Abstract
We first introduced the concept of the mTOR pathway’s involvement in congenital hyperinsulinism of infancy (CHI), based largely on morphoproteomic observations and clinical outcomes using sirolimus (rapamycin) as a therapeutic agent in infants refractory to octreotide and diazoxide treatment. Subsequent publications have verified the efficacy of such treatment in some cases but limited and variable in others. We present further evidence of a constant but variable role for the mTOR pathway in the biology of CHI and provide a strategy that allows for the short-term testing of sirolimus in individual CHI patients.
Keywords: Morphoproteomics, Biomedical analytics, Congenital hyperinsulinism of infancy, Sirolimus
The severe form of diffuse hyperinsulinemic hypoglycemia is mainly associated with mutations in ABCC8 and KCNJ11 that are unresponsive to diazoxide and/or octreotide therapy [1, 2]. This poses a threat to the infants with CHI not only by causing potential neurological damage leading to epilepsy, cerebral palsy and adverse neurological development in up to 40% of cases [3, 4] but also necessitating in some, near total pancreatectomy. Furthermore, 59% of such surgically treated patients can show persistent hyperinsulinemic hypoglycemia for up to 5 years post-surgery, and eventually diabetes mellitus will be manifested in all by the time they reach early adolescence [5].
The microanatomical characteristics of the diffuse form of CHI include non-proliferative, islet cell nucleomegaly [6]. In this context we have shown that the mammalian target of rapamycin (mTOR) protein, phosphorylated (p) on serine 2448, p-mTOR (Ser 2448) is overexpressed but variably on the plasmalemmal aspect of the acinar cells in CHI [7] and variably in the cytoplasm and nuclei of the insulin-producing islet cells, including those with karyomegaly [8] (Fig.1).
Fig. 1.

Pancreas of infant with CHI and paternal ABCC8 showing nucleomegaly (arrows) in islet cells, with insulin production (Frames a, H&E and b, beta cells with insulin), p-mTOR (Ser 2448) on the plasmalemmal aspect of the acinar cells and positivity in the islet cells with nucleomegaly (Frames c and d), p-Akt (Ser 473), expression in the islet cells with nucleomegaly (Frame e) and contrastively, the negative control (Frame f) (Original magnifications frames a-d and F ×400 and ×600 for frame e)
Biomedical analytics using Ingenuity Pathway Analysis and data mining of the National Library of Medicine’s Medline Data Base confirms the role of the mTOR pathway [8] in the biology of CHI with actionable therapeutic targets. A CHI network was constructed using the known CHI-associated genes described by Dunne and Banerjee’s associates [9]: GLUD1, SLC16A1, HADH, UCP2, KCNJ11, ABCC8, HNF1A, GCK, HNF4A.
The network showed that the MTOR-related molecules: MTOR, Raptor, Rictor, MTORC1 (but not MTORC2) interacted with all 9 CHI genes (Fig. 2). More than 300 interactions were identified (not shown due to complexity of image). Sirolimus modulated the MTOR group and thus, indirectly, the 9 CHI gene group. Sirolimus also increased expression of human UCP2 mRNA in the 9 CHI group [10].
Fig. 2.
