown in medium in presence with 10 ng/mL of Il-3 and CSF-1) as described (Bussmann et al., 2009). 18 hours after Cebpα expression vector infection, cells were cross-linked with 1% formaldehyde for 10 minutes, and then lysed in ChIP buffer composed by mixing SDS buffer (NaCl 100 mM, Tris-Cl pH 8.1 50 mM, EDTA pH 8.0 5 mM, NaN3 0.2%, SDS 0.5%) and Triton Dilution Buffer (Tris-Cl pH 8.6 100 mM, NaCl 100 mM, EDTA 5 mM, NaN3 0.2% and Triton X-100 5%) at the ratio 2:1. Sonication was performed in a Bioruptor (Diagenode) and soluble material containing fragmented chromatin was then incubated overnight with anti-Smad3 (Abcam, #ab28379) or IgG control antibodies respectively. Immunoprecipitates were recovered with 60 μL of Dynabeads® A or G (Life Technology) and washed 3 times with low salt buffer (HEPES pH 7.5, 50 mM, NaCl 140 mM, Triton X-100 1%), once with high salt buffer (HEPES pH 7.5, 50 mM, NaCl 500 mM, Triton X-100 1%) and decross-linked in freshly prepared elution buffer (1% SDS, NaHCO3 100 mM and NaCl 500 mM) overnight at 65°C on a shaker. Genomic DNA was eluted using PCR purification kit (QIAGEN). Primers are listed in Table S3.
Quantification and Statistical Analyses
A one or two-sided t test was performed using the GraphPad Prism software for Figures 1, 2, 4, and 5. One-sided tests were chosen for experiments where we expect unidirectional changes, such is for a ChIP-qPCR experiment, where enrichment is assessed for binding a given chromatin region over unbound regions. P values represent ∗ < 0.05; ∗∗ < 0.01; ∗∗∗ < 0.001. The number of experiments and biological samples used is specified in figure legends. Error bars represent standard deviation.
Data and Software Availability
The accession number for the RNA-seq, ChIP-seq, and ATAC-seq data reported in this paper is GEO: GSE85178.
Author Contributions
T.R. performed all iPSC reprogramming experiments. B.D.S., T.V.T., and S.C. contributed to the macrophage and adipocyte conversion experiments. U.P. and D.R.O. conducted iN experiments. J.A. and J.R.M. analyzed ChIP-seq, ATAC-seq, and RNA-seq data. E. Chantzoura generated the TNG MKOS ESC line. E. Cohen performed cloning for human cell reprogramming and optimized co-immunoprecipitation methods. M.B., D.F.K., L.T., W.T., and K.Y. contributed to the Cas9-mediated KO experiments. A.J. and S.L. performed multiplexed RNA-seq. T.R., T.G., M.P., and K.K. conceived the study and wrote the manuscript.
Acknowledgments
We thank F. Rossi and C. Cryer for assistance with flow cytometry, M. Foronda for comments on the manuscript, and B. Mattsson for help with illustrations. We also thank L. Robertson and J. Rennie for technical assistance and the Biomed unit staff for mouse husbandry. We thank Dr. Hideaki Nakajima for the C/EBPaER mice. This work was supported by the ERC (grants ROADTOIPS 261075 to K.K. and BRAINCELL 261063 to S.L. and iN-Brain 309712 to M.P.), the BBSRC (project grant BB/L023474/1 to K.K.), the Anne Rowling Regenerative Neurology Clinic (K.K.), the Swedish Research Council (grant STARGET to S.L. and grants 521-2012-5624 and 521-2013-3347 to M.P.), and the Wellcome Trust (grant WT098051 to K.Y.). T.R., L.T., J.A., and D.F.K. are funded by a Darwin Trust scholarship, a CMVM scholarship, and Principal’s Career Development scholarship from the University of Edinburgh, respectively. D.F.K. is supported by the BBSRC (EASTBIO doctoral training partnership). T.V.T. is supported by a Juan de la Cierva postdoctoral fellowship (MINECO, FJCI-2014-22946). B.D.S. was supported by an EMBO long-term fellowship (ALTF 1143-2015). K.K. is an MRC senior non-clinical fellow (MR/N008715/1). M.P. is a New York Stem Cell Foundation Robertson Investigator. We acknowledge support from the Spanish Ministry of Economy and Competitiveness, Centro de Excelencia Severo Ochoa 2013-2017, and the CERCA Program Generalitat de Catalunya.
Published: November 22, 2017
Footnotes
Supplemental Information includes six figures and three tables and can be found with this article online at https://doi.org/10.1016/j.stem.2017.10.013.
Supplemental Information
References
- Bertero A., Madrigal P., Galli A., Hubner N.C., Moreno I., Burks D., Brown S., Pedersen R.A., Gaffney D., Mendjan S. Activin/nodal signaling and NANOG orchestrate human embryonic stem cell fate decisions by controlling the H3K4me3 chromatin mark. Genes Dev. 2015;29:702–717. doi: 10.1101/gad.255984.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K.J., Simone A., Jou J., Gelboin-Burkhart C., Tran N., Sangar S., Li Y., Mu Y., Chen G., Yu D. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro J.D., Giresi P.G., Zaba L.C., Chang H.Y., Greenleaf W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann L.H., Schubert A., Vu Manh T.P., De Andres L., Desbordes S.C., Parra M., Zimmermann T., Rapino F., Rodriguez-Ubreva J., Ballestar E., Graf T. A robust and highly efficient immune cell reprogramming system. Cell Stem Cell. 2009;5:554–566. doi: 10.1016/j.stem.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- Chantzoura E., Skylaki S., Menendez S., Kim S.I., Johnsson A., Linnarsson S., Woltjen K., Chambers I., Kaji K. Reprogramming roadblocks are system dependent. Stem Cell Reports. 2015;5:350–364. doi: 10.1016/j.stemcr.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloufi S., Elling U., Hopfgartner B., Jung Y.L., Murn J., Ninova M., Hubmann M., Badeaux A.I., Euong Ang C., Tenen D. The histone chaperone CAF-1 safeguards somatic cell identity. Nature. 2015;528:218–224. doi: 10.1038/nature15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V.B., Wong E., Orlov Y.L., Zhang W., Jiang J. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Chipuk J.E., Cornelius S.C., Pultz N.J., Jorgensen J.S., Bonham M.J., Kim S.J., Danielpour D. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J. Biol. Chem. 2002;277:1240–1248. doi: 10.1074/jbc.M108855200. [DOI] [PubMed] [Google Scholar]
- Chronis C., Fiziev P., Papp B., Butz S., Bonora G., Sabri S., Ernst J., Plath K. Cooperative binding of transcription factors orchestrates reprogramming. Cell. 2017;168:442–459. doi: 10.1016/j.cell.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.L., Weintraub H., Lassar A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Di Stefano B., Sardina J.L., van Oevelen C., Collombet S., Kallin E.M., Vicent G.P., Lu J., Thieffry D., Beato M., Graf T. C/EBPα poises B cells for rapid reprogramming into induced pluripotent stem cells. Nature. 2014;506:235–239. doi: 10.1038/nature12885. [DOI] [PubMed] [Google Scholar]
- Di Stefano B., Collombet S., Jakobsen J.S., Wierer M., Sardina J.L., Lackner A., Stadhouders R., Segura-Morales C., Francesconi M., Limone F. C/EBPα creates elite cells for iPSC reprogramming by upregulating Klf4 and increasing the levels of Lsd1 and Brd4. Nat. Cell Biol. 2016;18:371–381. doi: 10.1038/ncb3326. [DOI] [PubMed] [Google Scholar]
- Feng X.H., Zhang Y., Wu R.Y., Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation. Genes Dev. 1998;12:2153–2163. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R., Desbordes S.C., Xie H., Tillo E.S., Pixley F., Stanley E.R., Graf T. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc. Natl. Acad. Sci. USA. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi Y., Shibata F., Ito M., Goto-Koshino Y., Sotomaru Y., Ito M., Kitamura T., Nakajima H. Comprehensive analysis of myeloid lineage conversion using mice expressing an inducible form of C/EBP alpha. EMBO J. 2006;25:3398–3410. doi: 10.1038/sj.emboj.7601199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funaba M., Mathews L.S. Identification and characterization of constitutively active Smad2 mutants: evaluation of formation of Smad complex and subcellular distribution. Mol. Endocrinol. 2000;14:1583–1591. doi: 10.1210/mend.14.10.0537. [DOI] [PubMed] [Google Scholar]