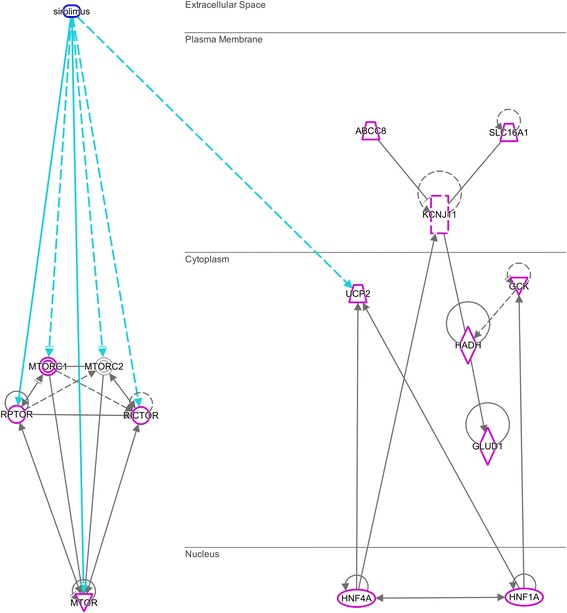
MTOR-related molecules (left) have more than 300 interactions (magenta highlights) with all 9 CHI genes (right) identified by Dunne and Banerjee [9] (interactions not shown due to density.) Direct interactions: solid lines; indirect interactions: dashed lines. Activation/expression: (−--➔), inhibition: (−--|), inhibits and acts upon: (−--|>)
Parenthetically, the contention by Banerjee and colleagues [4] that mTOR mRNA equates to mTOR gene in pancreases from normal, focal CHI, and diffuse CHIexpression could be accurate. Alternatively in the context of the morphoproteomic evidence of variable but constant activation and overexpression of the mTOR pathway in pancreases from diffuse CHI, it could represent the resultant of a steady state of transcription, translation and utilization of mTOR mRNA [11] following the integration of genomic, proteomic and pathway biology. Notably pathway ontology associated with the CHI disease network includes mTOR signaling [9]. Moreover, the clinical outcomes coincide with the findings of a variable therapeutic success in using sirolimus in the treatment of CHI [2, 12–18]. In the context of risk [3–5] versus benefit, we hold that severe diffuse CHI infants deserve a trial with an appropriate dose of sirolimus to see whether it is effective keeping in mind and monitoring for the potential immunosuppressive and adverse consequences of sirolimus [2]. We agree that sirolimus with or without other combinatorial therapies (octreotide, nifedipine, exendin-(9-39) and metformin) to counter the variable but constant activation of the mTOR biology in CHI should be explored in larger clinical trials.
Acknowledgements
The authors thank Pamela K Johnston, HT, ASCP for her technical expertise and Ms. Bheravi Patel for help with the graphics.
Funding
The authors have no financial relationships relevant to this article to disclose. Funding has been provided through the morphoproteomic initiative as part of the Harvey S. Rosenberg, M.D. Endowed Chair in Pathology and Laboratory Medicine.
Availability of data and materials
Please contact author for data requests.
Authors’ contributions
REB and MFM drafted the initial manuscript and co-wrote the final manuscript. SS and KH critically reviewed the manuscript and co-wrote the final manuscript. All authors approved the final manuscript as submitted.
Ethics approval and consent to participate
Not appropriate.
Consent for publication
All authors approved the final manuscript as submitted.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Senniappan S, Shanti B, James C, Hussain K. Hyperinsulinaemic hypoglycaemia: genetic mechanisms, diagnosis and management. J Inherit Metab Dis. 2012;35:589–601. doi: 10.1007/s10545-011-9441-2. [DOI] [PubMed] [Google Scholar]
- 2.Senniappan S, Alexandrescu S, Tatevian N, Shah P, Arya V, Flanagan S, Ellard S, Rampling D, Ashworth M, Brown RE, Hussain K. Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N Engl J Med. 2014;370(12):1131–1137. doi: 10.1056/NEJMoa1310967. [DOI] [PubMed] [Google Scholar]
- 3.Shah P, Demirbilek H, Hussain K. Persistant hyperinsulinaemic hypoglycemia in infancy. Semin Pediatr Surg. 2014;23(2):76–82. doi: 10.1053/j.sempedsurg.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee I, De Leon D, Dunne MJ. Exreme caution on the use of sirolimus for the congenital hyperinsulinism in infancy patient. Orphanet J Rare Dis. 2017;12(1):70. doi: 10.1186/s13023-017-0621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrand J, Caquard M, Arnoux JB, Laborde K, Velho G, Verkarre V, Rahier J, Brunelle F, Nihoul-Fekete C, Saudubray JM, Robert JJ, de Lonlay P. Glucose metabolism in 105 children and adolescents after pancreatectomy for congenital hyperinsulinism. Diabetes Care. 2012;35:198–203. doi: 10.2337/dc11-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han B, Newbould M, Batra G, Cheesman E, Craigie RJ, Mohamed Z, Rigby L, Padidela R, Skae M, Mironov A, Starborg T, Kadler KE, Cosgrove KE, Banerjee I, Dunne MJ. Enhanced islet cell Nucleomegaly defines diffuse congenital Hyperinsulinism in infancy but not other forms of the disease. Am J Clin Pathol. 