- Gaarenstroom T., Hill C.S. TGF-β signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin. Cell Dev. Biol. 2014;32:107–118. doi: 10.1016/j.semcdb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D., Soldner F., Cook E.G., Gao Q., Mitalipova M., Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3:346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H., Takahashi K., Ichisaka T., Aoi T., Kanagawa O., Nakagawa M., Okita K., Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida J.K., Blanchard J., Lam K., Son E.Y., Chung J.E., Egli D., Loh K.M., Carter A.C., Di Giorgio F.P., Koszka K. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima H., Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat. Rev. Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- Islam S., Kjällquist U., Moliner A., Zajac P., Fan J.B., Lönnerberg P., Linnarsson S. Highly multiplexed and strand-specific single-cell RNA 5′ end sequencing. Nat. Protoc. 2012;7:813–828. doi: 10.1038/nprot.2012.022. [DOI] [PubMed] [Google Scholar]
- Islam S., Zeisel A., Joost S., La Manno G., Zajac P., Kasper M., Lönnerberg P., Linnarsson S. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat. Methods. 2014;11:163–166. doi: 10.1038/nmeth.2772. [DOI] [PubMed] [Google Scholar]
- Kajimura S., Seale P., Kubota K., Lunsford E., Frangioni J.V., Gygi S.P., Spiegelman B.M. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Habas R., Katsuyama Y., Näär A.M., He X. A component of the ARC/Mediator complex required for TGF beta/Nodal signalling. Nature. 2002;418:641–646. doi: 10.1038/nature00969. [DOI] [PubMed] [Google Scholar]
- Kim S.I., Oceguera-Yanez F., Hirohata R., Linker S., Okita K., Yamada Y., Yamamoto T., Yamanaka S., Woltjen K. KLF4 N-terminal variance modulates induced reprogramming to pluripotency. Stem Cell Reports. 2015;4:727–743. doi: 10.1016/j.stemcr.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Yusa H., Li Y., Tan E.P., Velasco-Herrera Mdel.C., Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat. Biotechnol. 2014;32:267–273. doi: 10.1038/nbt.2800. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulessa H., Frampton J., Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9:1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Liang J., Ni S., Zhou T., Qing X., Li H., He W., Chen J., Li F., Zhuang Q. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E., Zunder E.R., Ng Y.H., Goronzy I.N., Nolan G.P., Wernig M. Early reprogramming regulators identified by prospective isolation and mass cytometry. Nature. 2015;521:352–356. doi: 10.1038/nature14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N., Hochedlinger K. Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr. Biol. 2009;19:1718–1723. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- McLean C.Y., Bristor D., Hiller M., Clarke S.L., Schaar B.T., Lowe C.B., Wenger A.M., Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S., Kojima T., Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Mullen A.C., Orlando D.A., Newman J.J., Lovén J., Kumar R.M., Bilodeau S., Reddy J., Guenther M.G., DeKoter R.P., Young R.A. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147:565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley J., Skylaki S., Iwabuchi K.A., Chantzoura E., Ruetz T., Johnsson A., Tomlinson S.R., Linnarsson S., Kaji K. High-resolution analysis with novel cell-surface markers identifies routes to iPS cells. Nature. 2013;499:88–91. doi: 10.1038/nature12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Pang Z.P., Yang N., Vierbuchen T., Ostermeier A., Fuentes D.R., Yang T.Q., Citri A., Sebastiano V., Marro S., Südhof T.C., Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M., Pfisterer U., Rylander D., Torper O., Lau S., Lundblad M., Grealish S., Parmar M. Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain. Sci. Rep. 2014;4:6330. doi: 10.1038/srep06330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U., Wood J., Nihlberg K., Hallgren O., Bjermer L., Westergren-Thorsson G., Lindvall O., Parmar M. Efficient induction of functional neurons from adult human fibroblasts. Cell Cycle. 2011;10:3311–3316. doi: 10.4161/cc.10.19.17584. [DOI] [PubMed] [Google Scholar]
- Quinlan A.R., Hall I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F., Dündar F., Diehl S., Grüning B.A., Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014;42:W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmer T., Diecke S., Grigoryan T., Quiroga-Negreira A., Birchmeier W., Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12:720–726. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohland N., Reich D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2012;22:939–946. doi: 10.1101/gr.128124.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes C.S., Stark R., Teschendorff A.E., Holmes K.A., Ali H.R., Dunning M.J., Brown G.D., Gojis O., Ellis I.O., Green A.R. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481:389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P., Golipour A., David L., Sung H.K., Beyer T.A., Datti A., Woltjen K., Nagy A., Wrana J.L. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Shi Y., Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shi Y., Wang Y.F., Jayaraman L., Yang H., Massagué J., Pavletich N.P. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- Soufi A., Donahue G., Zaret K.S. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thomas P.D., Campbell M.J., Kejariwal A., Mi H., Karlak B., Daverman R., Diemer K., Muruganujan A., Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Hamashima Y., Hanyu A., Kajimoto T., Saitoh M., Miyazono K., Node M., Imamura T. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005;96:791–800. doi: 10.1111/j.1349-7006.2005.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzelepis K., Koike-Yusa H., De Braekeleer E., Li Y., Metzakopian E., Dovey O.M., Mupo A., Grinkevich V., Li M., Mazan M. A CRISPR Dropout Screen Identifies Genetic Vulnerabilities and Therapeutic Targets in Acute Myeloid Leukemia. Cell Rep. 2016;17:1193–1205. doi: 10.1016/j.celrep.2016.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oevelen C., Collombet S., Vicent G., Hoogenkamp M., Lepoivre C., Badeaux A., Bussmann L., Sardina J.L., Thieffry D., Beato M. C/EBPα activates pre-existing and de novo macrophage enhancers during induced pre-B cell transdifferentiation and myelopoiesis. Stem Cell Reports. 2015;5:232–247. doi: 10.1016/j.stemcr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q., He W., Zhang X.H., Le H.V., Massagué J. Genome-wide impact of the BRG1 SWI/SNF chromatin remodeler on the transforming growth factor beta transcriptional program. J. Biol. Chem. 2008;283:1146–1155. doi: 10.1074/jbc.M707479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Ye M., Feng R., Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Xu J., Liu X., Jiang Y., Chu L., Hao H., Liua Z., Verfaillie C., Zweier J., Gupta K., Liu Z. MAPK/ERK signalling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J. Cell. Mol. Med. 2008;12(6A):2395–2406. doi: 10.1111/j.1582-4934.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa T., Sato Y., Matsumura Y., Kobayashi Y., Kawamura Y., Goshima N., Yamanaka S., Okita K. Screening of human cDNA library reveals two differentiation-related genes, HHEX and HLX, as promoters of early phase reprogramming toward pluripotency. Stem Cells. 2016;34:2661–2669. doi: 10.1002/stem.2436. [DOI] [PubMed] [Google Scholar]
- Zhang Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.