2016;145(6):757–768. doi: 10.1093/ajcp/aqw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandrescu S, Tatevian N, Olutoye O, Brown RE. Persistent hyperinsulinemic hypoglycemia of infancy: constitutive activation of the mTOR pathway with associated exocrine-islet transdifferentiation and therapeutic implications. Int J Clin Exp Pathol. 2010;3(7):691–705. [PMC free article] [PubMed] [Google Scholar]
- 8.Senniappan S, Brown RE, Hussain K. Genomic and morphoproteomic correlates implicate the IGF-1/mTOR/Akt pathway in the pathogenesis of diffuse congenital hyperinsulinism. Int J Clin Exp Pathol. 2016;9:548–562. [Google Scholar]
- 9.Stevens A, Cosgrove KE, Padidela R, Skae MS, Clayton PE, Banerjee I, Dunne MJ. Can network biology unravel the aetiology of congenital hyperinsulinism? Orphanet J Rare Dis. 2013;8:21. doi: 10.1186/1750-1172-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22(15):5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JJ, Biggin MD. Gene expression. Statistics requantitates the central dogma. Science. 2015;347(6226):1066–1067. doi: 10.1126/science.aaa8332. [DOI] [PubMed] [Google Scholar]
- 12.Shah P, Arya VB, Flanagan SE, Morgan K, Ellard S, Senniappan S, Hussain K. Sirolimus therapy in a patient with severe hyperinsulinaemic hypoglycaemia due to a compound heterozygous ABCC8 gene mutation. J Pediatr Endocrinol Metab. 2015;28(5–6):695–699. doi: 10.1515/jpem-2014-0371. [DOI] [PubMed] [Google Scholar]
- 13.Abraham MB, Shetty VB, Price G, Smith N, Md B, Siafarikas A, Resnick S, Whan E, Ellard S, Flanagan SE, Davis EA, Jones TW, Hussain K, Choong CS. Efficacy and safety of sirolimus in a neonate with persistent hypoglycaemia following neartotal pancreatectomy for hyperinsulinaemic hypoglycaemia. J Pediatr Endocrinol Metab. 2015;28(11–12):1391–1398. doi: 10.1515/jpem-2015-0094. [DOI] [PubMed] [Google Scholar]
- 14.Minute M, Patti G, Tornese G, Faleschini E, Zuiani C, Ventura A. Sirolimus therapy in congenital Hyperinsulinism: a successful experience beyond infancy. Pediatrics. 2015;136(5):e1373–e1376. doi: 10.1542/peds.2015-1132. [DOI] [PubMed] [Google Scholar]
- 15.Meder U, Bokodi G, Balogh L, Korner A, Szabo M, Pruhova S, Szabo AJ. Severe Hyperinsulinemic hypoglycemia in a neonate: response to Sirolimus therapy. Pediatrics. 2015;136(5):e1369–e1372. doi: 10.1542/peds.2014-4200. [DOI] [PubMed] [Google Scholar]
- 16.Guemes M, Shah P, Roženkova K, Gilbert C, Morgan K, Hussain K. Severe Hyperinsulinaemic Hypoglycaemia in Beckwith-Wiedemann syndrome due to paternal Uniparental Disomy of 11p15.5 managed with Sirolimus therapy. Horm Res Paediatr. 2016;85(5):353–357. doi: 10.1159/000443398. [DOI] [PubMed] [Google Scholar]
- 17.Unal S, Gonulal D, Ucakturk A, Siyah Bilgin B, Flanagan SE, Gurbuz F, Tayfun M, Elmaoğulları S, Araslı A, Demirel F, Ellard S, Hussain K. A novel homozygous mutation in the KCNJ11 gene of a neonate with congenital Hyperinsulinism and successful management with Sirolimus. J Clin Res Pediatr Endocrinol. 2016;8(4):478–481. doi: 10.4274/jcrpe.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szymanowski M, Estebanez MS, Padidela R, Han B, Mosinska K, Stevens A, Damaj L, Pihan-Le Bars F, Lascouts E, Reynaud R, Ferreira C, Bansept C, de Lonlay P, Saint-Martin C, Dunne MJ, Banerjee I, Arnoux JB. Mammalian target of rapamycin (mTOR) inhibitors for the treatment of severe congenital Hyperinsulinism: perspectives on limited therapeutic success. J Clin Endocrinol Metab. 2016;101(12):4719–4729. doi: 10.1210/jc.2016-2711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.


